Functions of the Thyroid-Stimulating Hormone on Key Developmental Features Revealed in a Series of Zebrafish Dyshormonogenesis Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance and Treatment
2.2. Establishment of Mutant Lines
2.3. RNA Extraction and qPCR
2.4. Whole-Mount Immunofluorescence
2.5. Protein Extraction and Western Blot Analysis
2.6. Analyses of Growth and Developmental Timing
2.7. Thyroid Hormone Quantification
2.8. Behavioral Assays
2.9. Micro-CT Scan
2.10. Statistical Analysis
3. Results
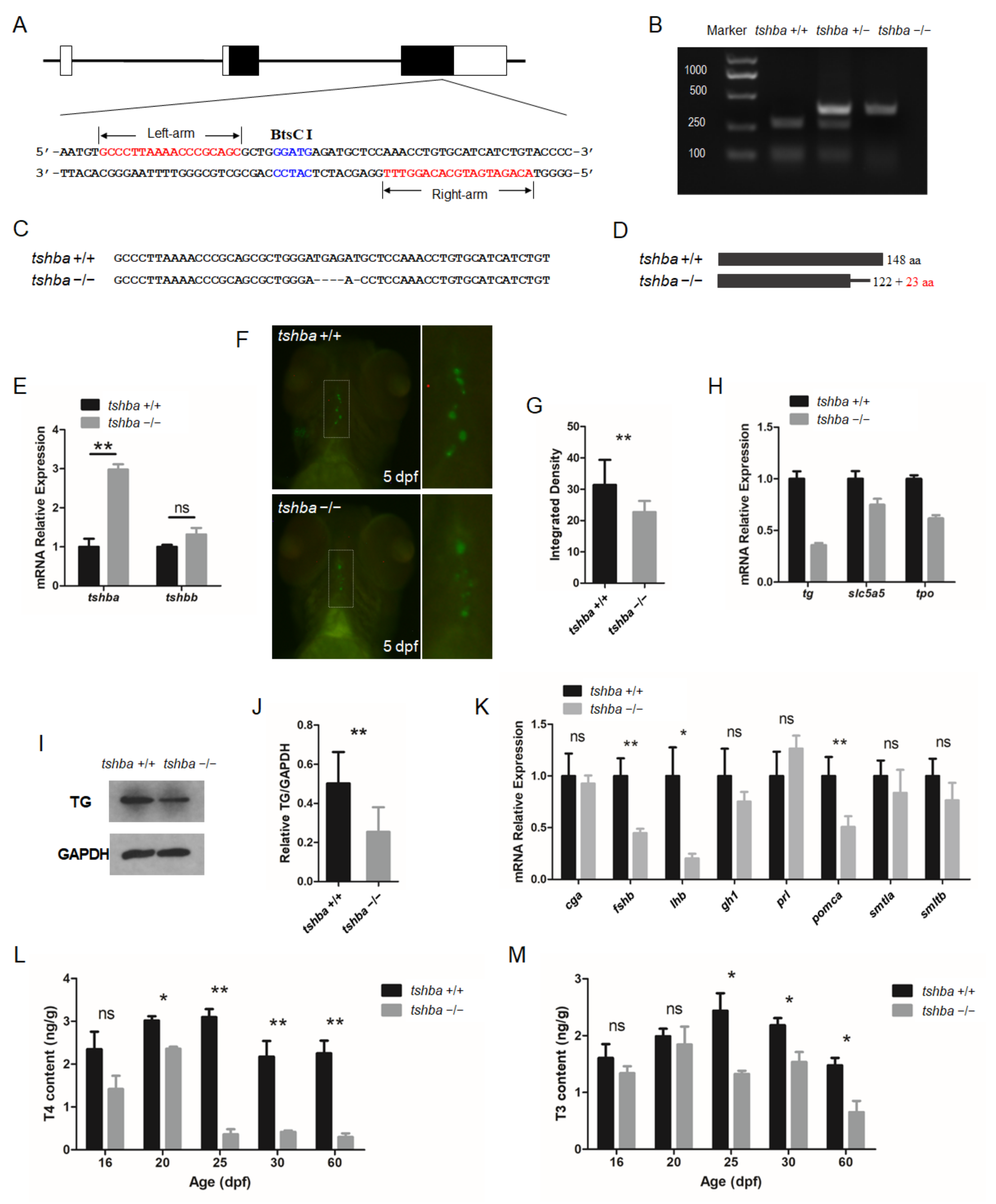
3.1. Generation of Tshba Mutant Zebrafish
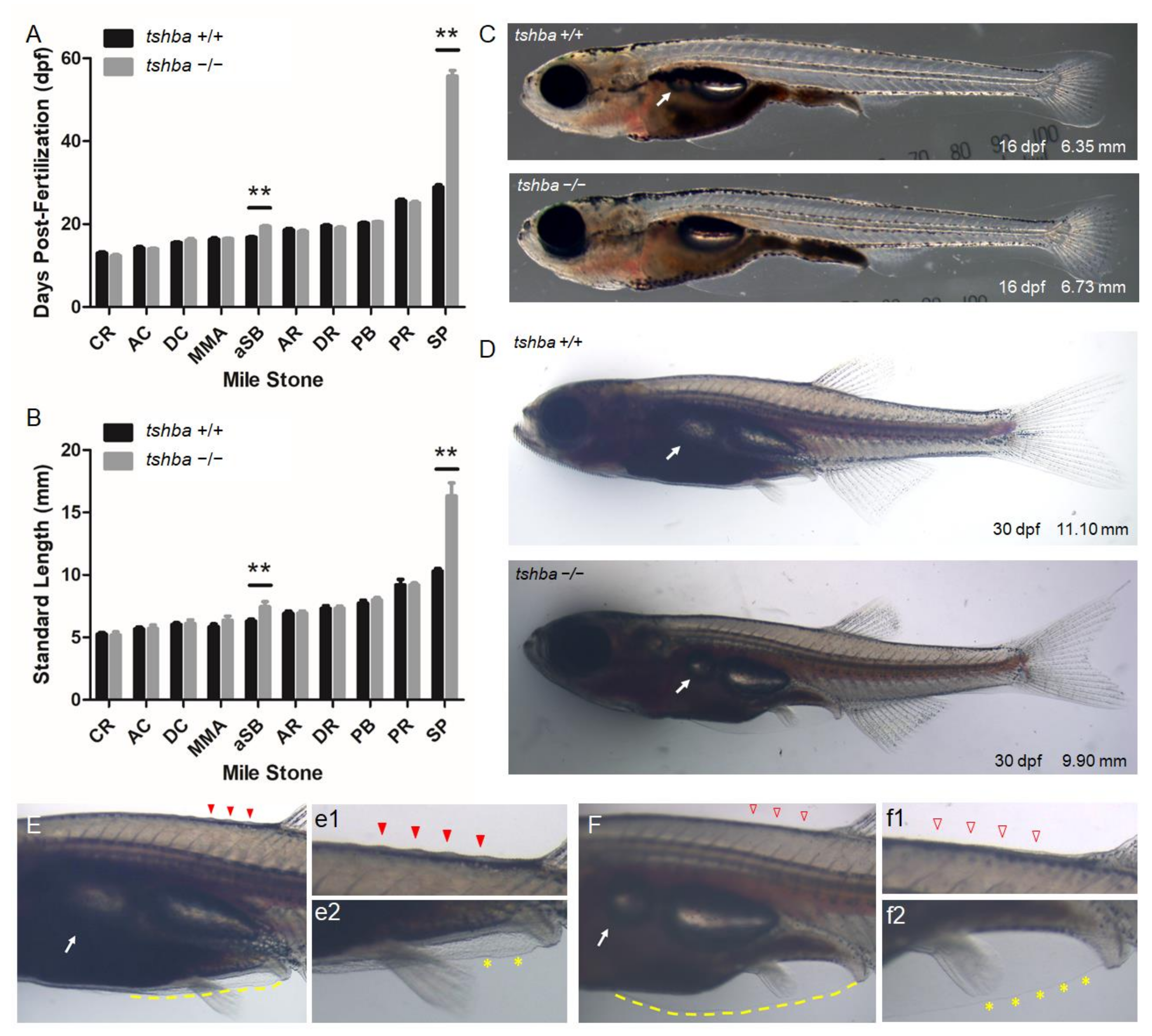
3.2. Growth Performance of the Tshba Mutant Zebrafish
3.3. Post-Embryonic Development of Tshba Mutant Zebrafish
3.4. Rescue Effects of the Supplemental Exogenous T3 Treatments on Tshba Mutants
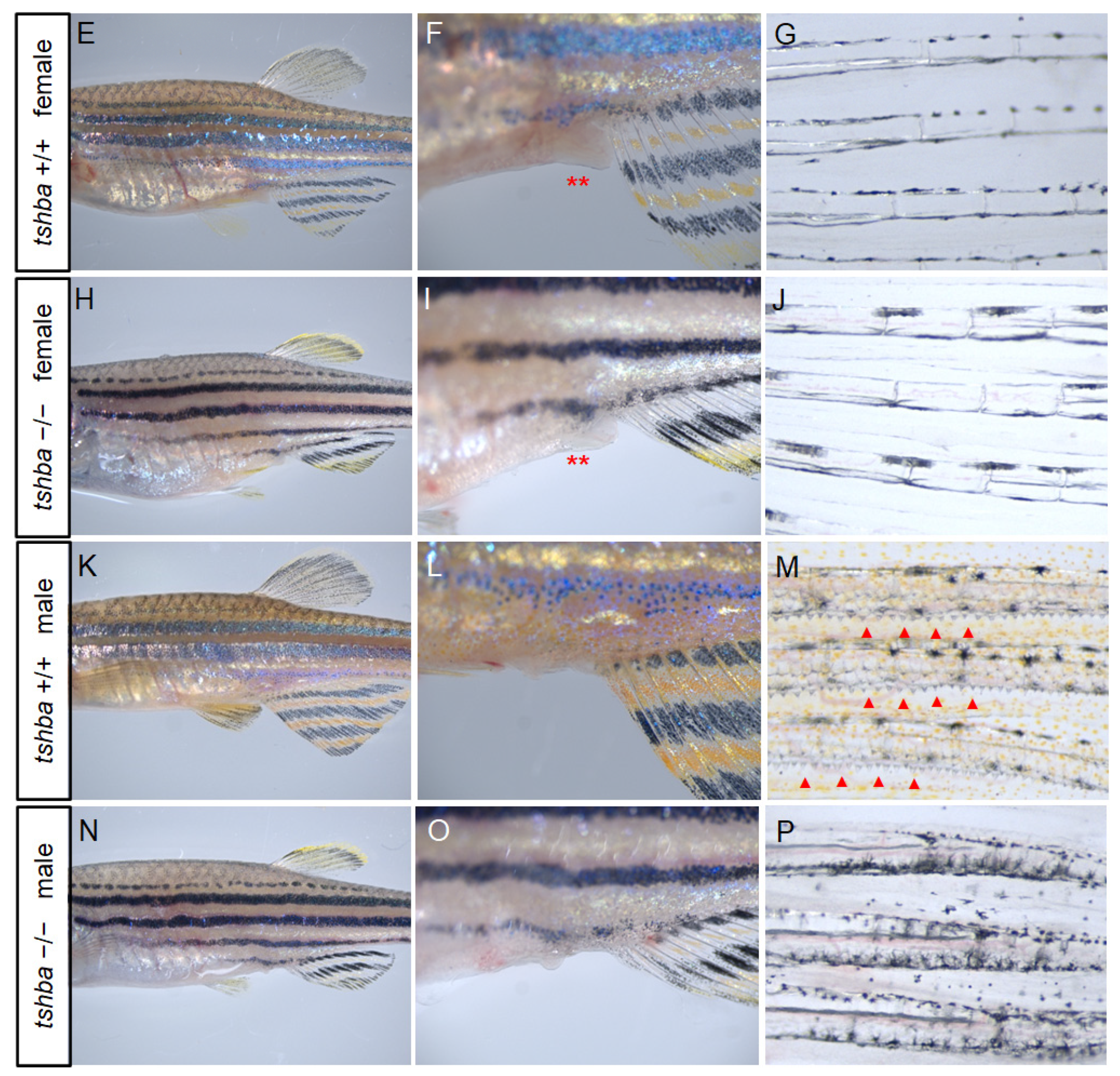
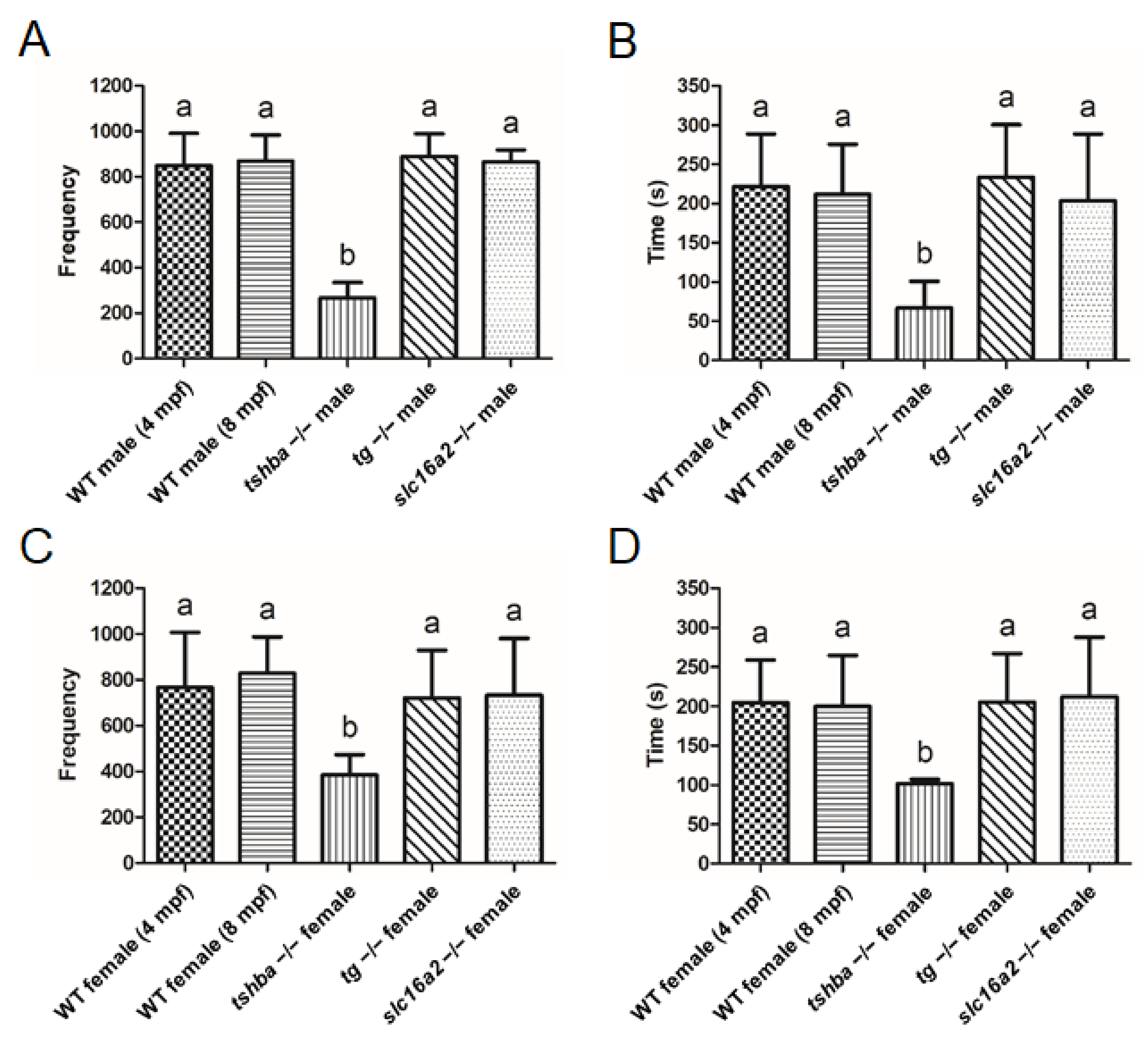
3.5. Reproductive Defects Observed in Tshba Mutant Zebrafish
3.6. Generation of tg Mutant Zebrafish
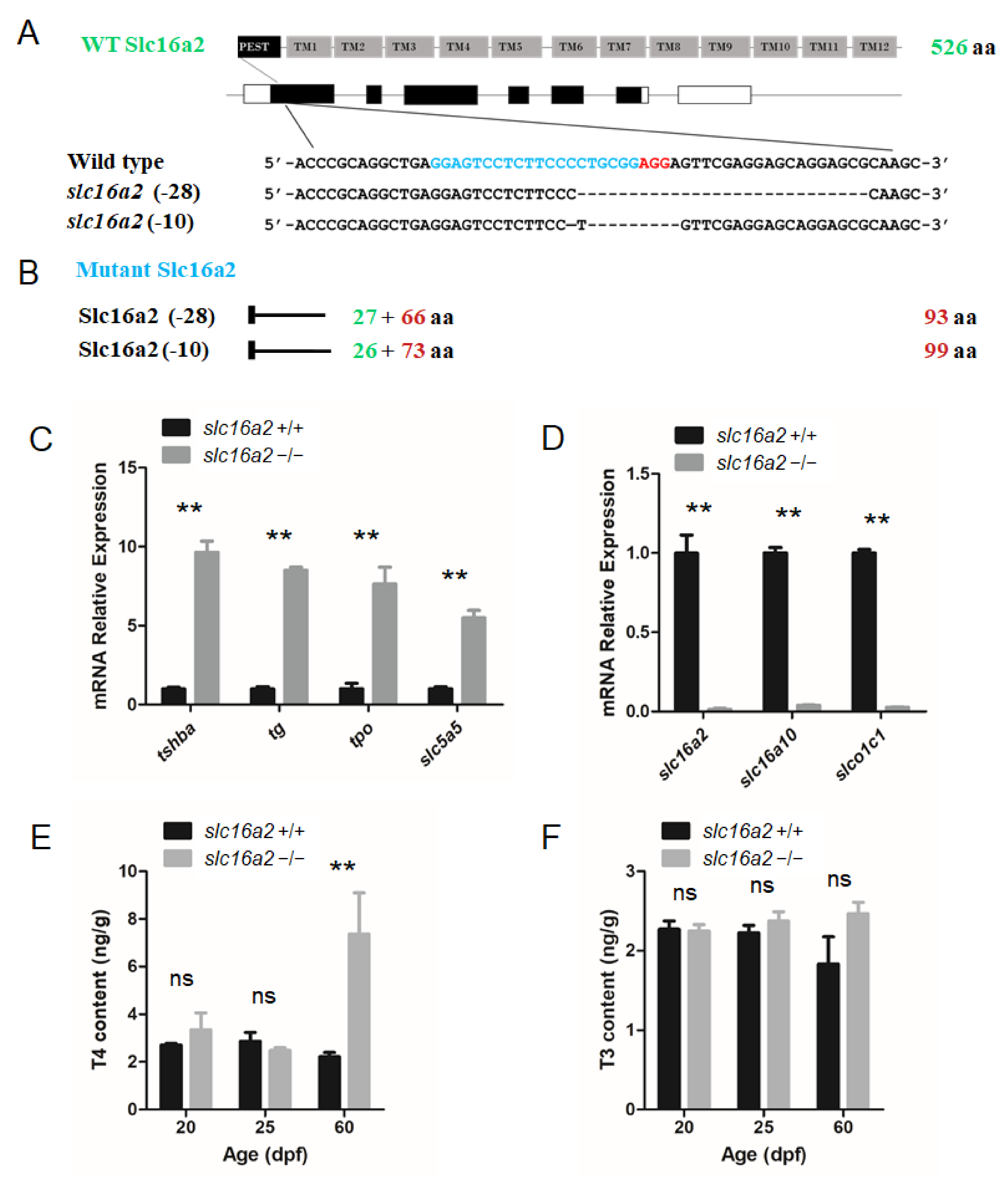
3.7. Generation of slc16a2 Mutant Zebrafish
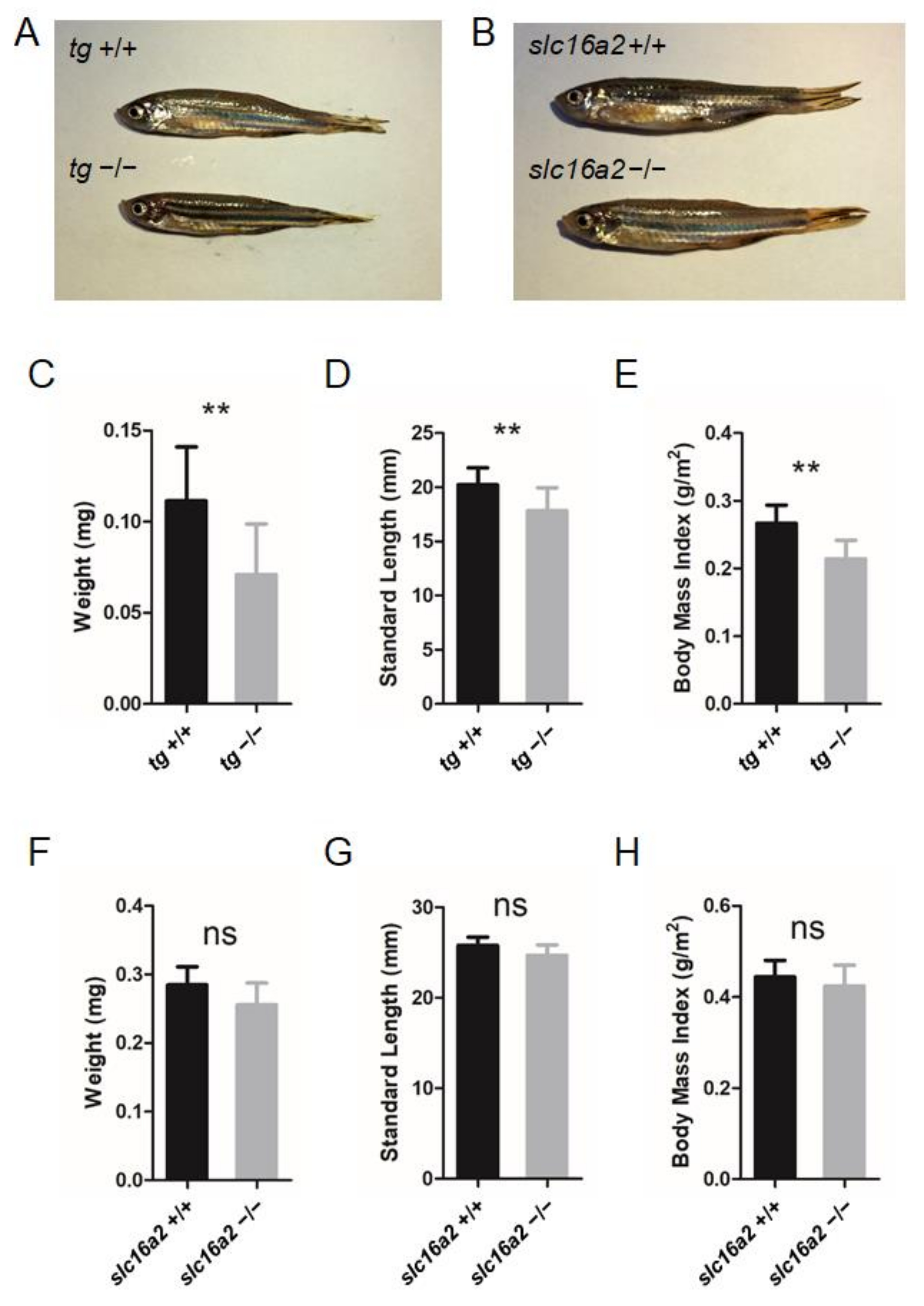
3.8. General Phenotype Observed in tg and slc16a2 Mutant Zebrafish
3.9. Divergent Effects of Reproduction in Various Zebrafish Hypothyroidism Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laudet, V. The Origins and Evolution of Vertebrate Metamorphosis. Curr. Biol. 2011, 21, R726–R737. [Google Scholar] [CrossRef]
- Dardente, H.; Hazlerigg, D.G.; Ebling, F.J.P. Thyroid Hormone and Seasonal Rhythmicity. Front. Endocrinol. 2014, 5, 19. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R. Role of Thyroid Hormone Receptor-α1 in Endochondral Ossification. Endocrinology 2014, 155, 2747–2750. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Jin, X.; He, J.; Yin, Z. Use of tsh beta:Egfp transgenic zebrafish as a rapid in vivo model for assessing thyroid-disrupting chemicals. Toxicol. Appl. Pharmacol. 2012, 262, 149–155. [Google Scholar] [CrossRef]
- Tu, W.; Xu, C.; Lu, B.; Lin, C.; Wu, Y.; Liu, W. Acute exposure to synthetic pyrethroids causes bioconcentration and disruption of the hypothalamus–pituitary–thyroid axis in zebrafish embryos. Sci. Total Environ. 2016, 542, 876–885. [Google Scholar] [CrossRef]
- Kim, P.S.; Lee, J.; Jongsamak, P.; Menon, S.; Li, B.; Hossain, S.A.; Bae, J.-H.; Panijpan, B.; Arvan, P. Defective Protein Folding and Intracellular Retention of Thyroglobulin-R19K Mutant as a Cause of Human Congenital Goiter. Mol. Endocrinol. 2008, 22, 477–484. [Google Scholar] [CrossRef]
- Friesema, E.C.; Grueters, A.; Biebermann, H.; Krude, H.; von Moers, A.; Reeser, M.; Barrett, T.; Mancilla, E.E.; Svensson, J.; Kester, M.H.; et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 2004, 364, 1435–1437. [Google Scholar] [CrossRef]
- Kalveram, L.; Kleinau, G.; Szymanska, K.; Scheerer, P.; Rivero-Mueller, A.; Grueters-Kieslich, A.; Biebermann, H. The pathogenic tsh beta-subunit variant c105vfs114x causes a modified signaling profile at tshr. Int. J. Mol. Sci. 2019, 20, 5564. [Google Scholar] [CrossRef]
- Arturi, F.; Chiefari, E.; Tumino, S.; Russo, D.; Squatrito, S.; Chazenbalk, G.; Persani, L.; Rapoport, B.; Filetti, S. Similarities and differences in the phenotype of members of an Italian family with hereditary non-autoimmune hyperthyroidism associated with an activating TSH receptor germline mutation. J. Endocrinol. Investig. 2002, 25, 696–701. [Google Scholar] [CrossRef]
- Böttcher, Y.; Eszlinger, M.; Tönjes, A.; Paschke, R. The genetics of euthyroid familial goiter. Trends Endocrinol. Metab. 2005, 16, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Ohye, H.; Fukata, S.; Hishinuma, A.; Kudo, T.; Nishihara, E.; Ito, M.; Kubota, S.; Amino, N.; Ieiri, T.; Kuma, K.; et al. A Novel Homozygous Missense Mutation of the Dual Oxidase 2 (DUOX2) Gene in an Adult Patient with Large Goiter. Thyroid 2008, 18, 561–566. [Google Scholar] [CrossRef]
- Ono, E.; Ariga, M.; Oshima, S.; Hayakawa, M.; Imai, M.; Ochiai, Y.; Mochizuki, H.; Namba, N.; Ozono, K.; Miyata, I. Three novel mutations of the mct8 (slc16a2) gene: Individual and temporal variations of endocrinological and radiological features. Clin. Pediatric Endocrinol. 2016, 25, 23–35. [Google Scholar] [CrossRef]
- Siffo, S.; Adrover, E.; Citterio, C.E.; Miras, M.B.; Balbi, V.A.; Chiesa, A.; Weill, J.; Sobrero, G.; González, V.G.; Papendieck, P.; et al. Molecular analysis of thyroglobulin mutations found in patients with goiter and hypothyroidism. Mol. Cell. Endocrinol. 2018, 473, 1–16. [Google Scholar] [CrossRef]
- Spitzweg, C.; Morris, J.C. Genetics and phenomics of hypothyroidism and goiter due to NIS mutations. Mol. Cell. Endocrinol. 2010, 322, 56–63. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, D.S.; Jones, R.A.; Miller, T.C. Thyrotropin in teleost fish. Gen. Comp. Endocrinol. 2009, 161, 83–89. [Google Scholar] [CrossRef]
- Lee, J.; Arvan, P. Repeat Motif-containing Regions within Thyroglobulin. J. Biol. Chem. 2011, 286, 26327–26333. [Google Scholar] [CrossRef]
- Mizokami, I.; Fukata, S.; Kogai, T.; Hishinuma, A.; Hamada, K.; Maruta, T.; Higashi, K.; Tajiri, J. Congenital primary hypothyroidism with the homozygous nonsense mutation p.K1374*in the thyroglobulin gene and a normal-sized thyroid gland on levothyroxine replacement. Intern. Med. 2019, 58, 2669–2673. [Google Scholar] [CrossRef]
- Sato, A.; Abe, K.; Yuzuriha, M.; Fujii, S.; Takahashi, N.; Hojo, H.; Teramoto, S.; Aoyama, H. A novel mutation in the thyroglobulin gene that causes goiter and dwarfism in Wistar Hannover GALAS rats. Mutat. Res. Mol. Mech. Mutagen. 2014, 762, 17–23. [Google Scholar] [CrossRef]
- Holzer, G.; Morishita, Y.; Fini, J.-B.; Lorin, T.; Gillet, B.; Hughes, S.; Tohmé, M.; Deléage, G.; Demeneix, B.; Arvan, P.; et al. Thyroglobulin Represents a Novel Molecular Architecture of Vertebrates. J. Biol. Chem. 2016, 291, 16553–16566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, X.; Lu, S.; Sang, N.; Li, G.; Xie, P.; Liu, C.; Zhang, L.; Xing, Y. Exposure to PFDoA causes disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae. Environ. Pollut. 2018, 235, 974–982. [Google Scholar] [CrossRef]
- Dumitrescu, A.M.; Liao, X.-H.; Weiss, R.E.; Millen, K.; Refetoff, S. Tissue-Specific Thyroid Hormone Deprivation and Excess in Monocarboxylate Transporter (Mct) 8-Deficient Mice. Endocrinology 2006, 147, 4036–4043. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, M.; Visser, T.J.; Mittag, J.; Horn, S.; Lukas, J.; Darras, V.M.; Raivich, G.; Bauer, K.; Heuer, H. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter. J. Clin. Investig. 2007, 117, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Zada, D.; Tovin, A.; Lerer-Goldshtein, T.; Vatine, G.; Appelbaum, L. Altered Behavioral Performance and Live Imaging of Circuit-Specific Neural Deficiencies in a Zebrafish Model for Psychomotor Retardation. PLoS Genet. 2014, 10, e1004615. [Google Scholar] [CrossRef] [PubMed]
- Admati, I.; Wasserman-Bartov, T.; Tovin, A.; Rozenblat, R.; Blitz, E.; Zada, D.; Lerer-Goldshtein, T.; Appelbaum, L. Neural Alterations and Hyperactivity of the Hypothalamic–Pituitary–Thyroid Axis in Oatp1c1 Deficiency. Thyroid 2020, 30, 161–174. [Google Scholar] [CrossRef]
- Chopra, K.; Ishibashi, S.; Amaya, E. Zebrafish duox mutations provide a model for human congenital hypothyroidism. Biol. Open 2019, 8, bio037655. [Google Scholar] [CrossRef]
- Han, C.R.; Holmsen, E.; Carrington, B.; Bishop, K.; Zhu, Y.J.; Starost, M.; Meltzer, P.; Sood, R.; Liu, P.; Cheng, S.-Y. Generation of Novel Genetic Models to Dissect Resistance to Thyroid Hormone Receptor α in Zebrafish. Thyroid 2020, 30, 314–328. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (danio rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Neff, K.L.; Argue, D.P.; Ma, A.C.; Lee, H.B.; Clark, K.J.; Ekker, S.C. Mojo Hand, a TALEN design tool for genome editing applications. BMC Bioinform. 2013, 14, S8. [Google Scholar] [CrossRef]
- Cermak, T.; Doyle, E.L.; Christian, M.; Wang, L.; Zhang, Y.; Schmidt, C.; Baller, J.A.; Somia, N.V.; Bogdanove, A.J.; Voytas, D. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011, 39, e82. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.; Aach, J.; Güell, M.; DiCarlo, J.; Norville, J.; Church, G.M. RNA-Guided Human Genome Engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Vanleuven, A.J.; Park, S.; Menke, D.B.; Lauderdale, J.D. A PAGE screening approach for identifying CRISPR-Cas9-induced mutations in zebrafish. Biotechniques 2018, 64, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Shang, G.; Wang, W.; Chen, X.; Lou, Q.; Zhai, G.; Li, D.; Du, Z.; Ye, Y.; Jin, X.; et al. Fatty acid oxidation in zebrafish adipose tissue is promoted by 1 alpha,25(oh)(2)d-3. Cell Rep. 2017, 19, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Thienpont, B.; Tingaud-Sequeira, A.; Prats, E.; Barata, C.; Babin, P.J.; Raldúa, D. Zebrafish Eleutheroembryos Provide a Suitable Vertebrate Model for Screening Chemicals that Impair Thyroid Hormone Synthesis. Environ. Sci. Technol. 2011, 45, 7525–7532. [Google Scholar] [CrossRef]
- Bradford, M.M. A novel method for protein estimation assay using brilliant blue g. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Parichy, D.M.; Elizondo, M.R.; Mills, M.G.; Gordon, T.N.; Engeszer, R.E. Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Dev. Dyn. 2009, 238, 2975–3015. [Google Scholar] [CrossRef]
- De Angelis, M.; Giesert, F.; Finan, B.; Clemmensen, C.; Müller, T.D.; Vogt-Weisenhorn, D.; Tschöp, M.H.; Schramm, K.-W. Determination of thyroid hormones in mouse tissues by isotope-dilution microflow liquid chromatography–mass spectrometry method. J. Chromatogr. B 2016, 1033–1034, 413–420. [Google Scholar] [CrossRef]
- Bussy, U.; Chung-Davidson, Y.W.; Li, K.; Fissette, S.D.; Buchinger, E.G.; Li, W.M. A validated lc-ms/ms method for thyroid hormone determination in sea lamprey (petromyzon marinus) plasma, gill, kidney and liver. J. Chromatogr. B 2017, 1041, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Singleman, C.; Holtzman, N.G. Growth and Maturation in the Zebrafish, Danio Rerio: A Staging Tool for Teaching and Research. Zebrafish 2014, 11, 396–406. [Google Scholar] [CrossRef]
- Aruldhas, M.; Valivullah, H.; Srinivasan, N.; Govindarajulu, P. Role of thyroid on testicular lipids in prepubertal, pubertal and adult rats. I. Hyperthyroidism. Biochim. Biophys. Acta (BBA) Gen. Subj. 1986, 881, 462–469. [Google Scholar] [CrossRef]
- Chang, J.; Wang, M.; Gui, W.; Zhao, Y.; Yu, L.; Zhu, G. Changes in Thyroid Hormone Levels during Zebrafish Development. Zool. Sci. 2012, 29, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Porazzi, P.; Calebiro, D.; Benato, F.; Tiso, N.; Persani, L. Thyroid gland development and function in the zebrafish model. Mol. Cell. Endocrinol. 2009, 312, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Sachs, L.M.; Shi, Y.-B. Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. Proc. Natl. Acad. Sci. USA 2000, 97, 13138–13143. [Google Scholar] [CrossRef]
- Schmidt, F.; Braunbeck, T. Alterations along the Hypothalamic-Pituitary-Thyroid Axis of the Zebrafish (Danio rerio) after Exposure to Propylthiouracil. J. Thyroid Res. 2011, 2011, 1–17. [Google Scholar] [CrossRef]
- Stinckens, E.; Vergauwen, L.; Ankley, G.T.; Blust, R.; Darras, V.M.; Villeneuve, D.L.; Witters, H.; Volz, D.C.; Knapen, D. An AOP-based alternative testing strategy to predict the impact of thyroid hormone disruption on swim bladder inflation in zebrafish. Aquat. Toxicol. 2018, 200, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stinckens, E.; Vergauwen, L.; Schroeder, A.L.; Maho, W.; Blackwell, B.; Witters, H.; Blust, R.; Ankley, G.T.; Covaci, A.; Villeneuve, D.L.; et al. Impaired anterior swim bladder inflation following exposure to the thyroid peroxidase inhibitor 2-mercaptobenzothiazole part II: Zebrafish. Aquat. Toxicol. 2016, 173, 204–217. [Google Scholar] [CrossRef]
- Vancamp, P.; Houbrechts, A.M.; Darras, V.M. Insights from zebrafish deficiency models to understand the impact of local thyroid hormone regulator action on early development. Gen. Comp. Endocrinol. 2019, 279, 45–52. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, E.; Van De Wiel, S.M.W.; Zethof, J.; Flik, G.; Klaren, P.; Arjona, F. Knockdown of Monocarboxylate Transporter 8 (mct8) Disturbs Brain Development and Locomotion in Zebrafish. Endocrinology 2014, 155, 2320–2330. [Google Scholar] [CrossRef] [PubMed]
- Heijlen, M.; Houbrechts, A.M.; Bagci, E.; Van Herck, S.L.; Kersseboom, S.; Esguerra, C.; Blust, R.; Visser, T.J.; Knapen, D.; Darras, V.M. Knockdown of Type 3 Iodothyronine Deiodinase Severely Perturbs Both Embryonic and Early Larval Development in Zebrafish. Endocrinology 2014, 155, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Houbrechts, A.M.; Vergauwen, L.; Bagci, E.; Van Houcke, J.; Heijlen, M.; Kulemeka, B.; Hyde, D.R.; Knapen, D.; Darras, V.M. Deiodinase knockdown affects zebrafish eye development at the level of gene expression, morphology and function. Mol. Cell. Endocrinol. 2016, 424, 81–93. [Google Scholar] [CrossRef]
- Opitz, R.; Maquet, E.; Zoenen, M.; Dadhich, R.; Costagliola, S. TSH Receptor Function Is Required for Normal Thyroid Differentiation in Zebrafish. Mol. Endocrinol. 2011, 25, 1579–1599. [Google Scholar] [CrossRef]
- Houbrechts, A.M.; Delarue, J.; Gabriëls, I.J.; Sourbron, J.; Darras, V.M. Permanent Deiodinase Type 2 Deficiency Strongly Perturbs Zebrafish Development, Growth, and Fertility. Endocrinology 2016, 157, 3668–3681. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Louro, B.; Trindade, M.; Power, D.; Campinho, M.A. Transcriptomics reveal an integrative role for maternal thyroid hormones during zebrafish embryogenesis. Sci. Rep. 2017, 7, 16657. [Google Scholar] [CrossRef]
- Trubiroha, A.; Gillotay, P.; Giusti, N.; Gacquer, D.; Libert, F.; Lefort, A.; Haerlingen, B.; De Deken, X.; Opitz, R.; Costagliola, S. A Rapid CRISPR/Cas-based Mutagenesis Assay in Zebrafish for Identification of Genes Involved in Thyroid Morphogenesis and Function. Sci. Rep. 2018, 8, 5647. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.D. The role of thyroid hormone in zebrafish and axolotl development. Proc. Natl. Acad. Sci. USA 1997, 94, 13011–13016. [Google Scholar] [CrossRef] [PubMed]
- Marians, R.C.; Ng, L.; Blair, H.C.; Unger, P.; Graves, P.N.; Davies, T.F. Nonlinear partial differential equations and applications: Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc. Natl. Acad. Sci. USA 2002, 99, 15776–15781. [Google Scholar] [CrossRef]
- Medeiros-Neto, G.; Herodotou, D.T.; Rajan, S.; Kommareddi, S.; Lacerda, L.; Sandrini, R.; Boguszewski, M.C.; Hollenberg, A.N.; Radovick, S.; Wondisford, F.E. A circulating, biologically inactive thyrotropin caused by a mutation in the beta subunit gene. J. Clin. Investig. 1996, 97, 1250–1256. [Google Scholar] [CrossRef]
- Szkudlinski, M.W.; Fremont, V.; Ronin, C.; Weintraub, B.D. Thyroid-Stimulating Hormone and Thyroid-Stimulating Hormone Receptor Structure-Function Relationships. Physiol. Rev. 2002, 82, 473–502. [Google Scholar] [CrossRef]
- Tonyushkina, K.N.; Shen, M.-C.; Ortiz-Toro, T.; Karlstrom, R.O. Embryonic exposure to excess thyroid hormone causes thyrotrope cell death. J. Clin. Investig. 2013, 124, 321–327. [Google Scholar] [CrossRef][Green Version]
- Alt, B.; Reibe, S.; Feitosa, N.M.; Elsalini, O.A.; Wendl, T.; Rohr, K.B. Analysis of origin and growth of the thyroid gland in zebrafish. Dev. Dyn. 2006, 235, 1872–1883. [Google Scholar] [CrossRef]
- McMenamin, S.; Bain, E.J.; McCann, A.E.; Patterson, L.B.; Eom, D.S.; Waller, Z.P.; Hamill, J.C.; Kuhlman, J.A.; Eisen, J.S.; Parichy, D.M. Thyroid hormone-dependent adult pigment cell lineage and pattern in zebrafish. Science 2014, 345, 1358–1361. [Google Scholar] [CrossRef]
- Campinho, M.A.; Saraiva, J.; Florindo, C.; Power, D. Maternal Thyroid Hormones Are Essential for Neural Development in Zebrafish. Mol. Endocrinol. 2014, 28, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Galindo, D.; Sweet, E.; DeLeon, Z.; Wagner, M.; DeLeon, A.; Carter, C.; McMenamin, S.; Cooper, W.J. Thyroid hormone modulation during zebrafish development recapitulates evolved diversity in danionin jaw protrusion mechanics. Evol. Dev. 2019, 21, 231–246. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, S.; Carter, C.; Cooper, W.J. Thyroid Hormone Stimulates the Onset of Adult Feeding Kinematics in Zebrafish. Zebrafish 2017, 14, 517–525. [Google Scholar] [CrossRef]
- Fay, R.R.; Popper, A.N. Acoustic Stimulation of the Ear of the Goldfish (Carassius Auratus). J. Exp. Biol. 1974, 61, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Di Jeso, B.; Arvan, P. Thyroglobulin From Molecular and Cellular Biology to Clinical Endocrinology. Endocr. Rev. 2015, 37, 2–36. [Google Scholar] [CrossRef]
- Citterio, C.E.; Morales, C.M.; Bouhours-Nouet, N.; Machiavelli, G.A.; Bueno, E.; Gatelais, F.; Coutant, R.; Gonzalez-Sarmiento, R.; Rivolta, C.M.; Targovnik, H.M. Novel compound heterozygous thyroglobulin mutations c.745+1g > a/c.7036+2t > a associated with congenital goiter and hypothyroidism in a vietnamese family. Identification of a new cryptic 5 splice site in the exon 6. Mol. Cell. Endocrinol. 2015, 404, 102–112. [Google Scholar] [CrossRef]
- Maniakas, A.; Davies, L.; Zafereo, M.E. Thyroid disease around the world. Otolaryngol. Clin. N. Am. 2018, 51, 631–642. [Google Scholar] [CrossRef]
- Cooper, D.S. Hyperthyroidism. Lancet 2003, 362, 459–468. [Google Scholar] [CrossRef]
- Franklyn, J.A.; Boelaert, K. Thyrotoxicosis. Lancet 2012, 379, 1155–1166. [Google Scholar] [CrossRef]
- Houbrechts, A.M.; Van Houcke, J.; Darras, V.M. Disruption of deiodinase type 2 in zebrafish disturbs male and female reproduction. J. Endocrinol. 2019, 241, 111–123. [Google Scholar] [CrossRef]
- Zhai, G.; Shu, T.; Xia, Y.; Lu, Y.; Shang, G.; Jin, X.; He, J.; Nie, P.; Yin, Z. Characterization of Sexual Trait Development in cyp17a1-Deficient Zebrafish. Endocrinology 2018, 159, 3549–3562. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Lu, Y.; Cheng, X.; Shi, C.; Lou, Q.; Jin, X.; He, J.; Zhai, G.; Yin, Z. Functions of the Thyroid-Stimulating Hormone on Key Developmental Features Revealed in a Series of Zebrafish Dyshormonogenesis Models. Cells 2021, 10, 1984. https://doi.org/10.3390/cells10081984
Song J, Lu Y, Cheng X, Shi C, Lou Q, Jin X, He J, Zhai G, Yin Z. Functions of the Thyroid-Stimulating Hormone on Key Developmental Features Revealed in a Series of Zebrafish Dyshormonogenesis Models. Cells. 2021; 10(8):1984. https://doi.org/10.3390/cells10081984
Chicago/Turabian StyleSong, Jia, Yao Lu, Xiaoxia Cheng, Chuang Shi, Qiyong Lou, Xia Jin, Jiangyan He, Gang Zhai, and Zhan Yin. 2021. "Functions of the Thyroid-Stimulating Hormone on Key Developmental Features Revealed in a Series of Zebrafish Dyshormonogenesis Models" Cells 10, no. 8: 1984. https://doi.org/10.3390/cells10081984
APA StyleSong, J., Lu, Y., Cheng, X., Shi, C., Lou, Q., Jin, X., He, J., Zhai, G., & Yin, Z. (2021). Functions of the Thyroid-Stimulating Hormone on Key Developmental Features Revealed in a Series of Zebrafish Dyshormonogenesis Models. Cells, 10(8), 1984. https://doi.org/10.3390/cells10081984





