Cep192, a Novel Missing Link between the Centrosomal Core and Corona in Dictyostelium Amoebae
Abstract
1. Introduction
2. Materials and Methods
2.1. Vector Construction
2.2. Fluorescence Microscopy
2.3. Electron Microscopy
2.4. Antibodies and Conjugates
2.5. Cell Culture
2.6. Other Methods
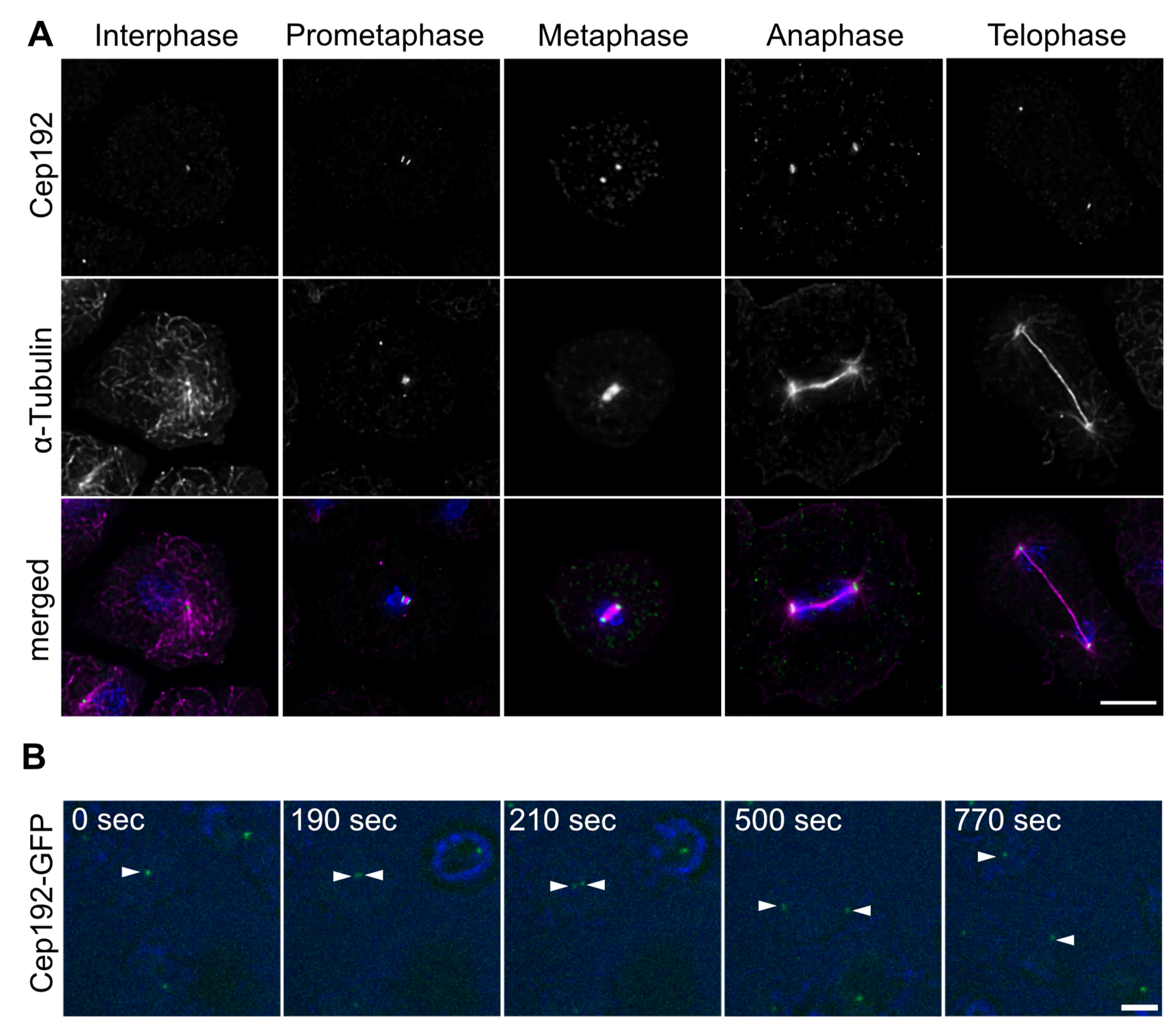
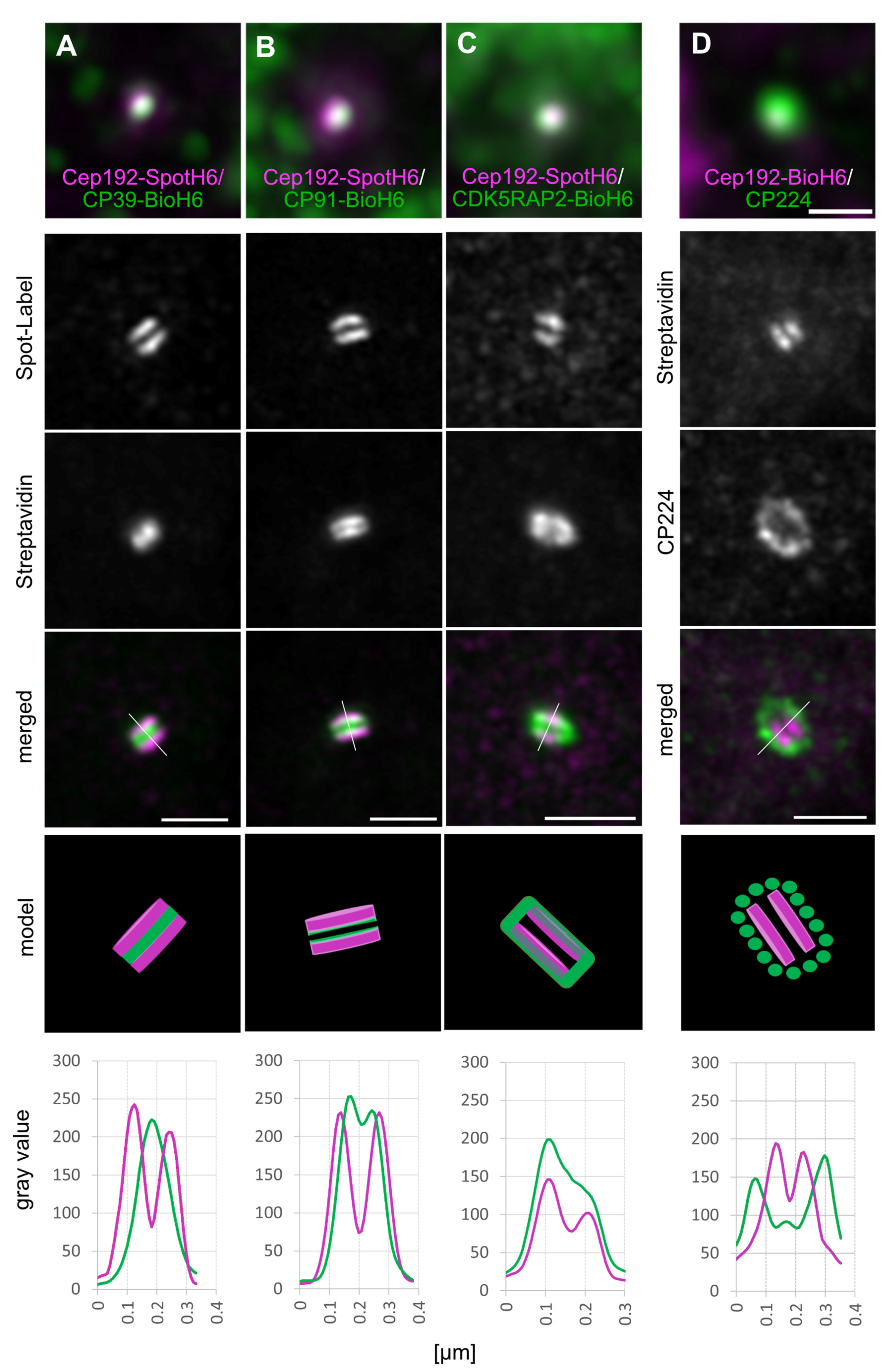
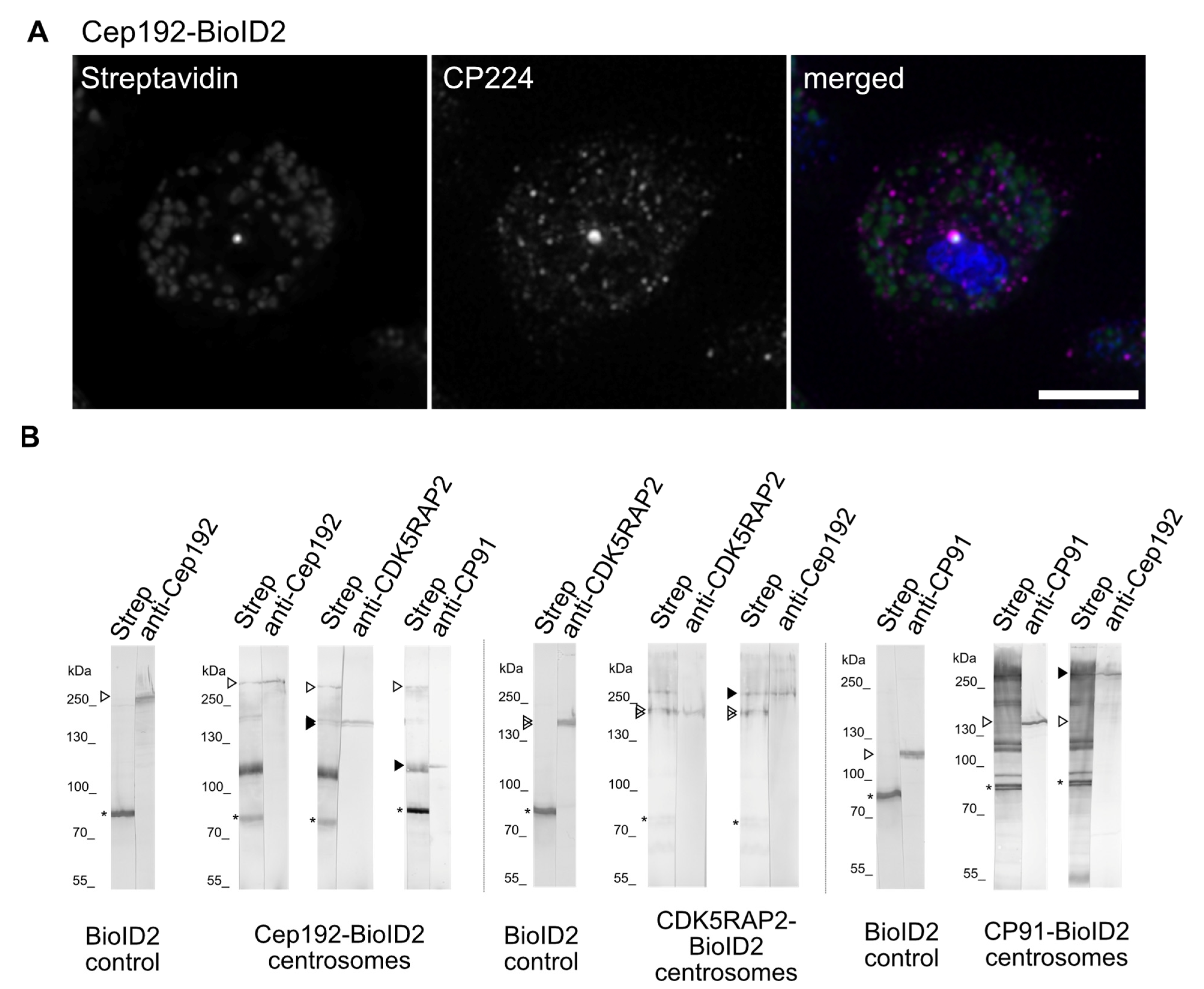
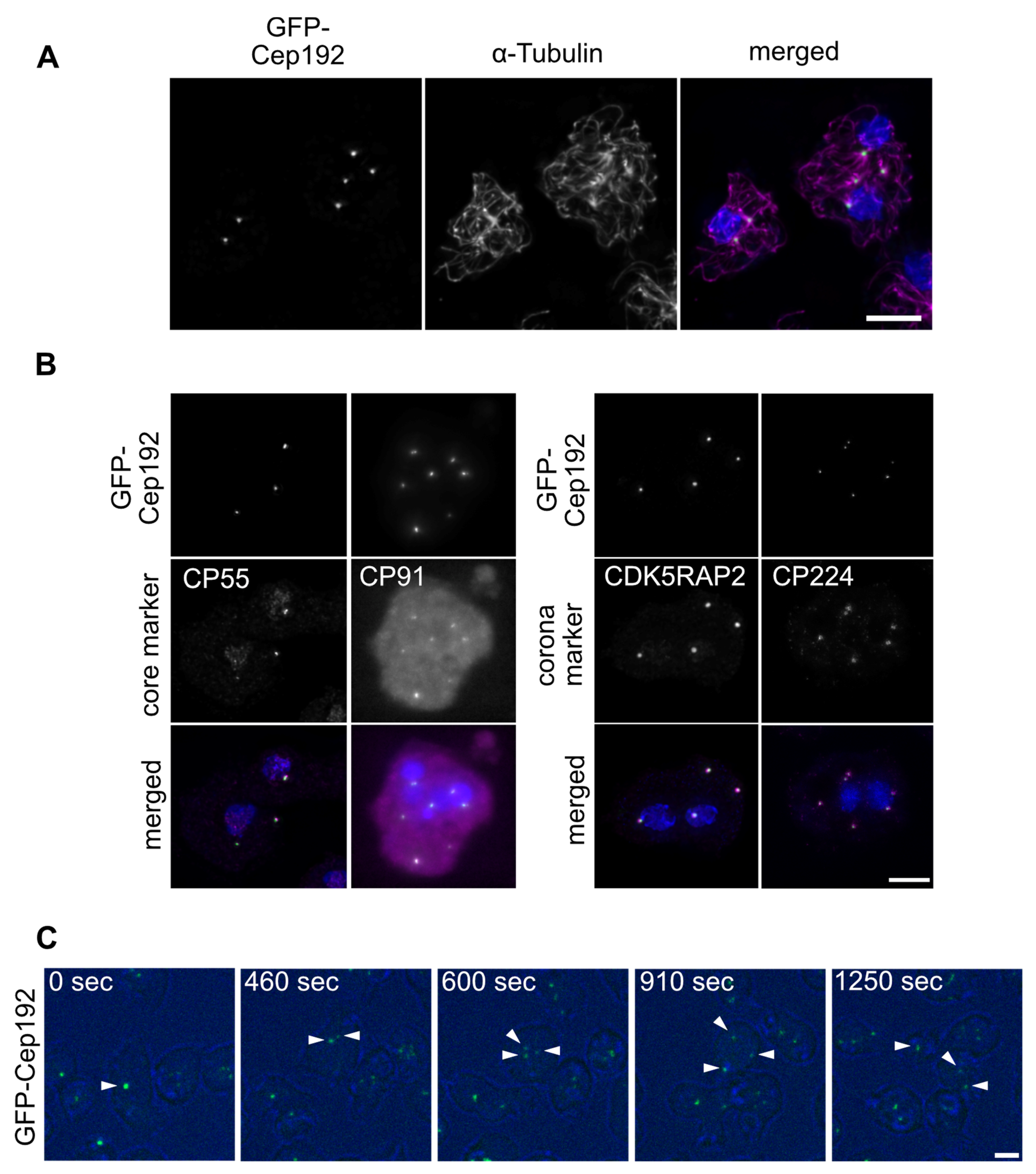
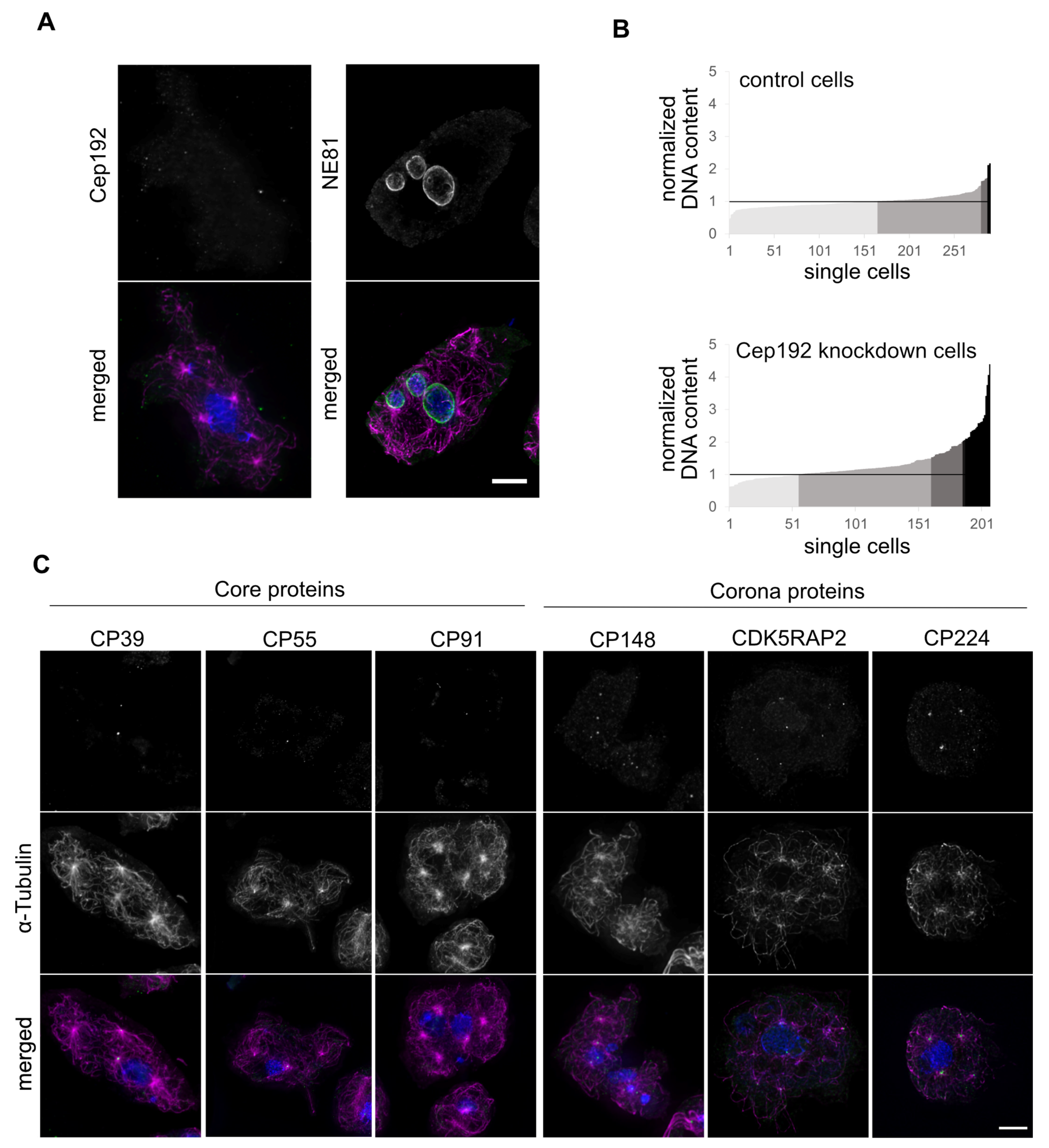
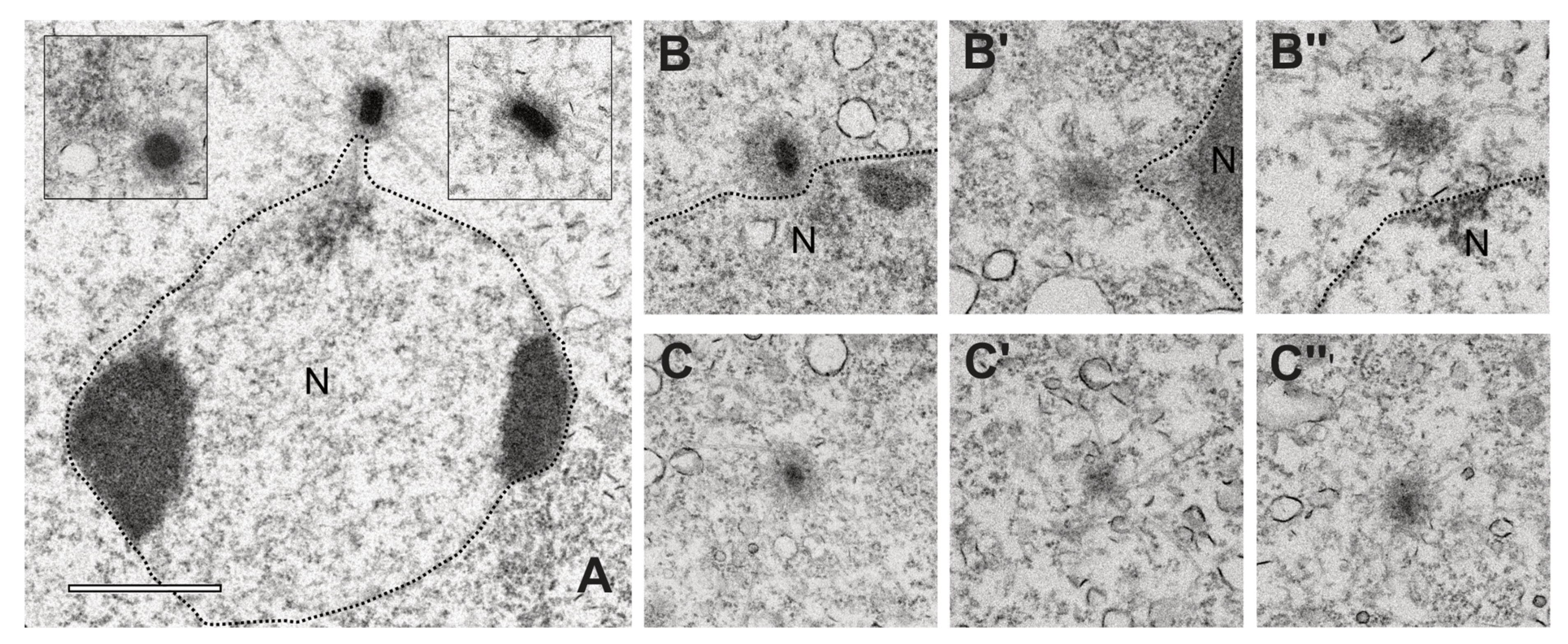
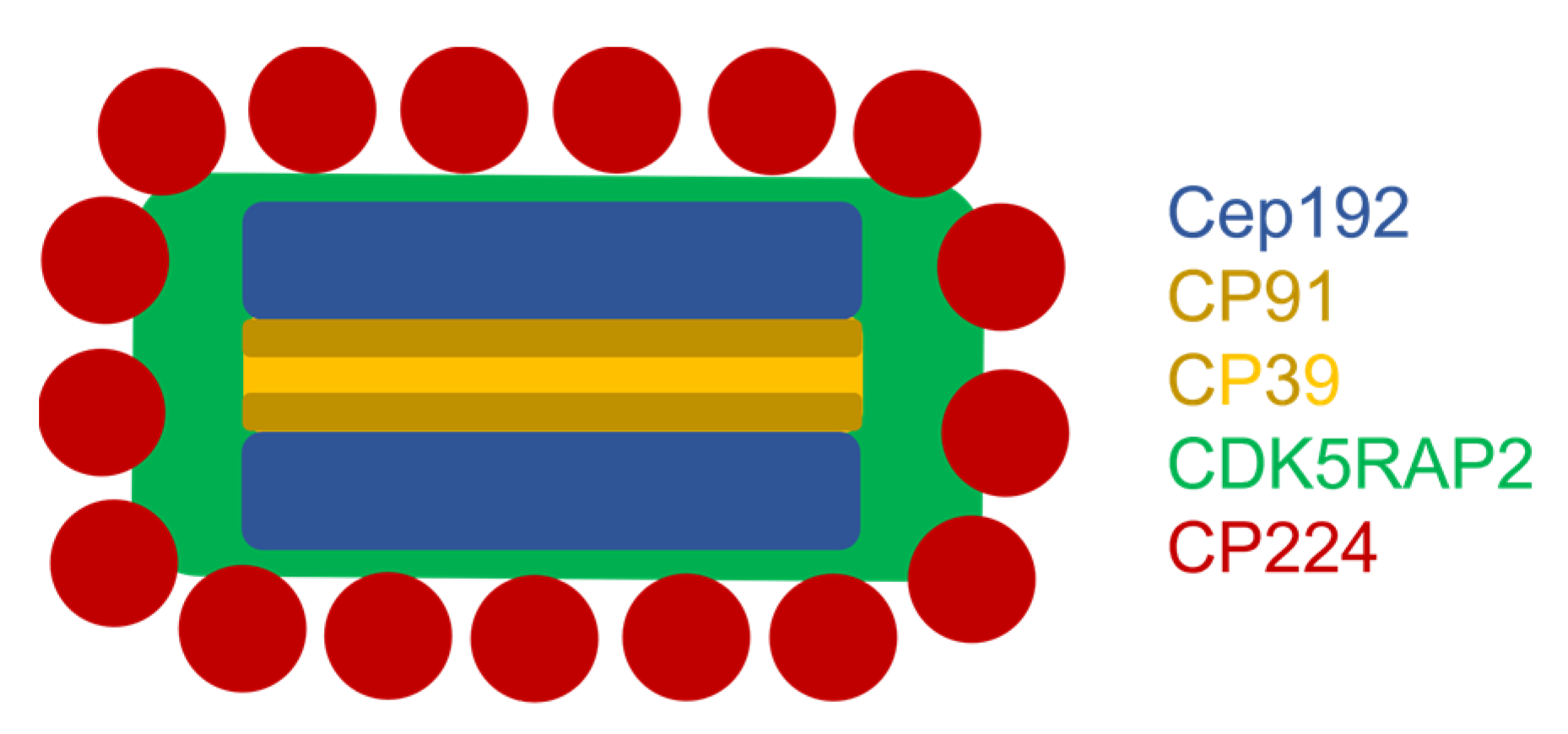
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gräf, R. Comparative biology of centrosomal structures in eukaryotes. Cells 2018, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Gräf, R.; Batsios, P.; Meyer, I. Evolution of centrosomes and the nuclear lamina: Amoebozoan assets. Eur. J. Cell Biol. 2015, 94, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Bettencourt-Dias, M. Centrosome remodelling in evolution. Cells 2018, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Uzbekov, R.E.; Avidor-Reiss, T. Principal postulates of centrosomal biology. Version 2020. Cells 2020, 9, 2156. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A.; Holland, A.J. Once and only once: Mechanisms of centriole duplication and their deregulation in disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 297–312. [Google Scholar] [CrossRef]
- Hoffmann, I. Centrosomes in mitotic spindle assembly and orientation. Curr. Opin. Struct. Biol. 2020, 66, 193–198. [Google Scholar] [CrossRef]
- Kuriyama, R.; Sato, C.; Fukui, Y.; Nishibayashi, S. In vitro nucleation of microtubules from microtubule-organizing center prepared from cellular slime mold. Cell Motil. 1982, 2, 257–272. [Google Scholar] [CrossRef]
- Roos, U.-P.; Guhl, B. Microtubules in interphase and mitosis of cellular slime molds. In Biomechanics of Active Movement and Deformation of Cells; Akkas, N., Ed.; Springer: Berlin, Germany, 1990. [Google Scholar]
- Omura, F.; Fukui, Y. Dictyostelium MTOC: Structure and linkage to the nucleus. Protoplasma 1985, 127, 212–221. [Google Scholar] [CrossRef]
- Tikhonenko, I.; Magidson, V.; Gräf, R.; Khodjakov, A.; Koonce, M.P. A kinesin-mediated mechanism that couples centrosomes to nuclei. Cell. Mol. Life Sci. 2013, 70, 1285–1296. [Google Scholar] [CrossRef]
- Roos, U.P. Fine structure of an organelle associated with the nucleus and cytoplasmic microtubules in the cellular slime mould polysphondylium violaceum. J. Cell Sci. 1975, 18, 315–326. [Google Scholar] [CrossRef]
- Ueda, M.; Schliwa, M.; Euteneuer, U. Unusual centrosome cycle in Dictyostelium: Correlation of dynamic behavior and structural changes. Mol. Biol. Cell 1999, 10, 151–160. [Google Scholar] [CrossRef]
- Meyer, I.; Peter, T.; Batsios, P.; Kuhnert, O.; Krüger-Genge, A.; Camurça, C.; Gräf, R. CP39, CP75 and CP91 Are major structural components of the Dictyostelium centrosome’s core structure. Eur. J. Cell Biol. 2017, 96, 119–130. [Google Scholar] [CrossRef]
- Kuhnert, O.; Baumann, O.; Meyer, I.; Gräf, R. CP55, a novel key component of centrosomal organization in Dictyostelium. Cell. Mol. Life Sci. 2012, 69, 3651–3664. [Google Scholar] [CrossRef]
- Putzler, S.; Meyer, I.; Gräf, R. CP91 is a component of the Dictyostelium centrosome involved in centrosome biogenesis. Eur. J. Cell Biol. 2016, 95, 124–135. [Google Scholar] [CrossRef]
- Kuhnert, O.; Baumann, O.; Meyer, I.; Gräf, R. Functional characterization of cp148, a novel key component for centrosome integrity in Dictyostelium. Cell. Mol. Life Sci. 2012, 69, 1875–1888. [Google Scholar] [CrossRef]
- Pitzen, V.; Askarzada, S.; Gräf, R.; Meyer, I. CDK5RAP2 is an essential scaffolding protein of the corona of the Dictyostelium centrosome. Cells 2018, 7, 32. [Google Scholar] [CrossRef]
- Kim, T.S.; Park, J.E.; Shukla, A.; Choi, S.; Murugan, R.N.; Lee, J.H.; Ahn, M.; Rhee, K.; Bang, J.K.; Kim, B.Y.; et al. Hierarchical recruitment of Plk4 and regulation of centriole biogenesis by two centrosomal scaffolds, Cep192 and Cep152. Proc. Natl. Acad. Sci. USA 2013, 110, E4849–E4857. [Google Scholar] [CrossRef]
- Conduit, P.T.; Brunk, K.; Dobbelaere, J.; Dix, C.I.; Lucas, E.P.; Raff, J.W. centrioles regulate centrosome size by controlling the rate of Cnn incorporation into the PCM. Curr. Biol. 2010, 20, 2178–2186. [Google Scholar] [CrossRef]
- Martens, H.; Novotny, J.; Oberstrass, J.; Steck, T.L.; Postlethwait, P.; Nellen, W. RNAi in Dictyostelium: The role of RNA-directed RNA polymerases and double-stranded RNase. Mol. Biol. Cell 2002, 13, 445–453. [Google Scholar] [CrossRef]
- Virant, D.; Traenkle, B.; Maier, J.; Kaiser, P.D.; Bodenhöfer, M.; Schmees, C.; Vojnovic, I.; Pisak-Lukáts, B.; Endesfelder, U.; Rothbauer, U. A peptide tag-specific nanobody enables high-quality labeling for DSTORM imaging. Nat. Commun. 2018, 9, 930. [Google Scholar] [CrossRef]
- Tagwerker, C.; Flick, K.; Cui, M.; Guerrero, C.; Dou, Y.; Auer, B.; Baldi, P.; Huang, L.; Kaiser, P. A tandem affinity tag for two-step purification under fully denaturing conditions: Application in ubiquitin profiling and protein complex identification combined with in vivocross-linking. Mol. Cell Proteom. 2006, 5, 737–748. [Google Scholar] [CrossRef]
- Cronan, J.E. Biotination of proteins in vivo. A post-translational modification to label, purify, and study proteins. J. Biol. Chem. 1990, 265, 10327–10333. [Google Scholar] [CrossRef]
- Faix, J.; Kreppel, L.; Shaulsky, G.; Schleicher, M.; Kimmel, A.R. A rapid and efficient method to generate multiple gene disruptions in Dictyostelium discoideum using a single selectable marker and the Cre-LoxP system. Nucleic Acids Res. 2004, 32, e143. [Google Scholar] [CrossRef]
- Batsios, P.; Baumann, O.; Gräf, R.; Meyer, I. Isolation of Dictyostelium nuclei for light and electron microscopy. Methods Mol. Biol. 2013, 983, 283–294. [Google Scholar] [CrossRef]
- Grafe, M.; Hofmann, P.; Batsios, P.; Meyer, I.; Gräf, R. In vivo assembly of a Dictyostelium lamin mutant induced by light, mechanical stress, and PH. Cells 2020, 9, 1834. [Google Scholar] [CrossRef]
- Fukui, Y.; Yumura, S.; Yumura, T.K. Agar-overlay immunofluorescence: High resolution studies of cytoskeletal components and their changes during chemotaxis. Methods Cell Biol. 1987, 28, 347–356. [Google Scholar]
- Samereier, M.; Meyer, I.; Koonce, M.P.; Gräf, R. Live cell-imaging techniques for analyses of microtubules in Dictyostelium. Methods Cell Biol. 2010, 97, 341–357. [Google Scholar]
- Tillberg, P.W.; Chen, F.; Piatkevich, K.D.; Zhao, Y.; Yu, C.C.; English, B.P.; Gao, L.; Martorell, A.; Suk, H.J.; Yoshida, F.; et al. Protein-retention expansion microscopy of cells and tissues labeled using standard fluorescent proteins and antibodies. Nat. Biotechnol. 2016, 34, 987–992. [Google Scholar] [CrossRef]
- Chozinski, T.J.; Halpern, A.R.; Okawa, H.; Kim, H.J.; Tremel, G.J.; Wong, R.O.; Vaughan, J.C. Expansion microscopy with conventional antibodies and fluorescent proteins. Nat. Methods 2016, 13, 485–488. [Google Scholar] [CrossRef]
- Grafe, M.; Batsios, P.; Meyer, I.; Lisin, D.; Baumann, O.; Goldberg, M.W.; Gräf, R. Supramolecular structures of the Dictyostelium lamin NE81. Cells 2019, 8, 162. [Google Scholar] [CrossRef]
- Gräf, R.; Daunderer, C.; Schliwa, M. Cell cycle-dependent localization of monoclonal antibodies raised against isolated Dictyostelium centrosomes. Biol. Cell 1999, 91, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Krüger, A.; Batsios, P.; Baumann, O.; Luckert, E.; Schwarz, H.; Stick, R.; Meyer, I.; Gräf, R. Characterization of NE81, the first lamin-like nucleoskeleton protein in a unicellular organism. Mol. Biol. Cell. 2012, 23, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Wehland, J.; Willingham, M.C.; Sandoval, I.V. A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin. II. Effects on cell movement, organization of microtubules, and intermediate filaments, and arrangement of golgi elements. J. Cell Biol. 1983, 97, 1476–1490. [Google Scholar] [CrossRef]
- Schulz, I.; Reinders, Y.; Sickmann, A.; Gräf, R. An improved method for Dictyostelium centrosome isolation. Methods Mol. Biol. 2006, 346, 479–489. [Google Scholar]
- Gräf, R.; Euteneuer, U.; Ueda, M.; Schliwa, M. Isolation of nucleation-competent centrosomes from Dictyostelium discoideum. Eur. J. Cell Biol. 1998, 76, 167–175. [Google Scholar] [CrossRef]
- Batsios, P.; Meyer, I.; Gräf, R. Proximity-dependent biotin identification (BioID) in Dictyostelium amoebae. Methods Enzymol. 2016, 569, 23–42. [Google Scholar] [CrossRef]
- Kim, D.I.; Jensen, S.C.; Noble, K.A.; Kc, B.; Roux, K.H.; Motamedchaboki, K.; Roux, K.J. An improved smaller biotin ligase for BioID proximity labeling. Mol Biol. Cell. 2016, 27, 1188–1196. [Google Scholar] [CrossRef]
- Reinders, Y.; Schulz, I.; Gräf, R.; Sickmann, A. Identification of novel centrosomal proteins in Dictyostelium discoideum by comparative proteomic approaches. J. Proteome Res. 2006, 5, 589–598. [Google Scholar] [CrossRef]
- Schulz, I.; Erle, A.; Gräf, R.; Krüger, A.; Lohmeier, H.; Putzler, S.; Samereier, M.; Weidenthaler, S. Identification and cell cycle-dependent localization of nine novel, genuine centrosomal components in Dictyostelium discoideum. Cell Motil. Cytoskelet. 2009, 66, 915–928. [Google Scholar] [CrossRef]
- Samereier, M.; Baumann, O.; Meyer, I.; Gräf, R. Analysis of Dictyostelium TACC reveals differential interactions with CP224 and unusual dynamics of Dictyostelium microtubules. Cell. Mol. Life Sci. 2011, 68, 275–287. [Google Scholar] [CrossRef]
- Peter, T. Molekulare Charakterisierung von CP75, Einem Neuen Centrosomalen Protein in Dictyostelium discoideum. Ph.D. Thesis, Universität Potsdam, Potsdam, Germany, 2016. [Google Scholar]
- Moens, P.B. Spindle and kinetochore morphology of Dictyostelium discoideum. J. Cell Biol. 1976, 68, 113–122. [Google Scholar] [CrossRef]
- Gambarotto, D.; Zwettler, F.U.; Le Guennec, M.; Schmidt-Cernohorska, M.; Fortun, D.; Borgers, S.; Heine, J.; Schloetel, J.-G.; Reuss, M.; Unser, M.; et al. Imaging cellular ultrastructures using expansion microscopy (U-ExM). Nat. Methods 2019, 16, 71–74. [Google Scholar] [CrossRef]
- Reth, M. Matching cellular dimensions with molecular sizes. Nat. Immunol. 2013, 14, 765–767. [Google Scholar] [CrossRef]
- Kim, D.I.; Birendra, K.C.; Zhu, W.; Motamedchaboki, K.; Doye, V.; Roux, K.J. Probing nuclear pore complex architecture with proximity-dependent biotinylation. Proc. Natl. Acad. Sci. USA 2014, 111, E2453–E2461. [Google Scholar] [CrossRef] [PubMed]
- Arslanhan, M.D.; Gulensoy, D.; Firat-Karalar, E.N. A proximity mapping journey into the biology of the mammalian centrosome/Cilium complex. Cells 2020, 9, 1390. [Google Scholar] [CrossRef]
- Gupta, G.D.; Coyaud, E.; Goncalves, J.; Mojarad, B.A.; Liu, Y.; Wu, Q.; Gheiratmand, L.; Comartin, D.; Tkach, J.M.; Cheung, S.W.; et al. A dynamic protein interaction landscape of the human centrosome-cilium interface. Cell 2015, 163, 1484–1499. [Google Scholar] [CrossRef] [PubMed]
- Firat-Karalar, E.N.; Stearns, T. Probing mammalian centrosome structure using BioID proximity-dependent biotinylation. Methods Cell Biol. 2015, 129, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Gräf, R.; Euteneuer, U.; Ho, T.H.; Rehberg, M. Regulated expression of the centrosomal protein DdCP224 affects microtubule dynamics and reveals mechanisms for the control of supernumerary centrosome number. Mol. Biol. Cell 2003, 14, 4067–4074. [Google Scholar] [CrossRef] [PubMed]
- Rehberg, M.; Gräf, R. Dictyostelium EB1 is a genuine centrosomal component required for proper spindle formation. Mol. Biol. Cell 2002, 13, 2301–2310. [Google Scholar] [CrossRef]
- Gräf, R. DdNek2, the first non-vertebrate homologue of human Nek2, is involved in the formation of microtubule-organizing centers. J. Cell Sci. 2002, 115, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Lawo, S.; Hasegan, M.; Gupta, G.D.; Pelletier, L. Subdiffraction imaging of centrosomes reveals higher-order organizational features of pericentriolar material. Nat. Cell Biol. 2012, 14, 1148–1158. [Google Scholar] [CrossRef]
- Fry, A.M.; Sampson, J.; Shak, C.; Shackleton, S. Recent advances in pericentriolar material organization: Ordered layers and scaffolding gels. F1000 Res. 2017, 6, 1622. [Google Scholar] [CrossRef]
- Mennella, V.; Keszthelyi, B.; McDonald, K.L.; Chhun, B.; Kan, F.; Rogers, G.C.; Huang, B.; Agard, D.A. Subdiffraction-resolution fluorescence microscopy reveals a domain of the centrosome critical for pericentriolar material organization. Nat. Cell Biol. 2012, 14, 1159–1168. [Google Scholar] [CrossRef]
- Mennella, V.; Agard, D.A.; Huang, B.; Pelletier, L. Amorphous no more: Subdiffraction view of the pericentriolar material architecture. Trends Cell Biol. 2014, 24, 188–197. [Google Scholar] [CrossRef]
- Joukov, V.; Walter, J.C.; De Nicolo, A. The Cep192-organized aurora a-plk1 cascade is essential for centrosome cycle and bipolar spindle assembly. Mol. Cell 2014, 55, 578–591. [Google Scholar] [CrossRef]
- Joukov, V.; De Nicolo, A. Aurora-PLK1 cascades as key signaling modules in the regulation of mitosis. Sci. Signal. 2018, 11, eaar4195. [Google Scholar] [CrossRef]
- Sukumaran, S.K.; Blau-Wasser, R.; Rohlfs, M.; Gallinger, C.; Schleicher, M.; Noegel, A.A. The centrosomal component CEP161 of Dictyostelium discoideum interacts with the hippo signaling pathway. Cell Cycle 2015, 14, 1024–1035. [Google Scholar] [CrossRef][Green Version]
- Carvalho-Santos, Z.; Machado, P.; Branco, P.; Tavares-Cadete, F.; Rodrigues-Martins, A.; Pereira-Leal, J.B.; Bettencourt-Dias, M. Stepwise evolution of the centriole-assembly pathway. J. Cell Sci. 2010, 123, 1414–1426. [Google Scholar] [CrossRef]
- Gomez-Ferreria, M.A.; Rath, U.; Buster, D.W.; Chanda, S.K.; Caldwell, J.S.; Rines, D.R.; Sharp, D.J. Human Cep192 is required for mitotic centrosome and spindle assembly. Curr. Biol. 2007, 17, 1960–1966. [Google Scholar] [CrossRef]
- Peel, N.; Stevens, N.R.; Basto, R.; Raff, J.W. Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr. Biol. 2007, 17, 834–843. [Google Scholar] [CrossRef]
- Stevens, N.R.; Dobbelaere, J.; Brunk, K.; Franz, A.; Raff, J.W. Drosophila Ana2 is a conserved centriole duplication factor. J. Cell Biol. 2010, 188, 313–323. [Google Scholar] [CrossRef]
- Arquint, C.; Nigg, E.A. The PLK4-STIL-SAS-6 module at the core of centriole duplication. Biochem. Soc. Trans. 2016, 44, 1253–1263. [Google Scholar] [CrossRef]
- O’Rourke, B.P.; Gomez-Ferreria, M.A.; Berk, R.H.; Hackl, A.M.U.; Nicholas, M.P.; O’Rourke, S.C.; Pelletier, L.; Sharp, D.J. Cep192 controls the balance of centrosome and non-centrosomal microtubules during interphase. PLoS ONE 2014, 9, e101001. [Google Scholar] [CrossRef]
- Koonce, M.P.; Khodjakov, A. Dynamic microtubules in Dictyostelium. J. Muscle Res. Cell Motil. 2002, 23, 613–619. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitzen, V.; Sander, S.; Baumann, O.; Gräf, R.; Meyer, I. Cep192, a Novel Missing Link between the Centrosomal Core and Corona in Dictyostelium Amoebae. Cells 2021, 10, 2384. https://doi.org/10.3390/cells10092384
Pitzen V, Sander S, Baumann O, Gräf R, Meyer I. Cep192, a Novel Missing Link between the Centrosomal Core and Corona in Dictyostelium Amoebae. Cells. 2021; 10(9):2384. https://doi.org/10.3390/cells10092384
Chicago/Turabian StylePitzen, Valentin, Sophia Sander, Otto Baumann, Ralph Gräf, and Irene Meyer. 2021. "Cep192, a Novel Missing Link between the Centrosomal Core and Corona in Dictyostelium Amoebae" Cells 10, no. 9: 2384. https://doi.org/10.3390/cells10092384
APA StylePitzen, V., Sander, S., Baumann, O., Gräf, R., & Meyer, I. (2021). Cep192, a Novel Missing Link between the Centrosomal Core and Corona in Dictyostelium Amoebae. Cells, 10(9), 2384. https://doi.org/10.3390/cells10092384






