Radiation-Induced Overexpression of TGFβ and PODXL Contributes to Colorectal Cancer Cell Radioresistance through Enhanced Motility
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Irradiation, and Drug Treatment
2.2. Patient Samples
2.3. RNAi Knockdown and Plasmid Transfection
2.4. Transwell Assay
2.5. Cell Viability and Clonogenic Assay
2.6. Flow Cytometry Analysis
2.7. Wound-Healing Assay
2.8. Western Blot Analysis
2.9. Immunofluorescence (IF) Staining
2.10. Immunohistochemistry (IHC) Staining
2.11. Bioinformatics Analysis
2.12. Spheroid Invasion Assays
2.13. Matrix Degradation Assay
2.14. Statistical Analysis
3. Results
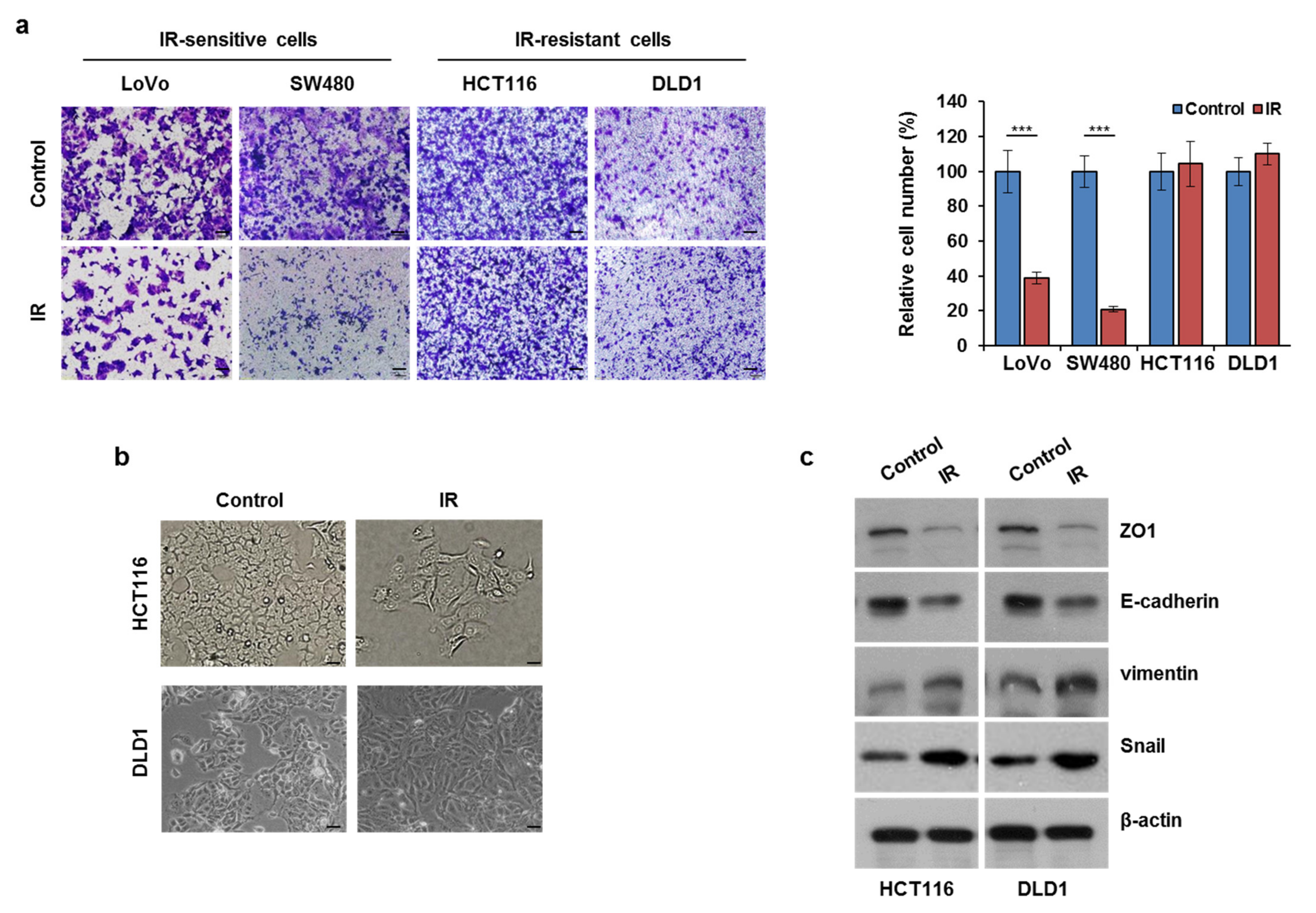
3.1. Ionizing Radiation (IR) Exposure Promoted CRC Cell Migration
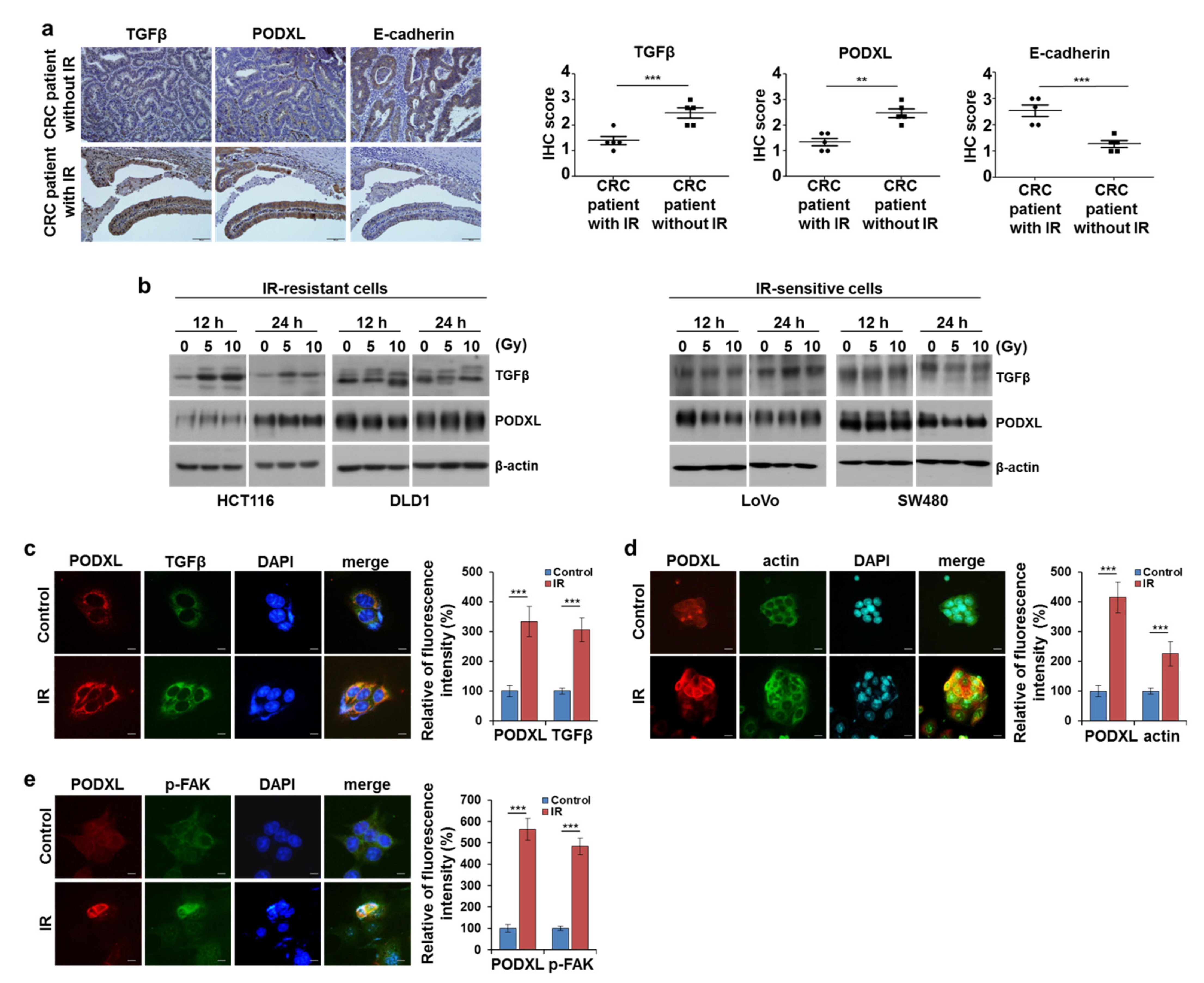
3.2. IR-Enhanced Migratory Potential through Upregulation of TGFβ and PODXL
3.3. Correlation between Upregulated PODXL and Poor Prognoses in CRC Patients
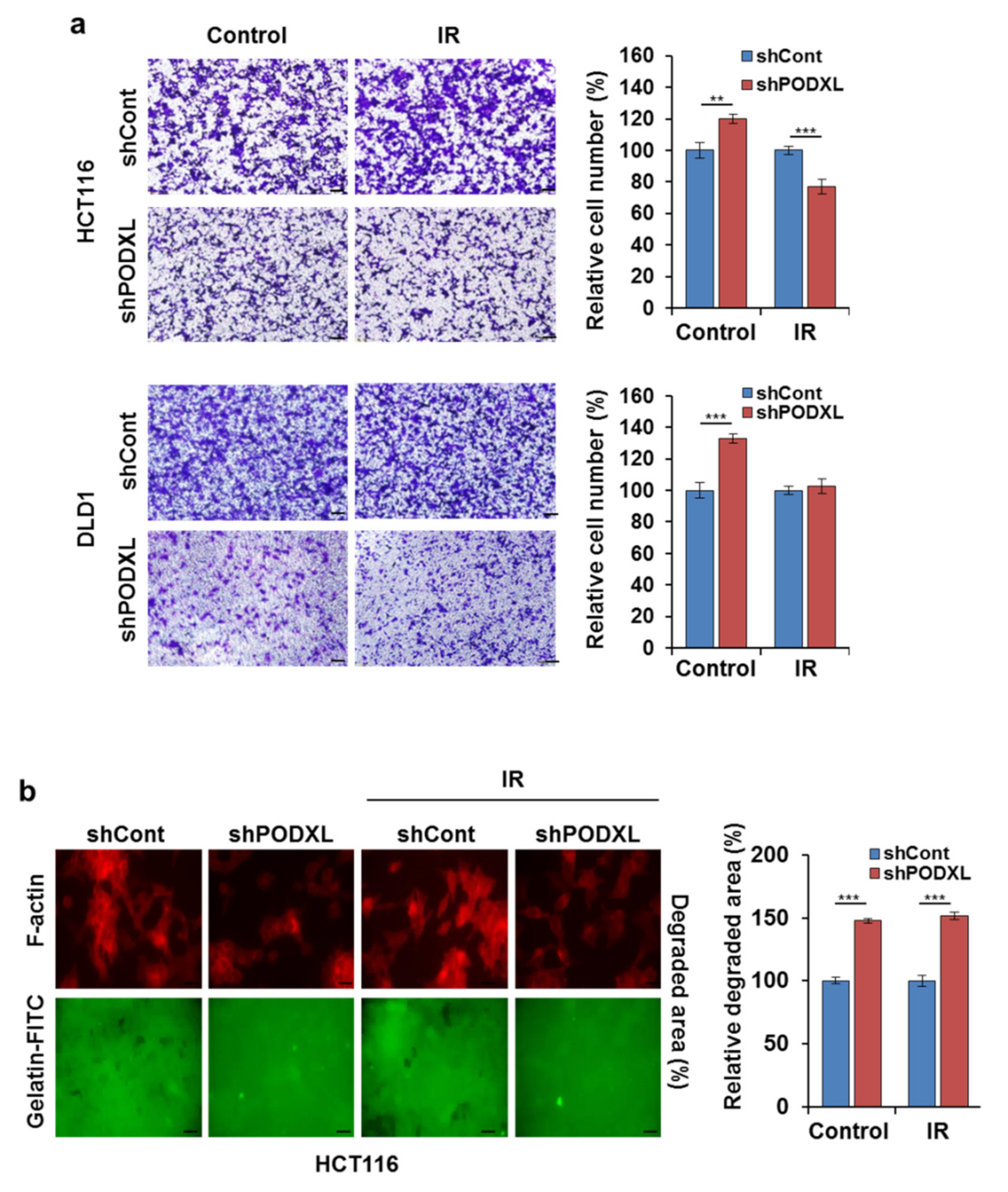
3.4. PODXL Knockdown Decreased Cell Motility through the Regulation of EMT-Related Gene Expression
3.5. PODXL Deletion Inhibited Invasiveness after IR Exposure
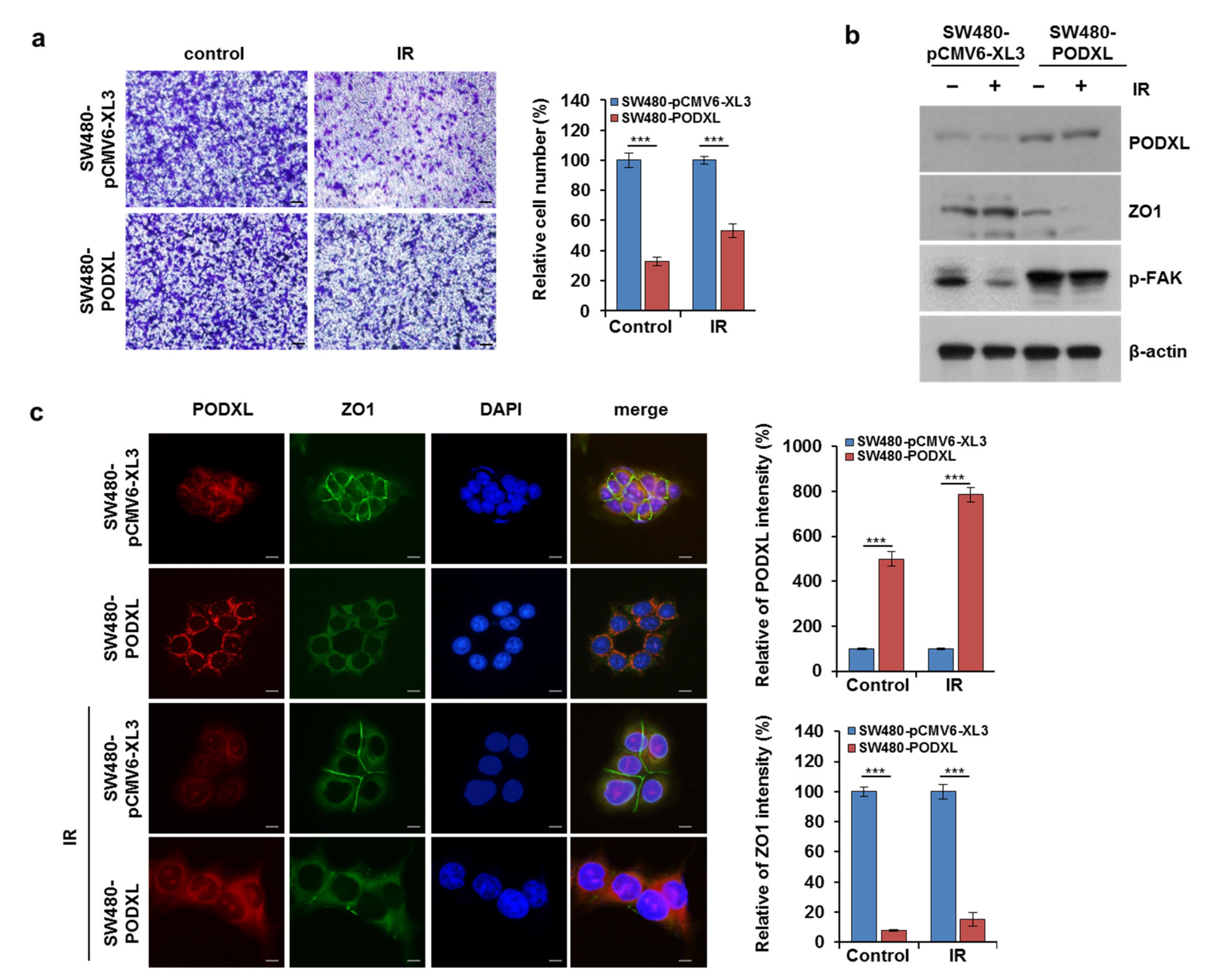
3.6. PODXL Overexpression Enhanced Cell Motility in IR-Sensitive CRC Cells
3.7. Galunisertib Enhanced IR-Antitumor Activity in CRC Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tauriello, D.V.; Calon, A.; Lonardo, E.; Batlle, E. Determinants of metastatic competency in colorectal cancer. Mol. Oncol. 2017, 11, 97–119. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.F.; Collette, L.; Calais, G.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Daban, A.; Bardet, E.; Beny, A.; Ollier, J.C. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006, 355, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Liersch, T.; Becker, H.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Graeven, U.; Arnold, D.; Lang-Welzenbach, M.; Raab, H.R.; et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: Initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. The Lancet. Oncology 2012, 13, 679–687. [Google Scholar] [CrossRef]
- von Essen, C.F. Radiation enhancement of metastasis: A review. Clin. Exp. Metastasis 1991, 9, 77–104. [Google Scholar] [CrossRef]
- Epanchintsev, A.; Shyamsunder, P.; Verma, R.S.; Lyakhovich, A. IL-6, IL-8, MMP-2, MMP-9 are overexpressed in Fanconi anemia cells through a NF-κB/TNF-α dependent mechanism. Mol. Carcinog. 2015, 54, 1686–1699. [Google Scholar] [CrossRef]
- Bataille, R.; Harousseau, J.L. Multiple myeloma. N. Engl. J. Med. 1997, 336, 1657–1664. [Google Scholar] [CrossRef]
- Neta, R.; Perlstein, R.; Vogel, S.N.; Ledney, G.D.; Abrams, J. Role of interleukin 6 (IL-6) in protection from lethal irradiation and in endocrine responses to IL-1 and tumor necrosis factor. J. Exp. Med. 1992, 175, 689–694. [Google Scholar] [CrossRef]
- Klein, B.; Bataille, R. Cytokine network in human multiple myeloma. Hematol. Oncol. Clin. N. Am. 1992, 6, 273–284. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad Signaling Pathways of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2017, 9, a022129. [Google Scholar] [CrossRef]
- Friedman, E.; Gold, L.I.; Klimstra, D.; Zeng, Z.S.; Winawer, S.; Cohen, A. High levels of transforming growth factor beta 1 correlate with disease progression in human colon cancer. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 1995, 4, 549–554. [Google Scholar]
- Tsushima, H.; Kawata, S.; Tamura, S.; Ito, N.; Shirai, Y.; Kiso, S.; Imai, Y.; Shimomukai, H.; Nomura, Y.; Matsuda, Y.; et al. High levels of transforming growth factor beta 1 in patients with colorectal cancer: Association with disease progression. Gastroenterology 1996, 110, 375–382. [Google Scholar] [CrossRef]
- Turtoi, A.; Blomme, A.; Debois, D.; Somja, J.; Delvaux, D.; Patsos, G.; Di Valentin, E.; Peulen, O.; Mutijima, E.N.; De Pauw, E.; et al. Organized proteomic heterogeneity in colorectal cancer liver metastases and implications for therapies. Hepatology 2014, 59, 924–934. [Google Scholar] [CrossRef]
- Dancea, H.C.; Shareef, M.M.; Ahmed, M.M. Role of Radiation-induced TGF-beta Signaling in Cancer Therapy. Mol. Cell. Pharmacol. 2009, 1, 44–56. [Google Scholar] [CrossRef]
- Binder, Z.A.; Siu, I.M.; Eberhart, C.G.; Ap Rhys, C.; Bai, R.Y.; Staedtke, V.; Zhang, H.; Smoll, N.R.; Piantadosi, S.; Piccirillo, S.G.; et al. Podocalyxin-like protein is expressed in glioblastoma multiforme stem-like cells and is associated with poor outcome. PLoS ONE 2013, 8, e75945. [Google Scholar] [CrossRef]
- Kaprio, T.; Hagström, J.; Fermér, C.; Mustonen, H.; Böckelman, C.; Nilsson, O.; Haglund, C. A comparative study of two PODXL antibodies in 840 colorectal cancer patients. BMC Cancer 2014, 14, 494. [Google Scholar] [CrossRef][Green Version]
- Jiang, J.; Liu, Y.; Fang, W.; Liu, F. Sperm-associated antigen 9 promotes astrocytoma cell invasion through the upregulation of podocalyxin. Mol. Med. Rep. 2014, 10, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Casey, G.; Neville, P.J.; Liu, X.; Plummer, S.J.; Cicek, M.S.; Krumroy, L.M.; Curran, A.P.; McGreevy, M.R.; Catalona, W.J.; Klein, E.A.; et al. Podocalyxin variants and risk of prostate cancer and tumor aggressiveness. Hum. Mol. Genet. 2006, 15, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Forse, C.L.; Yilmaz, Y.E.; Pinnaduwage, D.; O’Malley, F.P.; Mulligan, A.M.; Bull, S.B.; Andrulis, I.L. Elevated expression of podocalyxin is associated with lymphatic invasion, basal-like phenotype, and clinical outcome in axillary lymph node-negative breast cancer. Breast Cancer Res. Treat. 2013, 137, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Itai, S.; Yamada, S.; Kaneko, M.K.; Sano, M.; Nakamura, T.; Yanaka, M.; Handa, S.; Hisamatsu, K.; Nakamura, Y.; Furusawa, Y.; et al. Podocalyxin is crucial for the growth of oral squamous cell carcinoma cell line HSC-2. Biochem. Biophys. Rep. 2018, 15, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Johansson, M.E.; Wangefjord, S.; Gaber, A.; Nodin, B.; Kucharzewska, P.; Welinder, C.; Belting, M.; Eberhard, J.; Johnsson, A.; et al. Overexpression of podocalyxin-like protein is an independent factor of poor prognosis in colorectal cancer. Br. J. Cancer 2011, 105, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Fröse, J.; Chen, M.B.; Hebron, K.E.; Reinhardt, F.; Hajal, C.; Zijlstra, A.; Kamm, R.D.; Weinberg, R.A. Epithelial-Mesenchymal Transition Induces Podocalyxin to Promote Extravasation via Ezrin Signaling. Cell Rep. 2018, 24, 962–972. [Google Scholar] [CrossRef]
- Nielsen, J.S.; Graves, M.L.; Chelliah, S.; Vogl, A.W.; Roskelley, C.D.; McNagny, K.M. The CD34-related molecule podocalyxin is a potent inducer of microvillus formation. PLoS ONE 2007, 2, e237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, Z.; Wu, H.; Yu, Z.; Rong, Z.; Luo, Z.; Xu, Y.; Huang, K.; Qiu, Z.; Huang, C. PODXL, negatively regulated by KLF4, promotes the EMT and metastasis and serves as a novel prognostic indicator of gastric cancer. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2019, 22, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, H.; Shintani, Y.; Kanzaki, R.; Kawamura, T.; Funaki, S.; Minami, M.; Nagatomo, I.; Morii, E.; Okumura, M. Podocalyxin influences malignant potential by controlling epithelial-mesenchymal transition in lung adenocarcinoma. Cancer Sci. 2017, 108, 528–535. [Google Scholar] [CrossRef]
- Mladenov, E.; Magin, S.; Soni, A.; Iliakis, G. DNA double-strand break repair as determinant of cellular radiosensitivity to killing and target in radiation therapy. Front. Oncol. 2013, 3, 113. [Google Scholar] [CrossRef]
- Marie-Egyptienne, D.T.; Lohse, I.; Hill, R.P. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: Potential role of hypoxia. Cancer Lett. 2013, 341, 63–72. [Google Scholar] [CrossRef]
- Su, W.H.; Chuang, P.C.; Huang, E.Y.; Yang, K.D. Radiation-induced increase in cell migration and metastatic potential of cervical cancer cells operates via the K-Ras pathway. Am. J. Pathol. 2012, 180, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Anastasov, N.; Hirmer, E.; Klenner, M.; Ott, J.; Falkenberg, N.; Bao, X.; Mutschelknaus, L.; Moertl, S.; Combs, S.; Atkinson, M.J.; et al. MEK1 Inhibitor Combined with Irradiation Reduces Migration of Breast Cancer Cells Including miR-221 and ZEB1 EMT Marker Expression. Cancers 2020, 12, 3760. [Google Scholar] [CrossRef]
- Kim, A.; Shim, S.; Kim, Y.H.; Kim, M.J.; Park, S.; Myung, J.K. Inhibition of Y Box Binding Protein 1 Suppresses Cell Growth and Motility in Colorectal Cancer. Mol. Cancer Ther. 2020, 19, 479–489. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Jin, G.N.; Wang, W.; Chen, W.X.; Wu, Y.H.; Ai, X.; Chen, L.; Zhang, W.G.; Liang, H.F.; Laurence, A.; et al. Reduced expression of transcriptional intermediary factor 1 gamma promotes metastasis and indicates poor prognosis of hepatocellular carcinoma. Hepatology 2014, 60, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Fransvea, E.; Angelotti, U.; Antonaci, S.; Giannelli, G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology 2008, 47, 1557–1566. [Google Scholar] [CrossRef]
- Formenti, S.C.; Lee, P.; Adams, S.; Goldberg, J.D.; Li, X.; Xie, M.W.; Ratikan, J.A.; Felix, C.; Hwang, L.; Faull, K.F.; et al. Focal Irradiation and Systemic TGFβ Blockade in Metastatic Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 2493–2504. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, F.; Pal, A.; Pilones, K.A.; Demaria, S.; Hann, B.; Akhurst, R.J.; Babb, J.S.; Lonning, S.M.; DeWyngaert, J.K.; Formenti, S.C.; et al. TGFβ1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 6754–6765. [Google Scholar] [CrossRef] [PubMed]
- Hardee, M.E.; Marciscano, A.E.; Medina-Ramirez, C.M.; Zagzag, D.; Narayana, A.; Lonning, S.M.; Barcellos-Hoff, M.H. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β. Cancer Res. 2012, 72, 4119–4129. [Google Scholar] [CrossRef]
- Zhang, M.; Kleber, S.; Röhrich, M.; Timke, C.; Han, N.; Tuettenberg, J.; Martin-Villalba, A.; Debus, J.; Peschke, P.; Wirkner, U.; et al. Blockade of TGF-β signaling by the TGFβR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 2011, 71, 7155–7167. [Google Scholar] [CrossRef]
- Heby, M.; Elebro, J.; Nodin, B.; Jirström, K.; Eberhard, J. Prognostic and predictive significance of podocalyxin-like protein expression in pancreatic and periampullary adenocarcinoma. BMC Clin. Pathol. 2015, 15, 10. [Google Scholar] [CrossRef][Green Version]
- Heby, M.; Karnevi, E.; Elebro, J.; Nodin, B.; Eberhard, J.; Saukkonen, K.; Hagström, J.; Mustonen, H.; Seppänen, H.; Haglund, C.; et al. Additive clinical impact of epidermal growth factor receptor and podocalyxin-like protein expression in pancreatic and periampullary adenocarcinomas. Sci. Rep. 2020, 10, 10373. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Ezzati, P.; Wilkins, J.A. Requirement of podocalyxin in TGF-beta induced epithelial mesenchymal transition. PLoS ONE 2011, 6, e18715. [Google Scholar] [CrossRef]
- Herbertz, S.; Sawyer, J.S.; Stauber, A.J.; Gueorguieva, I.; Driscoll, K.E.; Estrem, S.T.; Cleverly, A.L.; Desaiah, D.; Guba, S.C.; Benhadji, K.A.; et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des. Dev. Ther. 2015, 9, 4479–4499. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kong, J.-S.; Lee, S.-S.; Kim, A. Radiation-Induced Overexpression of TGFβ and PODXL Contributes to Colorectal Cancer Cell Radioresistance through Enhanced Motility. Cells 2021, 10, 2087. https://doi.org/10.3390/cells10082087
Lee H, Kong J-S, Lee S-S, Kim A. Radiation-Induced Overexpression of TGFβ and PODXL Contributes to Colorectal Cancer Cell Radioresistance through Enhanced Motility. Cells. 2021; 10(8):2087. https://doi.org/10.3390/cells10082087
Chicago/Turabian StyleLee, Hyunjung, Joon-Seog Kong, Seung-Sook Lee, and Areumnuri Kim. 2021. "Radiation-Induced Overexpression of TGFβ and PODXL Contributes to Colorectal Cancer Cell Radioresistance through Enhanced Motility" Cells 10, no. 8: 2087. https://doi.org/10.3390/cells10082087
APA StyleLee, H., Kong, J.-S., Lee, S.-S., & Kim, A. (2021). Radiation-Induced Overexpression of TGFβ and PODXL Contributes to Colorectal Cancer Cell Radioresistance through Enhanced Motility. Cells, 10(8), 2087. https://doi.org/10.3390/cells10082087





