Dynamic Expression of Membrane Type 1-Matrix Metalloproteinase (Mt1-mmp/Mmp14) in the Mouse Embryo
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. β-Galactosidase Staining
2.3. Protein Extraction and Western Blot Analysis
2.4. Immunohistochemistry
2.5. Statistical Analysis
3. Results and Discussion
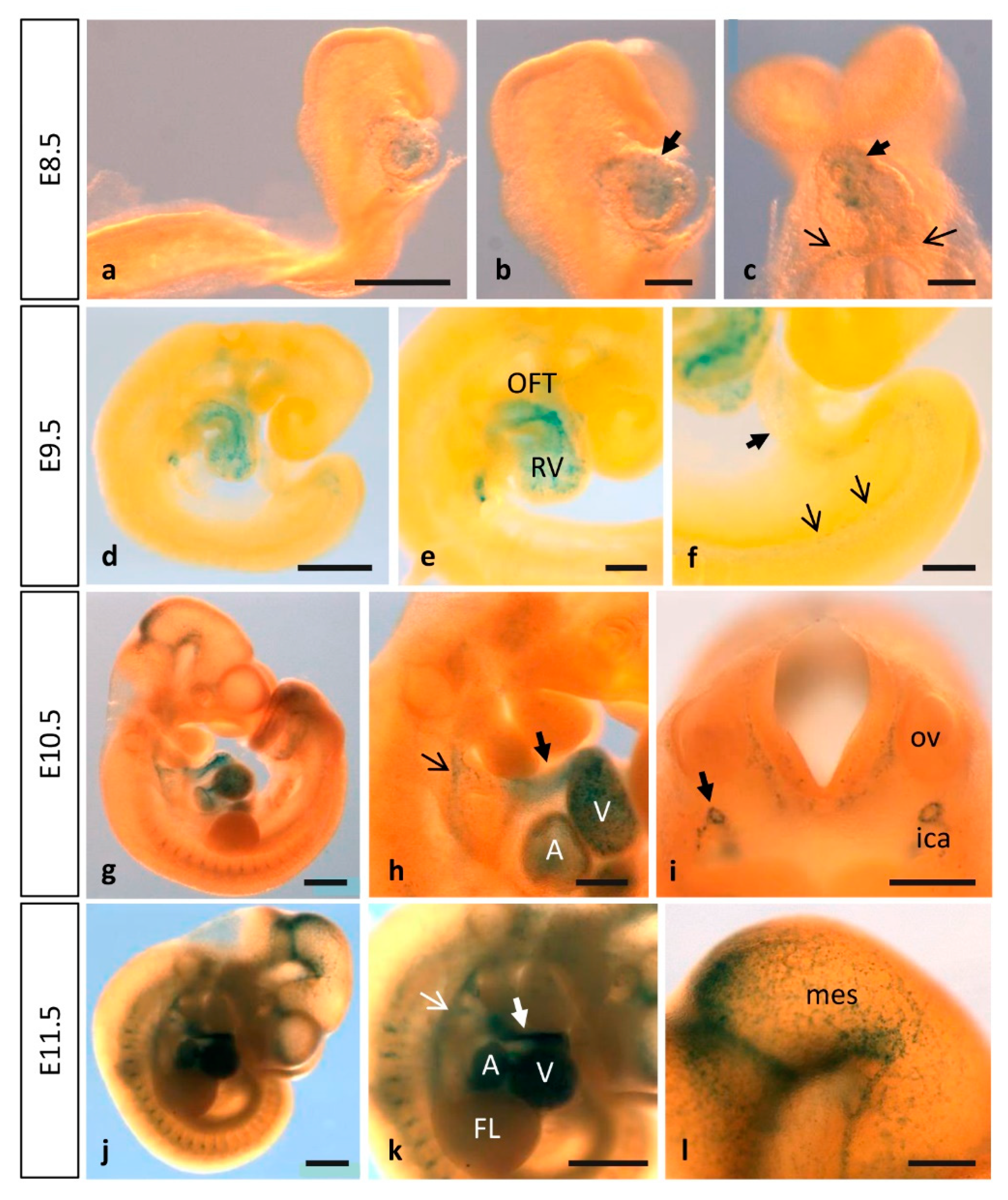
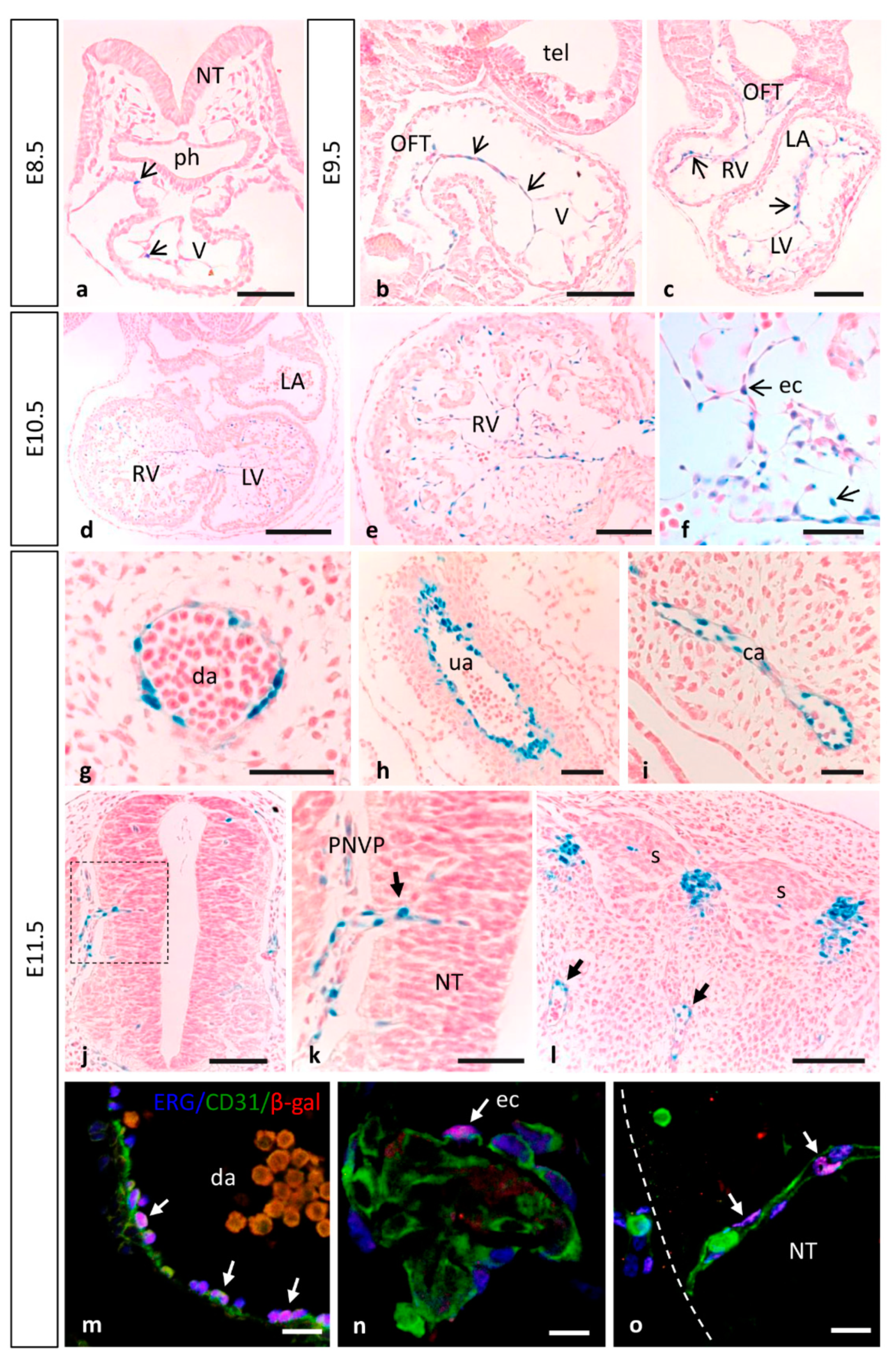
3.1. Dynamic Expression of MT1-MMP during Cardiovascular Development
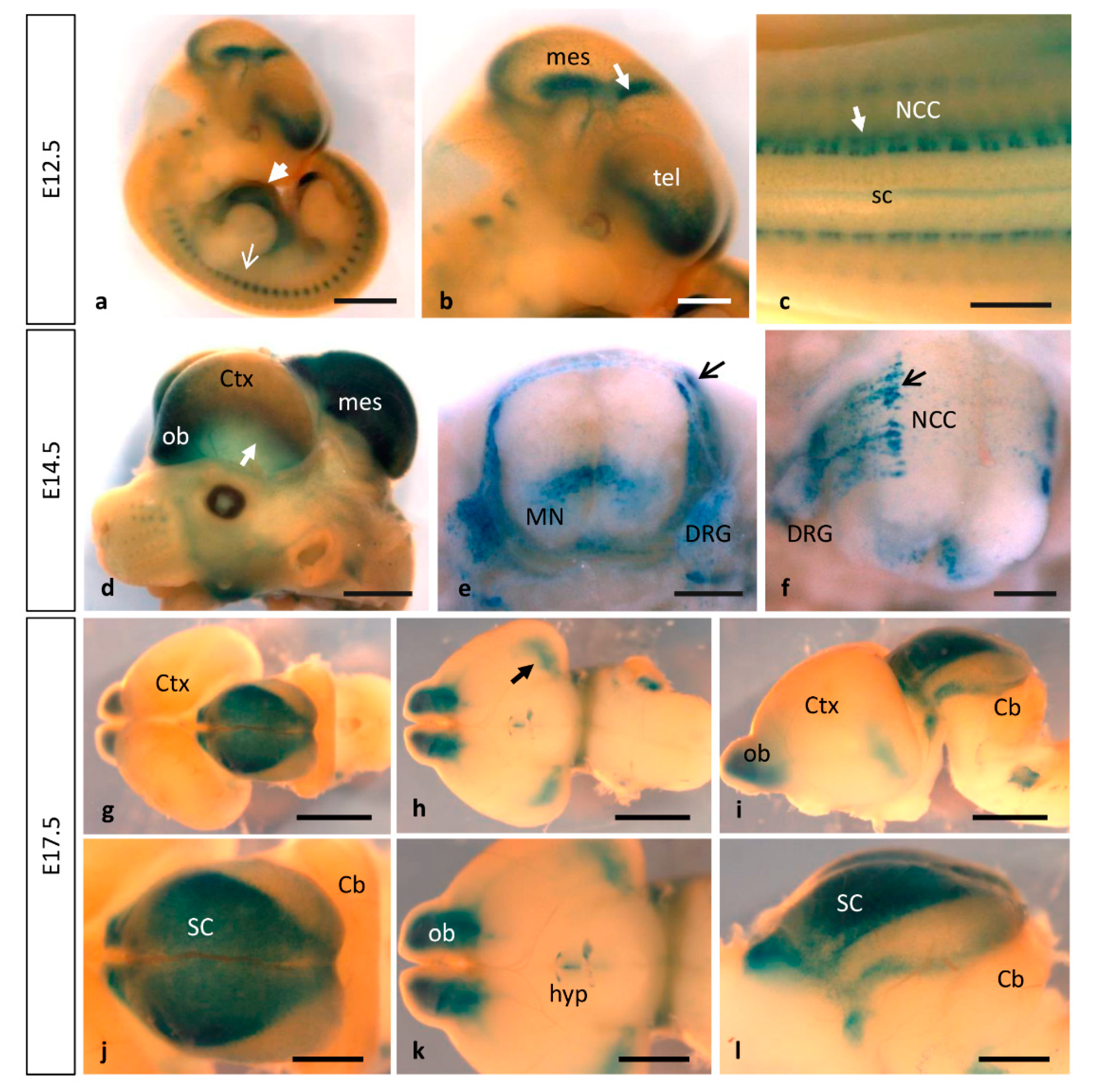
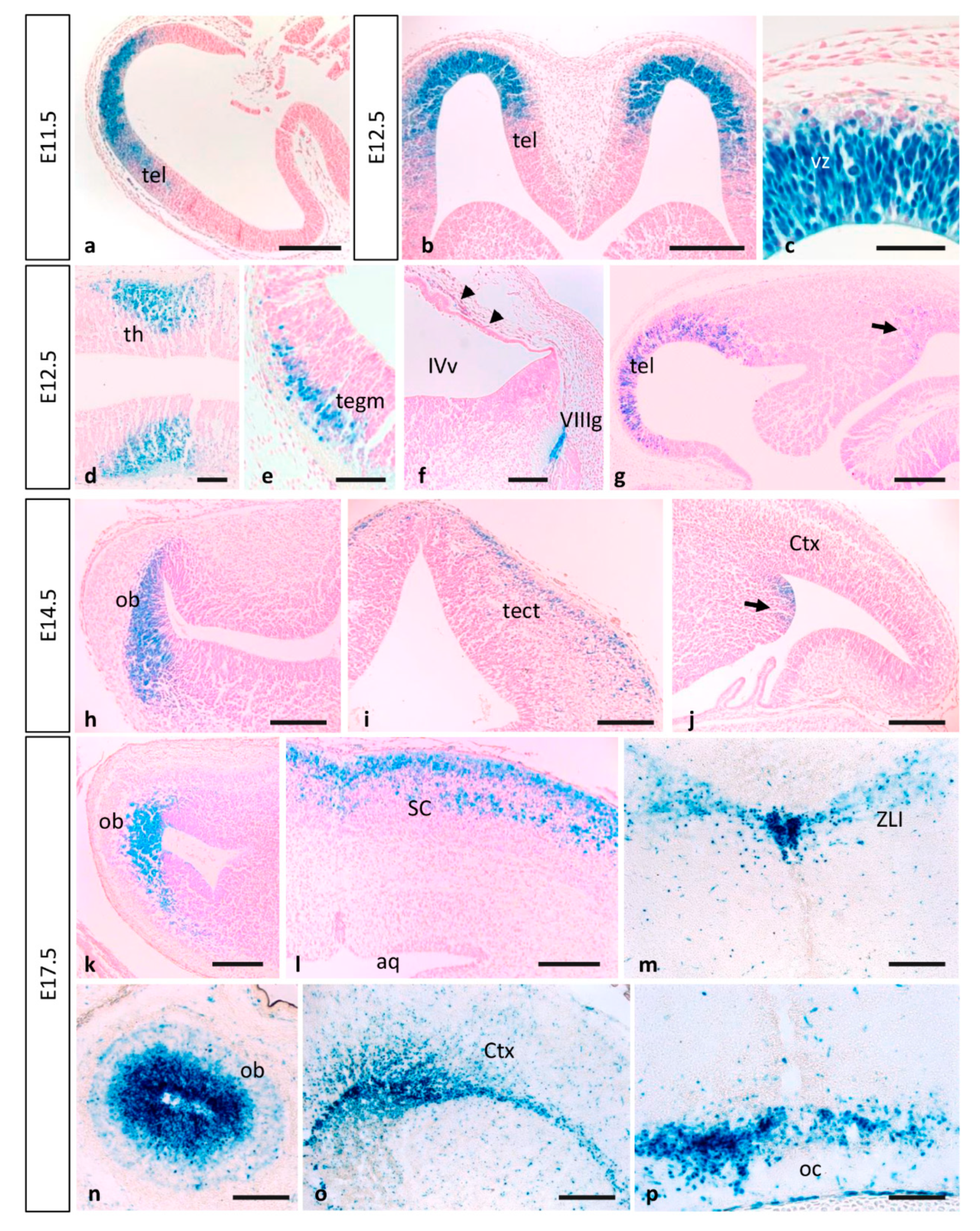
3.2. MT1-MMP Is Highly Expressed during Nervous System Development
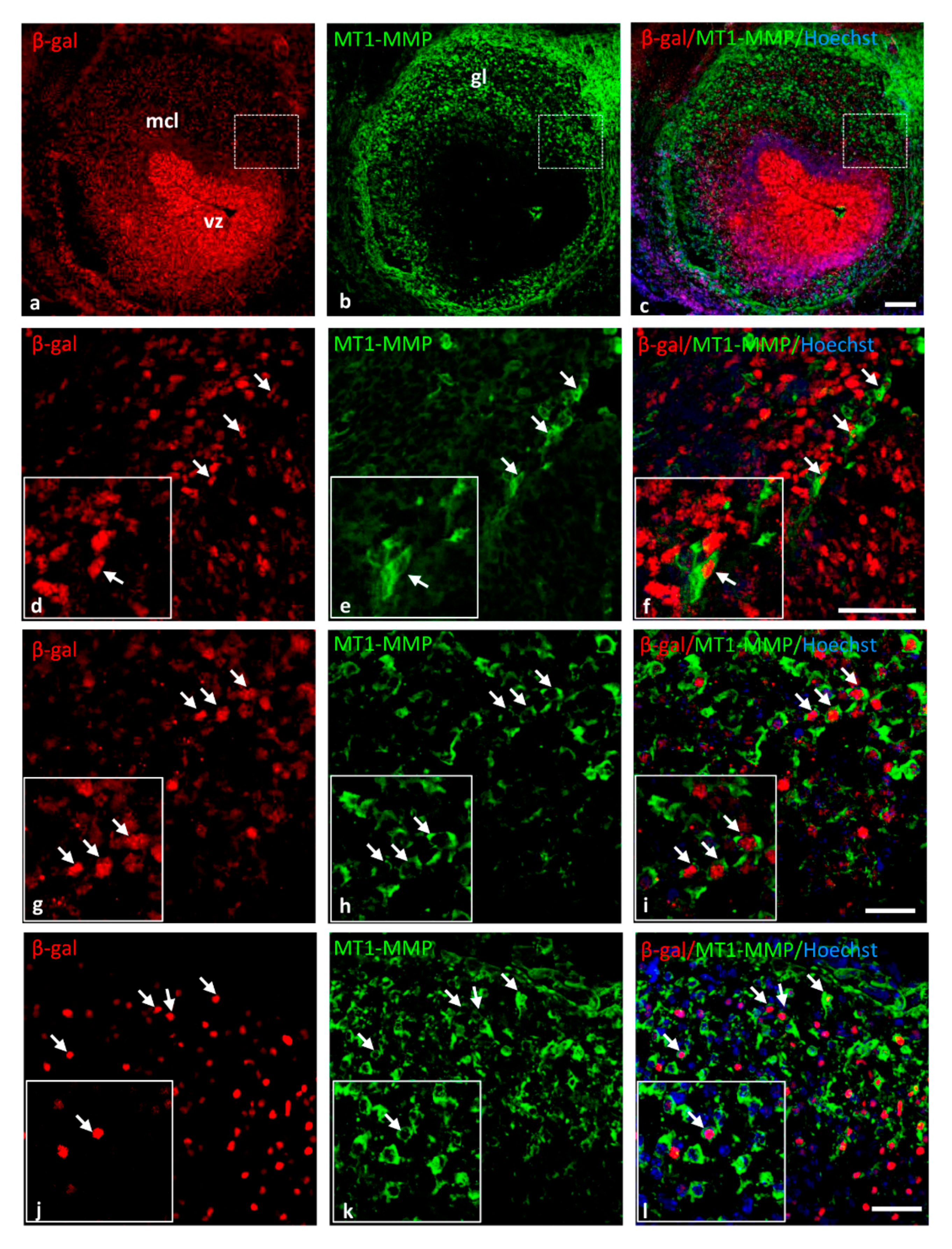

3.2.1. Olfactory System
3.2.2. Eye Development
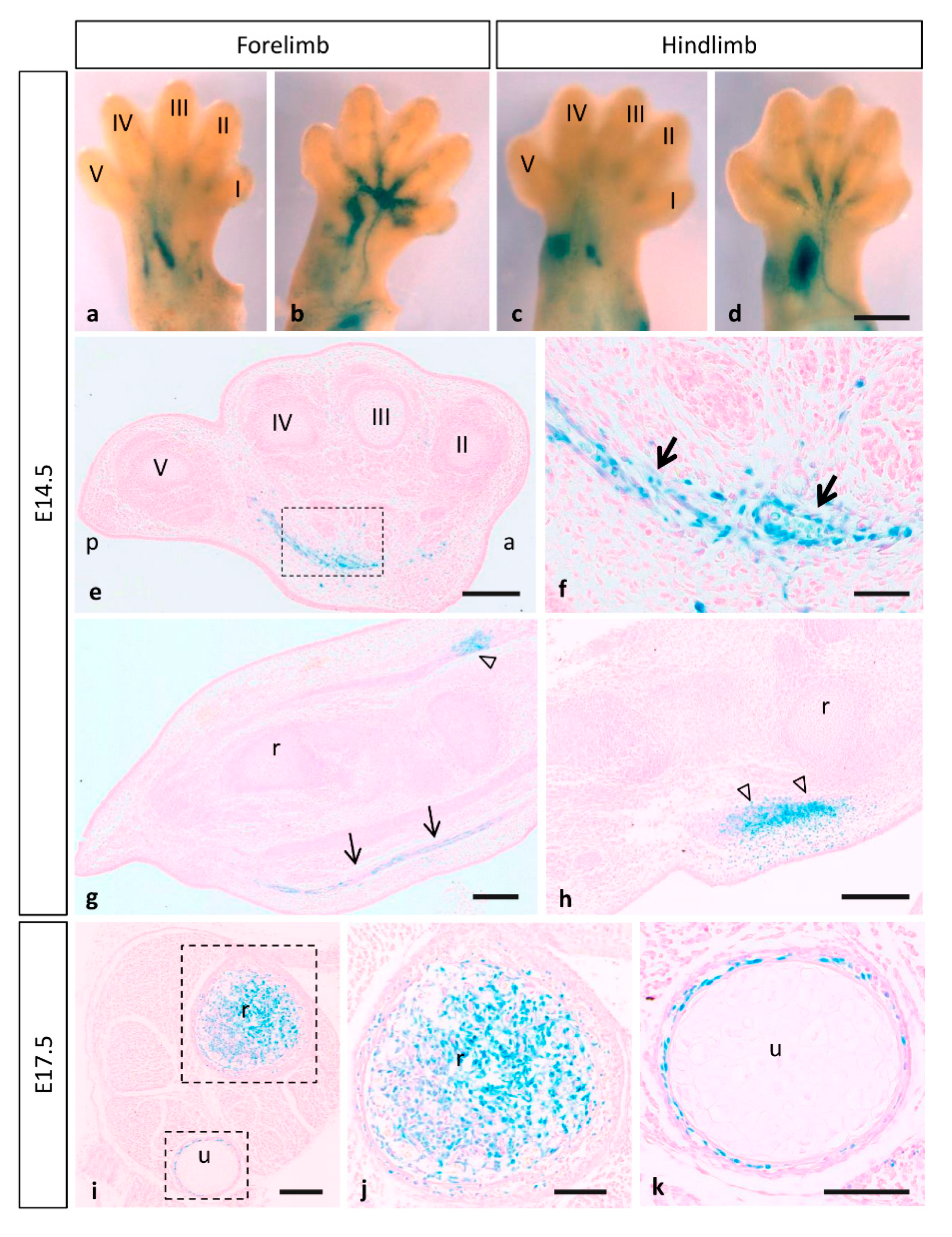
3.3. Expression of MT1-MMP during Limb Development
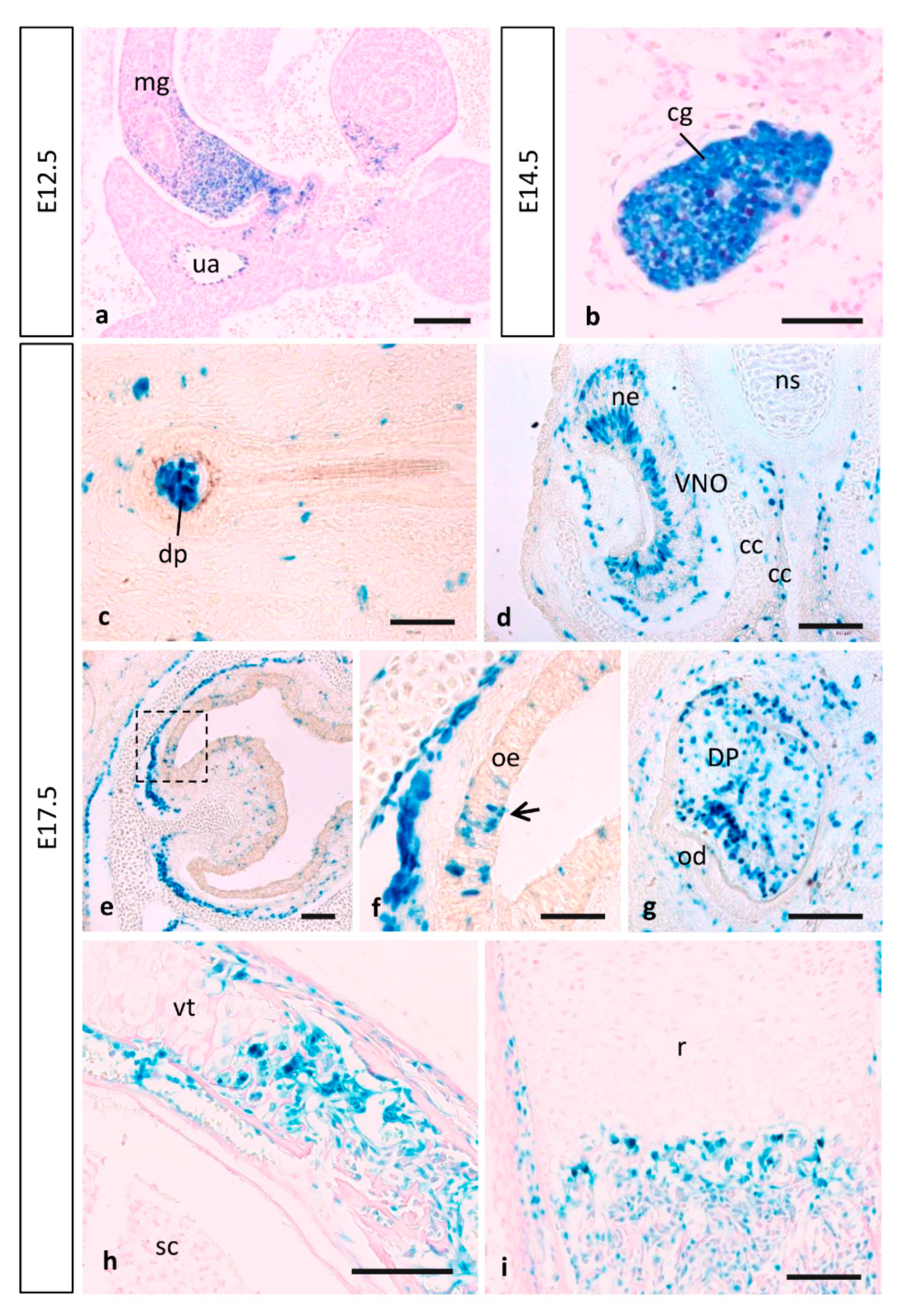
3.4. MT1-MMP Expression in Other Embryonic Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seiki, M. Membrane-type 1 matrix metalloproteinase: A key enzyme for tumor invasion. Cancer Lett. 2003, 194, 1–11. [Google Scholar] [CrossRef]
- Itoh, Y.; Seiki, M. MT1-MMP: A potent modifier of pericellular microenvironment. J. Cell. Physiol. 2006, 206, 1–8. [Google Scholar] [CrossRef]
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015, 44, 207–223. [Google Scholar] [CrossRef]
- Seiki, M. Membrane-type matrix metalloproteinases. APMIS 1999, 107, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Moracho, N.; Learte, A.I.R.; Muñoz-Sáez, E.; Marchena, M.A.; Cid, M.A.; Arroyo, A.G.; Sánchez-Camacho, C. Emerging roles of MT-MMPs in embryonic development. Dev. Dyn. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, Y.; Kane, K.T.; Collins, M.A.; Simeone, D.M.; Di Magliano, M.P.; Nguyen, K.T. CD44 Regulates Pancreatic Cancer Invasion through MT1-MMP. Mol. Cancer Res. 2015, 13, 9–15. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Takahashi, R.; Kondo, S.; Mizoguchi, A.; Adachi, E.; Sasahara, R.M.; Nishimura, S.; Imamura, Y.; Kitayama, H.; Alexander, D.B.; et al. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 2001, 107, 789–800. [Google Scholar] [CrossRef]
- Pahwa, S.; Stawikowski, M.; Fields, G. Monitoring and Inhibiting MT1-MMP during Cancer Initiation and Progression. Cancers 2014, 6, 416–435. [Google Scholar] [CrossRef]
- Shi, J.; Son, M.-Y.; Yamada, S.; Szabova, L.; Kahan, S.; Chrysovergis, K.; Wolf, L.; Surmak, A.; Holmbeck, K. Membrane-type MMPs enable extracellular matrix permissiveness and mesenchymal cell proliferation during embryogenesis. Dev. Biol. 2008, 313, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; McDill, B.W.; Li, S.-Z.; Deng, C.; Chang, C.-P.; Chen, F. Smad signaling in the neural crest regulates cardiac outflow tract remodeling through cell autonomous and non-cell autonomous effects. Dev. Biol. 2007, 311, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.B.; Cantrell, V.A.; Mundell, N.A.; Bennett, A.C.; Quick, R.E.; Jessen, J.R. VANGL2 regulates membrane trafficking of MMP14 to control cell polarity and migration. J. Cell Sci. 2012, 125, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Apte, S.; Soininen, R.; Cao, R.; Baaklini, G.Y.; Rauser, R.W.; Wang, J.; Cao, Y.; Tryggvason, K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. USA 2000, 97, 4052–4057. [Google Scholar] [CrossRef]
- Oblander, S.A.; Zhou, Z.; Galvez, B.G.; Starcher, B.; Shannon, J.M.; Durbeej, M.; Arroyo, A.G.; Tryggvason, K.; Apte, S. Distinctive functions of membrane type 1 matrix-metalloprotease (MT1-MMP or MMP-14) in lung and submandibular gland development are independent of its role in pro-MMP-2 activation. Dev. Biol. 2005, 277, 255–269. [Google Scholar] [CrossRef]
- Gifford, V.; Itoh, Y. MT1-MMP-dependent cell migration: Proteolytic and non-proteolytic mechanisms. Biochem. Soc. Trans. 2019, 47, 811–826. [Google Scholar] [CrossRef]
- Mori, H.; Tomari, T.; Koshikawa, N.; Kajita, M.; Itoh, Y.; Sato, H.; Tojo, H.; Yana, I.; Seiki, M. CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J. 2002, 21, 3949–3959. [Google Scholar] [CrossRef]
- Apte, S.S.; Fukai, N.; Beier, D.R.; Olsen, B.R. The Matrix Metalloproteinase-14 (MMP-14) gene is structurally distinct from other MMP genes and is co-expressed with the TIMP-2 gene during mouse embryogenesis. J. Biol. Chem. 1997, 272, 25511–25517. [Google Scholar] [CrossRef] [PubMed]
- Kheradmand, F.; Rishi, K.; Werb, Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J. Cell Sci. 2002, 115, 839–848. [Google Scholar] [CrossRef]
- Legallicier, B.; Trugnan, G.; Murphy, G.; Lelongt, B.; Ronco, P. Expression of the type IV collagenase system during mouse kidney development and tubule segmentation. J. Am. Soc. Nephrol. 2001, 12, 2358–2369. [Google Scholar] [CrossRef]
- Nuttall, R.K.; Sampieri, C.L.; Pennington, C.J.; Gill, S.E.; Schultz, G.A.; Edwards, D.R. Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett. 2004, 563, 129–134. [Google Scholar] [CrossRef]
- Ohtake, Y.; Tojo, H.; Seiki, M. Multifunctional roles of MT1-MMP in myofiber formation and morphostatic maintenance of skeletal muscle. J. Cell Sci. 2006, 119, 3822–3832. [Google Scholar] [CrossRef]
- Snyman, C.; Niesler, C.U. MMP-14 in skeletal muscle repair. J. Muscle Res. Cell Motil. 2015, 36, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Garmon, T.; Wittling, M.; Nie, S. MMP14 regulates cranial neural crest epithelial-to-mesenchymal transition and migration. Dev. Dyn. 2018, 247, 1083–1092. [Google Scholar] [CrossRef]
- Holmbeck, K.; Bianco, P.; Caterina, J.; Yamada, S.; Kromer, M.; Kuznetsov, S.A.; Mankani, M.; Robey, P.; Poole, A.; Pidoux, I.; et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 1999, 99, 81–92. [Google Scholar] [CrossRef]
- Malemud, C.J. Matrix metalloproteinases: Role in skeletal development and growth plate disorders. Front. Biosci. 2006, 11, 1702–1715. [Google Scholar] [CrossRef]
- Xu, H.; Snider, T.; Wimer, H.; Yamada, S.; Yang, T.; Holmbeck, K.; Foster, B. Multiple essential MT1-MMP functions in tooth root formation, dentinogenesis, and tooth eruption. Matrix Biol. 2016, 52, 266–283. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, J.; Qin, X.; Zheng, H.; Gao, B.; Liu, X.-G.; Jin, G.; Zhou, Z. MT1-MMP deficiency leads to defective ependymal cell maturation, impaired ciliogenesis, and hydrocephalus. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Byrne, L.C.; Zhou, Z.; Tryggvason, K.; Hökfelt, T.; Fetissov, S.O. Altered NPY and AgRP in membrane type-1 matrix metalloproteinase-deficient mice. NeuroReport 2004, 15, 569–574. [Google Scholar] [CrossRef]
- Gariano, R.F.; Hu, D.; Helms, J. Expression of angiogenesis-related genes during retinal development. Gene Expr. Patterns 2006, 6, 187–192. [Google Scholar] [CrossRef]
- Takano, A.; Hirata, A.; Inomata, Y.; Kawaji, T.; Nakagawa, K.; Nagata, S.; Tanihara, H. Intravitreal plasmin injection activates endogenous matrix metalloproteinase-2 in rabbit and human vitreous. Am. J. Ophthalmol. 2005, 140, 654–660. [Google Scholar] [CrossRef]
- Agapova, O.A.; Ricard, C.S.; Salvador-Silva, M.; Hernandez, M.R. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human optic nerve head astrocytes. Glia 2001, 33, 205–216. [Google Scholar] [CrossRef]
- Agapova, O.A.; Kaufman, P.L.; Lucarelli, M.J.; Gabelt, B.T.; Hernandez, M.R. Differential expression of matrix metalloproteinases in monkey eyes with experimental glaucoma or optic nerve transection. Brain Res. 2003, 967, 132–143. [Google Scholar] [CrossRef]
- de Groef, L.; Andries, L.; Lemmens, K.; van Hove, I.; Moons, L. Matrix metalloproteinases in the mouse retina: A comparative study of expression patterns and MMP antibodies. BMC Ophthalmol. 2015, 29, 187. [Google Scholar] [CrossRef]
- Blanco, M.J.; Rodríguez-Martín, I.; Learte, A.I.R.; Clemente, C.; Montalvo, M.G.; Seiki, M.; Arroyo, A.G.; Sánchez-Camacho, C. Developmental expression of membrane type 4-matrix metalloproteinase (Mt4-mmp/Mmp17) in the mouse embryo. PLoS ONE 2017, 12, e0184767. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, J.; Mori, H.; Ghajar, C.M.; Brownfield, U.; Galgoczy, R.; Bissell, M.J. Collective epithelial cell invasion overcomes mechanical barriers of collagenous extracellular matrix by a narrow tube-like geometry and MMP14-dependent local softening. Integr. Biol. 2011, 3, 1153–1166. [Google Scholar] [CrossRef]
- Mori, H.; Gjorevski, N.; Inman, J.L.; Bissell, M.J.; Nelson, C.M. Self-organization of engineered epithelial tubules by differential cellular motility. Proc. Natl. Acad. Sci. USA 2009, 106, 14890–14895. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Lo, A.T.; Inman, J.L.; Alcaraz, J.; Ghajar, C.M.; Mott, J.D.; Nelson, C.M.; Chen, C.S.; Zhang, H.; Bascom, J.L.; et al. Transmembrane/cytoplasmic, rather than catalytic, domains of Mmp14 signal to MAPK activation and mammary branching morphogenesis via binding to integrin 1. Development 2013, 140, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Simian, M.; Hirai, Y.; Navre, M.; Werb, Z.; Lochter, A.; Bissell, M.J. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development 2001, 128, 3117–3131. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Feinberg, T.Y.; Rowe, R.G.; Saunders, T.L.; Weiss, S.J. Functional roles of MMP14 and MMP15 in early postnatal mammary gland development. Development 2016, 143, 3956–3968. [Google Scholar] [CrossRef]
- Szabova, L.; Yamada, S.S.; Holmbeck, K.; Birkedal-Hansen, H. Expression pattern of four membrane-type matrix metalloproteinases in the normal and diseased mouse mammary gland. J. Cell. Physiol. 2005, 205, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Camargo, K.; Gomes, J.; Loddi, M.; de Sordi, R.; Costa-Ayub, C.; Soares, M.D.M. MT1-MMP and its potential role in the vertebrate intestinal morphogenesis. Acta Histochem. 2016, 118, 729–735. [Google Scholar] [CrossRef]
- Tanney, D.C.; Feng, L.; Pollock, A.S.; Lovett, D.H. Regulated expression of matrix metalloproteinases and TIMP in nephrogenesis. Dev. Dyn. 1998, 213, 121–129. [Google Scholar] [CrossRef]
- Díez-Torre, A.; Díaz-Núñez, M.; Eguizábal, C.; Silván, U.; Aréchaga, J. Evidence for a role of matrix metalloproteinases and their inhibitors in primordial germ cell migration. Andrology 2013, 1, 779–786. [Google Scholar] [CrossRef]
- Riggins, K.S.; Mernaugh, G.; Su, Y.; Quaranta, V.; Koshikawa, N.; Seiki, M.; Pozzi, A.; Zent, R. MT1-MMP-mediated basement membrane remodeling modulates renal development. Exp. Cell Res. 2010, 316, 2993–3005. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Olofsson, J.I.; Wahlberg, P.; Ny, T. Distinct Expression of Gelatinase A [Matrix Metalloproteinase (MMP)-2], Collagenase-3 (MMP-13), Membrane Type MMP 1 (MMP-14), and Tissue Inhibitor of MMPs Type 1 Mediated by physiological signals during formation and regression of the rat corpus luteum1. Endocrinology 1999, 140, 5330–5338. [Google Scholar] [CrossRef]
- Liu, K.; Wahlberg, P.; Hagglund, A.C.; Ny, T. Expression pattern and functional studies of matrix degrading proteases and their inhibitors in the mouse corpus luteum. Mol. Cell. Endocrinol. 2003, 205, 131–140. [Google Scholar] [CrossRef]
- Li, R.; Vannitamby, A.; Yue, S.S.K.; Handelsman, D.; Hutson, J. Mouse minipuberty coincides with gonocyte transformation into spermatogonial stem cells: A model for human minipuberty. Reprod. Fertil. Dev. 2017, 29, 2430–2436. [Google Scholar] [CrossRef]
- Churchill, J.A.; Buraundi, S.; Farmer, P.J.; Li, R.; Southwell, B.R.; Hutson, J.M.; Balic, A. Gubernaculum as icebreaker: Do matrix metalloproteinases in rodent gubernaculum and inguinal fat pad permit testicular descent? J. Pediatr. Surg. 2011, 46, 2353–2357. [Google Scholar] [CrossRef]
- Yana, I.; Sagara, H.; Takaki, S.; Takatsu, K.; Nakamura, K.; Nakao, K.; Katsuki, M.; Taniguchi, S.-I.; Aoki, T.; Sato, H.; et al. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J. Cell Sci. 2007, 120, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.J.; Learte, A.I.; Marchena, M.A.; Muñoz-Sáez, E.; Cid, M.A.; Rodríguez-Martín, I.; Sánchez-Camacho, C. Tracing gene expression through detection of beta-galactosidase activity in whole mouse embryos. JoVE J. Vis. Exp. 2018, 136, e57785. [Google Scholar] [CrossRef]
- Gálvez, B.G.; Matías-Román, S.; Albar, J.P.; Sánchez-Madrid, F.; Arroyo, A.G. Membrane type 1-matrix metalloproteinase is activated during migration of human endothelial cells and modulates endothelial motility and matrix remodeling. J. Biol. Chem. 2001, 276, 37491–37500. [Google Scholar] [CrossRef]
- Lehti, K.; Allen, E.; Birkedal-Hansen, H.; Holmbeck, K.; Miyake, Y.; Chun, T.H.; Weiss, S.J. An MT1-MMP-PDGF receptor- axis regulates mural cell investment of the microvasculature. Genes Dev. 2005, 19, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lui, K.O.; Zhou, B. Endocardial cell plasticity in cardiac development, diseases and regeneration. Circ. Res. 2018, 122, 774–789. [Google Scholar] [CrossRef]
- Luxán, G.; D’Amato, G.; MacGrogan, D.; de La Pompa, J.L. Endocardial notch signaling in cardiac development and disease. Circ. Res. 2016, 118, e1–e18. [Google Scholar] [CrossRef]
- Brauer, P.R. MMPs-role in cardiovascular development and disease. Front. Biosci. 2006, 11, 447–478. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.M.; Jackson, K.J.; Bushnell, K.M.; McGuire, P.G. Spatial and temporal expression of the 72-kDa type IV collagenase (MMP-2) correlates with development and differentiation of valves in the embryonic avian heart. Dev. Dyn. 1997, 209, 261–268. [Google Scholar] [CrossRef]
- Song, W.; Jackson, K.; McGuire, P.G. Degradation of type IV collagen by matrix metalloproteinases is an important step in the epithelial-mesenchymal transformation of the endocardial cushions. Dev. Biol. 2000, 227, 606–617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shelton, E.L.; Yutzey, K.E. Tbx20 regulation of endocardial cushion cell proliferation and extracellular matrix gene expression. Dev. Biol. 2007, 302, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.H.; Brauer, P.R. Synthetic matrix metalloproteinase inhibitor decreases early cardiac neural crest migration in chicken embryos. Dev. Dyn. 2002, 224, 441–449. [Google Scholar] [CrossRef]
- Arroyo, A.; Genis, L.; Gonzalo, P.; Matias-Roman, S.; Pollan, A.; Galvez, B. Matrix Metalloproteinases: New routes to the use of MT1-MMP as a therapeutic target in angiogenesis-related disease. Curr. Pharm. Des. 2007, 13, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Koziol, A.; Martin-Alonso, M.; Clemente, C.; Gonzalo, P.; Arroyo, A.G. Site-specific cellular functions of MT1-MMP. Eur. J. Cell Biol. 2012, 91, 889–895. [Google Scholar] [CrossRef]
- Alonso, M.M.; García-Redondo, A.B.; Guo, D.; Camafeita, E.; Martinez, F.; Alfranca, A.; Mendez-Barbero, N.; Pollán, Á.; Sanchez, M.S.; Denhardt, D.T.; et al. Deficiency of MMP17/MT4-MMP proteolytic activity predisposes to aortic aneurysm in mice. Circ. Res. 2015, 117, e13–e26. [Google Scholar] [CrossRef]
- Hiraoka, N.; Allen, E.; Apel, I.J.; Gyetko, M.R.; Weiss, S.J. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell 1998, 95, 365–377. [Google Scholar] [CrossRef]
- Chun, T.-H.; Sabeh, F.; Ota, I.; Murphy, H.; McDonagh, K.T.; Holmbeck, K.; Birkedal-Hansen, H.; Allen, E.D.; Weiss, S.J. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J. Cell Biol. 2004, 167, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Handsley, M.M.; Edwards, D.R. Metalloproteinases and their inhibitors in tumor angiogenesis. Int. J. Cancer 2005, 115, 849–860. [Google Scholar] [CrossRef] [PubMed]
- van Hinsbergh, V.W.; Koolwijk, P. Endothelial sprouting and angiogenesis: Matrix metalloproteinases in the lead. Cardiovasc. Res. 2008, 78, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.E.; Sacharidou, A.; Stratman, A.N.; Mayo, A.M.; Fisher, S.; Mahan, R.D.; Davis, M.J.; Davis, G.E. MT1-MMP- and Cdc42-dependent signaling co-regulate cell invasion and tunnel formation in 3D collagen matrices. J. Cell Sci. 2009, 122, 4558–4569. [Google Scholar] [CrossRef]
- Davis, G.E.; Stratman, A.N.; Sacharidou, A.; Koh, W. Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting. Int. Rev. Cell Mol. Biol. 2011, 288, 101–165. [Google Scholar] [CrossRef]
- Welch-Reardon, K.M.; Ehsan, S.M.; Wang, K.; Wu, N.; Newman, A.C.; Romero-Lopez, M.; Fong, A.H.; George, S.C.; Edwards, R.A.; Hughes, C.C.W. Angiogenic sprouting is regulated by endothelial cell expression of Slug. J. Cell Sci. 2014, 127, 2017–2028. [Google Scholar] [CrossRef]
- Koziol, A.; Gonzalo, P.; Mota, A.; Pollán, Á.; Lorenzo, C.; Colomé, N.; Montaner, D.; Dopazo, J.; Arribas, J.; Canals, F.; et al. The protease MT1-MMP drives a combinatorial proteolytic program in activated endothelial cells. FASEB J. 2012, 26, 4481–4494. [Google Scholar] [CrossRef]
- Burri, P.H.; Djonov, V. Intussusceptive angiogenesis—The alternative to capillary sprouting. Mol. Asp. Med. 2002, 23, S1–S27. [Google Scholar] [CrossRef]
- Esteban, S.; Clemente, C.; Koziol, A.; Gonzalo, P.; Rius, C.; Martinez, F.; Linares, P.M.; Chaparro, M.; Urzainqui, A.; Andrés, V.; et al. Endothelial MT1-MMP targeting limits intussusceptive angiogenesis and colitis via TSP1/nitric oxide axis. EMBO Mol. Med. 2020, 12, e10862. [Google Scholar] [CrossRef]
- Genís, L.; Gonzalo, P.; Tutor, A.S.; Galvez, B.G.; Martínez-Ruiz, A.; Zaragoza, C.; Lamas, S.; Tryggvason, K.; Apte, S.; Arroyo, A.G. Functional interplay between endothelial nitric oxide synthase and membrane type 1 matrix metalloproteinase in migrating endothelial cells. Blood 2007, 110, 2916–2923. [Google Scholar] [CrossRef]
- Sobolewski, K.; Bankowski, E.; Chyczewski, L.; Jaworski, S. Collagen and glycosaminoglycans of Wharton’s jelly. Neonatology 1997, 71, 11–21. [Google Scholar] [CrossRef]
- Filippov, S.; Koenig, G.C.; Chun, T.-H.; Hotary, K.B.; Ota, I.; Bugge, T.H.; Roberts, J.D.; Fay, W.P.; Birkedal-Hansen, H.; Holmbeck, K.; et al. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J. Exp. Med. 2005, 202, 663–671. [Google Scholar] [CrossRef]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Dave, J.M.; Abbey, C.A.; Duran, C.L.; Seo, H.; Johnson, G.A.; Bayless, K.J. Hic-5 mediates the initiation of endothelial sprouting by regulating a key surface metalloproteinase. J. Cell Sci. 2016, 129, 743–756. [Google Scholar] [CrossRef]
- Nandadasa, S.; Nelson, C.M.; Apte, S.S. ADAMTS9-Mediated Extracellular Matrix Dynamics Regulates Umbilical Cord Vascular Smooth Muscle Differentiation and Rotation. Cell Rep. 2015, 11, 1519–1528. [Google Scholar] [CrossRef]
- Hammoud, L.; Walsh, L.A.; Damjanovski, S. Cloning and developmental characterization of Xenopus laevis membrane type-3 matrix metalloproteinase (MT3-MMP). Biochem. Cell Biol. 2006, 84, 167–177. [Google Scholar] [CrossRef]
- Tomlinson, M.L.; Guan, P.; Morris, R.; Fidock, M.D.; Rejzek, M.; García-Morales, C.; Field, R.; Wheeler, G.N. A Chemical genomic approach identifies matrix metalloproteinases as playing an essential and specific role in Xenopus Melanophore migration. Chem. Biol. 2009, 16, 93–104. [Google Scholar] [CrossRef]
- Roth, L.; Kalev-Altman, R.; Monsonego-Ornan, E.; Sela-Donenfeld, D. A new role of the membrane-type matrix metalloproteinase 16 (MMP16/MT3-MMP) in neural crest cell migration. Int. J. Dev. Biol. 2017, 61, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Leigh, N.R.; Schupp, M.-O.; Li, K.; Padmanabhan, V.; Gastonguay, A.; Wang, L.; Chun, C.Z.; Wilkinson, G.A.; Ramchandran, R. Mmp17b is essential for proper neural crest cell migration in vivo. PLoS ONE 2013, 8, e76484. [Google Scholar] [CrossRef]
- Suzuki, J.; Osumi, N. Neural crest and placode contributions to olfactory development. Curr. Top. Dev. Biol. 2015, 111, 351–374. [Google Scholar] [CrossRef]
- Cheung, M.; Chaboissier, M.-C.; Mynett, A.; Hirst, E.; Schedl, A.; Briscoe, J. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev. Cell. 2005, 8, 179–192. [Google Scholar] [CrossRef]
- Brandl, M.; Seidler, B.; Haller, F.; Adamski, J.; Schmid, R.M.; Saur, D.; Schneider, G. IKKα controls canonical TGF -SMAD signaling to regulate genes expressing SNAIL and SLUG during EMT in Panc1 cells. J. Cell Sci. 2010, 123, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Shields, M.A.; Dangi-Garimella, S.; Krantz, S.B.; Bentrem, D.J.; Munshi, H.G. Pancreatic Cancer Cells Respond to Type I Collagen by Inducing Snail Expression to Promote Membrane Type 1 Matrix Metalloproteinase-dependent Collagen Invasion. J. Biol. Chem. 2011, 286, 10495–10504. [Google Scholar] [CrossRef]
- Shields, M.A.; Krantz, S.B.; Bentrem, D.J.; Dangi-Garimella, S.; Munshi, H.G. Interplay between beta1-integrin and Rho signaling regulates differential scattering and motility of pancreatic cancer cells by snail and Slug proteins. J. Biol. Chem. 2012, 287, 6218–6229. [Google Scholar] [CrossRef]
- Ota, I.; Li, X.-Y.; Hu, Y.; Weiss, S.J. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc. Natl. Acad. Sci. USA 2009, 106, 20318–20323. [Google Scholar] [CrossRef]
- Tsukatani, T.; Fillmore, H.L.; Hamilton, H.R.; Holbrook, E.H.; Costanzo, R.M. Matrix metalloproteinase expression in the olfactory epithelium. NeuroReport 2003, 14, 1135–1140. [Google Scholar] [CrossRef][Green Version]
- Strasser, G.A.; Kaminker, J.S.; Tessier-Lavigne, M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood 2010, 115, 5102–5110. [Google Scholar] [CrossRef] [PubMed]
- Aplin, A.C.; Zhu, W.H.; Fogel, E.; Nicosia, R.F. Vascular regression and survival are differentially regulated by MT1-MMP and TIMPs in the aortic ring model of angiogenesis. Am. J. Physiol. Cell Physiol. 2009, 297, C471–C480. [Google Scholar] [CrossRef]
- Gaublomme, D.; Buyens, T.; De Groef, L.; Stakenborg, M.; Janssens, E.; Ingvarsen, S.Z.; Porse, A.; Behrendt, N.; Moons, L. Matrix metalloproteinase 2 and membrane type 1 matrix metalloproteinase co-regulate axonal outgrowth of mouse retinal ganglion cells. J. Neurochem. 2014, 129, 966–979. [Google Scholar] [CrossRef]
- Janssens, E.; Gaublomme, D.; de Groef, L.; Darras, V.M.; Arckens, L.; Delorme, N.; Claes, F.; van Hove, I.; Moons, L. Matrix metalloproteinase 14 in the Zebrafish: An eye on retinal and retinotectal development. PLoS ONE 2013, 8, e52915. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, K.; Bollaerts, I.; Bhumika, S.; de Groef, L.; Van Houcke, J.; Darras, V.M.; van Hove, I.; Moons, L. Matrix metalloproteinases as promising regulators of axonal regrowth in the injured adult zebrafish retinotectal system. J. Comp. Neurol. 2016, 524, 1472–1493. [Google Scholar] [CrossRef]
- Kinoh, H.; Sato, H.; Tsunezuka, Y.; Takino, T.; Kawashima, A.; Okada, Y.; Seiki, M. MT-MMP, the cell surface activator of proMMP-2 (pro-gelatinase A), is expressed with its substrate in mouse tissue during embryogenesis. J. Cell Sci. 1996, 109, 953–959. [Google Scholar] [CrossRef]
- Sato, T.; del Carmen Ovejero, M.; Hou, P.; Heegaard, A.M.; Kumegawa, M.; Foged, N.T.; Delaissé, J.M. Identification of the membrane-type matrix metalloproteinase MT1-MMP in osteoclasts. J. Cell Sci. 1997, 110, 589–596. [Google Scholar] [CrossRef]
- Page-McCaw, A. Remodeling the model organism: Matrix metalloproteinase functions in invertebrates. Semin. Cell Dev. Biol. 2008, 19, 14–23. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Larsen, L.; Engsig, M.T.; Lou, H.; Ferreras, M.; Lochter, A.; Delaisse, J.-M.; Foged, N.T. Matrix Metalloproteinase-dependent activation of latent transforming growth factor-β controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J. Biol. Chem. 2002, 277, 44061–44067. [Google Scholar] [CrossRef]
- Gonzalo, P.; Guadamillas, M.C.; Hernández-Riquer, M.V.; Pollán, Á.; Grande-García, A.; Bartolome, R.A.; Vasanji, A.; Ambrogio, C.; Chiarle, R.; Teixidó, J.; et al. MT1-MMP is required for myeloid cell fusion via regulation of Rac1 signaling. Dev. Cell 2010, 18, 77–89. [Google Scholar] [CrossRef]
- Ortega, N.; Behonick, D.J.; Werb, Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Brand-Saberi, B. Genetic and epigenetic control of skeletal muscle development. Ann. Anat. 2005, 187, 199–207. [Google Scholar] [CrossRef]
- El Fahime, E.; Torrente, Y.; Caron, N.J.; Bresolin, M.D.; Tremblay, J.P. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp. Cell Res. 2000, 258, 279–287. [Google Scholar] [CrossRef]
- Yeung, C.-Y.C.; Zeef, L.A.H.; Lallyett, C.; Lu, Y.; Laird, E.; Kadler, K.E. Chick tendon fibroblast transcriptome and shape depend on whether the cell has made its own collagen matrix. Sci. Rep. 2015, 5, 13555. [Google Scholar] [CrossRef]
- Magnusson, S.P.; Langberg, H.; Kjaer, M. The pathogenesis of tendinopathy: Balancing the response to loading. Nat. Rev. Rheumatol. 2010, 6, 262–268. [Google Scholar] [CrossRef]
- Kalson, N.S.; Lu, Y.; Taylor, S.H.; Starborg, T.; Holmes, D.F.; Kadler, K.E. A structure-based extracellular matrix expansion mechanism of fibrous tissue growth. eLife 2015, 4, e05958. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.H.; Yeung, C.-Y.C.; Kalson, N.S.; Lu, Y.; Zigrino, P.; Starborg, T.; Warwood, S.; Holmes, D.F.; Canty-Laird, E.G.; Mauch, C.; et al. Matrix metalloproteinase 14 is required for fibrous tissue expansion. eLife 2015, 4, e09345. [Google Scholar] [CrossRef]
- Chan, Z.C.-K.; Kwan, H.L.R.; Wong, Y.S.; Jiang, Z.; Zhou, Z.; Tam, K.W.; Chan, Y.-S.; Chan, C.B.; Lee, C.W. Site-directed MT1-MMP trafficking and surface insertion regulate AChR clustering and remodeling at developing NMJs. eLife 2020, 9, e54379. [Google Scholar] [CrossRef]
- Ahn, Y. Signaling in tooth, hair, and mammary placodes. Curr. Top. Dev. Biol. 2015, 111, 421–459. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Hayashi, S. Contribution of neural crest cells in tooth development and the possibility of tooth regeneration. J. Oral Biosci. 2004, 46, 509–518. [Google Scholar] [CrossRef]
- Bartlett, J.D.; Zhou, Z.; Skobe, Z.; Dobeck, J.M.; Tryggvason, K. Delayed tooth eruption in membrane type-1 matrix metalloproteinase deficient mice. Connect. Tissue Res. 2003, 44, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Kurakata, H.; Oka, M.; Matsubara, Y.; Niwa, T.; Utsunomiya, H.; Fujishiro, M.; Miki, K.; Fukamachi, H.; Kubota, S.; Ichinose, M. Developmentally regulated expression of matrix metalloproteinases during fetal rat colon morphogenesis. Dev. Growth Differ. 2007, 50, 41–48. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Sáez, E.; Moracho, N.; Learte, A.I.R.; Arroyo, A.G.; Sánchez-Camacho, C. Dynamic Expression of Membrane Type 1-Matrix Metalloproteinase (Mt1-mmp/Mmp14) in the Mouse Embryo. Cells 2021, 10, 2448. https://doi.org/10.3390/cells10092448
Muñoz-Sáez E, Moracho N, Learte AIR, Arroyo AG, Sánchez-Camacho C. Dynamic Expression of Membrane Type 1-Matrix Metalloproteinase (Mt1-mmp/Mmp14) in the Mouse Embryo. Cells. 2021; 10(9):2448. https://doi.org/10.3390/cells10092448
Chicago/Turabian StyleMuñoz-Sáez, Emma, Natalia Moracho, Ana I. R. Learte, Alicia G. Arroyo, and Cristina Sánchez-Camacho. 2021. "Dynamic Expression of Membrane Type 1-Matrix Metalloproteinase (Mt1-mmp/Mmp14) in the Mouse Embryo" Cells 10, no. 9: 2448. https://doi.org/10.3390/cells10092448
APA StyleMuñoz-Sáez, E., Moracho, N., Learte, A. I. R., Arroyo, A. G., & Sánchez-Camacho, C. (2021). Dynamic Expression of Membrane Type 1-Matrix Metalloproteinase (Mt1-mmp/Mmp14) in the Mouse Embryo. Cells, 10(9), 2448. https://doi.org/10.3390/cells10092448






