Time-Dependent Serial Changes of Antigen-Presenting Cell Subsets in the Ocular Surface Are Distinct between Corneal Sterile Inflammation and Allosensitization in a Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Mouse Model of PT or Corneal Transplantation
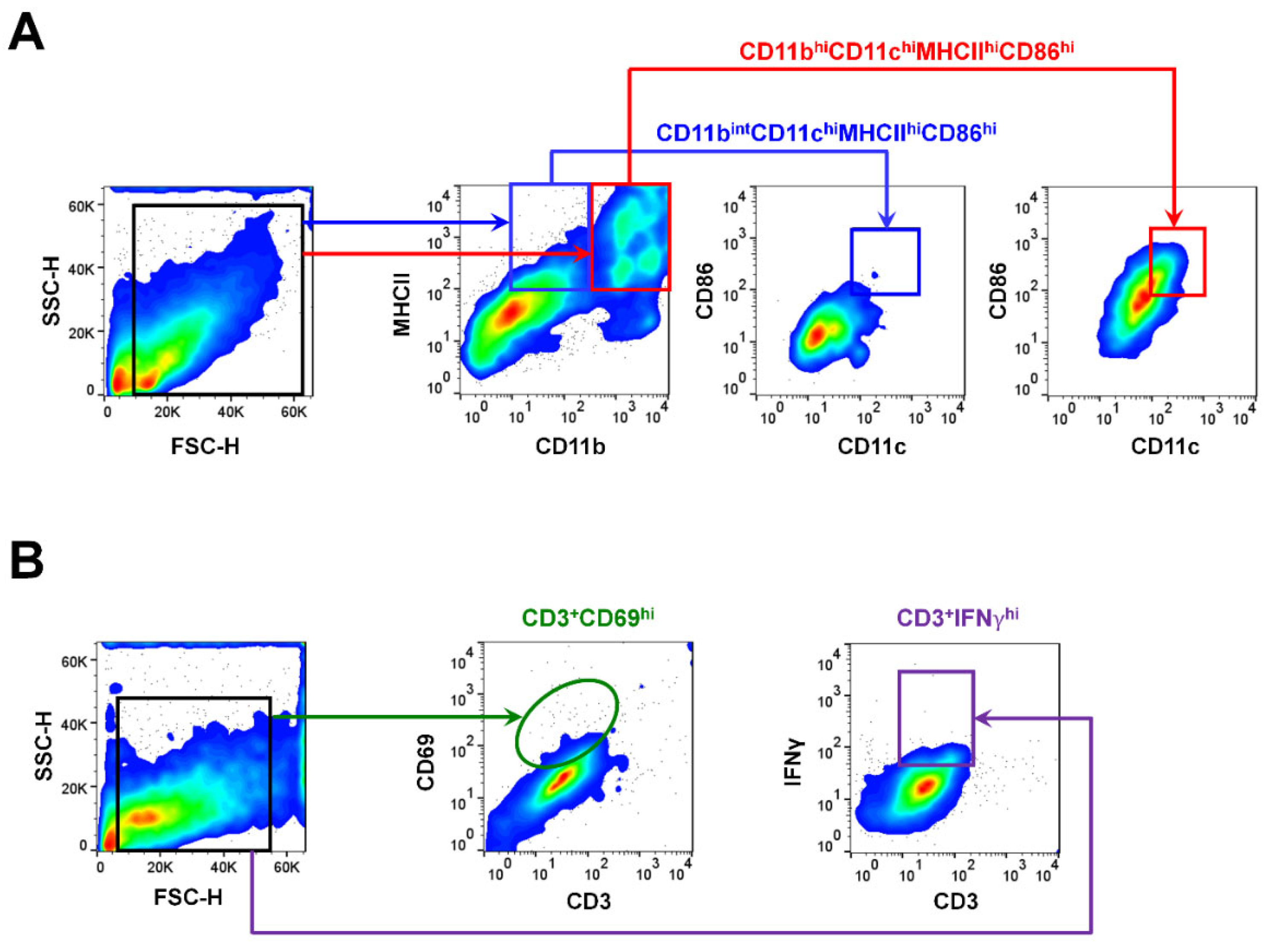
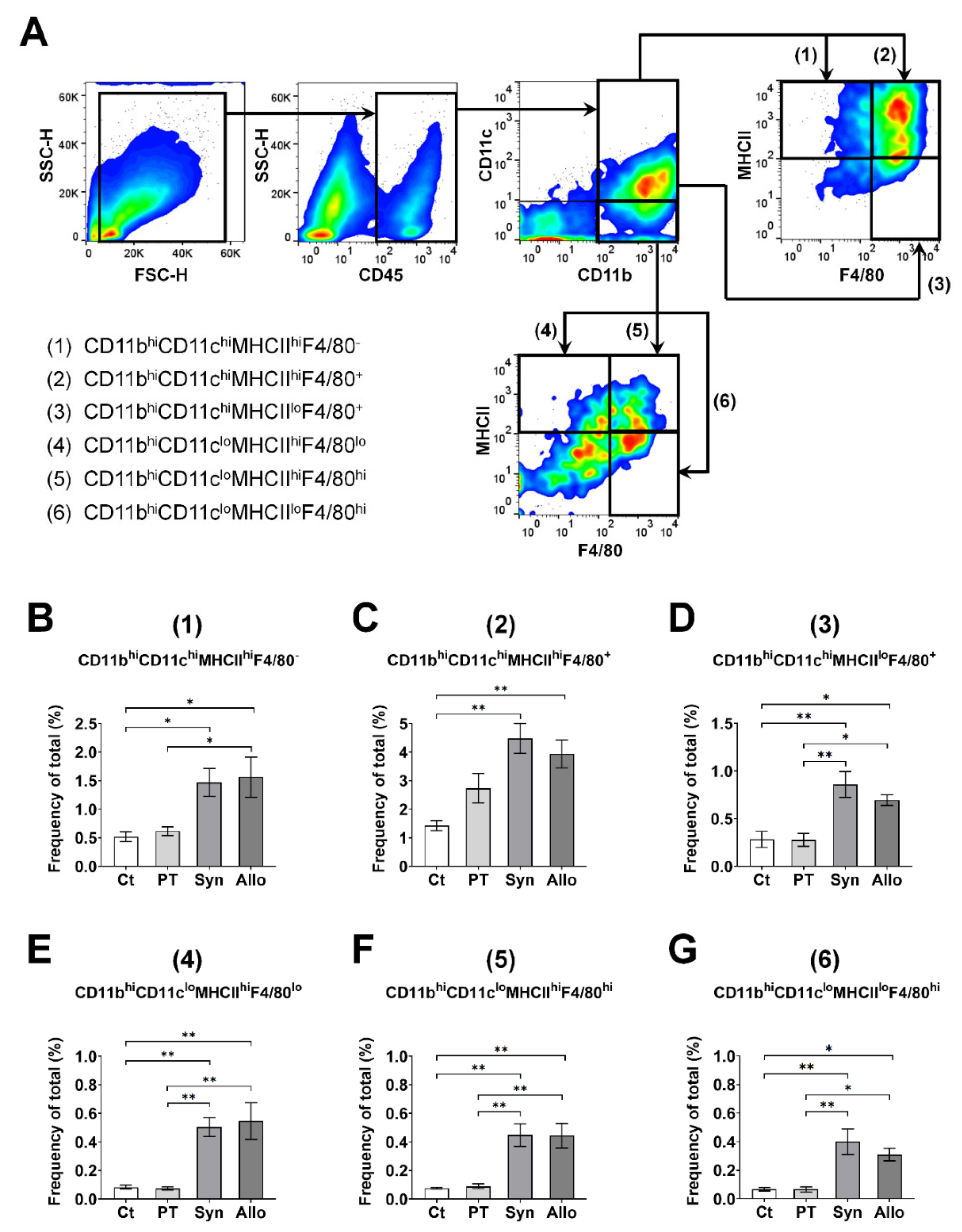
2.3. Gating Strategies for APCs and T Cells Using Flow Cytometry
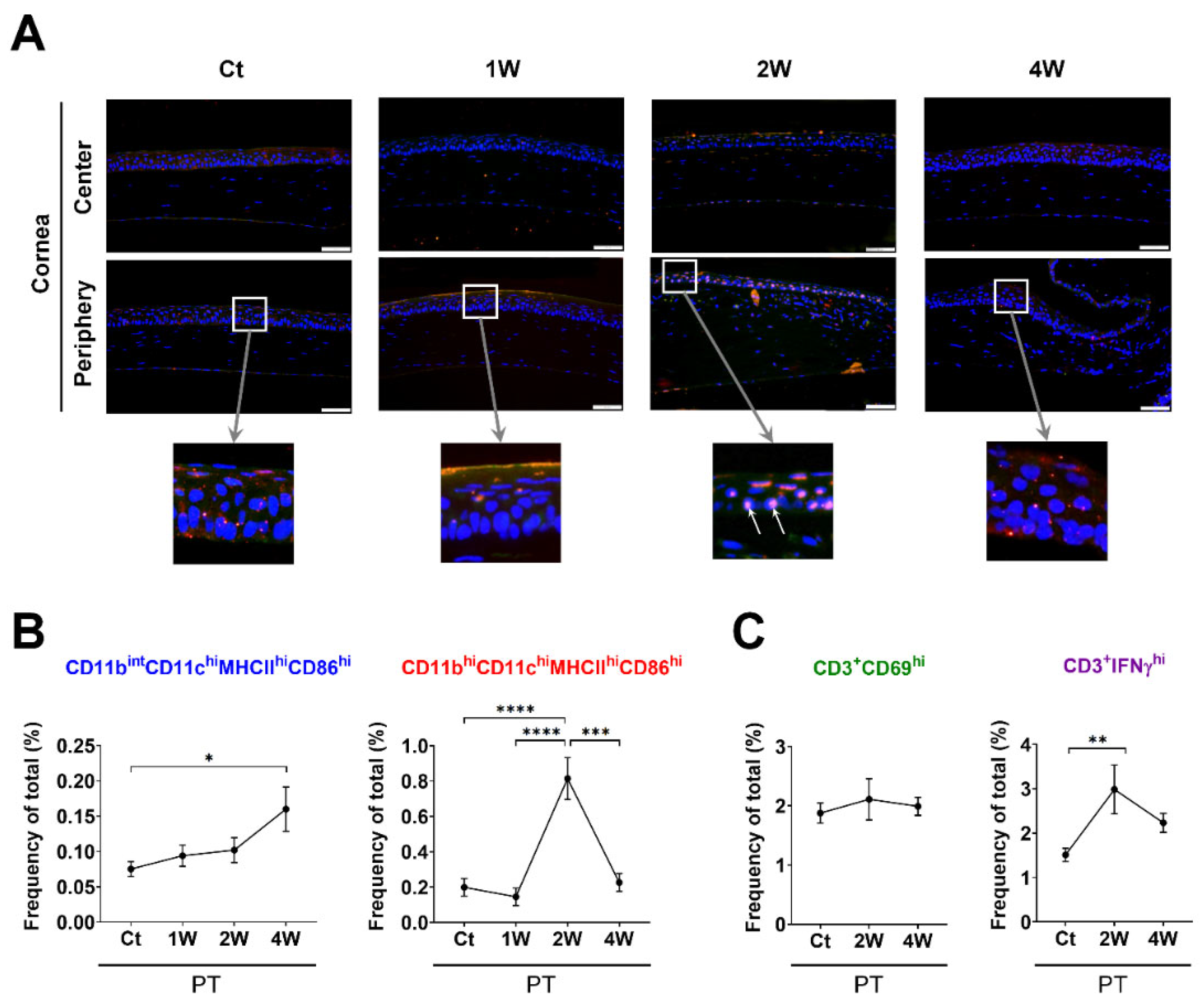
2.4. Corneal Histology for Evaluating the Distribution of MHCIIhi-Expressing Cells
2.5. Regression Analysis for Evaluating the Relationship between T-Cells and APC Subsets
2.6. Statistical Analysis
3. Results
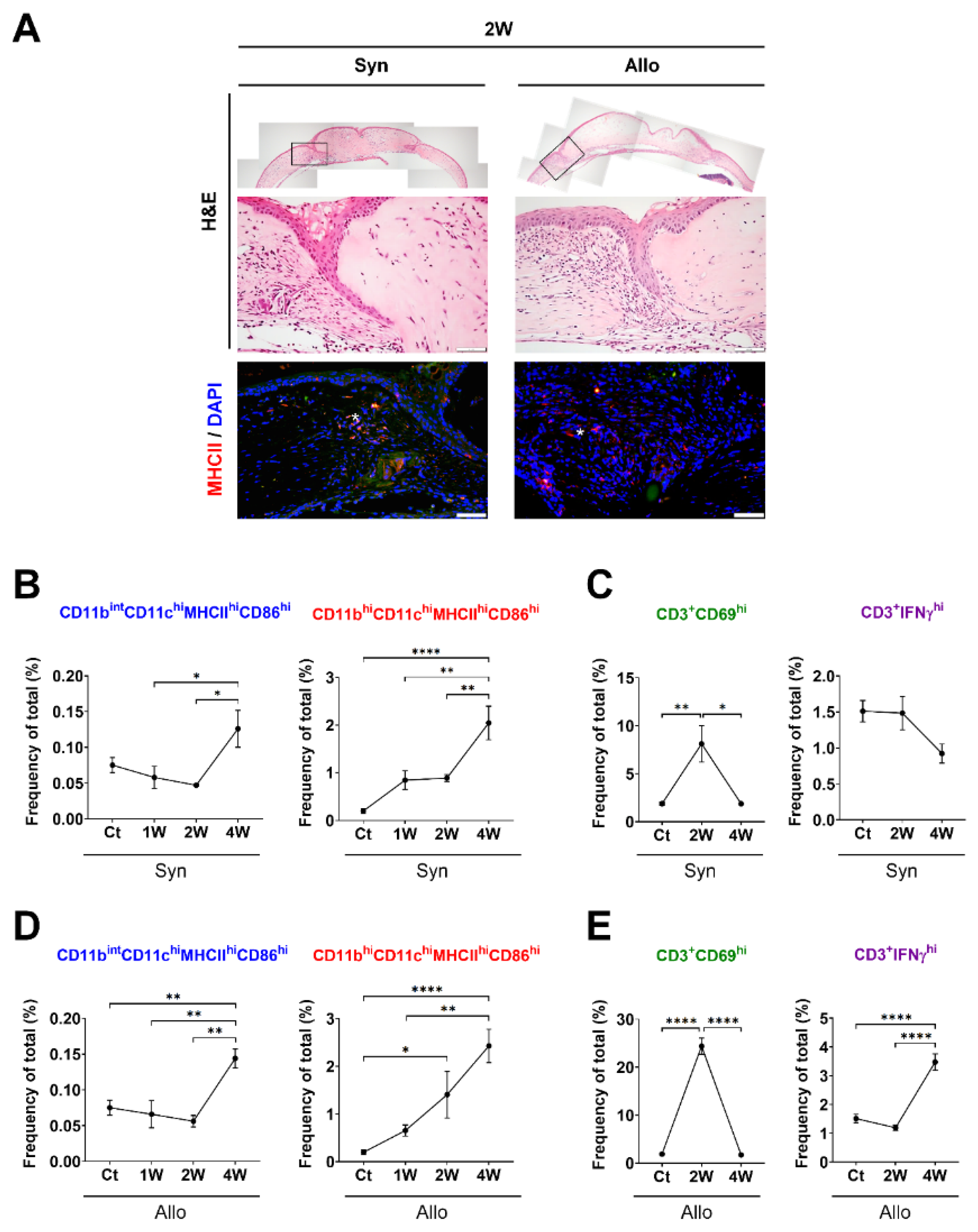
3.1. Time-Dependent Serial Changes of APC and T-Cell Subsets in Response to Sterile Inflammation or Allosensitization in the Ocular Surface
3.2. Regression Analysis of the T-Cell Subset Proportions Related to the APC Subsets in the Ocular Surface
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hori, J.; Yamaguchi, T.; Keino, H.; Hamrah, P.; Maruyama, K. Immune privilege in corneal transplantation. Prog. Retin. Eye Res. 2019, 72, 100758. [Google Scholar] [CrossRef]
- Medawar, P.B. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 1948, 29, 58–69. [Google Scholar]
- Streilein, J.W. Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 2003, 3, 879–889. [Google Scholar] [CrossRef]
- Muraine, M.; Sanchez, C.; Watt, L.; Retout, A.; Brasseur, G. Long-term results of penetrating keratoplasty. A 10-year-plus retrospective study. Graefes Arch. Clin. Exp. Ophthalmol. 2003, 241, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.W.; Price, M.O.; Bowers, P.J.; Price, F.W. Long-term graft survival after penetrating keratoplasty. Ophthalmology 2003, 110, 1396–1402. [Google Scholar] [CrossRef]
- Hamrah, P.; Liu, Y.; Zhang, Q.; Dana, M.R. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest. Ophthalmol. Vis. Sci. 2003, 44, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, S.E.; Gardner, P.J.; Ribeiro, J.; Cristante, E.; Sampson, R.D.; Luhmann, U.F.; Ali, R.R.; Bainbridge, J.W. Flow cytometric analysis of inflammatory and resident myeloid populations in mouse ocular inflammatory models. Exp. Eye Res. 2016, 151, 160–170. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, K.W.; Ryu, J.S.; Jeong, H.J.; Lee, S.M.; Kim, M.K. Bilateral effect of the unilateral corneal nerve cut on both ocular surface and lacrimal gland. Invest. Ophthalmol. Vis. Sci. 2019, 60, 430–441. [Google Scholar] [CrossRef]
- Lee, H.S.; Amouzegar, A.; Dana, R. Kinetics of corneal antigen presenting cells in experimental dry eye disease. BMJ Open Ophthalmol. 2017, 1, e000078. [Google Scholar] [CrossRef]
- Forrester, J.V.; Xu, H.; Kuffova, L.; Dick, A.D.; McMenamin, P.G. Dendritic cell physiology and function in the eye. Immunol. Rev. 2010, 234, 282–304. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. Corneal transplantation and immune privilege. Int. Rev. Immunol. 2013, 32, 57–67. [Google Scholar] [CrossRef]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef]
- Misharin, A.V.; Morales-Nebreda, L.; Mutlu, G.M.; Budinger, G.R.; Perlman, H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol. 2013, 49, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Nguyen, A.; Gommerman, J.L. Dendritic cell subsets in intestinal immunity and inflammation. J. Immunol. 2020, 204, 1075–1083. [Google Scholar] [CrossRef]
- Yu, Y.R.; O’Koren, E.G.; Hotten, D.F.; Kan, M.J.; Kopin, D.; Nelson, E.R.; Que, L.; Gunn, M.D. A protocol for the comprehensive flow cytometric analysis of immune cells in normal and inflamed murine non-lymphoid tissues. PLoS ONE 2016, 11, e0150606. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.J.; Seyed-Razavi, Y.; Yamaguchi, T.; Ortiz, G.; Sendra, V.G.; Harris, D.L.; Jamali, A.; Hamrah, P. Multiphoton intravital microscopy of mandibular draining lymph nodes: A mouse model to study corneal immune responses. Front. Immunol. 2020, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.H.; Ryu, J.S.; Moon, J.; Kim, M.K. Association between aging-dependent gut microbiome dysbiosis and dry eye severity in C57BL/6 male mouse model: A pilot study. BMC Microbiol. 2021, 21, 106. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, S.Y.; Jeong, H.J.; Kim, H.J.; Kim, M.K.; Oh, J.Y. Glucocorticoids induce corneal allograft tolerance through expansion of monocytic myeloid-derived suppressor cells. Am. J. Transplant. 2018, 18, 3029–3037. [Google Scholar] [CrossRef]
- Cibrian, D.; Sanchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef]
- Caraher, E.M.; Parenteau, M.; Gruber, H.; Scott, F.W. Flow cytometric analysis of intracellular IFN-gamma, IL-4 and IL-10 in CD3(+)4(+) T-cells from rat spleen. J. Immunol. Methods 2000, 244, 29–40. [Google Scholar] [CrossRef]
- McMenamin, P.G.; Crewe, J. Endotoxin-induced uveitis. Kinetics and phenotype of the inflammatory cell infiltrate and the response of the resident tissue macrophages and dendritic cells in the iris and ciliary body. Invest. Ophthalmol. Vis. Sci. 1995, 36, 1949–1959. [Google Scholar] [PubMed]
- Kuffova, L.; Lumsden, L.; Vesela, V.; Taylor, J.A.; Filipec, M.; Holan, V.; Dick, A.D.; Forrester, J.V. Kinetics of leukocyte and myeloid cell traffic in the murine corneal allograft response. Transplantation 2001, 72, 1292–1298. [Google Scholar] [CrossRef]
- Liu, J.; Xue, Y.; Dong, D.; Xiao, C.; Lin, C.; Wang, H.; Song, F.; Fu, T.; Wang, Z.; Chen, J.; et al. CCR2(−) and CCR2(+) corneal macrophages exhibit distinct characteristics and balance inflammatory responses after epithelial abrasion. Mucosal Immunol. 2017, 10, 1145–1159. [Google Scholar] [CrossRef]
- Wakim, L.M.; Waithman, J.; van Rooijen, N.; Heath, W.R.; Carbone, F.R. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 2008, 319, 198–202. [Google Scholar] [CrossRef]
- Zanna, M.Y.; Yasmin, A.R.; Omar, A.R.; Arshad, S.S.; Mariatulqabtiah, A.R.; Nur-Fazila, S.H.; Mahiza, M.I.N. Review of dendritic cells, their role in clinical immunology, and distribution in various animal species. Int. J. Mol. Sci. 2021, 22, 8044. [Google Scholar] [CrossRef]
- Meredith, M.M.; Liu, K.; Darrasse-Jeze, G.; Kamphorst, A.O.; Schreiber, H.A.; Guermonprez, P.; Idoyaga, J.; Cheong, C.; Yao, K.H.; Niec, R.E.; et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J. Exp. Med. 2012, 209, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Shortman, K.; Liu, Y.J. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2002, 2, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar] [CrossRef] [PubMed]
- Vander Lugt, B.; Khan, A.A.; Hackney, J.A.; Agrawal, S.; Lesch, J.; Zhou, M.; Lee, W.P.; Park, S.; Xu, M.; DeVoss, J.; et al. Transcriptional programming of dendritic cells for enhanced MHC class II antigen presentation. Nat. Immunol. 2014, 15, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Tubo, N.J.; Jenkins, M.K. TCR signal quantity and quality in CD4(+) T cell differentiation. Trends Immunol. 2014, 35, 591–596. [Google Scholar] [CrossRef]
- Yoon, C.H.; Choi, H.J.; Kim, M.K. Corneal xenotransplantation: Where are we standing? Prog. Retin. Eye Res. 2021, 80, 100876. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y. Immune mechanisms of corneal allograft rejection. Curr. Eye Res. 2007, 32, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Bharat, A. Role of monocytes and macrophages in regulating immune response following lung transplantation. Curr. Opin. Organ Transplant. 2016, 21, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Coronella, F.; Satta, G.M.; Fossarello, M. A novel technique of contrast-enhanced optical coherence tomography imaging in evaluation of clearance of lipids in human tears. PLoS ONE 2014, 9, e109843. [Google Scholar] [CrossRef][Green Version]
- Napoli, P.E.; Coronella, F.; Satta, G.M.; Galantuomo, M.S.; Fossarello, M. Evaluation of the adhesive properties of the cornea by means of optical coherence tomography in patients with meibomian gland dysfunction and lacrimal tear deficiency. PLoS ONE 2014, 9, e115762. [Google Scholar] [CrossRef][Green Version]
- Nioi, M.; Napoli, P.E.; Mayerson, S.M.; Fossarello, M.; d’Aloja, E. Optical coherence tomography in forensic sciences: A review of the literature. Forensic Sci. Med. Pathol. 2019, 15, 445–452. [Google Scholar] [CrossRef]
- Seyed-Razavi, Y.; Lopez, M.J.; Mantopoulos, D.; Zheng, L.; Massberg, S.; Sendra, V.G.; Harris, D.L.; Hamrah, P. Kinetics of corneal leukocytes by intravital multiphoton microscopy. FASEB J. 2019, 33, 2199–2211. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-W.; Lee, H.-J.; Kim, H.-J.; Kim, M.-K. Time-Dependent Serial Changes of Antigen-Presenting Cell Subsets in the Ocular Surface Are Distinct between Corneal Sterile Inflammation and Allosensitization in a Murine Model. Cells 2021, 10, 2210. https://doi.org/10.3390/cells10092210
Kim K-W, Lee H-J, Kim H-J, Kim M-K. Time-Dependent Serial Changes of Antigen-Presenting Cell Subsets in the Ocular Surface Are Distinct between Corneal Sterile Inflammation and Allosensitization in a Murine Model. Cells. 2021; 10(9):2210. https://doi.org/10.3390/cells10092210
Chicago/Turabian StyleKim, Kyoung-Woo, Hyun-Ju Lee, Hyeon-Ji Kim, and Mee-Kum Kim. 2021. "Time-Dependent Serial Changes of Antigen-Presenting Cell Subsets in the Ocular Surface Are Distinct between Corneal Sterile Inflammation and Allosensitization in a Murine Model" Cells 10, no. 9: 2210. https://doi.org/10.3390/cells10092210
APA StyleKim, K.-W., Lee, H.-J., Kim, H.-J., & Kim, M.-K. (2021). Time-Dependent Serial Changes of Antigen-Presenting Cell Subsets in the Ocular Surface Are Distinct between Corneal Sterile Inflammation and Allosensitization in a Murine Model. Cells, 10(9), 2210. https://doi.org/10.3390/cells10092210





