Supra-Optimal Temperature: An Efficient Approach for Overaccumulation of Starch in the Green Alga Parachlorella kessleri
Abstract
1. Introduction
2. Materials and Methods
2.1. Organism and Culture
2.2. Mineral Nutrient Medium
2.3. Culture Conditions
2.4. Synchronization of Cultures
2.5. Management of Deuterated Cultures
2.6. Measurement of Light Intensity
2.7. Assessment of Cell Division Curves
2.8. Dry Matter Determination
2.9. Cell Volume and Number
2.10. Quantum Yield Measurement
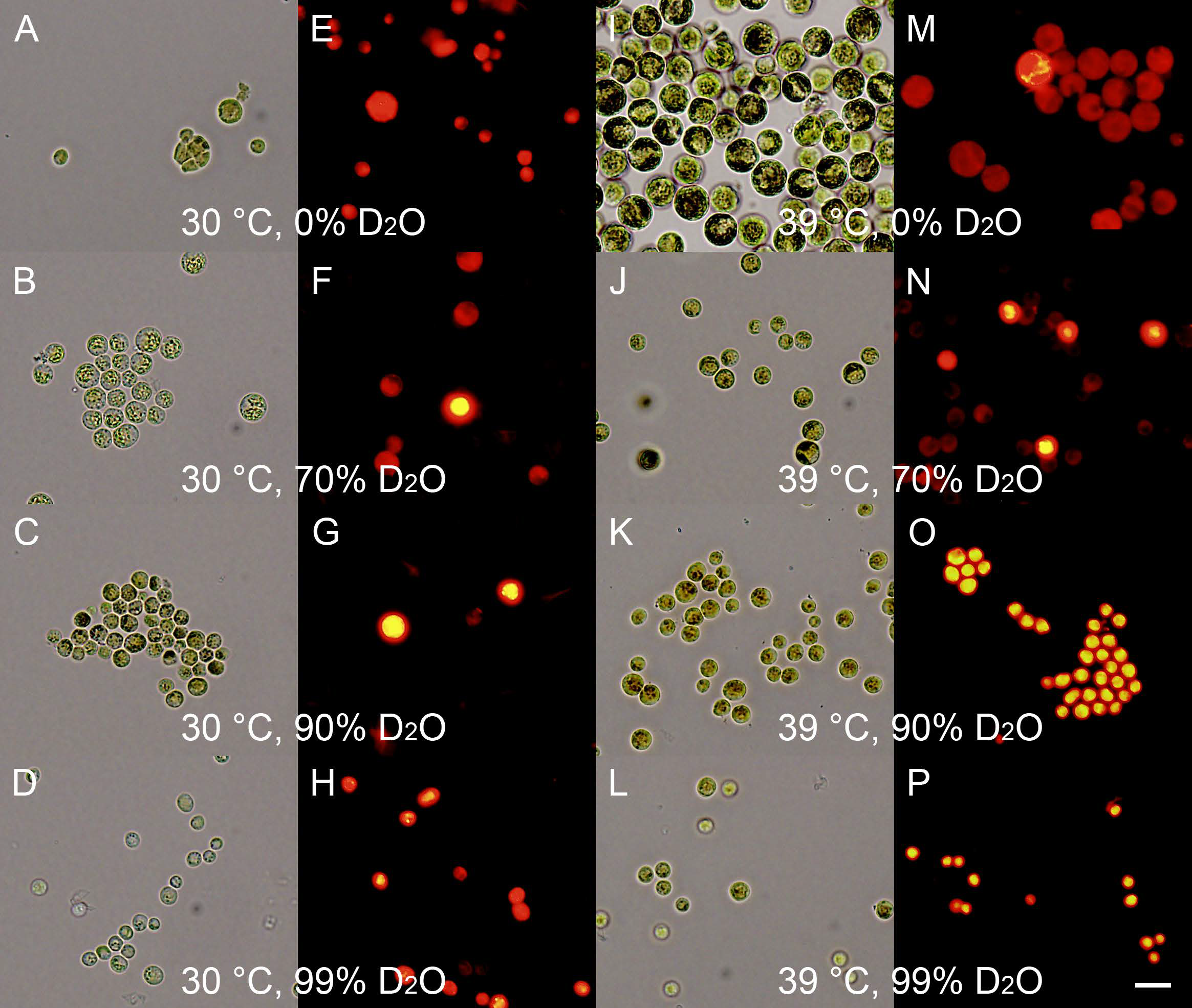
2.11. Neutral Lipids and Starch Staining
2.12. Neutral Lipids Quantification
2.13. Estimation of Bulk RNA, DNA, and Proteins
2.13.1. Total Nucleic Acids Extraction
2.13.2. DNA and RNA Determination
2.13.3. Protein Determination
2.14. Starch Analyses
2.15. Statistical Analysis
3. Results
3.1. Growth in Normal Water
3.1.1. Reproductive Events
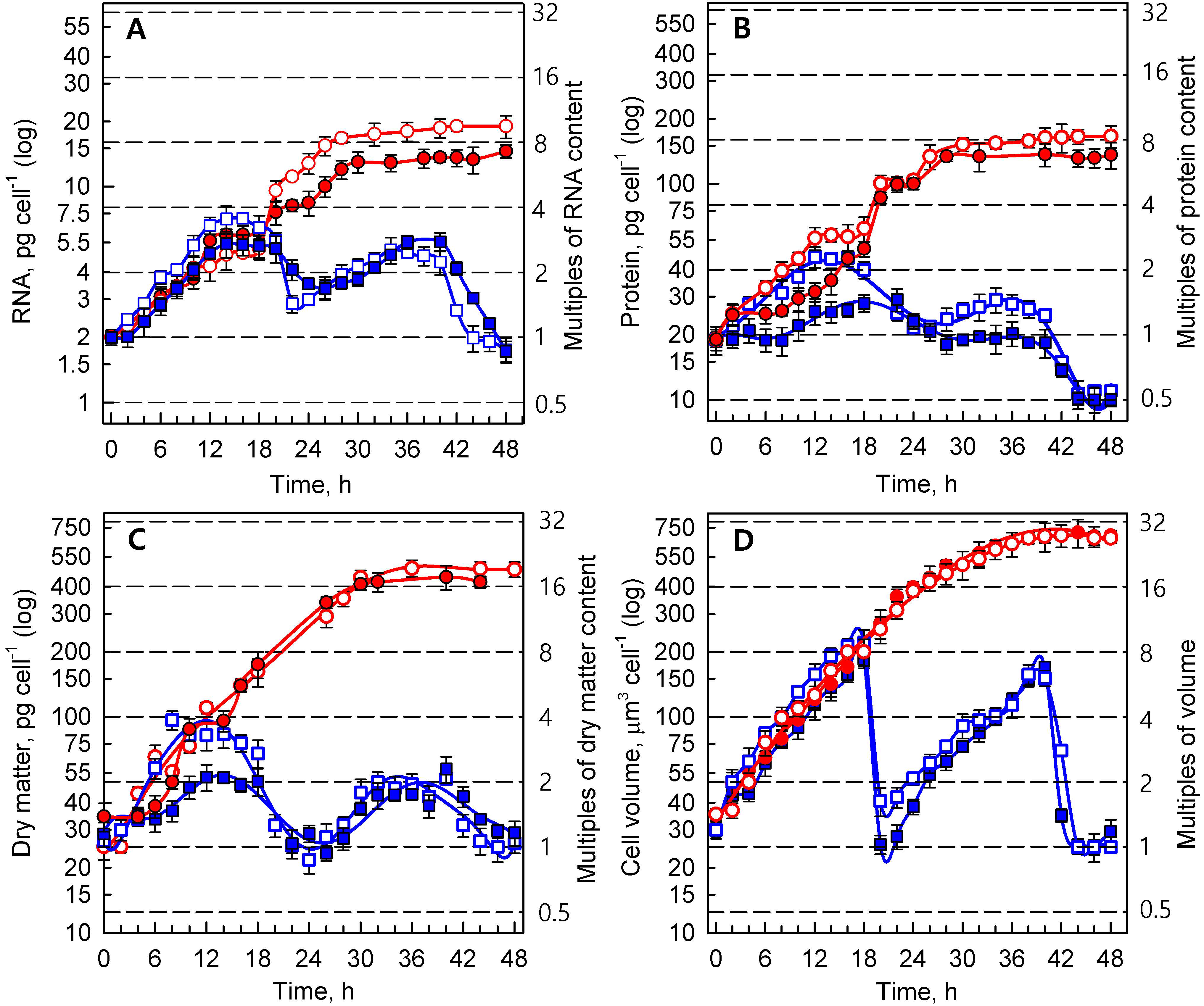
3.1.2. Growth Processes
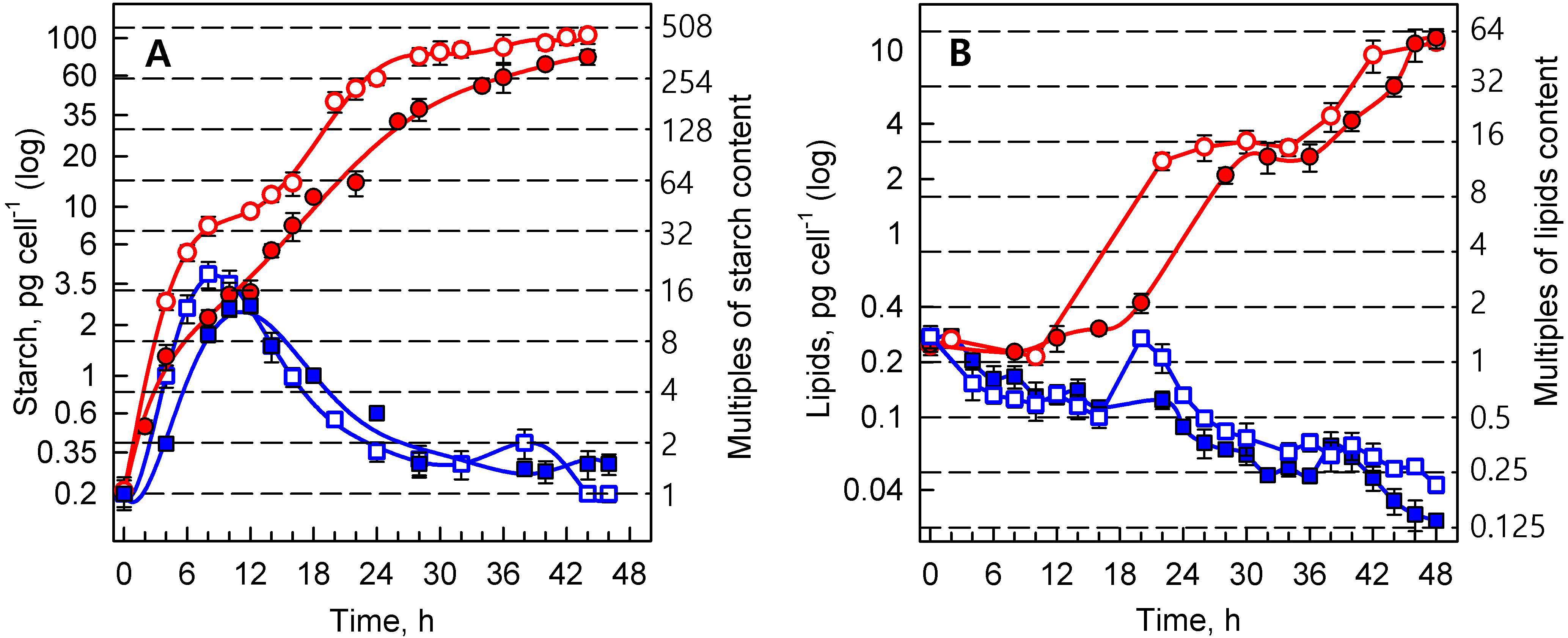
3.1.3. Energy Reserves
3.2. Growth in Deuterated Water
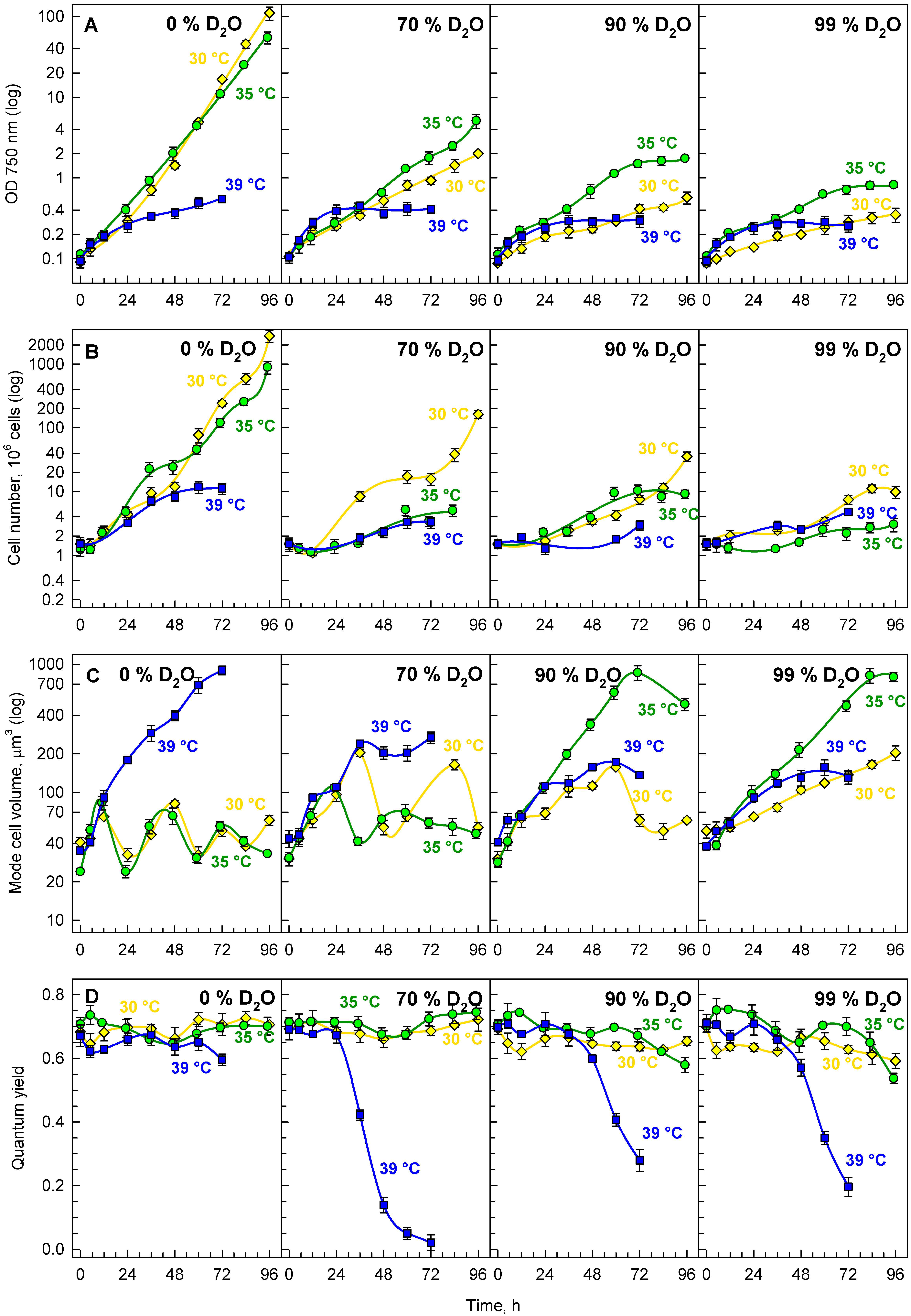
3.2.1. Growth and Division
3.2.2. Energy Reserves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zachleder, V.; Bišová, K.; Vítová, M.; Štěpán, K.; Hendrychová, J. Variety of cell cycle patterns in the alga Scenedesmus quadricauda (Chlorophyta) as revealed by application of illumination regimes and inhibitors. Eur. J. Phycol. 2002, 37, 361–371. [Google Scholar] [CrossRef]
- Oldenhof, H.; Zachleder, V.; van den Ende, H. Blue- and red-light regulation of the cell cycle in Chlamydomonas reinhardtii (Chlorophyta). Eur. J. Phycol. 2006, 41, 313–320. [Google Scholar] [CrossRef]
- Oldenhof, H.; Zachleder, V.; van den Ende, H. Blue light delays commitment to cell division in Chlamydomonas reinhardtii. Plant Biol. 2004, 6, 689–695. [Google Scholar] [CrossRef]
- Munzner, P.; Voigt, J. Blue light regulation of cell division in Chlamydomonas reinhardtii. Plant Physiol. 1992, 99, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.; Munzner, P. The Chlamydomonas cell-cycle is regulated by a light dark- responsive cell-cycle switch. Planta 1987, 172, 463–472. [Google Scholar] [CrossRef]
- McAteer, M.; Donnan, L.; John, P.C.L. The timing of division in Chlamydomonas. New Phytol. 1985, 99, 41–56. [Google Scholar] [CrossRef]
- Donnan, L.; Carvill, E.P.; Gilliland, T.J.; John, P.C.L. The cell-cycles of Chlamydomonas and Chlorella. New Phytol. 1985, 99, 1–40. [Google Scholar] [CrossRef]
- Donnan, L.; John, P.C.L. Cell cycle control by timer and sizer in Chlamydomonas. Nature 1983, 304, 630–633. [Google Scholar] [CrossRef]
- Zachleder, V.; Šetlík, I. Timing of events in overlapping cell reproductive sequences and their mutual interactions in the alga Scenedesmus quadricauda. J. Cell Sci. 1990, 97, 631–638. [Google Scholar] [CrossRef]
- Los, D.A.; Zachleder, V.; Kuptsova, E.S.; Ksenofontov, A.L.; Markelova, A.G.; Shapiguzov, Y.M.; Semenenko, V.E. Effect of the spectral composition of light on replication of chloroplast DNA and division of chloroplast nucleoids in the green-alga Dunaliella salina. Russ. Plant Physiol. 1990, 37, 791–798. [Google Scholar]
- Morimura, Y. Synchronous culture of Chlorella. I. Kinetic analysis of the life cycle of Chlorella ellipsoidea as affected by changes of temperature and light intensity. Plant Cell Physiol. 1959, 1, 49–62. [Google Scholar]
- Bisova, K.; Zachleder, V. Cell-cycle regulation in green algae dividing by multiple fission. J. Exp. Bot. 2014, 65, 2585–2602. [Google Scholar] [CrossRef]
- Vítová, M.; Bišová, K.; Hlavová, M.; Kawano, S.; Zachleder, V.; Čížková, M. Chlamydomonas reinhardtii: Duration of its cell cycle and phases at growth rates affected by temperature. Planta 2011, 234, 599–608. [Google Scholar] [CrossRef]
- Zachleder, V.; van den Ende, H. Cell cycle events in the green alga Chlamydomonas eugametos and their control by environmental factors. J. Cell Sci. 1992, 102, 469–474. [Google Scholar] [CrossRef]
- Zachleder, V.; Ivanov, I.; Vítová, M.; Bišová, K. Effects of cyclin-dependent kinase activity on the coordination of growth and the cell cycle in green algae at different temperatures. J. Exp. Bot. 2019, 70, 845–858. [Google Scholar] [CrossRef]
- Bišová, K.; Hendrychová, J.; Cepák, V.; Zachleder, V. Cell growth and division processes are differentially sensitive to cadmium in Scenedesmus quadricauda. Folia Microbiol. 2003, 48, 805–816. [Google Scholar] [CrossRef]
- Zachleder, V.; Ivanov, I.; Vítová, M.; Bišová, K. Cell cycle arrest by supraoptimal temperature in the alga Chlamydomonas reinhardtii. Cells 2019, 8, 1237–1257. [Google Scholar] [CrossRef] [PubMed]
- Šetlík, I.; Zachleder, V.; Doucha, J.; Berková, E.; Bartoš, J. The nature of temperature block in the sequence of reproductive processes in Chlorella vulgaris BEIJERINCK. Arch. Hydrobiol. Suppl. 49 Algol. Stud. 1975, 14, 70–104. [Google Scholar]
- Krienitz, L.; Hegewald, E.H.; Hepperle, D.; Huss, V.A.R.; Rohr, T.; Wolf, M. Phylogenetic relationship of Chlorella and Parachlorella gen nov (Chlorophyta, Trebouxiophyceae). Phycologia 2004, 43, 529–542. [Google Scholar] [CrossRef]
- Řezanka, T.; Podojil, M. The very long-chain fatty-acids of the green-alga Chlorella kessleri. Lipids 1984, 19, 472–473. [Google Scholar] [CrossRef]
- Ota, S.; Matsuda, T.; Takeshita, T.; Yamazaki, T.; Kazama, Y.; Abe, T.; Kawano, S. Phenotypic spectrum of Parachlorella kessleri (Chlorophyta) mutants produced by heavy-ion irradiation. Bioresour. Technol. 2013, 149, 432–438. [Google Scholar] [CrossRef]
- Ota, S.; Yoshihara, M.; Yamazaki, T.; Takeshita, T.; Hirata, A.; Konomi, M.; Oshima, K.; Hattori, M.; Bišová, K.; Zachleder, V.; et al. Deciphering the relationship among phosphate dynamics, electron-dense body and lipid accumulation in the green alga Parachlorella kessleri. Sci. Rep. 2016, 6, 25731. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.; Teixeira, J.; Dragone, G.; Vicente, A.A.; Kawano, S.; Bišová, K.; Přibyl, P.; Zachleder, V.; Vítová, M. Relationship between starch and lipid accumulation induced by nutrient depletion and replenishment in the microalga Parachlorella kessleri. Bioresour. Technol. 2013, 144, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Sato, A.; Watanabe, K.; Hirata, A.; Takeshita, T.; Ota, S.; Sato, N.; Zachleder, V.; Tsuzuki, M.; Kawano, S. Sequential accumulation of starch and lipid induced by sulfur deficiency in Chlorella and Parachlorella species. Bioresour. Technol. 2013, 129, 150–155. [Google Scholar] [CrossRef]
- Takeshita, T.; Ivanov, I.N.; Oshima, K.; Ishii, K.; Kawamoto, H.; Ota, S.; Yamazaki, T.; Hirata, A.; Kazama, Y.; Abe, T.; et al. Comparison of lipid productivity of Parachlorella kessleri heavy-ion beam irradiation mutant PK4 in laboratory and 150-L mass bioreactor, identification and characterization of its genetic variation. Algal Res. 2018, 35, 416–426. [Google Scholar] [CrossRef]
- Taleb, A.; Legrand, J.; Takache, H.; Taha, S.; Pruvost, J. Investigation of lipid production by nitrogen-starved Parachlorella kessleri under continuous illumination and day/night cycles for biodiesel application. J. Appl. Phycol. 2017. [Google Scholar] [CrossRef]
- Rathod, J.P.; Prakash, G.; Pandit, R.; Lali, A.M. Agrobacterium-mediated transformation of promising oil-bearing marine algae Parachlorella kessleri. Photosynth. Res. 2013, 118, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Tsuzuki, M.; Kawaguchi, A. Glycerolipid synthesis in Chlorella kessleri 11h - I. Existence of a eukaryotic pathway. Biochim. Biophys. Acta 2003, 1633, 27–34. [Google Scholar] [CrossRef]
- Saleh, M.M.; Matorin, D.N.; Zayadan, B.K.; Todorenko, D.A.; Lukashov, E.P.; Gaballah, M.M. Differentiation between two strains of microalga Parachlorella kessleri using modern spectroscopic method. Bot. Stud. 2014, 55, 53. [Google Scholar] [CrossRef]
- Zachleder, V.; Ivanov, I.N.; Kselíková, V.; Bialevich, V.; Vítová, M.; Ota, S.; Takeshita, T.; Kawano, S.; Bišová, K. Characterization of growth and cell cycle events as affected by light intensity in the green alga Parachlorella kessleri, as a new model for cell cycle research. Biomolecules 2021, 11, 891. [Google Scholar] [CrossRef] [PubMed]
- Lien, T.; Knutsen, G. Synchronous growth of Chlamydomonas reinhardtii (Chlorophyceae): A review of optimal conditions. J. Phycol. 1979, 15, 191–200. [Google Scholar] [CrossRef]
- Takeshita, T.; Ota, S.; Yamazaki, T.; Hirata, A.; Zachleder, V.; Kawano, S. Starch and lipid accumulation in eight strains of six Chlorella species under comparatively high light intensity and aeration culture conditions. Bioresour. Technol. 2014, 158, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Torres-Romero, I.; Kong, F.; Legeret, B.; Beisson, F.; Peltier, G.; Li-Beisson, Y. Chlamydomonas cell cycle mutant crcdc5 over-accumulates starch and oil. Biochimie 2019. [Google Scholar] [CrossRef]
- Hirokawa, T.; Hata, M.; Takeda, H. Correlation between the starch level and the rate of starch synthesis during the development cycle of Chlorella ellipsoidea. Plant Cell Physiol. 1982, 23, 813–820. [Google Scholar]
- Sorokin, C. Changes in photosynthetic activity in the course of cell development in Chlorella. Physiol. Plant. 1957, 10, 659–666. [Google Scholar] [CrossRef]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae-novel highly efficient starch producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Izumo, A.; Fujiwara, S.; Oyama, Y.; Satoh, A.; Fujita, N.; Nakamura, Y.; Tsuzuki, M. Physicochemical properties of starch in Chlorella change depending on the CO2 concentration during growth: Comparison of structure and properties of pyrenoid and stroma starch. Plant Sci. 2007, 172, 1138–1147. [Google Scholar] [CrossRef]
- Ivanov, I.N.; Zachleder, V.; Vítová, M.; Barbosa, M.J.; Bišová, K. Starch production in Chlamydomonas reinhardtii through supraoptimal temperature in a pilot-scale photobioreactor. Cells 2021, 10. [Google Scholar] [CrossRef]
- Li, X.; Přibyl, P.; Bišová, K.; Kawano, S.; Cepák, V.; Zachleder, V.; Čížková, M.; Brányiková, I.; Vítová, M. The microalga Parachlorella kessleri––a novel highly efficient lipid producer. Biotechnol. Bioeng. 2013, 110, 97–107. [Google Scholar] [CrossRef]
- Přibyl, P.; Cepák, V.; Zachleder, V. Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2012, 94, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Feng, J.; Lv, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S. Physiological changes of Parachlorella kessleri ty02 in lipid accumulation under nitrogen stress. Int. J. Environ. Res. Public Health 2019, 16. [Google Scholar] [CrossRef]
- You, Z.; Zhang, Q.; Peng, Z.; Miao, X. Lipid droplets mediate salt stress tolerance in Parachlorella kessleri. Plant Physiol. 2019, 181, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Zachleder, V.; Vítová, M.; Hlavová, M.; Moudříková, Š.; Mojzeš, P.; Heumann, H.; Becher, J.R.; Bišová, K. Stable isotope compounds - production, detection, and application. Biotechnol. Adv. 2018, 36, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. Deuterium: Discovery and applications in organic chemistry; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Lehmann, W.D. A timeline of stable isotopes and mass spectrometry in the life sciences. Mass Spectrom. Rev. 2017, 36, 58–85. [Google Scholar] [CrossRef]
- Kinetic isotope effects. Available online: https://chem.libretexts.org/@go/page/1685 (accessed on 10 June 2021).
- Hirakura, Y.; Sugiyama, T.; Takeda, M.; Ikeda, M.; Yoshioka, T. Deuteration as a tool in investigating the role of protons in cell signaling. Biochim. Biophys. Acta 2011, 1810, 218–225. [Google Scholar] [CrossRef]
- Salomonsson, L.; Branden, G.; Brzezinski, P. Deuterium isotope effect of proton pumping in cytochrome c oxidase. Biochim. Biophys. Acta 2008, 1777, 343–350. [Google Scholar] [CrossRef]
- De Kouchkovsky, Y.; Haraux, F.; Sigalat, C. Effect of hydrogen-deuterium exchange on energy-coupled processes in thylakoids. FEBS Lett. 1982, 139, 245–249. [Google Scholar] [CrossRef]
- Evans, B.R.; Bali, G.; Reeves, D.T.; O’Neill, H.M.; Sun, Q.; Shah, R.; Ragauskas, A.J. Effect of D2O on growth properties and chemical structure of annual ryegrass (Lolium multiflorum). J. Agr. Food Chem. 2014, 62, 2595–2604. [Google Scholar] [CrossRef]
- Sacchi, G.A.; Cocucci, M. Effects of deuterium oxide on growth, proton extrusion, potassium influx, and in vitro plasma membrane activities in maize root segments. Plant Physiol. 1992, 100, 1962–1967. [Google Scholar] [CrossRef][Green Version]
- Saha, S.K.; Hayes, J.; Moane, S.; Murray, P. Tagging of biomolecules with deuterated water (D2O) in commercially important microalgae. Biotechnol. Lett. 2013, 35, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Gireesh, T.; Jayadeep, A.; Rajasekharan, K.N.; Menon, V.P.; Vairamany, M.; Tang, G.; Nair, P.P.; Sudhakaran, P.R. Production of deuterated b-carotene by metabolic labelling of Spirulina platensis. Biotechnol. Lett. 2001, 23, 447–449. [Google Scholar] [CrossRef]
- Cargnin, S.; Serafini, M.; Pirali, T. A primer of deuterium in drug design. Future Med. Chem. 2019, 11, 2039–2042. [Google Scholar] [CrossRef]
- DeWitt, S.H.; Maryanoff, B.E. Deuterated drug nolecules: Focus on FDA-approved deutetrabenazine. Biochemistry 2018, 57, 472–473. [Google Scholar] [CrossRef]
- Raffa, R.B.; Pergolizzi, J.V.; Taylor, R. The First Approved “Deuterated” Drug: A Short Review of the Concept. Pharmacol. Pharm. 2018, 09, 440–446. [Google Scholar] [CrossRef]
- Schmidt, C. First deuterated drug approved. Nat. Biotechnol. 2017, 35, 493–494. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.I.; Crespi, H.L.; Mohan, V.; Katz, J.J. Isolation of fully deuterated metabolites from Scenedesmus obliquus grown in deuterium oxide. J. Pharm. Sci. 1961, 50, 425–429. [Google Scholar] [CrossRef]
- Hattori, A.; Crespi, H.L.; Katz, J.J. Effect of side-chain deuteration on protein stability. Biochemistry 1965, 4, 1213–1225. [Google Scholar] [CrossRef]
- Closs, D.L.; Katz, J.J.; Pennington, M.R.; Thomas, H.R.; Strain, J. Hydrogen exchange at methine and C-10 positions in chlorophyll. Amer. Chem. Soc. 1963, 85, 3809. [Google Scholar] [CrossRef]
- Doucha, J.; Lívanský, K. Productivity, CO2/O2 exchange and hydraulics in outdoor open high density microalgal (Chlorella sp.) photobioreactors operated in a Middle and Southern European climate. J. Appl. Phycol. 2006, 18, 811–826. [Google Scholar] [CrossRef]
- Takeshita, T.; Takeda, K.; Ota, S.; Yamazaki, T.; Kawano, S. A simple method for measuring the starch and ĺipid contents in the cell of microalgae. Cytologia 2015, 80, 475–481. [Google Scholar] [CrossRef]
- Wanka, F. Die bestimmung der nucleinsäuren in Chlorella pyrenoidosa. Planta 1962, 58, 594–619. [Google Scholar] [CrossRef]
- Lukavský, J.; Tetík, K.; Vendlová, J. Extraction of nucleic acid from the alga Scenedesmus quadricauda. Arch. Hydrobiol. Suppl. 41 Algol. Stud. 1973, 9, 416–426. [Google Scholar]
- Decallonne, J.R.; Weyns, C.J. A shortened procedure of the diphenylamine reaction for measurement of deoxyribonucleic acid by using light activation. Anal. Biochem. 1976, 74, 448–456. [Google Scholar] [CrossRef]
- Zachleder, V. Optimization of nucleic acids assay in green and blue-green algae: Extraction procedures and the light-activated reaction for DNA. Arch. Hydrobiol. Suppl. 67 Algol. Stud. 1984, 36, 313–328. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of light intensity and quality on growth rate and composition of Chlorella vulgaris. Plants 2019, 9. [Google Scholar] [CrossRef]
- Barnett, J.Z.; Foy, J.; Malone, R.; Rusch, K.A.; Gutierrez-Wing, M.T. Impact of light quality on a native Louisiana Chlorella vulgaris/Leptolyngbya sp. co-culture. Eng. Life. Sci. 2017, 17, 678–685. [Google Scholar] [CrossRef][Green Version]
- Borowitzka, M.A. Energy from microalgae: A short history. In Algae for biofuels and energy; Borowitzka, M.A., Moheimani, N.R., Eds.; Springer: Dordrecht, The Netherlands; New York, NY, USA; London, UK, 2013; Volume Developments in applied phycology 5, pp. 1–16. [Google Scholar]
- Borowitzka, M.A.; Raven, J.A.; Beardall, J. The physiology of microalgae, 6 ed.; Borowitzka, M.A., Raven, J.A., Beardall, J., Eds.; Springer International Publishing Switzerland: Cham, Germany; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2016; p. 681. [Google Scholar] [CrossRef]
- Schuler, L.; Greque de Morais, E.; Trovao, M.; Machado, A.; Carvalho, B.; Carneiro, M.; Maia, I.; Soares, M.; Duarte, P.; Barros, A.; et al. Isolation and characterization of novel Chlorella vulgaris mutants with low chlorophyll and improved protein contents for food applications. Front. Bioeng. Biotechnol. 2020, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Al Jabri, H.; Taleb, A.; Touchard, R.; Saadaoui, I.; Goetz, V.; Pruvost, J. Cultivating microalgae in desert conditions: Evaluation of the effect of light-temperature summer conditions on the growth and metabolism of Nannochloropsis QU130. Appl. Sci. 2021, 11. [Google Scholar] [CrossRef]
- Levin, G.; Kulikovsky, S.; Liveanu, V.; Eichenbaum, B.; Meir, A.; Isaacson, T.; Tadmor, Y.; Adir, N.; Schuster, G. The desert green algae Chlorella ohadii thrives at excessively high light intensities by exceptionally enhancing the mechanisms that protect photosynthesis from photoinhibition. Plant J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Treves, H.; Raanan, H.; Kedem, I.; Murik, O.; Keren, N.; Zer, H.; Berkowicz, S.M.; Giordano, M.; Norici, A.; Shotland, Y.; et al. The mechanisms whereby the green alga Chlorella ohadii, isolated from desert soil crust, exhibits unparalleled photodamage resistance. New Phytol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Treves, H.; Raanan, H.; Finkel, O.M.; Berkowicz, S.M.; Keren, N.; Shotland, Y.; Kaplan, A. A newly isolated Chlorella sp. from desert sand crusts exhibits a unique resistance to excess light intensity. FEMS Microbiol. Ecol. 2013, 86, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Hemme, D.; Veyel, D.; Muhlhaus, T.; Sommer, F.; Juppner, J.; Unger, A.K.; Sandmann, M.; Fehrle, I.; Schonfelder, S.; Steup, M.; et al. Systems-wide analysis of acclimation responses to long-term heat stress and recovery in the photosynthetic model organism Chlamydomonas reinhardtii. Plant Cell 2014, 26, 4270–4297. [Google Scholar] [CrossRef] [PubMed]
- Semenenko, V.E.; Vladimirova, M.G.; Orleanskaya, O.B. Physiological characteristics of Chlorella sp. K under conditions of high extremal temperatures I. Uncoupling effect of extreme temperatures on the cellular functions of Chlorella. Russian J. Plant Physiol. 1967, 14, 612–625. [Google Scholar]
- Zachleder, V.; Brányiková, I. Starch overproduction by means of algae. In Algal biorefineries; Bajpai, R.K., Prokop, A., Zappi, M., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2014; Volume 1: Cultivation of cells and products, pp. 217–240. [Google Scholar]
- Cedergreen, N.; Streibig, J.C.; Kudsk, P.; Mathiassen, S.K.; Duke, S.O. The occurrence of hormesis in plants and algae. Dose Response 2006, 5, 150–162. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef]

| Mean Light Intensity (μmol photons m−2 s−1) | |||||
|---|---|---|---|---|---|
| Incident Light Intensity | 110 μmol photons m−2 s−1 | 500 μmol photons m−2 s−1 | |||
| Temperature | 30 °C | 40 °C | 30 °C | 40 °C | |
| Time (h) | 0 | 70 ± 5 | 75 ± 5 | 332 ± 15 | 332 ± 12 |
| 24 | 56 ± 5 | 54 ± 7 | 274 ± 10 | 261 ± 10 | |
| 42 | 50 ± 5 | 50 ± 5 | 186 ± 10 | 220 ± 10 | |
| 48 | 50 ± 5 | 50 ± 5 | 177 ± 10 | 220 ± 10 | |
| Concentration of Deuterated Water (%) | Temperature (°C) | Mass Doubling Time (h) | % of Control | Cell Number Doubling Time (h) | % of Control |
|---|---|---|---|---|---|
| 0 | 30 | 9.58 | 100.00 | 8.85 | 100.00 |
| 35 | 10.66 | 100.00 | 9.99 | 100.00 | |
| 39 | 28.18 | 100.00 | 24.84 | 100.00 | |
| 70 | 30 | 22.97 | 239.67 | 14.18 | 160.25 |
| 35 | 17.01 | 159.54 | 28.90 | 289.35 | |
| 39 | 36.48 | 129.46 | 63.35 | 255.00 | |
| 90 | 30 | 32.23 | 336.30 | 21.00 | 237.37 |
| 35 | 24.01 | 225.27 | 36.35 | 363.91 | |
| 39 | 43.92 | 155.86 | 73.63 | 296.38 | |
| 99 | 30 | 42.34 | 441.87 | 35.35 | 399.54 |
| 35 | 35.32 | 331.37 | 92.33 | 924.32 | |
| 39 | 50.61 | 179.58 | 63.51 | 255.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zachleder, V.; Kselíková, V.; Ivanov, I.N.; Bialevich, V.; Vítová, M.; Ota, S.; Takeshita, T.; Kawano, S.; Bišová, K. Supra-Optimal Temperature: An Efficient Approach for Overaccumulation of Starch in the Green Alga Parachlorella kessleri. Cells 2021, 10, 1806. https://doi.org/10.3390/cells10071806
Zachleder V, Kselíková V, Ivanov IN, Bialevich V, Vítová M, Ota S, Takeshita T, Kawano S, Bišová K. Supra-Optimal Temperature: An Efficient Approach for Overaccumulation of Starch in the Green Alga Parachlorella kessleri. Cells. 2021; 10(7):1806. https://doi.org/10.3390/cells10071806
Chicago/Turabian StyleZachleder, Vilém, Veronika Kselíková, Ivan N. Ivanov, Vitali Bialevich, Milada Vítová, Shuhei Ota, Tsuyoshi Takeshita, Shigeyuki Kawano, and Kateřina Bišová. 2021. "Supra-Optimal Temperature: An Efficient Approach for Overaccumulation of Starch in the Green Alga Parachlorella kessleri" Cells 10, no. 7: 1806. https://doi.org/10.3390/cells10071806
APA StyleZachleder, V., Kselíková, V., Ivanov, I. N., Bialevich, V., Vítová, M., Ota, S., Takeshita, T., Kawano, S., & Bišová, K. (2021). Supra-Optimal Temperature: An Efficient Approach for Overaccumulation of Starch in the Green Alga Parachlorella kessleri. Cells, 10(7), 1806. https://doi.org/10.3390/cells10071806







