A Molecular Pinball Machine of the Plasma Membrane Regulates Plant Growth—A New Paradigm
Abstract
1. Introduction—Brief Historical Perspective
2. The Origin of Ion Gradients
3. Plasma Membrane Dynamics
4. Proton Pumps
5. Indirect Evidence for the Role of AGPs in Ca2+ Homeostasis
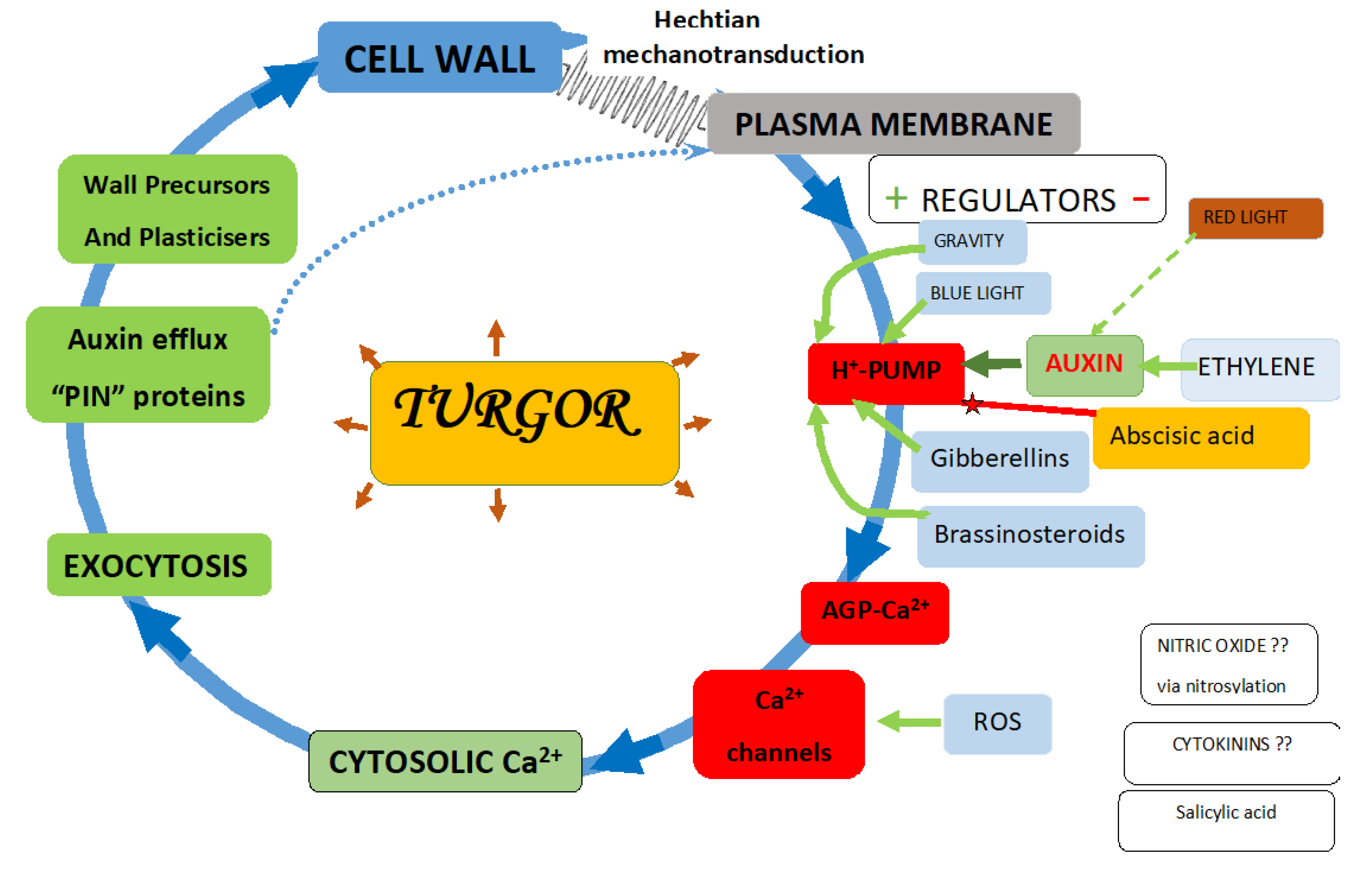
6. Direct Evidence for AGP Regulation of Ca2+ Homeostasis—A New Paradigm
- AGP glucuronic acid is essential for growth.
- AGP glucuronic acid enables AGP-Ca2+ binding.
- AGP-Ca2+ binding is the source of cytosolic Ca2+.
- Specific Ca2+ channels facilitate its influx.
- Auxin triggers a rapid increase in cytosolic Ca2+.
- Ca2+ waves are essential for root growth.
- AGP adaptation to salt stress also involves upregulation of their genes.
6.1. AGP Glucuronic Acid Is Essential for Growth
6.2. AGP Glucuronic Acid Enables AGP-Ca2+ Binding
6.3. AGP-Ca2+ Binding Is a Major Source of Cytosolic Ca2+
6.4. Auxin Increases Cytosolic Ca2+
6.5. Ca2+ ATPase Recycles Cytosolic Ca2+
6.6. Ca2+ Waves Are Essential for Root Growth
6.7. AGPs Respond to Salt Stress
7. The Quest for Key Regulators of Plant Growth
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Dedication
References
- Lamport, D.T.A.; Northcote, D.H. Hydroxyproline in primary cell walls of higher plants. Nature 1960, 188, 665–666. [Google Scholar] [CrossRef]
- Sanger, F. Selected Papers of Frederick Sanger; World Scientific: London, UK, 1996; Volume 1. [Google Scholar]
- Hill, R. Oxygen Evolved by Isolated Chloroplasts. Nature 1937, 139, 881–882. [Google Scholar] [CrossRef]
- Bendall, D.S. Robert Hill 2 April 1899–15 March 1991. Biogr. Mems. Fell. R. Soc. 1994, 40, 142–170. [Google Scholar] [CrossRef]
- Lamport, D.T.A. Oxygen fixation into hydroxyproline of plant cell wall protein. J. Biol. Chem. 1963, 238, 1438–1440. [Google Scholar] [CrossRef]
- Dunwoodie, S.L. The Role of Hypoxia in Development of the Mammalian Embryo. Dev. Cell 2009, 17, 755–773. [Google Scholar] [CrossRef]
- Lamport, D.T.A. The isolation and partial characterization of hydroxyproline-rich glycopeptides obtained by enzymic degradation of primary cell walls. Biochemistry 1969, 8, 1155–1163. [Google Scholar] [CrossRef]
- Jermyn, M.A.; Yeow, Y.M. A class of lectins present in the tissues of seed plants. Aust. J. Plant Physiol. 1975, 2, 501–531. [Google Scholar] [CrossRef]
- Lamport, D.T.A. Cell wall metabolism. Ann. Rev. Plant Physiol. 1970, 21, 235–270. [Google Scholar] [CrossRef]
- Mitchell, P. Approaches to the analysis of specific membrane transport. In Biological Structure and Function; 1st IUB/IUBS Intern. Symp., Stockholm, Sweden; Academic Press: London, UK, 1961; pp. 581–603. [Google Scholar]
- Pope, D.G. Relationships between hydroxyproline-containing proteins secreted into the cell wall and medium by suspension-cultured Acer pseudoplatanus cells. Plant Physiol. 1977, 59, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Mort, A.J.; Lamport, D.T.A. Anhydrous hydrogen fluoride deglycosylates glycoproteins. Analyt. Biochem. 1977, 82, 289–309. [Google Scholar] [CrossRef]
- Gendler, S.J.; Burchell, J.M.; Duhig, T.; Lamport, D.T.A.; White, R.; Parker, M.; Taylor-Papadimitriou, J. Cloning the cDNA coding for differentiation and tumour- associated mucin glycoproteins expressed by human mammary epithelium. Proc. Natl. Acad. Sci. USA 1987, 84, 6060–6064. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Varner, J.E. Isolation and characterization of cDNA clones for carrot extensin and a proline-rich 33-kDa protein. Proc. Natl. Acad. Sci. USA 1985, 82, 4399–4403. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.T.A. Hydroxyproline-O-glycosidic linkage of the plant cell wall glycoprotein extensin. Nature 1967, 216, 1322–1324. [Google Scholar] [CrossRef]
- Tan, L.; Varnai, P.; Lamport, D.T.A.; Yuan, C.; Xu, J.; Qiu, F.; Kieliszewski, M.J. Plant O-Hydroxyproline Arabinogalactans Are Composed of Repeating Trigalactosyl Subunits with Short Bifurcated Side Chains. J. Biol. Chem. 2010, 285, 24575–24583. [Google Scholar] [CrossRef]
- Lopez-Hernandez, F.; Tryfona, T.; Rizza, A.; Yu, X.L.; Harris, M.O.B.; Webb, A.A.R.; Kotake, T.; Dupree, P. Calcium Binding by Arabinogalactan Polysaccharides Is Important for Normal Plant Development. Plant Cell 2020, 32, 3346–3369. [Google Scholar] [CrossRef]
- Zhang, Y.; Held, M.A.; Showalter, A.M. Elucidating the roles of three β-glucuronosyltransferases (GLCATs) acting on arabinogalactan-proteins using a CRISPR-Cas9 multiplexing approach in Arabidopsis. BMC Plant Biol. 2020, 20, 221. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, O.O.; Held, M.A.; Showalter, A.M. Three β-Glucuronosyltransferase Genes Involved in Arabinogalactan Biosynthesis Function in Arabidopsis Growth and Development. Plants 2021, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, C.C.; Froyd, K.D.; Schill, G.P.; Murphy, D.M.; Bui, T.P.; Dean-Day, J.M.; Weinzierl, B.; Dollner, M.; Diskin, G.S.; et al. Sea spray aerosol concentration modulated by sea surface temperature. Proc. Natl. Acad. Sci. USA 2021, 118, e2020583118. [Google Scholar] [CrossRef]
- Dobson, C.M.; Ellison, G.B.; Tuck, A.F.; Vaida, V. Atmospheric aerosols as prebiotic chemical reactors. Proc. Natl. Acad. Sci. USA 2000, 97, 11864. [Google Scholar] [CrossRef]
- Stoeckenius, W. The Purple Membrane of Salt-loving Bacteria. Sci. Am. 1976, 234, 38–46. [Google Scholar] [CrossRef]
- Haruta, M.; Sussman, M.R. The effect of a genetically reduced plasma membrane protonmotive force on vegetative growth of Arabidopsis. Plant Physiol. 2012, 158, 1158–1171. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, S.; Friml, J. Calcium: The missing link in auxin action. Plants 2013, 2, 650–675. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. David Keilin’s Respiratory Chain Concept and Its Chemiosmotic Consequences. In Nobel Lectures in Chemistry 1971–1980; World Scientific: Singapore, 1993; pp. 295–330. [Google Scholar]
- Abrahams, J.P.; Leslie, A.G.W.; Lutter, R.; Walker, J.E. Structure at 2.8 A resolution of F1·ATPase from bovine heart mitochondria. Nature 1994, 370, 621–628. [Google Scholar] [CrossRef]
- Falhof, J.; Pedersen, J.T.; Fuglsang, J.T.; Palmgren, M. Plasma Membrane H+-ATPase Regulation in the Center of Plant Physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef]
- Pacheco-Villalobos, D.; Diaz-Moeeno, M.; van der Schuren, A.; Tamaki, T.; Kang, Y.H.; Gujas, B.; Novak, O.; Jaspert, N.; Li, Z.; Wolf, S.; et al. The Effects of High Steady State Auxin Levels on Root Cell Elongation in Brachypodium. Plant Cell 2016, 28, 1009–1024. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, M.; Runions, A.; Prusinkiewicz, P. Auxin-driven patterning with unidirectional fluxes. J. Exp. Bot. 2015, 66, 5083–5102. [Google Scholar] [CrossRef] [PubMed]
- Leyser, O. Auxin Signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Habets, M.E.; Offringa, R. PIN-driven polar auxin transport in plant developmental plasticity: A key target for environmental and endogenous signals. New Phytol. 2014, 203, 362–377. [Google Scholar] [CrossRef]
- Hayashi, Y.; Takahashi, K.; Inoue, S.; Kinoshita, T. Abscisic Acid Suppresses Hypocotyl Elongation by Dephosphorylating Plasma Membrane H+-ATPase in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 845–853. [Google Scholar] [CrossRef]
- De Vries, J.; Curtis, B.A.; Gould, S.B.; Archibald, J.M. Embryophyte stress signaling evolved in the algal progenitors of land plants. Proc. Natl. Acad. Sci. USA 2018, 115, E3471–E3486. [Google Scholar] [CrossRef] [PubMed]
- Folta, K.M.; Lieg, E.G.; Durham, T.; Spalding, E.P. Primary Inhibition of Hypocotyl Growth and Phototropism Depend Differently on PhototropinMediated Increases in Cytoplasmic Calcium Induced by Blue Light. Plant Physiol. 2003, 133, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qi, Z.; Berkowitz, G.A. Teaching an Old Hormone New Tricks: Cytosolic Ca2+ Elevation Involvement in Plant Brassinosteroid Signal Transduction Cascades. Plant Physiol. 2013, 163, 555–565. [Google Scholar] [CrossRef]
- Swarup, R.; Perry, P.; Hagenbeek, D.; Van Der Straeten, D.; Beemster, G.T.S.; Sandberg, G.; Bhalerao, R.; Ljung, K.; Bennett, M. Ethylene Upregulates Auxin Biosynthesis in Arabidopsis Seedlings to Enhance Inhibition of Root Cell Elongation. Plant Cell 2007, 19, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Muday, G.K.; Rahman, A.; Binder, B.M. Auxin and ethylene: Collaborators or competitors? Trends Plant Sci. 2012, 17, 1360–1385. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.T.A.; Tan, L.; Held, M.A.; Kieliszewksi, M.J. The Role of the Primary Cell Wall in Plant Morphogenesis. Int. J. Mol. Sci. 2018, 19, 2674. [Google Scholar] [CrossRef] [PubMed]
- Rubery, P.H.; Sheldrake, A.R. Carrier-mediated Auxin Transport. Planta 1974, 118, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Zazimalova, E.; Murphy, A.S.; Yang, H.; Hoyerova, K.; Hosek, P. Auxin Transporters—Why So Many? Cold Spring Harb. Perspect. Biol. 2010, 2, a001552. [Google Scholar] [CrossRef]
- Rayle, D.L.; Cleland, R.E. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.P.; Day, S.; Roberts, K. A set of cell surface glycoproteins forms an early marker of cell position, but not cell type, in the root apical meristem of Daucus carota L. Development 1989, 106, 56. [Google Scholar] [CrossRef]
- Silva, J.; Ferraz, R.; Dupree, P.; Showalter, A.M.; Coimbra, S. Three Decades of Advances in Arabinogalactan-Protein Biosynthesis. Front. Plant Sci. 2020, 11, 610377. [Google Scholar] [CrossRef]
- Hromadova, D.; Soukup, A.; Tylova, E. Arabinogalactan Proteins in Plant Roots—An Update on Possible Functions. Front. Plant Sci. 2021, 12, 674010. [Google Scholar] [CrossRef] [PubMed]
- Ligrone, R.; Vaughn, K.C.; Renzaglia, K.S.; Knox, J.P.; Duckett, J.G. Diversity in the distribution of polysaccharide and glycoprotein epitopes in the cell walls of bryophytes: New evidence for the multiple evolution of water-conducting cells. New Phytol. 2002, 156, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Kuczak, M.; Kurcznska, E. Cell Wall Composition as a Marker of the Reprogramming of the Cell Fate on the Example of a Daucus carota (L.) Hypocotyl in Which Somatic Embryogenesis Was Induced. Int. J. Mol. Sci. 2020, 21, 8126. [Google Scholar] [CrossRef] [PubMed]
- Rafinska, K.; Niedojadlo, K.; Swidzinski, M.; Niedojadlo, J.; Bednarska-Kozakiewicz, E. Spatial and Temporal Distribution of Arabinogalactan Proteins during Larix decidua Male Gametophyte and Ovule Interaction. Int. J. Mol. Sci. 2021, 22, 4298. [Google Scholar] [CrossRef] [PubMed]
- Moller, B.; Weijers, D. Auxin Control of Embryo Patterning. Cold Spring Harb. Perspect. Biol. 2009, 1, a001545. [Google Scholar] [CrossRef] [PubMed]
- Van Hengel, A.J.; van Kammen, A.; de Vries, S.C. A relationship between seed development, Arabinogalactan-proteins (AGPs) and the AGP mediated promotion of somatic embryogenesis. Physiol. Plant 2002, 114, 637–644. [Google Scholar] [CrossRef]
- Pilarska, M.; Knox, J.P.; Konieczny, R. Arabinogalactan-protein and pectin epitopes in relation to an extracellular matrix surface network and somatic embryogenesis and callogenesis in Trifolium nigrescens Viv. Plant Cell Tissue Organ. Cult. 2013, 115, 35–44. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Tan, L.; Held, M.A.; Kieliszewksi, M.J. Phyllotaxis Turns Over a New Leaf—A New Hypothesis. Int. J. Mol. Sci. 2020, 21, 1145. [Google Scholar] [CrossRef]
- Leszczuk, A.; Kalaitzis, P.; Blazakis, K.; Zdunek, A. The role of arabinogalactan proteins (AGPs) in fruit ripening—A review. Hortic. Res. 2020, 7, 176. [Google Scholar] [CrossRef]
- Tan, L.; Qiu, F.; Lamport, D.T.A.; Kieliszewski, M.J. Structure of a Hydroxyproline (Hyp)-Arabinogalactan Polysaccharide from Repetitive Ala-Hyp Expressed in Transgenic Nicotiana tabacum. J. Biol. Chem. 2004, 279, 13156–13165. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Varnai, P. Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytol. 2013, 197, 58–64. [Google Scholar] [CrossRef]
- Qi, W.; Fong, C.; Lamport, D.T.A. Gum arabic glycoprotein is a twisted hairy rope: A new model based on O-galactosylhydroxyproline as the polysaccharide attachment site. Plant Physiol. 1991, 96, 848–855. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Komalavilas, P.; Zhu, J.; Nothnagel, E.A. Arabinogalactan-proteins from the suspension culture medium and plasma membrane of rose cells. J. Biol. Chem. 1991, 266, 15956–15965. [Google Scholar] [CrossRef]
- Behera, S.; Zhaolong, X.; Luoni, L.; Bonza, M.C.; Doccula, F.G.; De Michelis, M.I.; Morris, M.J.; Schwarzlander, M.; Costa, A. Cellular Ca2+ Signals Generate Defined pH Signatures in Plants. Plant Cell 2018, 30, 2704–2719. [Google Scholar] [CrossRef]
- De Vriese, K.; Himschoot, E.; Dunser, K.; Nguyen, L.; Drozdzecki, A.; Costa, A.; Nowack, M.K.; Kleine-Vehn, J.; Audenaert, D.; Beeckman, T.; et al. Identification of Novel Inhibitors of Auxin-Induced Ca2+ Signaling via a Plant-Based Chemical Screen. Plant Physiol. 2019, 180, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Junge, W.; Nelson, N. ATP Synthase. Annu. Rev. Biochem. 2015, 84, 631–657. [Google Scholar] [CrossRef] [PubMed]
- Rahmati Ishka, M.; Brown, E.; Rosenberg, A.; Romanowsky, S.; Davis, J.A.; Choi, W.G.; Harper, J.F. Arabidopsis Ca2+-ATPases 1, 2, and 7 in the endoplasmic reticulum contribute to growth and pollen fitness. Plant Physiol. 2021, 185, 1966–1985. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Kieliszewksi, M.J.; Showalter, A.M. Salt-stress upregulates periplasmic arabinogalactan-proteins: Using salt-stress to analyse AGP function. New Phytol. 2006, 169, 479–492. [Google Scholar] [CrossRef]
- Green, A.E.; Unsworth, R.K.F.; Chadwick, M.A.; Jones, P.J.S. Historical Analysis Exposes Catastrophic Seagrass Loss for the United Kingdom. Front. Plant Sci. 2021, 12, 629962. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, L.; Shafee, T.; Johnson, K.L.; Bacic, A.; Classen, B. Arabinogalactan-proteins of Zostera marina L. contain unique glycan structures and provide insight into adaption processes to saline environments. Nat. Sci. Rep. 2020, 10, 8232. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Palmgren, M. The quest for the central players governing pollen tube growth and guidance. Plant Physiol. 2021, 185, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Edel, K.H.; Marchadier, E.; Brownlee, C.; Kudla, J.; Hetherington, A.M. The Evolution of Calcium-Based Signalling in Plants. Curr. Biol. 2017, 27, R667–R679. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J.; Hedrich, R. Stretch-activated chloride, potassium, and calcium channels coexisting in plasma membranes of guard cells of Vicia faba L. Planta 1991, 186, 143–153. [Google Scholar] [CrossRef]
- Pickard, B.G. Delivering Force and Amplifying Signals in Plant Mechanosensing. Curr. Top. Plant Membr. 2007, 58, 361–392. [Google Scholar]
- Mousavi, S.A.R.; Dubin, A.E.; Zeng, W.Z.; Coombs, A.M.; Do, K.; Ghadiri, D.A.; Keenan, W.T.; Ge, C.; Zhao, Y.; Patapoutian, A. PIEZO ion channel is required for root mechanotransduction in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2021, 118, e2102188118. [Google Scholar] [CrossRef]
- Tan, L.; Eberhard, S.; Pattathil, S.; Warder, C.; Glushka, J.; Yuan, C.; Hao, Z.; Zhu, X.; Avci, U.; Miller, J.S.; et al. An Arabidopsis Cell Wall Proteoglycan Consists of Pectin and Arabinoxylan Covalently Linked to an Arabinogalactan Protein. Plant Cell 2013, 25, 270–287. [Google Scholar] [CrossRef]
- Sato, C.S.; Byerrum, R.U.; Albersheim, P.; Bonner, J. Metabolism of methionine and pectin esterification in a plant tissue. J. Biol. Chem. 1958, 233, 128. [Google Scholar] [CrossRef]
- McQueen-Mason, S.; Cosgrove, D.J. Disruption of hydrogen bonding between plant cell wall polymers by proteins that induce wall extension. Proc. Natl. Acad. Sci. USA 1994, 91, 6574–6578. [Google Scholar] [CrossRef]
- Dyson, R.J.; Band, L.R.; Jensen, O.E. A model of crosslink kinetics in the expanding plant cell wall: Yield stress and enzyme action. J. Theor. Biol. 2012, 307, 125–136. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Catalysts of plant cell wall loosening. F100Research 2016, 5, 1–13. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Northcote, D.H. The use of tissue cultures for the study of plant cell walls. Biochem. J. 1960, 76, 52P. [Google Scholar]
- Lamport, D.T.A. Cell suspension cultures of higher plants, isolation and growth energetics. Exp. Cell Res. 1964, 33, 195–206. [Google Scholar] [CrossRef]
- Aspinall, G.O.; Malloy, J.A.; Craig, J.W.T. Extracellular polysaccharides from suspension-cultured sycamore cells. Can. J. Biochem. 1969, 47, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Tryfona, T.; Liang, H.-C.; Kotake, T.; Kaneko, S.; Marsh, J.; Ichinose, H.; Lovegrove, A.; Tsumaraya, Y.; Shewry, P.R.; Dupree, P. Carbohydrate structural analysis of wheat flour arabinogalactan protein. Carbohydr. Res. 2010, 345, 2656. [Google Scholar] [CrossRef] [PubMed]
| Amino Acid | Sycamore | Tomato | Sphaerocarpos | Ginkgo | Tomato Cell Wall |
|---|---|---|---|---|---|
| Hyp | 29 | 31 | 30 | 28 | 30 |
| Pro | 6 | nd | nd | 1 | 8 |
| Asp | 11 | 5 | 15 | 25 | 8 |
| Thr | 14 | 12 | 17 | 16 | 6 |
| Ser | 19 | 19 | 20 | 27 | 15 |
| Glu | 8 | 6 | 10 | 20 | 9 |
| Gly | 9 | 9 | 10 | 14 | 8 |
| Ala | 20 | 26 | 27 | 28 | 7 |
| Val | 6 | 7 | 15 | 9 | 8 |
| Cys | 6 | 4 | 4 | 5 | 0 |
| Met | 0 | 1 | 3 | 2 | 1 |
| Ile | 4 | 2 | 1 | 4 | 5 |
| Leu | 6 | 4 | 8 | 10 | 9 |
| Tyr | 1 | 0.5 | 1 | 2 | 3 |
| Phe | 3 | 0.5 | 2 | 3 | 3 |
| Lys | 7 | 4 | 3 | 6 | 11 |
| His | 1 | 0.5 | 1 | 2 | 2 |
| Arg | 2 | 1 | 1 | 2 | 4 |
| Galactose | [++++] | 740 | [++++] | [++++] | 150 |
| Arabinose | [++++] | 540 | [++++] | [++++] | 165 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamport, D.T.A.; Tan, L.; Kieliszewski, M.J. A Molecular Pinball Machine of the Plasma Membrane Regulates Plant Growth—A New Paradigm. Cells 2021, 10, 1935. https://doi.org/10.3390/cells10081935
Lamport DTA, Tan L, Kieliszewski MJ. A Molecular Pinball Machine of the Plasma Membrane Regulates Plant Growth—A New Paradigm. Cells. 2021; 10(8):1935. https://doi.org/10.3390/cells10081935
Chicago/Turabian StyleLamport, Derek T. A., Li Tan, and Marcia J. Kieliszewski. 2021. "A Molecular Pinball Machine of the Plasma Membrane Regulates Plant Growth—A New Paradigm" Cells 10, no. 8: 1935. https://doi.org/10.3390/cells10081935
APA StyleLamport, D. T. A., Tan, L., & Kieliszewski, M. J. (2021). A Molecular Pinball Machine of the Plasma Membrane Regulates Plant Growth—A New Paradigm. Cells, 10(8), 1935. https://doi.org/10.3390/cells10081935






