1. Introduction
Skeletal muscles contribute to ~40% of total body mass, and, aside from being the motors that drive basic movements, also play a critical role in breathing, whole-body metabolism, and maintaining a high quality of life [
1,
2]. Indeed, the average individual will lose 30–40% of their muscle mass by the age of 80, and this loss in muscle mass (i.e., sarcopenia) is associated with disability, loss of independence, and an increased risk of morbidity and mortality, as well as an estimated USD 18.5 billion in annual healthcare costs in the United States alone [
3,
4,
5]. Accordingly, the development of therapies that can restore, maintain, and/or increase muscle mass will be of great clinical and fiscal significance. However, to develop such therapies, we will first need to establish a comprehensive understanding of the mechanisms that regulate the mass of this vital tissue.
Over the last century, it has become apparent that an increase in muscle mass can be induced by a variety of different stimuli, with one of the most widely recognized being progressive resistance exercise (PRE) [
6,
7]. For instance, numerous studies have shown that 8–16 weeks of PRE can lead to a 5–20% increase in skeletal muscle mass/volume, along with an even greater increase in strength [
8,
9,
10]. The PRE-induced increase in muscle mass is also associated with improvements in markers of mental, metabolic, and cardiovascular health, as well as a reduction in body fat and the risk of developing/dying from aging-related diseases [
11,
12,
13,
14]. In other words, the health-related benefits of PRE go well beyond an increase in muscularity, yet the mechanisms that drive these effects remain far from defined.
During the last 50 years, a variety of different animal models have been used to develop a better understanding of how PRE induces an increase in muscle mass. The majority of these models have involved the use of rodents, with earlier models largely relying on rats and more recent models relying on mice [
15,
16,
17,
18,
19]. Mouse models are now viewed as being particularly advantageous because they are amenable to the wide array of genetic inventions that are often paramount to mechanistic studies. With this point in mind, Murach et al. (2020) recently summarized the currently available mouse models and, as highlighted in their review, most models use chronic forms of mechanical overload (e.g., the synergist ablation model) and/or only focus on one or two muscles within a single limb [
20]. Indeed, we are not aware of any mouse models that have been shown to induce an increase in the mass of numerous muscles throughout the body. This is an important point because the most robust health-related benefits of PRE would be expected to come from interventions that impact all of the major muscle groups. Thus, one of the primary goals of this study was to develop a mouse model that mimics a human PRE training paradigm and induces an increase in the mass of numerous muscles throughout the body.
In order to develop a model that accurately mimics a human PRE training paradigm, we referred to the American College of Sports Medicine (ACSM) recommendations for progression models of resistance exercise [
6]. Specifically, we focused on the recommendations for training paradigms that are intended to induce muscular growth in novice human subjects (i.e., untrained individuals with no prior resistance exercise experience). For such individuals, the ACSM recommends that all major muscle groups be trained two to three times per week, with an emphasis placed on multi-joint exercises that involve 8–12 repetitions per set, 1–2 min of rest between sets, and a total of one to three maximal-intensity sets of each exercise per training session. Moreover, the resistance employed during each training session should be progressively increased as the individual’s strength improves. Over the last decade, these recommendations have been widely accepted, and, therefore, served as the foundation for the training paradigm that was implemented in our mouse model.
In addition to inducing an increase in muscle mass, we also wanted to develop a mouse model that could elicit some of the classic types of responses that are known to occur when humans engage in PRE. For instance, numerous studies have shown that a bout of PRE leads to an acute increase in the rate of protein synthesis and the activation of signaling through growth regulatory molecules, such as the mechanistic target of rapamycin complex 1 (mTORC1) [
21,
22]. It has also been shown that the PRE-induced increase in muscle mass can be attributed to an increase in the cross-sectional area of muscle fibers (i.e., hypertrophy), and/or an increase in the length of muscle fascicles/fibers [
23,
24]. Moreover, these changes are often accompanied by an increase in the number of myonuclei per fiber, as well as a fast-to-slow transition in the composition of the Type II fibers [
25,
26]. Accordingly, we used a variety of different assays to determine whether these types of responses were elicited by our model, which we refer to as “weight pulling”.
2. Materials and Methods
2.1. Animals and Ethical Approval
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Madison (#V005375) or the William S. Middleton Memorial Veterans Hospital (Assurance ID: D16-00403). Male C57BL/6J (Jackson Laboratories, Bar Harbor, MA, USA) mice at 9–10 weeks of age were randomly assigned to one of the experimental interventions described below, given food (5001 Rodent Laboratory Chow, Purinal Mills, Gray Summit, MO, USA) and water ad libitum (unless otherwise indicated), and kept in a room that was maintained at 25 °C with a 12–h light cycle (lights off at 6:00 p.m.–6:00 a.m.). During terminal collections, the mice were anesthetized with 1–5% isoflurane mixed in oxygen and euthanized by cervical dislocation under anesthesia.
2.2. Weight Pulling Model Components
The custom-built components of the weight pulling model are described in
Figure 1 and included a 120 cm long weight pulling track that was constructed out of impermeable plastic lumber (BestPlus, Somerville, MA, USA) and lined with grip support material (Con-Tact Brand #05F-C6B0B-06, Pomona, CA, USA). The custom-built components also included a weight pulling cart (
Figure 1B,C). The base of this cart was built from the parts that were contained within a toy car kit (eBay, sold by Sktflyer, item #370838782830, China), and these parts allowed for the construction of a 6.8 cm wide × 8.0 cm long wheelbase. An 11.4 cm long section of an 80-well microfuge tube rack was then cut and secured to the top of the wheelbase with strength molding tape (3M, #03609, Saint Paul, MN, USA). Superglue was also used to attach a 10 cm long piece of 1.5 mm thick nylon string to the front corners of the wheelbase. Combined, these components were defined as the “unweighted cart” and weighed a total of 70 g (
Figure 1B). To construct the “weighted cart”, strength molding tape was used to secure microfuge tubes to the bottom corners of an acrylic box (5 cm wide × 12.7 cm long × 10.2 cm tall, shopPOPdisplays, #CS001, Little Falls, NJ, USA) and the tubes were placed in a position that aligned with the wells of the microfuge tube rack. The weighted cart was then formed by placing these tubes into the wells of the unweighted cart and, as needed, the box was filled with various amounts of lead weight (Eagle Claw, Denver, CO, USA) (
Figure 1C).
2.3. Training Paradigm
2.3.1. Acclimation
All mice were provided with one acclimation session. During this session, the unweighted cart was attached to the mouse’s tail by interweaving a 10 cm long strip of vinyl tape (3M, #80610833842, Saint Paul, MN, USA) around the tail and the nylon string of the cart (
Figure 1D). With the unweighted cart attached to the tail, the mice were placed at the start line on the weight pulling track and then familiarized with pulling the cart to the end of the 50 cm long weight pulling lane (
Figure 1A). At the end of the lane, the mice encountered a resting house and, upon reaching the resting house, they were given 1 min to recover before being returned to the start line on the weight pulling track. Initially, the mice were motivated to pull the unweighted cart by touching their rear fur close to the lumbar vertebrae region. If the mice failed to make forward progress after being touched 3 times (with a 1 s interval between each touch), then an additional incentive was provided by delivering a 1 mA shock to the lumbar vertebrae region/tail (Precision Animal Shocker, Coulbourn Instruments, #H13–15, Lehigh Valley, PA, USA). The familiarization process was repeated a minimum of 8 times, and until the mouse voluntarily traversed the entire length of the weight pulling lane three consecutive times.
2.3.2. Weighted Pulling Paradigm
The weighted pulling (WP) training paradigm was initiated two days after the completion of the acclimation session. The first training session in this paradigm was aimed at determining the mouse’s maximal pulling load and consisted of ~10–12 sets with 2 min of rest between each set. During this procedure, the cart was attached to the tail as described above, and the mice were motivated to pull the cart by touching their rear fur. If the mice did not make forward progress after being touched 3 times (with a 3 s interval between each touch), an additional 1 mA shock was delivered. The first set during this session consisted of pulling the unweighted cart and then the next three sets consisted of pulling the weighted cart with a total load of 300, 600, and then 750 g. After completing these sets, an additional 50 g was added to the weighted cart and the mouse completed another set. The process of adding 50 g to the weighted cart was repeated until a load was reached during which the mouse failed to traverse the entire length of the weight pulling lane. Failure during this session was defined as the inability of the mouse to make forward progress after 3 touches and 1 shock. Upon reaching failure, assistance (i.e., gentle pushing of the cart in a manner that would be analogous to a spotter helping a human complete a final repetition during resistance exercise) was provided so that the mouse could traverse the remaining length of weight pulling lane without the need for a further touch/shock incentive. At this point, the training session was complete, and the highest load successfully pulled along the entire length of the lane was defined as the mouse’s maximal pulling load.
Three days after the first training session, the mice initiated the remainder of the WP training paradigm. The training sessions during this period were performed three times per week for a total of 36 sessions (i.e., on Monday, Wednesday, Friday, or Tuesday, Thursday, Saturday). External motivation during these sessions consisted of touching the rear fur as described above, and if the mice did not make forward progress after 5 touches (with a 3 s interval between each touch), an additional 1 mA shock was delivered. A total of 2 min of rest was given between each set, and each training session began with a warm-up set that only involved pulling the unweighted cart. The warm-up set was then followed by sets with loads representing 50, 75, 85, 90, 95, and 100% of the mouse’s previous maximal pulling load, respectively. During all subsequent sets, an additional 15 g was added to the weighted cart, and this cycle was repeated until a load was reached during which the mouse failed to traverse the entire length of the weight pulling lane. Failure during this portion of the training paradigm was defined as the inability of the mouse to make forward progress after 5 consecutive touches followed by 1 shock and then 2 additional touches. Upon reaching failure, assistance (as described above) was provided so that the mouse could traverse the remaining length of the weight pulling lane without the need for further touch/shock incentive.
2.3.3. Control (Unweighted) Paradigm
To appropriately control for the stresses that are incurred during the WP paradigm (e.g., taping of the cart to the tail, touches, shocks, etc.), the control mice performed a total of 37 control training sessions. Each control training session was performed at the same time as a WP training session (6:00–9:00 p.m.) and each control mouse was always paired with a WP mouse. The control session began by attaching an unweighted cart to the tail (as described above), and then the mouse performed the same number of sets as the paired WP mouse, but additional weight was never added to the cart. Moreover, before each set, the control mouse was placed on a smooth surface and subjected to the same number of touches/shocks that were experienced by the paired WP mouse during each of its respective sets.
2.4. Rotarod, Body Composition, and Isometric Grip Strength
Prior to the animal’s final training session, rotarod performance (a marker of motor coordination) was assessed with Rotamex 5 System (Columbus Instruments, Columbus, OH, USA). To assess performance, an acclimation session was performed 1 day before the actual testing session and consisted of three familiarization trials that were separated by 15 min of rest. Each familiarization trial lasted 1 min and used preprogrammed settings of 0.3 RPM (increment speed), 10 (increment seconds), 5 RPM (start speed), and 40 RPM (end speed). At 24 h after the acclimation session, rotarod performance was measured during three trials that were separated by 15 min of rest. During each trial, the system was set at 1 RPM (increment speed), 8 (increment seconds), 5 RPM (start speed), and 40 RPM (end speed). The average time to fall (i.e., latency) during all three trials was determined and used as the marker of rotarod performance.
At 48 h after the final training session, body composition was determined in awake mice using an EchoMRI body composition analyzer. Once completed, isometric grip strength was assessed with a dual-range force sensor (Vernier, Beaverton, OR, USA) that was connected to a custom-built horizontal platform (6 cm wide × 12 cm long) that had been covered with the same grip support material that was used to line the base of the weight pulling lane (Con-Tact Brand #05F-C6B0B-06, Pomona, CA, USA). During the grip strength measurements, the mice were first allowed to grasp the matting with all four paws. Then, while keeping the torso parallel with the platform, the mouse’s tail was slowly pulled away from the force sensor until the mouse could no longer maintain its grip. The highest force generated was recorded and the procedure was repeated 3 times with 5 min of rest between each recording. The highest force measured during all three recordings was recorded as the animal’s isometric grip strength. Importantly, all of the measurements described in this section were performed by blinded investigators.
2.5. Terminal Collections after 13-Weeks of Training
Terminal collections were performed 60–96 h after the final training session. During this procedure, all potentially identifiable information (e.g., tail markings, etc.) was masked, and then the animals were weighed and subsequently anesthetized with isoflurane. The extensor digitorum longus (EDL), flexor digitorum longus (FDL), and soleus (SOL) muscles from both the left and right hindlimb were then weighed, submerged in optimal cutting temperature compound (OCT, Tissue-Tek; Sakura Finetek, The Netherlands) at resting length, and frozen in liquid nitrogen-chilled isopentane. At this point, the mice were euthanized by cervical dislocation, a photograph that included both a scale bar and the musculature of the left forelimb was obtained, and then additional skeletal muscles from both the left and right side of the body were collected, including the: tibialis anterior (TA); gastrocnemius (GAST); plantaris (PLT); tibialis posterior (TP); quadriceps (QUAD); semitendinosus (ST); teres major (TM); pectoralis major (PEC); lateral head of the triceps brachii (Tri-Lat); long head of the triceps brachii (Tri-Long); short head of the biceps brachii (Bi-Short); and the forearm flexor complex (FF), which consisted of the flexor carpi ulnaris, flexor carpi radialis, flexor digitorum superficialis, and all three heads of the flexor digitorum profundus. In addition to skeletal muscles, tissues including the heart, as well as the left and right tibias, epididymal fat pads, and adrenal glands were also collected post-mortem. All of the collection procedures were performed by blinded investigators.
2.6. Immunohistochemistry
Mid-belly cross-sections (10 μm thick) from muscles frozen in OCT were taken with a cryostat and fixed for 10 min at room temperature with 1% paraformaldehyde dissolved in PBS (for myonuclei staining) or for 10 min with −20 °C acetone (for fiber type staining). Fixed sections were washed with PBS for 15 min and then blocked for 20 min at room temp in buffer A (0.5% Triton X-100, 0.5% BSA dissolved in PBS).
Myonuclei staining was performed as previously described [
27]. Briefly, blocked samples were incubated for 1 h at room temp in buffer A containing mouse anti-dystrophin IgG1 (1:20, #NCL-DYS2, Novocastra/Leica, Buffalo Grove, IL, USA). The sections were then washed 3 times for 15 min with PBS and incubated in buffer A containing Alexa 488 goat anti-mouse IgG1 (1:500, #115-545-205, Jackson Immunoresearch, West Grove, PA, USA) for 1 h at room temp. Sections were then washed with PBS 3 times for 15 min and incubated with PBS containing Hoechst (1:5000, #561908, BD Pharmingen, Lincoln Park, NJ, USA) for 5 min before a final series of washes with PBS.
For fiber type staining, blocked samples were subjected to a 1 h incubation at room temp in buffer A containing rabbit anti-laminin (1:500, #L9393, MilliporeSigma, Burlington, MA, USA), mouse IgG2b anti-Type I MHC (1:100, # BA-D5-s, Developmental Studies Hybridoma Bank, Iowa City, IA, USA), mouse IgG1 anti-Type IIA MHC (1:100, #SC-71-s, Developmental Studies Hybridoma Bank, Iowa City, IA, USA), and mouse IgM anti-Type IIB MHC (1:10, #BF-F3-s, Developmental Studies Hybridoma Bank, Iowa City, IA, USA). The sections were then washed 3 times for 15 min with PBS and incubated for 1 h at room temp in buffer A containing Alexa 568 goat anti-rabbit IgG (1:5000, #A11011, Invitrogen, Waltham, MA, USA), Alexa 647 goat anti-mouse IgG2b (1:100, #115-605-207, Jackson Immunoresearch, West Grove, PA, USA), Alexa 488 goat anti-mouse IgG1 (1:3000, #115-545-205, Jackson Immunoresearch, West Grove, PA, USA), and Alexa 350 goat anti-mouse IgM (1:500, #A-31552, Invitrogen, Waltham, MA, USA). The sections were then subjected to a final series of washes with PBS. All washed sections were mounted in a layer of ProLong Gold anti-fade mounting medium (Invitrogen, Waltham, MA, USA), overlaid with a coverslip (Thermo Fisher Scientific, Waltham, MA, USA), and then whole muscle cross-sections were imaged by a blinded investigator with a 10X objective on a BZ-X700 Keyence microscope and 4 different filters (DAPI, GFP, TRITC, CY5) (Keyence, Itasca, IL, USA).
2.7. Image Analyses
For fiber cross-sectional area measurements, images of the whole muscle cross-section were analyzed and the cross-sectional areas (CSA) of all qualified fibers of each fiber type (Type I, IIA, IIB, and non-Type I, -Type IIA, or -Type IIB, which were defined as Type IIX) were counted and measured using our fiber cross-sectional area CellProfiler pipeline, which was based on modifications of the Muscle2View pipeline [
28] (see
Supplemental Methods for more details). For myonuclei and interstitial nuclei identification, our myonuclei CellProfiler pipeline that was based on modifications of the Muscle2View pipeline was employed [
28]. In this case, the whole muscle cross-section was analyzed and the average fiber CSA, total fiber number, total number of myonuclei, as well as total nuclei were determined (see
Supplemental Methods for more details). The distinction between myonuclei and interstitial nuclei was achieved by defining nuclei that resided within the dystrophin layer of individual myofibers as myonuclei, whereas those that resided outside of the dystrophin layer were designated as interstitial nuclei.
2.8. Acute Changes in Protein Phosphorylation and Protein Synthesis
The rate of protein synthesis and the phosphorylation state of various proteins were assessed in the FDL muscles from a subset of the mice. Specifically, all of the mice in this subset performed the acclimation procedure and 3 sessions of training with WP. Prior to the fourth and final training session, the mice were fasted for 2 h, and then one cohort was subjected to WP while the other cohort was subjected to the control training paradigm (i.e., they experienced the same number of touch/shock cycles as the mice that were subjected to WP, but they only pulled an unweighted cart). At the end of this training session, the mice were returned to a cage that contained water but no food. At 30 min after completing the training session, the mice were given an intraperitoneal (IP) injection of 0.04 μmol/g bodyweight of puromycin (MilliporeSigma, Burlington, MA, USA) to measure the rate of protein synthesis as previously described [
29]. At exactly 30 min after the injection of puromycin (i.e., 1 h post-training), the FDL muscle from the left leg of each mouse was collected, immediately frozen in liquid nitrogen, and stored at −80 °C. Of note, this experimental approach was used because it helped to minimize the potentially confounding effects of stress that occur during the initial WP sessions (e.g., stress that is due to a lack of experience with the handling procedures that occur during WP, as well as the damage/stress that occurs when naive muscles are subjected to a bout of intense exercise). Most importantly, the only difference between the WP and control group was whether the mice pulled a weighted vs. unweighted cart during the final training session (i.e., whether they performed the WP or control training paradigm). Thus, our experimental design allowed for a clear interpretation of the acute responses that are elicited by WP, rather than the responses that could have accumulated throughout several control vs. WP training sessions.
2.9. Western Blot Analysis
Frozen muscles were homogenized in ice-cold buffer B [40 mM Tris (pH 7.5), 1 mM EDTA, 5 mM EGTA, 0.5% Triton X-100, 25 mM β-glycerolphosphate, 25 mM NaF, 1 mM Na3VO4, 10 μg/mL leupeptin, and 1 mM PMSF] with a Polytron (PT 1200 E, Kinematica, Luceme, Switzerland), and then the homogenates were centrifuged at 6000 g for 1 min to remove bubbles and confirm complete homogenization. The entire sample was then thoroughly vortexed to resuspend the insoluble material, and an aliquot of the whole homogenate was subjected to further analysis. Specifically, the concentration of protein in the whole homogenates was determined with the DC protein assay kit (Bio-Rad, Hercules, CA, USA). Equal amounts of protein from each sample were dissolved in Laemmli buffer, subjected to SDS-PAGE, and transferred to a PVDF membrane as previously described [
30]. At this point, the total protein levels on the membranes that were destined for the analysis of puromycin-labeled peptides (i.e., protein synthesis) were detected with “No-Stain” protein labeling reagent, as detailed in the manufacturer’s protocol (Invitrogen, Waltman, MA, USA). All membranes were then blocked with 5% milk-TBST and incubated with primary and secondary antibodies as previously described [
30]. All blots were imaged with a UVP Autochemi system (UVP, Upland, CA, USA) along with the use of either regular enhanced chemiluminescence (ECL) reagent (Pierce, Rockford, IL) or ECL-prime (Amersham, Piscataway, NJ, USA). After imaging, some blots were incubated in stripping buffer [100 mM B-Mercaptoethanol, 2% SDS, and 62.5 mM Tris (pH 6.8)] for 15 min at 50 °C. These membranes were then blocked with 5% milk-TBST, incubated with new primary and secondary antibodies, and imaged as described above. In all cases, once appropriate images had been captured, Coomassie Blue staining was performed on the PVDF membrane and used to verify equal protein loading/transfer across the membranes. Images were quantified with ImageJ (
https://imagej.nih.gov/nih-image/, accessed on 16 August 2021).
2.10. Western Blot Antibodies
Antibodies targeting 4E-BP1 (1:1000, #9644), eEF2 (1:3000, #2332), phosphorylated eEF2(56) (1:1000, #2331), eIF4B (1:1000, #3592), phosphorylated eIF4B (422) (1:1000, #3591), MKK4 (1:1000, #3346), phosphorylated MKK4 (257) (1:1000, #4514), p38 (1:1000, #9212), phosphorylated p38 (180/182) (1:1000, #9211), p70S6K (1:1000, #9234), phosphorylated p70S6K (389) (1:1000, #2708), S6 (1:1000, #2217), and phosphorylated S6 (240/244) (1:1000, #5364) were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-puromycin (1:5000, 12D10) was purchased from MilliporeSigma (Burlington, MA, USA). Peroxidase-labeled anti-mouse IgG2a (1:20,000, 115-035-206) was obtained from Jackson ImmunoResearch Laboratories, Inc (West Grove, PA, USA). Peroxidase-labeled anti-rabbit (1:5000, PI-1000) was obtained from Vector Labs Inc. (Burlingame, CA, USA).
2.11. Statistical Analyses
Statistical significance was determined by using the unpaired
t-tests, one-way ANOVA, repeated measures one-way ANOVA, two-way ANOVA, or repeated measures two-way ANOVA. For all ANOVA-based analyses, multiple post hoc comparisons were FDR corrected with the two-stage step-up method of Benjamini, Krieger, and Yekutieli. Differences between groups were considered significant when
p ≤ 0.05, or
q ≤ 0.05 (i.e., when an FDR correction was employed). Prior to performing statistical analyses, the datasets were searched for outliers and individual data points that resided more than 3 standard deviations away from the mean were removed. All statistical analyses were then performed by GraphPad Prism 9, and the type of statistical analysis that was performed for each experiment is described in the figure legends. A detailed summary of the data and all of the associated statistical outcomes can be found in the
Supplemental File Entitled “Statistical Summary”.
4. Discussion
As stated in the introduction, one of the primary goals of this study was to develop a mouse model that mimics a human PRE training paradigm and induces an increase in the mass of numerous muscles throughout the body. As detailed in the results, the basic characteristics of the WP training paradigm were well aligned with the ACSM’s recommendation for models of PRE that are aimed at inducing muscular growth in humans (i.e., major muscle groups should be trained two to three times per week with an emphasis placed on multi-joint exercises that involve 8–12 repetitions per set, 1–2 min of rest between sets, and a total of one to three maximal-intensity sets of each exercise per training session). Moreover, the training paradigm fulfilled the ACSM’s recommendation of progressively increasing resistance as the individual’s strength improved [
6]. Hence, our goal of developing a mouse model that mimics a human PRE training paradigm was accomplished.
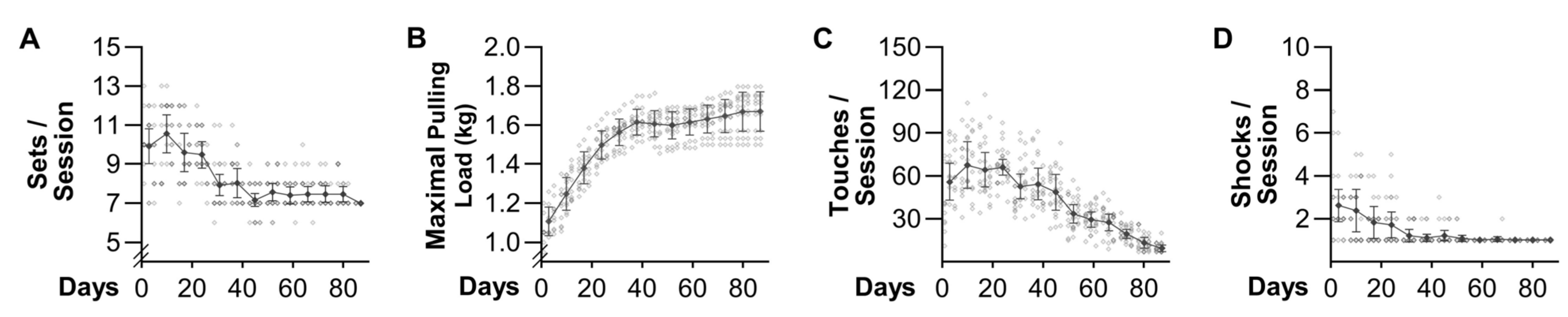
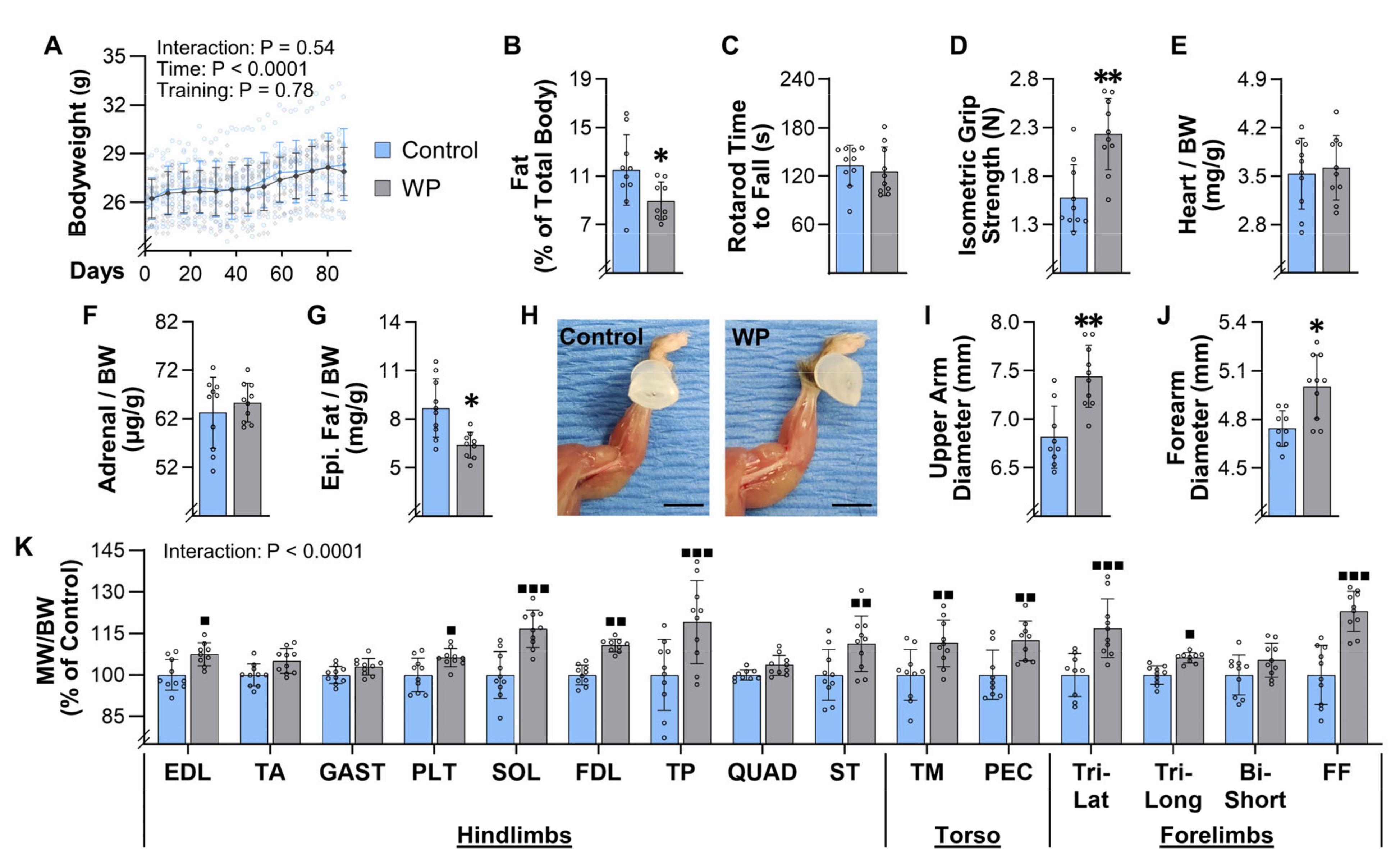
The results presented in
Figure 3 indicate that we also accomplished our goal of developing a mouse model that can induce an increase in the mass of numerous muscles throughout the body. Indeed, we observed a 6–23% increase in the mass of 10 of the 15 different muscles that were examined, which is very similar to the 5–20% increase in mass/volume that has been reported in humans after 8–16 weeks of PRE [
8,
9,
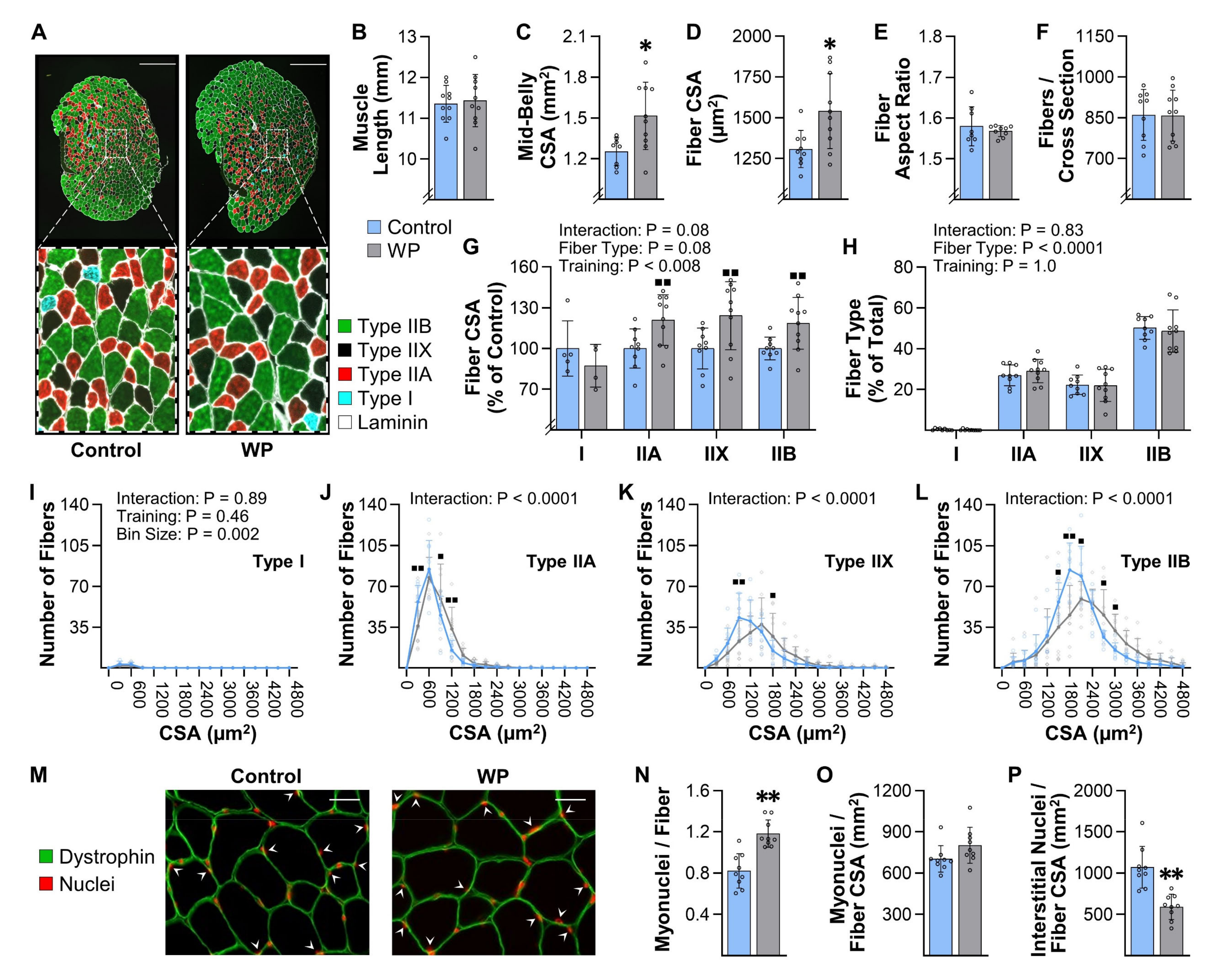
10]. We also determined that our model can be used to gain insight into the long-term adaptations that lead to the increases in mass. For instance, we demonstrated that WP can be used to study the mechanisms via which PRE induces hypertrophy, myonuclear accretion, and fiber type switching (
Figure 4 and
Figure 6). Moreover, the results in
Figure 5 suggest that WP could also potentially be used to study how PRE induces the longitudinal growth of fibers.
WP not only induced an increase in the mass of numerous skeletal muscles, but it also led to whole-body level changes that are commonly observed with PRE. For instance, in humans, it has been shown that PRE can lead to a decrease in body fat percentage [
11,
46,
47]. Consistent with these reports, we found that WP led to a 26% reduction in the mass of the epidydimal fats pads and a 22% reduction in the percentage of the total body mass that was composed of fat (
Figure 3B,G). This is noteworthy because the induction of whole-body level changes suggests that WP could serve as an effective model for defining the mechanisms via which PRE induces system-wide improvements in metabolic and cardiovascular health, and how PRE can act as an effective countermeasure against aging-related diseases, such as sarcopenia and cancer [
11,
12,
13,
14].
Another major strength of the weight pulling model is that, aside from being capable of inducing the classic long-term adaptations that occur in humans (e.g., hypertrophy, myonuclear accretion), it also recapitulates the acute responses that are thought to drive these adaptations. For instance, current dogma asserts that PRE induces the activation of mTORC1-dependent signaling events, and that these events, in turn, promote an increase in the rate of protein synthesis and the concomitant increase in muscle mass [
48,
49,
50]. However, support for this paradigm has largely come from studies that used the drug rapamycin to inhibit mTORC1 signaling and/or a model of chronic mechanical overload (e.g., synergist ablation) to induce an increase in muscle mass [
22,
30,
51,
52,
53]. With the advent of the weight pulling model, it will now be possible to test this paradigm within the confines of a physiologically relevant stimulus. Moreover, the weight pulling model will allow for the use of established genetic inventions that are often paramount to mechanistic studies. As a case in point, we previously developed mice with a skeletal muscle-specific and inducible knockout of Raptor/mTORC1 [
30], and these mice could be used to determine whether signaling through mTORC1 in the skeletal muscle is necessary for the WP-induced increase in protein synthesis, muscle mass, and strength, as well as system-wide changes, such as the reduction in body fat, etc.
Like all models, the weight pulling model has unique strengths, but it also has limitations. In our opinion, the greatest limitation is that, unlike other recently described mouse models of PRE, the weight pulling model requires a significant amount of hands-on time from investigators (~20 h per week are required for an investigator to train 10 control and 10 WP mice) [
16,
34]. However, it is important to consider that approximately the same amount of time would be required for an investigator to train an equal number of human subjects (personal communication from Dr. Stuart Phillips, McMaster University). Moreover, we suspect that the bulk of the long-term adaptations reported in this study would have been detected after just 6 weeks of training, rather than the 13 weeks that were employed. The basis for this argument stems from the data in
Figure 2B, which reveal that the gains in strength substantially plateaued after the 6th week of training (i.e., 90% of the total increase in maximal pulling load occurred during the first 6 weeks of training). According to Sale 1998, muscle and strength adaptations plateau at the same time after the onset of PRE [
31]. If this point holds true in the weight pulling model, then transitioning from 6–13 weeks of training would have only led to a ~10% greater increase in variables such as muscle mass (e.g., a 10% increase in mass after 6 weeks vs. an 11% increase after 13 weeks). Thus, we strongly suspect that the long-term adaptations observed in this study could have been achieved with as little as 120 h of investigator time. However, even if the full 13 weeks of training is necessary, the translatable nature of the outcomes could still be well worth the time that is required in order to obtain them.
5. Conclusions
The results of this study describe a cost-effective mouse model of PRE that is based on a full-body/multi-joint exercise along with a training paradigm that mimics human PRE (three training sessions per week, 8–12 repetitions per set, 2 min of rest between sets, approximately two maximal-intensity sets per session, last set taken to failure, and progressive increase in loading that is based on the individual’s performance). We demonstrate that WP is capable of inducing an increase in the mass of numerous muscles throughout the body, and that the increase in mass is associated with the same type of long-term adaptations that are known to occur in humans (fiber hypertrophy, myonuclear accretion, and, in some instances, a fast-to-slow transition in the composition of the Type II fibers). Moreover, we demonstrate that WP can induce the same type of acute responses that are thought to drive these long-term adaptations (e.g., activation of signaling through mTORC1 and the induction of protein synthesis). When taken together, the results of our study suggest that WP is a highly translatable mouse model of human PRE. Hence, we propose that our model will not only help investigators to gain better insight into the mechanisms via which PRE induces an increase in muscle mass, but will also open the door for new types of studies that are aimed at determining how PRE leads to improvements in overall health and disease prevention.