Presynaptic AMPA Receptors in Health and Disease
Abstract
1. Introduction
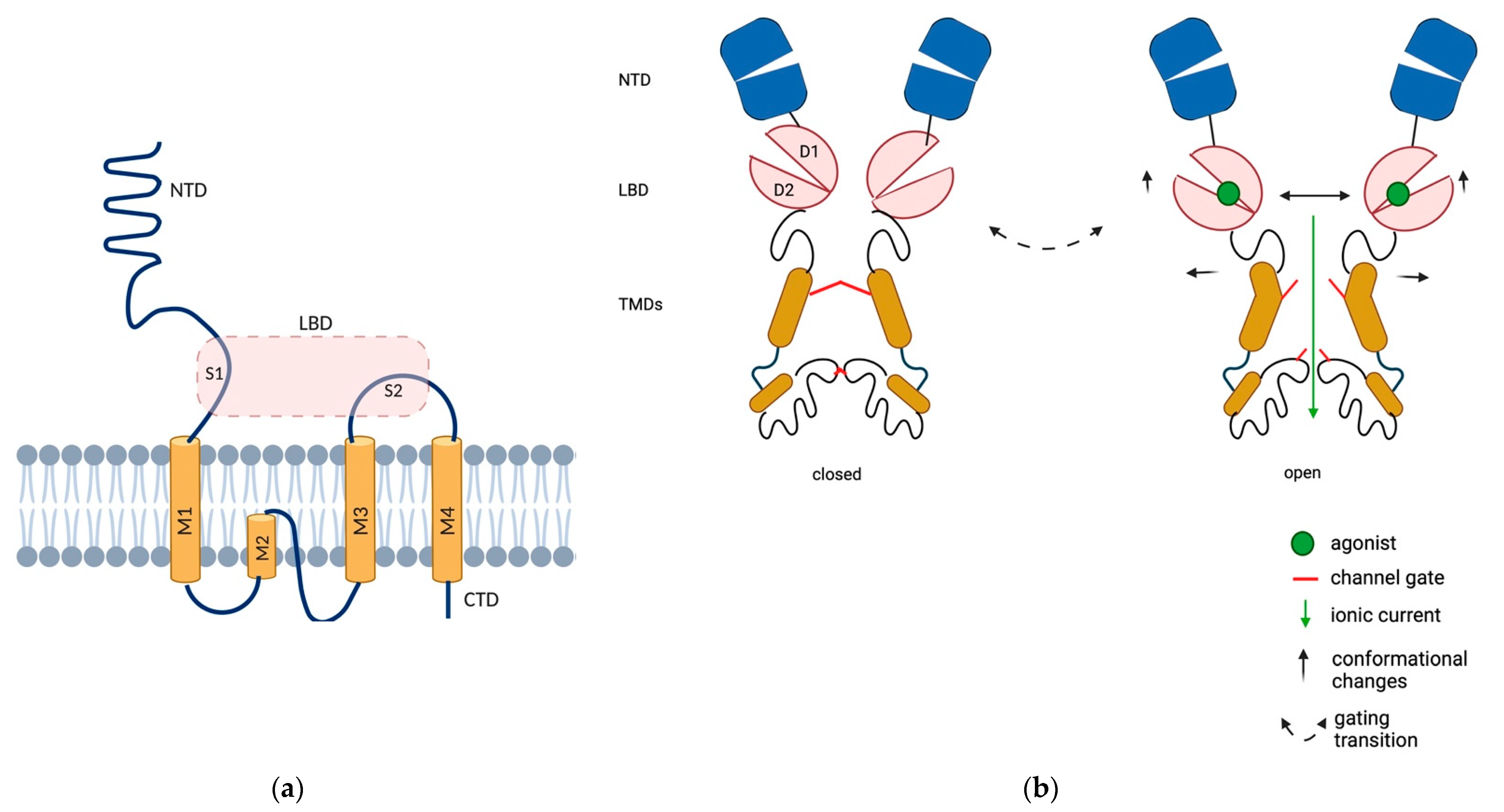
2. AMPAR Localization at Presynapse
3. Physiological Functions of Presynaptic AMPAR
3.1. AMPAR as Regulator of Neurotransmitter Release
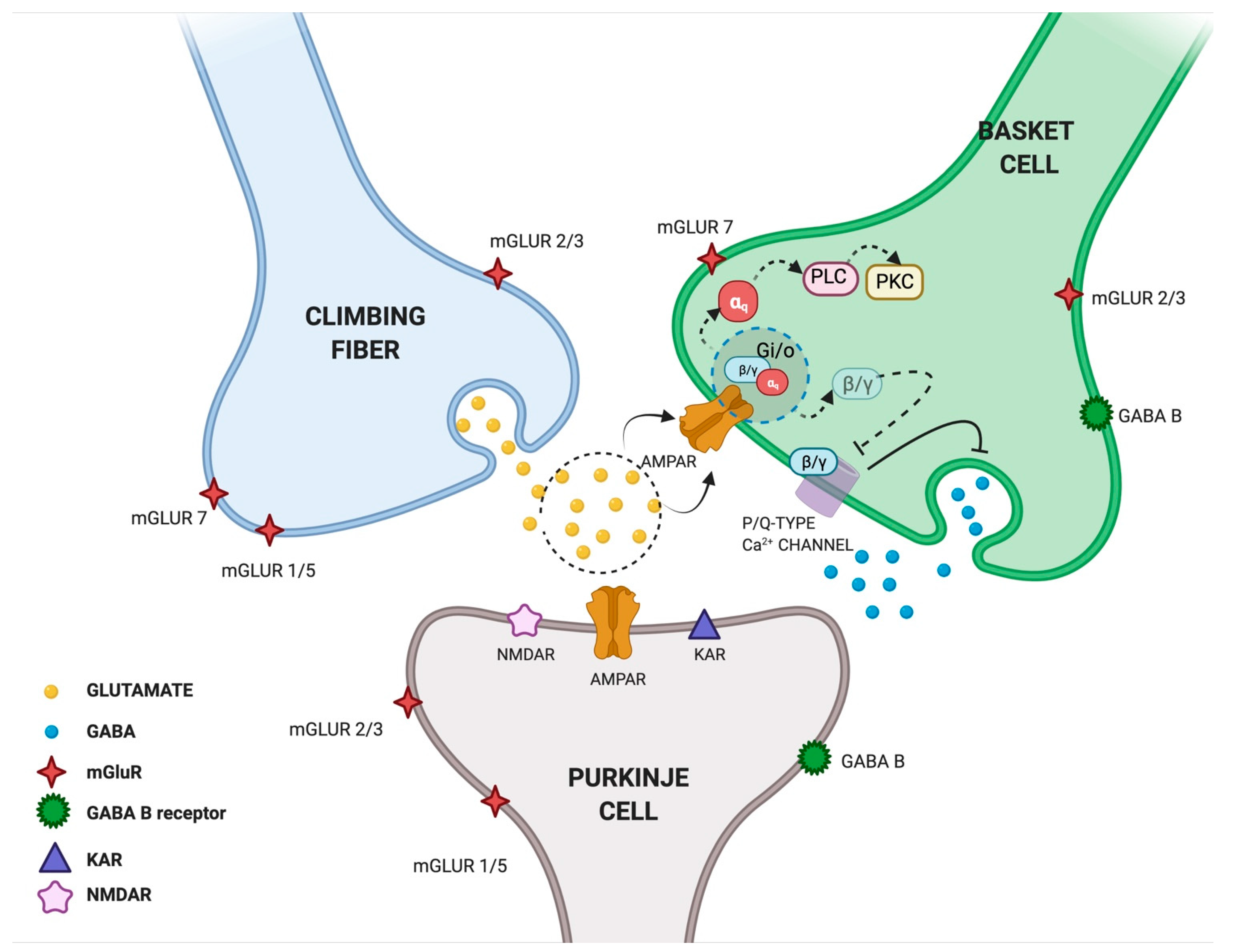
3.1.1. Glutamate
3.1.2. GABA
3.1.3. DA, NA, 5HT and ACh
3.2. AMPAR as Regulator of Axonal Development
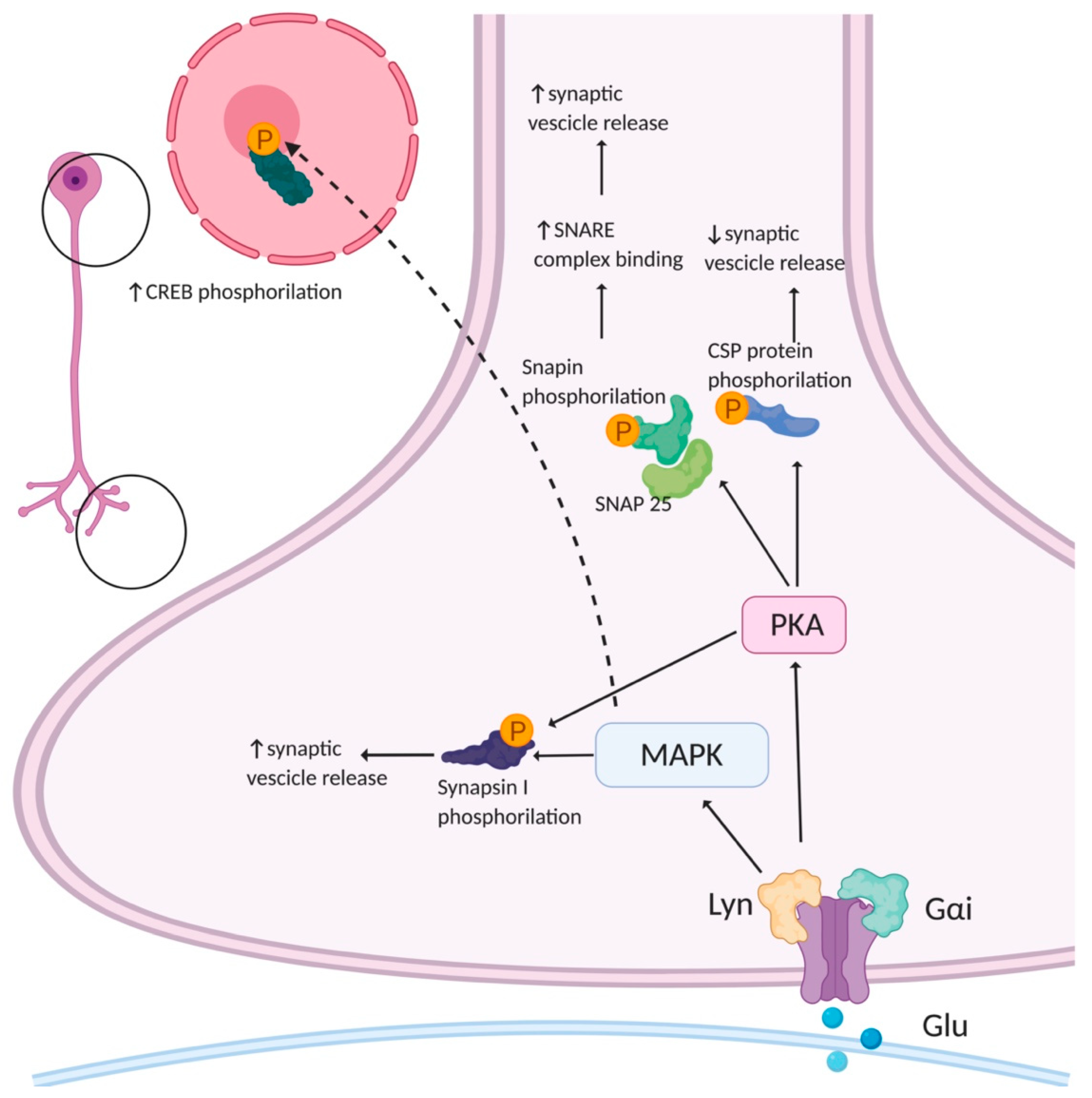
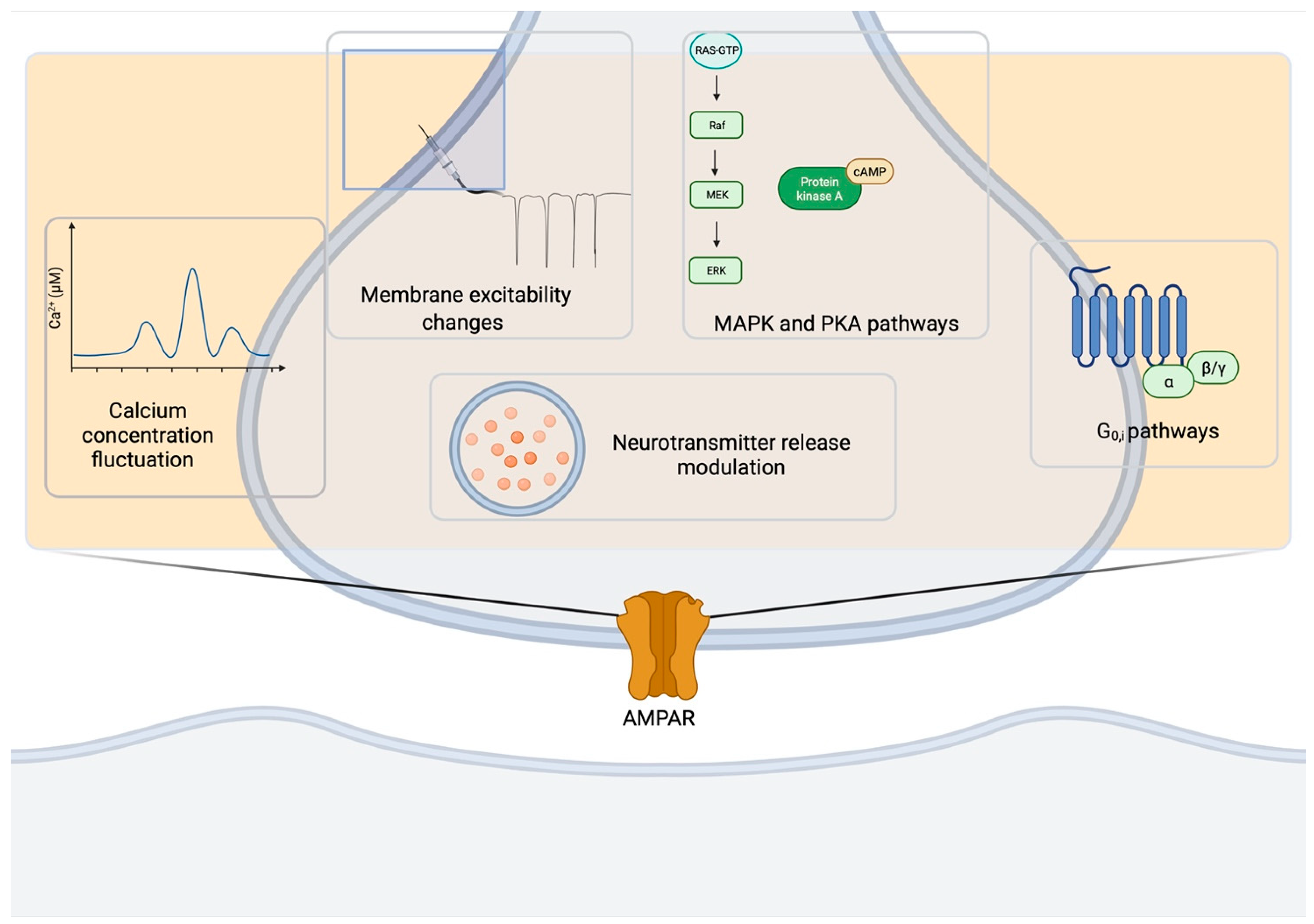
3.3. Signaling Pathways of Presynaptic AMPAR
3.4. Presynaptic AMPAR as Regulator of Synaptic Plasticity
4. Pathological Roles of Presynaptic AMPAR
4.1. Involvement of Presynaptic AMPAR in Axonal Pathology
4.2. Presynaptic AMPAR in Pain Transmission
4.3. Presynaptic AMPAR in the Auditory System
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-AP | 4-aminopyridine |
| 5HT | serotonin |
| ACh | acetylcholine |
| AMPARs | α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors |
| ATP | adenosine triphosphate |
| BCs | basket cells |
| BDNF | brain-derived neurotrophic factor |
| CaM | calmodulin |
| cAMP | cyclic adenosine monophosphate |
| CF | climbing fiber |
| CNQX | 6-cyano-7-nitroquinoxaline-2,3-dione |
| CNS | central nervous system |
| CSP | cysteine string protein |
| CTD | C-terminal cytoplasmic domain |
| DA | dopamine |
| DNQX | 6,7-dinitroquinoxaline-2,3-dione |
| DRG | dorsal root ganglion |
| eIPSCs | evoked inhibitory postsynaptic currents |
| GRIA | Glutamate Receptor Ionotropic AMPA types |
| iGluRs | ionotropic glutamate receptors |
| IPSCs | inhibitory postsynaptic currents |
| KA | kainic acid |
| KARs | kainate receptors |
| LBD | ligand binding domain |
| LM | light microscopy |
| LTD | long-term depression |
| LTP | long-term potentiation |
| MAPK | mitogen-associated protein kinase |
| mGluRs | metabotropic glutamate receptors |
| mIPSPs | miniature inhibitory postsynaptic currents |
| MLIs | molecular layer interneurons |
| NA | noradrenaline |
| NBQX | 2,3-dioxo-6-nitro-7-sulfamoyl-benzo[f]quinoxaline |
| NK1 | Neurokinin-1 |
| NK1R+ | Neurokinin-1 receptor expressing |
| NMDARs | N-methyl-d-aspartate receptor |
| NSSP | non-synaptic synaptosome fractions |
| NTD | N-terminal domain |
| P | postnatal day |
| PAD | primary afferent depolarization |
| PAFs | primary afferent fibers |
| PCs | Purkinje cells |
| PICK1 | protein interacting with C kinase 1 |
| PKA | protein kinase A |
| SNAP-25 | synaptosomal-associated protein-25 |
| SNARE | soluble N-ethylmaleimide–sensitive factor attachment protein receptor |
| SV | synaptic vesicle |
| TCN | trigeminal caudal nucleus |
| TEM | transmission electron microscopy |
| TMD | transmembrane domain |
| V-ATPase | vacuolar H+-ATPase |
| vGLUT | vesicular glutamate transporter |
| VSCCs | voltage-sensitive Ca2+ channel |
References
- Torgner, I.; Kvamme, E. Synthesis of Transmitter Glutamate and the Glial-Neuron Interrelationship. Mol. Chem. Neuropathol. 1990, 12, 11–17. [Google Scholar] [CrossRef]
- Farsi, Z.; Jahn, R.; Woehler, A. Proton Electrochemical Gradient: Driving and Regulating Neurotransmitter Uptake. BioEssays 2017, 39, 1600240. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [PubMed]
- Möykkynen, T.; Coleman, S.K.; Semenov, A.; Keinänen, K. The N-Terminal Domain Modulates α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid (AMPA) Receptor Desensitization. J. Biol. Chem. 2014, 289, 13197–13205. [Google Scholar] [CrossRef] [PubMed]
- Greger, I.H.; Watson, J.F.; Cull-Candy, S.G. Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron 2017, 94, 713–730. [Google Scholar] [CrossRef]
- Burnashev, N.; Villarroel, A.; Sakmann, B. Dimensions and Ion Selectivity of Recombinant AMPA and Kainate Receptor Channels and Their Dependence on Q/R Site Residues. J. Physiol. 1996, 496, 165–173. [Google Scholar] [CrossRef]
- Malinow, R. AMPA Receptor Trafficking and Long-Term Potentiation. Philosophical Transactions of the Royal Society of London. Ser. B Biol. Sci. 2003, 358, 707–714. [Google Scholar] [CrossRef]
- Pei, W.; Huang, Z.; Wang, C.; Han, Y.; Park, J.S.; Niu, L. Flip and Flop: A Molecular Determinant for AMPA Receptor Channel Opening. Biochemistry 2009, 48, 3767–3777. [Google Scholar] [CrossRef]
- Kim, K.S.; Yan, D.; Tomita, S. Assembly and Stoichiometry of the AMPA Receptor and Transmembrane Ampa Receptor Regulatory Protein Complex. J. Neurosci. 2010, 30, 1064–1072. [Google Scholar] [CrossRef]
- Henley, J.M.; Wilkinson, K.A. Synaptic AMPA Receptor Composition in Development, Plasticity and Disease. Nat. Rev. Neurosci. 2016, 17, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Wenthold, R.J.; Petralia, R.S.; Niedzielski, A.S. Evidence for Multiple AMPA Receptor Complexes in Hippocampal CA1/CA2 Neurons. J. Neurosci. 1996, 16, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Reimers, J.M.; Milovanovic, M.; Wolf, M.E. Quantitative Analysis of AMPA Receptor Subunit Composition in Addiction-Related Brain Regions. Brain Res. 2011, 1367, 223–233. [Google Scholar] [CrossRef]
- Shi, S.-H.; Hayashi, Y.; Esteban, J.A.; Malinow, R. Subunit-Specific Rules Governing AMPA Receptor Trafficking to Synapses in Hippocampal Pyramidal Neurons. Cell 2001, 105, 331–343. [Google Scholar] [CrossRef]
- Kamalova, A.; Nakagawa, T. AMPA Receptor Structure and Auxiliary Subunits. J. Physiol. 2021, 599, 453–469. [Google Scholar] [CrossRef]
- Fabian-Fine, R.; Volknandt, W.; Fine, A.; Stewart, M.G. Age-dependent Pre-and Postsynaptic Distribution of AMPA Receptors at Synapses in CA3 Stratum Radiatum of Hippocampal Slice Cultures Compared with Intact Brain. Eur. J. Neurosci. 2000, 12, 3687–3700. [Google Scholar] [CrossRef] [PubMed]
- Schenk, U.; Verderio, C.; Benfenati, F.; Matteoli, M. Regulated Delivery of AMPA Receptor Subunits to the Presynaptic Membrane. EMBO J. 2003, 22, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Feligioni, M.; Holman, D.; Haglerod, C.; Davanger, S.; Henley, J.M. Ultrastructural Localisation and Differential Agonist-induced Regulation of AMPA and Kainate Receptors Present at the Presynaptic Active Zone and Postsynaptic Density. J. Neurochem. 2006, 99, 549–560. [Google Scholar] [CrossRef]
- Rossi, B.; Maton, G.; Collin, T. Calcium-permeable Presynaptic AMPA Receptors in Cerebellar Molecular Layer Interneurones. J. Physiol. 2008, 586, 5129–5145. [Google Scholar] [CrossRef]
- Lu, C.R.; Hwang, S.J.; Phend, K.D.; Rustioni, A.; Valtschanoff, J.G. Primary Afferent Terminals in Spinal Cord Express Presynaptic AMPA Receptors. J. Neurosci. 2002, 22, 9522–9529. [Google Scholar] [CrossRef] [PubMed]
- Ouardouz, M.; Coderre, E.; Zamponi, G.W.; Hameed, S.; Yin, X.; Trapp, B.D.; Stys, P.K. Glutamate Receptors on Myelinated Spinal Cord Axons: II. AMPA and GluR5 Receptors. Ann. Neurol. 2009, 65, 160–166. [Google Scholar] [CrossRef]
- Bernard, V.; Somogyi, P.; Bolam, J.P. Cellular, Subcellular, and Subsynaptic Distribution of AMPA-Type Glutamate Receptor Subunits in the Neostriatum of the Rat. J. Neurosci. 1997, 17, 819–833. [Google Scholar] [CrossRef]
- Fujiyama, F.; Kuramoto, E.; Okamoto, K.; Hioki, H.; Furuta, T.; Zhou, L.; Nomura, S.; Kaneko, T. Presynaptic Localization of an AMPA-type Glutamate Receptor in Corticostriatal and Thalamostriatal Axon Terminals. Eur. J. Neurosci. 2004, 20, 3322–3330. [Google Scholar] [CrossRef]
- Takago, H.; Nakamura, Y.; Takahashi, T. G Protein-Dependent Presynaptic Inhibition Mediated by AMPA Receptors at the Calyx of Held. Proc. Natl. Acad. Sci. USA 2005, 102, 7368–7373. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Furuta, A.; Blackstone, C.D. AMPA Receptor Protein in Developing Rat Brain: Glutamate Receptor-1 Expression and Localization Change at Regional, Cellular, and Subcellular Levels with Maturation. Neuroscience 1998, 83, 917–928. [Google Scholar] [CrossRef]
- Wyszynski, M.; Kim, E.; Dunah, A.W.; Passafaro, M.; Valtschanoff, J.G.; Serra-Pagès, C.; Streuli, M.; Weinberg, R.J.; Sheng, M. Interaction between GRIP and Liprin-α/SYD2 Is Required for AMPA Receptor Targeting. Neuron 2002, 34, 39–52. [Google Scholar] [CrossRef]
- Zhen, M.; Jin, Y. The Liprin Protein SYD-2 Regulates the Differentiation of Presynaptic Termini in C. Elegans. Nature 1999, 401, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Pittaluga, A.; Feligioni, M.; Longordo, F.; Luccini, E.; Raiteri, M. Trafficking of Presynaptic AMPA Receptors Mediating Neurotransmitter Release: Neuronal Selectivity and Relationships with Sensitivity to Cyclothiazide. Neuropharmacology 2006, 50, 286–296. [Google Scholar] [CrossRef]
- Haglerød, C.; Kapic, A.; Boulland, J.L.; Hussain, S.; Holen, T.; Skare, Ø.; Laake, P.; Ottersen, O.P.; Haug, F.M.S.; Davanger, S. Protein Interacting with C Kinase 1 (PICK1) and GluR2 Are Associated with Presynaptic Plasma Membrane and Vesicles in Hippocampal Excitatory Synapses. Neuroscience 2009, 158, 242–252. [Google Scholar] [CrossRef]
- Haglerød, C.; Hussain, S.; Nakamura, Y.; Xia, J.; Haug, F.-M.; Ottersen, O.P.; Henley, J.M.; Davanger, S. Presynaptic PICK1 Facilitates Trafficking of AMPA-Receptors between Active Zone and Synaptic Vesicle Pool. Neuroscience 2017, 344, 102–112. [Google Scholar] [CrossRef]
- Patel, D.R.; Croucher, M.J. Evidence for a Role of Presynaptic AMPA Receptors in the Control of Neuronal Glutamate Release in the Rat Forebrain. Eur. J. Pharmacol. 1997, 332, 143–151. [Google Scholar] [CrossRef]
- Patel, D.R.; Young, A.M.J.; Croucher, M.J. Presynaptic α-Amino-3-Hydroxy-5-Methyl-4-Isoxazole Propionate Receptor-Mediated Stimulation of Glutamate and GABA Release in the Rat Striatum in Vivo: A Dual-Label Microdialysis Study. Neuroscience 2001, 102, 101–111. [Google Scholar] [CrossRef]
- Lee, C.J.; Bardoni, R.; Tong, C.K.; Engelman, H.S.; Joseph, D.J.; Magherini, P.C.; MacDermott, A.B. Functional Expression of AMPA Receptors on Central Terminals of Rat Dorsal Root Ganglion Neurons and Presynaptic Inhibition of Glutamate Release. Neuron 2002, 35, 135–146. [Google Scholar] [CrossRef]
- Keller, B.U.; Hollmann, M.; Heinemann, S.; Konnerth, A. Calcium Influx through Subunits GluR1/GluR3 of Kainate/AMPA Receptor Channels Is Regulated by CAMP Dependent Protein Kinase. EMBO J. 1992, 11, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Goraya, T.A.; Cooper, D.M.F. Ca2+-Calmodulin-Dependent Phosphodiesterase (PDE1): Current Perspectives. Cell. Signal. 2005, 17, 789–797. [Google Scholar] [CrossRef]
- Dohovics, R.; Janáky, R.; Varga, V.; Hermann, A.; Saransaari, P.; Oja, S.S. Regulation of Glutamatergic Neurotransmission in the Striatum by Presynaptic Adenylyl Cyclase-Dependent Processes. Neurochem. Int. 2003, 42, 1–7. [Google Scholar] [CrossRef]
- Bureau, I.; Mulle, C. Potentiation of GABAergic Synaptic Transmission by AMPA Receptors in Mouse Cerebellar Stellate Cells: Changes during Development. J. Physiol. 1998, 509, 817–831. [Google Scholar] [CrossRef]
- Fiszman, M.L.; Erdélyi, F.; Szabó, G.; Vicini, S. Presynaptic AMPA and Kainate Receptors Increase the Size of GABAergic Terminals and Enhance GABA Release. Neuropharmacology 2007, 52, 1631–1640. [Google Scholar] [CrossRef][Green Version]
- Satake, S.; Saitow, F.; Yamada, J.; Konishi, S. Synaptic Activation of AMPA Receptors Inhibits GABA Release from Cerebellar Interneurons. Nat. Neurosci. 2000, 3, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Satake, S.I.; Saitow, F.; Rusakov, D.; Konishi, S. AMPA Receptor-Mediated Presynaptic Inhibition at Cerebellar GABAergic Synapses: A Characterization of Molecular Mechanisms. Eur. J. Neurosci. 2004, 19, 2464–2474. [Google Scholar] [CrossRef]
- Rusakov, D.A.; Saitow, F.; Lehre, K.P.; Konishi, S. Modulation of Presynaptic Ca2+ Entry by AMPA Receptors at Individual GABAergic Synapses in the Cerebellum. J. Neurosci. 2005, 25, 4930–4940. [Google Scholar] [CrossRef]
- Engelman, H.S.; Anderson, R.L.; Daniele, C.; MacDermott, A.B. Presynaptic Alpha-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid (AMPA) Receptors Modulate Release of Inhibitory Amino Acids in Rat Spinal Cord Dorsal Horn. Neuroscience 2006, 139, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Malva, J.O.; Carvalho, A.P.; Carvalho, C.M. Modulation of Dopamine and Noradrenaline Release and of Intracellular Ca2+ Concentration by Presynaptic Glutamate Receptors in Hippocampus. Br. J. Pharmacol. 1994, 113, 1439–1447. [Google Scholar] [CrossRef]
- Desce, J.M.; Godeheu, G.; Galli, T.; Artaud, F.; Cheramy, A.; Glowinski, J. Presynaptic Facilitation of Dopamine Release through D,L-α-Amino-3- Hydroxy-5-Methyl-4-Isoxazole Propionate Receptors on Synaptosomes from the Rat Striatum. J. Pharmacol. Exp. Ther. 1991, 259, 692–698. [Google Scholar] [PubMed]
- Imperato, A.; Honore, T.; Jensen, L.H. Dopamine Release in the Nucleus Caudatus and in the Nucleus Accumbens Is under Glutamatergic Control through Non-NMDA Receptors: A Study in Freely-Moving Rats. Brain Res. 1990, 530, 223–228. [Google Scholar] [CrossRef]
- Langer, S.Z. Presynaptic Regulation of the Release of Catecholamines. Pharmacol. Rev. 1980, 32, 337–362. [Google Scholar] [CrossRef]
- Lupp, A.; Lücking, C.H.; Koch, R.; Jackisch, R.; Feuerstein, T.J. Inhibitory Effects of the Antiparkinsonian Drugs Memantine and Amantadine on N-Methyl-D-Aspartate-Evoked Acetylcholine Release in the Rabbit Caudate Nucleus in Vitro. J. Pharmacol. Exp. Ther. 1992, 263, 717–724. [Google Scholar] [PubMed]
- Jin, S.; Fredholm, B.B. Role of NMDA, AMPA and Kainate Receptors in Mediating Glutamate- and 4-AP-Induced Dopamine and Acetylcholine Release from Rat Striatal Slices. Neuropharmacology 1994, 33, 1039–1048. [Google Scholar] [CrossRef]
- Morari, M.; Sbrenna, S.; Marti, M.; Caliari, F.; Bianchi, C.; Beani, L. NMDA and Non-NMDA Ionotropic Glutamate Receptors Modulate Striatal Acetylcholine Release via Pre- and Postsynaptic Mechanisms. J. Neurochem. 2002, 71, 2006–2017. [Google Scholar] [CrossRef]
- Giovannini, M.G.; Camilli, F.; Mundula, A.; Bianchi, L.; Colivicchi, M.A.; Pepeu, G. Differential Regulation by N-Methyl-D-Aspartate and NON-N-Methyl-D-Aspartate Receptoors of Acetylcholine Release from the Rat Striatum In Vivo. Neuroscience 1995, 65, 409–415. [Google Scholar] [CrossRef]
- Desce, J.M.; Godf, G.; Galli, T.; Glovvinski, J. L-Glutamate-Evoked Release of Dopamine from Synaptosomes of the Rat Striatum: Involvement Of AMPA And N-Methyl-D-Aspartate Receptors. Neuroscience 1992, 47, 333–339. [Google Scholar] [CrossRef]
- Partin, K.M.; Patneau, D.K.; Winters, C.A.; Mayer, M.L.; Buonanno, A. Selective Modulation of Desensitization at AMPA versus Kainate Receptors by Cyclothiazide and Concanavalin A. Neuron 1993, 11, 1069–1082. [Google Scholar] [CrossRef]
- Pittaluga, A.; Thellung, S.; Maura, G.; Raiteri, M.; Genova, U.; Cembrano, V. Characterization of Two Central AMPA-Preferring Receptors Having Distinct Location, Function and Pharmacology. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1994, 349, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.; Schmitz, V.; Böing, C.; Göthert, M. Stimulation of Serotonin Release in the Rat Brain Cortex by Activation of Ionotropic Glutamate Receptors and Its Modulation via A2-Heteroreceptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1995, 352, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Pittaluga, A.; Bonfanti, A.; Raiteri, M. Differential Desensitization of Ionotropic Non-NMDA Receptors Having Distinct Neuronal Location and Function. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1997, 356, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ziv, N.E.; Smith, S.J. Evidence for a Role of Dendritic Filopodia in Synaptogenesis and Spine Formation. Neuron 1996, 17, 91–102. [Google Scholar] [CrossRef]
- Jontes, J.D.; Smith, S.J. Filopodia, Spines, and the Generation of Synaptic Diversity. Neuron 2000, 27, 11–14. [Google Scholar] [CrossRef]
- Hering, H.; Sheng, M.; Medical, H.H. Dendritic Spines: Structure, Function and Regulation. Nat. Rev. Neurosci. 2001, 2, 880–888. [Google Scholar] [CrossRef]
- Matus, A. Growth of Dendritic Spines: A Continuing Story. Curr. Opin. Neurobiol. 2005, 15, 67–72. [Google Scholar] [CrossRef]
- Ozcan, A.S. Filopodia: A Rapid Structural Plasticity Substrate for Fast Learning. Front. Synaptic Neurosci. 2017, 9, 12. [Google Scholar] [CrossRef]
- McKinney, R.A.; Capogna, M.; Dürr, R.; Gähwiler, B.H.; Thompson, S.M. Miniature Synaptic Events Maintain Dendritic Spines via AMPA Receptor Activation. Nat. Neurosci. 1999, 2, 44–49. [Google Scholar] [CrossRef]
- Fischer, M.; Kaech, S.; Knutti, D.; Matus, A. Rapid Actin-Based Plasticity in Dendritic Spines. Neuron 1998, 20, 847–854. [Google Scholar] [CrossRef]
- Kaech, S.; Fischer, M.; Doll, T.; Matus, A. Isoform Specificity in the Relationship of Actin to Dendritic Spines. J. Neurosci. 1997, 17, 9565–9572. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Kaech, S.; Wagner, U.; Brinkhaus, H.; Matus, A. Glutamate Receptors Regulate Actin-Based Plasticity in Dendritic Spines. Nat. Neurosci. 2000, 3, 887–894. [Google Scholar] [CrossRef]
- Chang, S.; de Camilli, P. Glutamate Regulates Actin-Based Motility in Axonal Filopodia. Nat. Neurosci. 2001, 4, 787–793. [Google Scholar] [CrossRef]
- Wang, Y.; Durkin, J.P. α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid, but Not N-Methyl- D-Aspartate, Activates Mitogen-Activated Protein Kinase through G-Protein Βγ Subunits in Rat Cortical Neurons. J. Biol. Chem. 1995, 270, 22783–22787. [Google Scholar] [CrossRef] [PubMed]
- Perkinton, M.S.; Sihra, T.S.; Williams, R.J. Ca2+-Permeable AMPA Receptors Induce Phosphorylation of CAMP Response Element-Binding Protein through a Phosphatidylinositol 3-Kinase-Dependent Stimulation of the Mitogen-Activated Protein Kinase Signaling Cascade in Neurons. J. Neurosci. 1999, 19, 5861–5874. [Google Scholar] [CrossRef]
- Wang, Y.; Small, D.L.; Stanimirovic, D.B.; Morley, P.; Durkin, J.P. AMPA Receptor-Mediated Regulation of a G(i)-Protein in Cortical Neurons. Nature 1997, 389, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Umemori, H.; Mishina, M.; Yamamoto, T. The AMPA Receptor Interacts with and Signals through the Protein Tyrosine Kinase Lyn. Nature 1999, 397, 72–76. [Google Scholar] [CrossRef]
- Kawai, F.; Sterling, P. AMPA Receptor Activates a G-Protein That Suppresses a CGMP-Gated Current. J. Neurosci. 1999, 19, 2954–2959. [Google Scholar] [CrossRef]
- Marin, P.; Fagni, L.; Torrens, Y.; Alcaraz, G.; Couraud, F.; Bockaert, J.; Glowinski, J.; Prémont, J. Ampa Receptor Activation Induces Association of G-Beta Protein with the Alpha Subunit of the Sodium Channel in Neurons. Eur. J. Neurosci. 2001, 14, 1953–1960. [Google Scholar] [CrossRef]
- Schenk, U.; Menna, E.; Kim, T.; Passafaro, M.; Chang, S.; de Camilli, P.; Matteoli, M. A Novel Pathway for Presynaptic Mitogen-Activated Kinase Activation via AMPA Receptors. J. Neurosci. 2005, 25, 1654–1663. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jovanovic, J.N.; Benfenati, F.; Siow, Y.L.; Sihra, T.S.; Sanghera, J.S.; Pelech, S.L.; Greengard, P.; Czernik, A.J. Neurotrophins Stimulate Phosphorylation of Synapsin I by MAP Kinase and Regulate Synapsin I-Actin Interactions. Proc. Natl. Acad. Sci. USA 1996, 93, 3679–3683. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Chang, L. Mammalian MAP Kinase Signaling Cascades. Nature 2001, 410, 37–40. [Google Scholar]
- Hilfiker, S.; Pieribone, V.A.; Czernik, A.J.; Kao, H.-T.; Augustine, G.J.; Greengard, P. Synapsins as Regulators of Neurotransmitter Release. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1999, 354, 269–279. [Google Scholar] [CrossRef]
- Matsubara, M.; Kusubata, M.; Ishiguro, K.; Uchida, T.; Titani, K.; Taniguchi, H. Site-Specific Phosphorylation of Synapsin I by Mitogen-Activated Protein Kinase and Cdk5 and Its Effects on Physiological Functions. J. Biol. Chem. 1996, 271, 21108–21113. [Google Scholar] [CrossRef]
- Jovanovic, J.N.; Czernik, A.J.; Fienberg, A.A.; Greengard, P.; Sihra, T.S. Synapsins as Mediators of BDNF-Enhanced Neurotransmitter Release. Nat. Neurosci. 2000, 3, 323–329. [Google Scholar] [CrossRef]
- Chi, P.; Greengard, P.; Ryan, T.A. Synaptic Vesicle Mobilization Is Regulated by Distinct Synapsin I Phosphorylation Pathways at Different Frequencies. Neuron 2003, 38, 69–78. [Google Scholar] [CrossRef]
- Chi, P.; Greengard, P.; Ryan, T.A. Synapsin Dispersion and Reclustering during Synaptic Activity. Nat. Neurosci. 2001, 4, 1187–1193. [Google Scholar] [CrossRef]
- Schenk, U.; Matteoli, M. Presynaptic AMPA Receptors: More than Just Ion Channels? Biol. Cell 2004, 96, 257–260. [Google Scholar] [CrossRef]
- Evans, G.J.O.; Morgan, A. Phosphorylation-Dependent Interaction of the Synaptic Vesicle Proteins Cysteine String Protein and Synaptotagmin I. Biochem. J. 2002, 364, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Chheda, M.G.; Ashery, U.; Thakur, P.; Rettig, J.; Sheng, Z.-H. Phosphorylation of Snapin by PKA Modulates Its Interaction with the SNARE Complex. Nat. Cell Biol. 2001, 3, 331. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Calakos, N. Presynaptic Long-Term Plasticity. Front. Synaptic Neurosci. 2013, 5, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Akins, M.R.; Berk-Rauch, H.E.; Kwan, K.Y.; Mitchell, M.E.; Shepard, K.A.; Korsak, L.I.T.; Stackpole, E.E.; Warner-Schmidt, J.L.; Sestan, N.; Cameron, H.A. Axonal Ribosomes and MRNAs Associate with Fragile X Granules in Adult Rodent and Human Brains. Hum. Mol. Genet. 2017, 26, 192–209. [Google Scholar] [CrossRef]
- Thieffry, M.; Bruner, J. Direct Evidence for a Presynaptic Action of Glutamate at a Crayfish Neuromuscular Junction. Brain Res. 1978, 156, 402–406. [Google Scholar] [CrossRef]
- Garcia-Munoz, M.; Young, S.J.; Groves, P.M. Terminal Excitability of the Corticostriatal Pathway. II. Regulation by Glutamate Receptor Stimulation. Brain Res. 1991, 551, 207–215. [Google Scholar] [CrossRef]
- Kullmann, D.M.; Erdemli, G.; Asztély, F. LTP of AMPA and NMDA Receptor-Mediated Signals: Evidence for Presynaptic Expression and Extrasynaptic Glutamate Spill-Over. Neuron 1996, 17, 461–474. [Google Scholar] [CrossRef]
- Calabresi, P.; Centonze, D.; Gubellini, P.; Marfia, G.A.; Bernardi, G. Glutamate-Triggered Events Inducing Corticostriatal Long-Term Depression. J. Neurosci. 1999, 19, 6102–6110. [Google Scholar] [CrossRef]
- Garcia-Munoz, M.; Patino, P.; Masliah, E.; Young, S.J.; Groves, P.M. Glutamate-Dependent Long-Term Presynaptic Changes in Corticostriatal Excitability. Neuroscience 1996, 73, 109–119. [Google Scholar] [CrossRef]
- Granzotto, A.; Canzoniero, L.M.T.; Sensi, S.L. A Neurotoxic Ménage-à-Trois: Glutamate, Calcium, and Zinc in the Excitotoxic Cascade. Front. Mol. Neurosci. 2020, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What Is the Evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef]
- Mattson, M.P. Excitotoxicity. In Stress: Physiology, Biochemistry, and Pathology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 125–134. [Google Scholar]
- Gill, S.S.; Pulido, O.M. Glutamate Receptors in Peripheral Tissues: Current Knowledge, Future Research, and Implications for Toxicology. Toxicol. Pathol. 2001, 29, 208–223. [Google Scholar] [CrossRef]
- Kopach, O.; Voitenko, N. Spinal AMPA Receptors: Amenable Players in Central Sensitization for Chronic Pain Therapy? Channels 2021, 15, 284–297. [Google Scholar] [CrossRef]
- Currò, D.; Navarra, P.; Samengo, I.; Martire, M. P2X7 Receptors Exert a Permissive Effect on the Activation of Presynaptic AMPA Receptors in Rat Trigeminal Caudal Nucleus Glutamatergic Nerve Terminals. J. Headache Pain 2020, 21, 1–10. [Google Scholar]
- Takago, H.; Oshima-Takago, T. Pre-and Postsynaptic Ionotropic Glutamate Receptors in the Auditory System of Mammals. Hear. Res. 2018, 362, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, A.; Laake, J.H.; Davanger, S.; Usami, S.; Ottersen, O.P. Organization of AMPA Receptor Subunits at a Glutamate Synapse: A Quantitative Immunogold Analysis of Hair Cell Synapses in the Rat Organ of Corti. J. Neurosci. 1996, 16, 4457–4467. [Google Scholar] [CrossRef]
- Sanchez-Vives, M.V.; Barbero-Castillo, A.; Perez-Zabalza, M.; Reig, R. GABAB Receptors: Modulation of Thalamocortical Dynamics and Synaptic Plasticity. Neuroscience 2021, 456, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Lovinger, D.M. Presynaptic Modulation by Endocannabinoids. Pharmacol. Neurotransm. Release 2008, 184, 435–477. [Google Scholar]
- Upreti, C.; Zhang, X.; Alford, S.; Stanton, P.K. Role of Presynaptic Metabotropic Glutamate Receptors in the Induction of Long-Term Synaptic Plasticity of Vesicular Release. Neuropharmacology 2013, 66, 31–39. [Google Scholar] [CrossRef][Green Version]
- Falcón-Moya, R.; Rodríguez-Moreno, A. Metabotropic Actions of Kainate Receptors Modulating Glutamate Release. Neuropharmacology 2021, 197, 108696. [Google Scholar] [CrossRef]
- Brasier, D.J.; Feldman, D.E. Synapse-Specific Expression of Functional Presynaptic NMDA Receptors in Rat Somatosensory Cortex. J. Neurosci. 2008, 28, 2199–2211. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, E.; Feuerstein, T. Human Presynaptic Receptors. Pharmacol. Ther. 2017, 172, 1–21. [Google Scholar] [CrossRef]
- Talay, R.S.; Liu, Y.; Michael, M.; Li, A.; Friesner, I.D.; Zeng, F.; Sun, G.; Chen, Z.S.; Zhang, Q.; Wang, J. Pharmacological Restoration of Anti-Nociceptive Functions in the Prefrontal Cortex Relieves Chronic Pain. Prog. Neurobiol. 2021, 201, 102001. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, Q.; Liu, Y.; Sun, G.; Li, A.; Talay, R.S.; Wang, J. AMPAkines Potentiate the Corticostriatal Pathway to Reduce Acute and Chronic Pain. Mol. Brain 2021, 14, 1–14. [Google Scholar] [CrossRef]
- Su, C.; Lin, H.Y.; Yang, R.; Xu, D.; Lee, M.; Pawlak, N.; Norcini, M.; Sideris, A.; Recio-Pinto, E.; Huang, D. AMPAkines Target the Nucleus Accumbens to Relieve Postoperative Pain. Anesthesiology 2016, 125, 1030–1043. [Google Scholar] [CrossRef][Green Version]
- Gordillo-Salas, M.; Pascual-Antón, R.; Ren, J.; Greer, J.; Adell, A. Antidepressant-like Effects of CX717: A Positive Allosteric Modulator of AMPA Receptors. Mol. Neurobiol. 2020, 57, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Radin, D.P.; Johnson, S.; Purcell, R.; Lippa, A.S. Effects of Chronic Systemic Low-Impact Ampakine Treatment on Neurotrophin Expression in Rat Brain. Biomed. Pharmacother. 2018, 105, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Lauterborn, J.C.; Lynch, G.; Vanderklish, P.; Arai, A.; Gall, C.M. Positive Modulation of AMPA Receptors Increases Neurotrophin Expression by Hippocampal and Cortical Neurons. J. Neurosci. 2000, 20, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Seese, R.R.; Le, A.A.; Wang, K.; Cox, C.D.; Lynch, G.; Gall, C.M. A TrkB Agonist and Ampakine Rescue Synaptic Plasticity and Multiple Forms of Memory in a Mouse Model of Intellectual Disability. Neurobiol. Dis. 2020, 134, 104604. [Google Scholar] [CrossRef] [PubMed]
| Neurotransmitter | Preparation | Methods | AMPAR Effect | Reference(s) |
|---|---|---|---|---|
| Glutamate | Rat forebrain slices | Loading of brain slices with [3H]D-aspartate and measurement of [3H]D-aspartate release in basal conditions and after administration of either L-glutamate, or racemic (R,S)-AMPA | L-glutamate and racemic (R,S)-AMPA dose-dependently enhanced [3H]-D-aspartate release | [31] |
| Rat neostriatum, in vivo analysis | Intracerebral microdialysis | AMPA application increased Ca2+-dependent efflux of both [3H]-glutamate and [14C]-GABA in a dose-dependent manner | [32] | |
| PAFs of DRG neurons | Recording of receptor-mediated depolarization of the central terminals | Inhibition of glutamate release from primary afferent terminals in the superficial layers of the dorsal horn Induction of PAD | [33] | |
| GABA | Cerebellar MLIs | Single-cell RT-PCR | Increase in GABAergic synaptic transmission | [19] |
| Cerebellar interneurons | Measurement of GABA release after AMPAR activation by domoate | Potentiation of GABAergic synaptic activity in stellate cells | [37] | |
| Primary cultures of cerebellar neurons derived from GFP-GAD65 transgenic mice | Measurement of GABA release after AMPAR activation by AMPA and kainate Whole-cell recordings | Increase in the size of GABAergic terminals of interneurons Increase in spontaneous synaptic GABA release | [38] | |
| Presynaptic terminals of interneuron BC | Recording of IPSCs from PCs and measurement of intracellular Ca2+ fluctuations at BC terminals | Inhibition of GABAergic transmission through AMPAR-mediated activation of GTP-binding proteins (Gi/o proteins) and inhibition of the activity of P/Q-type Ca2+ channel | [39,40,41] | |
| Superficial dorsal horn of the rat spinal cord at second postnatal week Dorsal horn slices prepared from P27–P30 rat spinal cord | Recording of eIPSCs and mIPSPs | Increase in spontaneous GABA release Increase in mIPSP frequency Modulation of pain signaling | [42] | |
| DA and NA | Purified synaptosomes from rat striatum | Superfusion with [3H]-tyrosine and measurement of newly synthesized [3H]DA release | Kainate and quisqualate facilitate DA release | [44,51,52] |
| Hyppocampal synaptosomes | Superfusion system and a fluorimetric assay | AMPARs activation increase DA and NA release Increase in NA release in a Ca2+-dependent manner | [43] | |
| Rat hippocampus synaptosomes | Application of increasing AMPA concentrations | Release of preloaded [3H]NA in a Ca2+-dependent manner | [53] | |
| Hippocampal noradrenergic axon terminals | Activation of AMPAR | Induction of NA release | [28] | |
| 5HT | Rat brain cortex slices | Preincubation with [3H]5HT and superfusion in the presence of 6-nitroquipazine, Mg2+, KA or AMPA | Increase in 5HT release | [54] |
| Hippocampal synaptosomes | Superfusion with AMPA | Ca2+-dependent DNQX-sensitive increase in 5HT release | [55] | |
| ACh | Slices of rabbit caudate nucleus | Incubation with [3H]ACh and stimulation with NMDA, AMPA, L-glutamate or KA | Increase in ACh efflux | [47] |
| Rat striatal slices | Incubation with [3H]ACh and treatment with 4-AP | Increase in ACh release | [48] | |
| Rat striatal slices and synaptosomes | Stimulation of NMDA and non-NMDA ionotropic receptors Application of AMPA | Facilitation of striatal Ach release Inhibition of ACh release | [49] | |
| Striatum of moving rats | Local administration of AMPA through a transversal microdialysis probe | Decrease in ACh output | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanetti, L.; Regoni, M.; Ratti, E.; Valtorta, F.; Sassone, J. Presynaptic AMPA Receptors in Health and Disease. Cells 2021, 10, 2260. https://doi.org/10.3390/cells10092260
Zanetti L, Regoni M, Ratti E, Valtorta F, Sassone J. Presynaptic AMPA Receptors in Health and Disease. Cells. 2021; 10(9):2260. https://doi.org/10.3390/cells10092260
Chicago/Turabian StyleZanetti, Letizia, Maria Regoni, Elena Ratti, Flavia Valtorta, and Jenny Sassone. 2021. "Presynaptic AMPA Receptors in Health and Disease" Cells 10, no. 9: 2260. https://doi.org/10.3390/cells10092260
APA StyleZanetti, L., Regoni, M., Ratti, E., Valtorta, F., & Sassone, J. (2021). Presynaptic AMPA Receptors in Health and Disease. Cells, 10(9), 2260. https://doi.org/10.3390/cells10092260









