Housekeeping Genes for Parkinson’s Disease in Humans and Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Mice
2.3. Modeling of Parkinson-like Phenotype with MPTP
2.4. Expression Analysis
RNA Isolation
2.5. Analysis of Changes in Relative Levels of mRNAs
2.6. Statistical Analysis
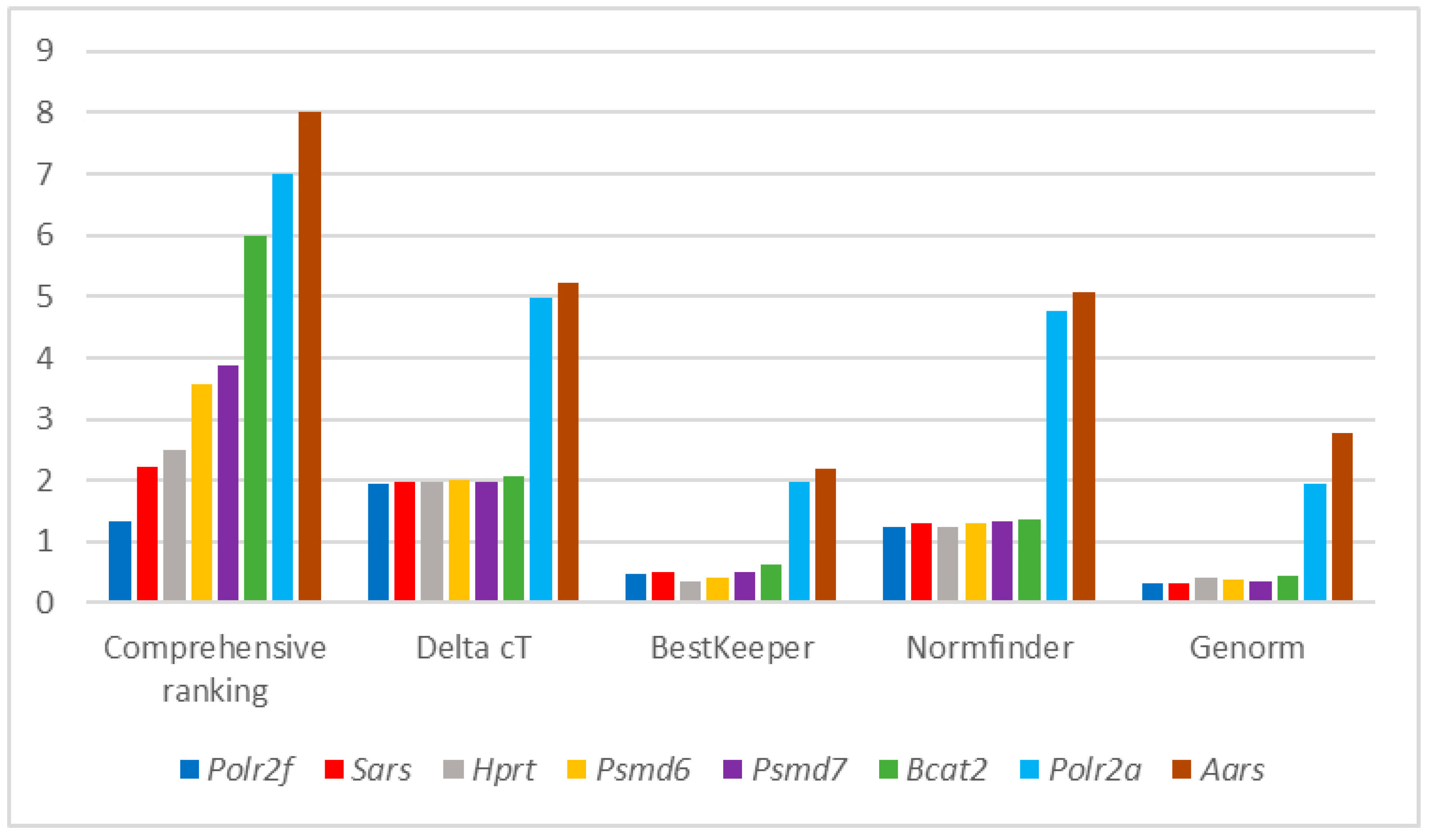
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Guilder, H.D.; Vrana, K.E.; Freeman, W.M. Twenty-five years of quantitative PCR for gene expression analysis. BioTechniques 2008, 44, 619–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dheda, K.; Huggett, J.F.; Bustin, S.A.; Johnson, M.A.; Rook, G.; Zumla, A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. BioTechniques 2004, 37, 112–114, 116, 118–119. [Google Scholar] [CrossRef] [Green Version]
- Chervoneva, I.; Li, Y.; Schulz, S.; Croker, S.; Wilson, C.; Waldman, S.A.; Hyslop, T. Selection of optimal reference genes for normalization in quantitative RT-PCR. BMC Bioinform. 2010, 11, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thellin, O.; ElMoualij, B.; Heinen, E.; Zorzi, W. A decade of improvements in quantification of gene expression and internal standard selection. Biotechnol. Adv. 2009, 27, 323–333. [Google Scholar] [CrossRef]
- Takagi, S.; Ohashi, K.; Utoh, R.; Tatsumi, K.; Shima, M.; Okano, T. Suitable reference genes for the analysis of direct hyperplasia in mice. Biochem. Biophys. Res. Commun. 2008, 377, 1259–1264. [Google Scholar] [CrossRef]
- Tatsumi, K.; Ohashi, K.; Taminishi, S.; Okano, T.; Yoshioka, A.; Shima, M. Reference gene selection for real-time RT-PCR in regenerating mouse livers. Biochem. Biophys. Res. Commun. 2008, 374, 106–110. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, C.L.; Jensen, J.L.; Orntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef]
- Kin, K.; Yasuhara, T.; Kameda, M.; Date, I. Animal Models for Parkinson’s Disease Research: Trends in the 2000s. Int. J. Mol. Sci. 2019, 20, 5402. [Google Scholar] [CrossRef] [Green Version]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [Green Version]
- The Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011.
- Suslov, O.; Steindler, D.A. PCR inhibition by reverse transcriptase leads to an overestimation of amplification efficiency. Nucleic Acids Res. 2005, 33, e181. [Google Scholar] [CrossRef] [PubMed]
- NCBI Database. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 15 July 2019).
- Primer3. Available online: http://bioinfo.ut.ee/primer3 (accessed on 15 July 2019).
- Primer-BLAST. Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 15 July 2019).
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Maurer-Morelli, C.V.; de Vasconcellos, J.F.; Reis-Pinto, F.C.; Rocha Cde, S.; Domingues, R.R.; Yasuda, C.L.; Tedeschi, H.; De Oliveira, E.; Cendes, F.; Lopes-Cendes, I. A comparison between different reference genes for expression studies in human hippocampal tissue. J. Neurosci. Methods 2012, 208, 44–47. [Google Scholar] [CrossRef]
- Penna, I.; Vella, S.; Gigoni, A.; Russo, C.; Cancedda, R.; Pagano, A. Selection of candidate housekeeping genes for normalization in human postmortem brain samples. Int. J. Mol. Sci. 2011, 12, 5461–5470. [Google Scholar] [CrossRef] [Green Version]
- Gebhardt, F.M.; Scott, H.A.; Dodd, P.R. Housekeepers for accurate transcript expression analysis in Alzheimer’s disease autopsy brain tissue. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2010, 6, 465–474. [Google Scholar] [CrossRef]
- Swijsen, A.; Nelissen, K.; Janssen, D.; Rigo, J.M.; Hoogland, G. Validation of reference genes for quantitative real-time PCR studies in the dentate gyrus after experimental febrile seizures. BMC Res. Notes 2012, 5, 685. [Google Scholar] [CrossRef] [Green Version]
- Harrison, P.J.; Laatikainen, L.M.; Tunbridge, E.M.; Eastwood, S.L. Human brain weight is correlated with expression of the ‘housekeeping genes’ beta-2-microglobulin (beta2M) and TATA-binding protein (TBP). Neuropathol. Appl. Neurobiol. 2010, 36, 498–504. [Google Scholar] [CrossRef]
- He, Y.X.; Zhang, Y.; Yang, Q.; Wang, C.; Su, G. Selection of suitable reference genes for reverse transcription-quantitative polymerase chain reaction analysis of neuronal cells differentiated from bone mesenchymal stem cells. Mol. Med. Rep. 2015, 12, 2291–2300. [Google Scholar] [CrossRef]
- Warrington, J.A.; Nair, A.; Mahadevappa, M.; Tsyganskaya, M. Comparison of human adult and fetal expression and identification of 535 housekeeping/maintenance genes. Physiol. Genom. 2000, 2, 143–147. [Google Scholar] [CrossRef]
- Chapman, J.R.; Waldenstrom, J. With Reference to Reference Genes: A Systematic Review of Endogenous Controls in Gene Expression Studies. PLoS ONE 2015, 10, e0141853. [Google Scholar] [CrossRef]
- Hoerndli, F.J.; Toigo, M.; Schild, A.; Gotz, J.; Day, P.J. Reference genes identified in SH-SY5Y cells using custom-made gene arrays with validation by quantitative polymerase chain reaction. Anal. Biochem. 2004, 335, 30–41. [Google Scholar] [CrossRef]
- Alieva, A.K.; Filatova, E.V.; Karabanov, A.V.; Illarioshkin, S.N.; Slominsky, P.A.; Shadrina, M.I. Potential Biomarkers of the Earliest Clinical Stages of Parkinson’s Disease. Parkinson’s Dis. 2015, 2015, 294396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrents, D.; Suyama, M.; Zdobnov, E.; Bork, P. A genome-wide survey of human pseudogenes. Genome Res. 2003, 13, 2559–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCBI-Gene/ACTB Pseudogenes. Available online: https://www.ncbi.nlm.nih.gov/gene/?Term=related_functional_gene_60%5Bgroup%5D (accessed on 8 June 2021).
- NCB-Gene/GUSB Pseudogenes. Available online: https://www.ncbi.nlm.nih.gov/gene/?Term=related_functional_gene_2990%5Bgroup%5D (accessed on 8 June 2021).
- NCBI-Gene/PPIA Pseudogenes. Available online: https://www.ncbi.nlm.nih.gov/gene/?Term=related_functional_gene_5478%5Bgroup%5D (accessed on 8 June 2021).
- NCBI-Gene/YWHAZ Pseudogenes. Available online: https://www.ncbi.nlm.nih.gov/gene/?Term=related_functional_gene_7534%5Bgroup%5D (accessed on 8 June 2021).
- NCBI-Gene/RPL30 Pseudogenes. Available online: https://www.ncbi.nlm.nih.gov/gene/?Term=related_functional_gene_6156%5Bgroup%5D (accessed on 8 June 2021).
- NCBI-Gene/GAPDH Pseudogenes. Available online: https://www.ncbi.nlm.nih.gov/gene/?Term=related_functional_gene_2597%5Bgroup%5D (accessed on 8 June 2021).
- BioGPS. Available online: http://biogps.org/#goto=genereport&id=567 (accessed on 8 June 2021).
- Panina, Y.; Germond, A.; Masui, S.; Watanabe, T.M. Validation of Common Housekeeping Genes as Reference for qPCR Gene Expression Analysis During iPS Reprogramming Process. Sci. Rep. 2018, 8, 8716. [Google Scholar] [CrossRef] [PubMed]
- Panina, Y.; Germond, A.; Watanabe, T.M. Analysis of the stability of 70 housekeeping genes during iPS reprogramming. Sci. Rep. 2020, 10, 21711. [Google Scholar] [CrossRef]
- Salari, S.; Bagheri, M. In vivo, in vitro and pharmacologic models of Parkinson’s disease. Physiol. Res. 2019, 68, 17–24. [Google Scholar] [CrossRef]
- German, D.C.; Nelson, E.L.; Liang, C.L.; Speciale, S.G.; Sinton, C.M.; Sonsalla, P.K. The neurotoxin MPTP causes degeneration of specific nucleus A8, A9 and A10 dopaminergic neurons in the mouse. Neurodegener. A J. Neurodegener. Disord. Neuroprot. Neuroregener. 1996, 5, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Meredith, G.E.; Totterdell, S.; Potashkin, J.A.; Surmeier, D.J. Modeling PD pathogenesis in mice: Advantages of a chronic MPTP protocol. Parkinsonism Relat. Disord. 2008, 14 (Suppl. 2), S112–S115. [Google Scholar] [CrossRef] [Green Version]
- Ugrumov, M.V.; Khaindrava, V.G.; Kozina, E.A.; Kucheryanu, V.G.; Bocharov, E.V.; Kryzhanovsky, G.N.; Kudrin, V.S.; Narkevich, V.B.; Klodt, P.M.; Rayevsky, K.S.; et al. Modeling of presymptomatic and symptomatic stages of parkinsonism in mice. Neuroscience 2011, 181, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, R.; Gerstein, M. Genomics: Protein fossils live on as RNA. Nature 2008, 453, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Vanniarajan, A. Implication of Pseudo Reference Genes in Normalization of Data from Reverse Transcription-Quantitative PCR. Gene 2020, 757, 144948. [Google Scholar] [CrossRef] [PubMed]
| Gene Rank 2 | Mice | Humans | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole Brain | Cortex | Cerebellum | Striatum | Substantia Nigra | Blood | Peripheral Blood | ||||||||
| HKG | Score 1 | HKG | Score | HKG | Score | HKG | Score | HKG | Score | HKG | Score | HKG | Score | |
| 1 | Psmd7 | 1.57 | Aars | 1.68 | Psmd7 | 1.86 | Psmd7 | 1.00 | Psmd7 | 1.32 | Sars | 1.57 | SARS1 | 1.57 |
| 2 | Aars | 2.21 | Psmd7 | 2.06 | Psmd6 | 2.63 | Aars | 2.21 | Aars | 1.68 | Polr2f | 2.38 | PSMD6 | 2.11 |
| 3 | Polr2a | 2.45 | Polr2f | 2.21 | Hprt | 3.13 | Hprt | 3.31 | Polr2a | 2.83 | Polr2a | 2.71 | HPRT1 | 3.16 |
| 4 | Bcat2 | 2.99 | Psmd6 | 3.83 | Aars | 3.31 | Polr2a | 3.31 | Bcat2 | 3.87 | Hprt | 3.98 | POLR2A | 3.22 |
| 5 | Psmd6 | 3.98 | Bcat2 | 4.53 | Polr2f | 4.30 | Sars | 4.56 | Sars | 4.61 | Psmd7 | 4.16 | PSMA5 | 3.94 |
| 6 | Hprt | 6.24 | Hprt | 4.95 | Bcat2 | 4.79 | Bcat2 | 5.92 | Polr2f | 6.09 | Psmd6 | 4.92 | PSMD7 | 4.56 |
| 7 | Sars | 7.24 | Polr2a | 6.44 | Polr2a | 5.66 | Psmd6 | 6.48 | Psmd6 | 6.24 | Bcat2 | 5.14 | AARS1 | 7.00 |
| 8 | Polr2f | 7.44 | Sars | 8.00 | Sars | 5.73 | Polr2f | 8.00 | Hprt | 8.00 | Aars | 8.00 | ||
| Gene Rank 2 | Mice | Humans | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole Brain | Cortex | Cerebellum | Striatum | Substantia Nigra | Blood | Peripheral Blood | ||||||||
| HKG | Score 1 | HKG | Score | HKG | Score | HKG | Score | HKG | Score | HKG | Score | HKG | Score | |
| 1 | Psmd6 | 1.86 | Bcat2 | 1.32 | Aars | 1.78 | Sars | 1.50 | Hprt | 1.50 | Hprt | 1.78 | POLR2A | 1.41 |
| 2 | Aars | 2.00 | Polr2f | 2.06 | Polr2f | 1.86 | Psmd7 | 2.21 | Psmd7 | 2.00 | Polr2f | 1.86 | PSMD6 | 2.45 |
| 3 | Polr2f | 2.11 | Aars | 2.63 | Psmd6 | 2.45 | Aars | 3.25 | Bcat2 | 3.00 | Psmd6 | 2.45 | PSMA5 | 2.82 |
| 4 | Psmd7 | 3.08 | Psmd6 | 3.46 | Polr2a | 2.63 | Polr2a | 3.46 | Psmd6 | 3.83 | Psmd7 | 2.83 | PSMD7 | 3.98 |
| 5 | Sars | 4.16 | Polr2a | 5.00 | Bcat2 | 5.38 | Bcat2 | 4.60 | Aars | 4.43 | Sars | 4.61 | SARS | 4.23 |
| 6 | Bcat2 | 6.24 | Psmd7 | 5.09 | Psmd7 | 5.48 | Hprt | 4.61 | Polr2f | 5.12 | Bcat2 | 5.73 | HPRT1 | 4.30 |
| 7 | Polr2a | 7.24 | Sars | 7.24 | Sars | 6.96 | Psmd6 | 5.96 | Sars | 5.44 | Polr2a | 7.00 | AARS1 | 6.00 |
| 8 | Hprt | 7.44 | Hprt | 7.44 | Hprt | 7.74 | Polr2f | 7.20 | Polr2a | 8.00 | Aars | 8.00 | ||
| Gene Rank 2 | Mice | Humans | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole Brain | Cortex | Cerebellum | Striatum | Substantia Nigra | Peripheral Blood | Peripheral Blood | ||||||||
| HKG | S-Score 1 | HKG | S-Score | HKG | S-Score | HKG | S-Score | HKG | S-Score | HKG | S-Score | HKG | S-Score | |
| 1 | Aars | 4.21 | Polr2f | 4.27 | Psmd6 | 5.08 | Psmd7 | 3.21 | Psmd7 | 3.32 | Polr2f | 4.24 | PSMD6 | 4.56 |
| 2 | Psmd7 | 4.65 | Aars | 4.31 | Aars | 5.09 | Aars | 5.46 | Aars | 6.11 | Hprt | 5.76 | POLR2A | 4.63 |
| 3 | Psmd6 | 5.84 | Bcat2 | 5.85 | Polr2f | 6.16 | Sars | 6.06 | Bcat2 | 6.87 | Sars | 6.18 | SARS1 | 5.80 |
| 4 | Bcat2 | 9.23 | Psmd7 | 7.15 | Psmd7 | 7.34 | Polr2a | 6.77 | Hprt | 9.50 | Psmd7 | 6.99 | PSMA5 | 6.76 |
| 5 | Polr2f | 9.55 | Psmd6 | 7.29 | Polr2a | 8.29 | Hprt | 7.92 | Sars | 10.05 | Psmd6 | 7.37 | HPRT1 | 7.46 |
| 6 | Polr2a | 9.69 | Polr2a | 11.44 | Bcat2 | 10.17 | Bcat2 | 10.52 | Psmd6 | 10.07 | Polr2a | 9.71 | PSMD7 | 8.54 |
| 7 | Sars | 11.40 | Hprt | 12.39 | Hprt | 10.87 | Psmd6 | 12.44 | Polr2a | 10.83 | Bcat2 | 10.87 | AARS1 | 13.00 |
| 8 | Hprt | 13.68 | Sars | 15.24 | Sars | 12.69 | Polr2f | 15.20 | Polr2f | 11.21 | Aars | 16.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alieva, A.K.; Filatova, E.V.; Rudenok, M.M.; Slominsky, P.A.; Shadrina, M.I. Housekeeping Genes for Parkinson’s Disease in Humans and Mice. Cells 2021, 10, 2252. https://doi.org/10.3390/cells10092252
Alieva AK, Filatova EV, Rudenok MM, Slominsky PA, Shadrina MI. Housekeeping Genes for Parkinson’s Disease in Humans and Mice. Cells. 2021; 10(9):2252. https://doi.org/10.3390/cells10092252
Chicago/Turabian StyleAlieva, Anelya Kh., Elena V. Filatova, Margarita M. Rudenok, Petr A. Slominsky, and Maria I. Shadrina. 2021. "Housekeeping Genes for Parkinson’s Disease in Humans and Mice" Cells 10, no. 9: 2252. https://doi.org/10.3390/cells10092252
APA StyleAlieva, A. K., Filatova, E. V., Rudenok, M. M., Slominsky, P. A., & Shadrina, M. I. (2021). Housekeeping Genes for Parkinson’s Disease in Humans and Mice. Cells, 10(9), 2252. https://doi.org/10.3390/cells10092252








