Sex-Specific Differences in Autophagic Responses to Experimental Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Transient Cerebral Ischemia Model
2.3. 3-Methyladenine (3MA) Administration
2.4. Ovariectomy and Estrogen Supplementation
2.5. Histological Assessment
2.6. Subcellular Fractionation
2.7. Western Blot
2.8. Neuronal Culture
2.9. Longitudinal Imaging and Survival Analysis
2.10. Statistics
3. Results
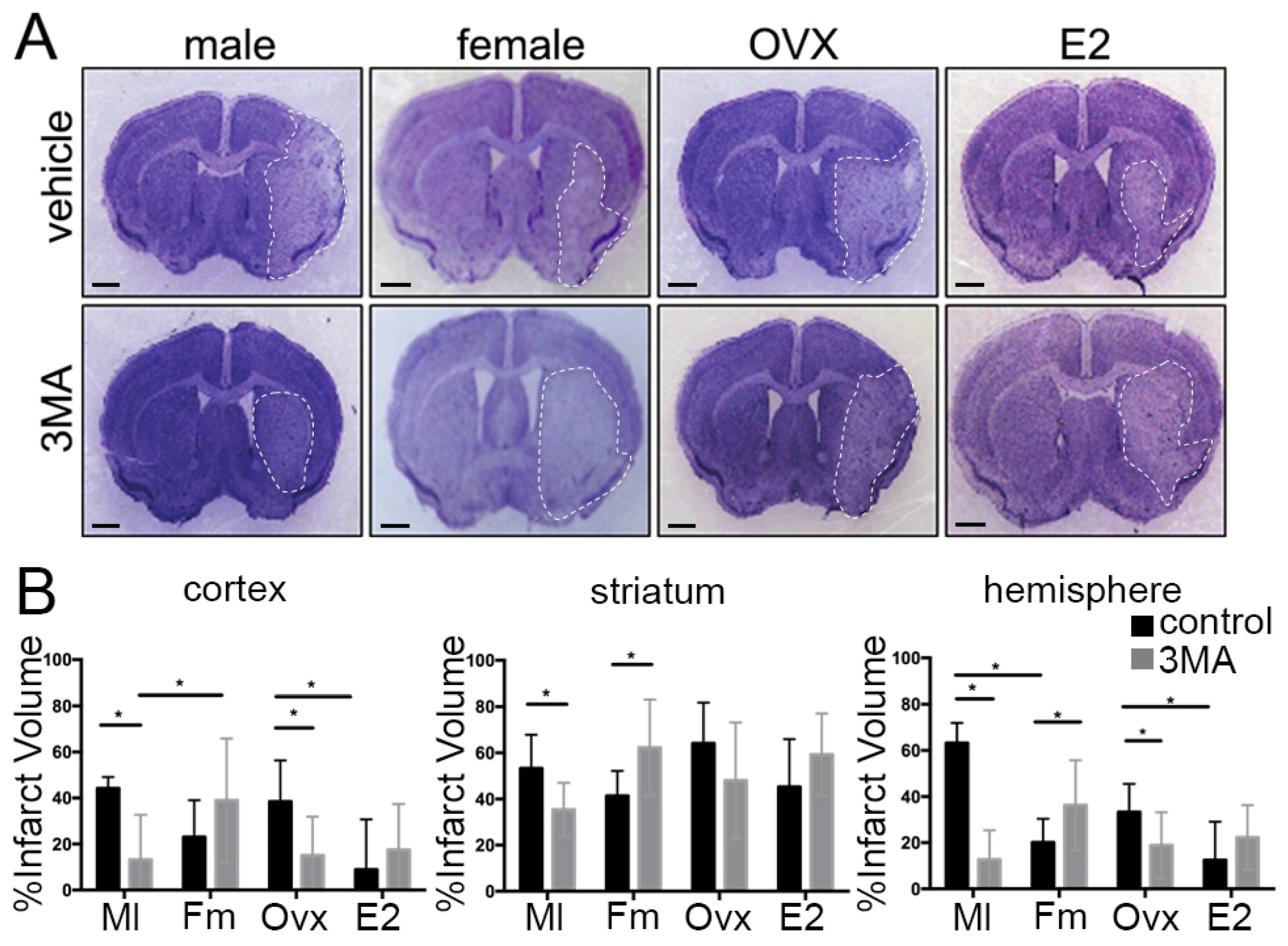
3.1. Sex Differences in Infarct Size Following Administration of 3MA
3.2. The Effect of Ovarian Hormones on Autophagy
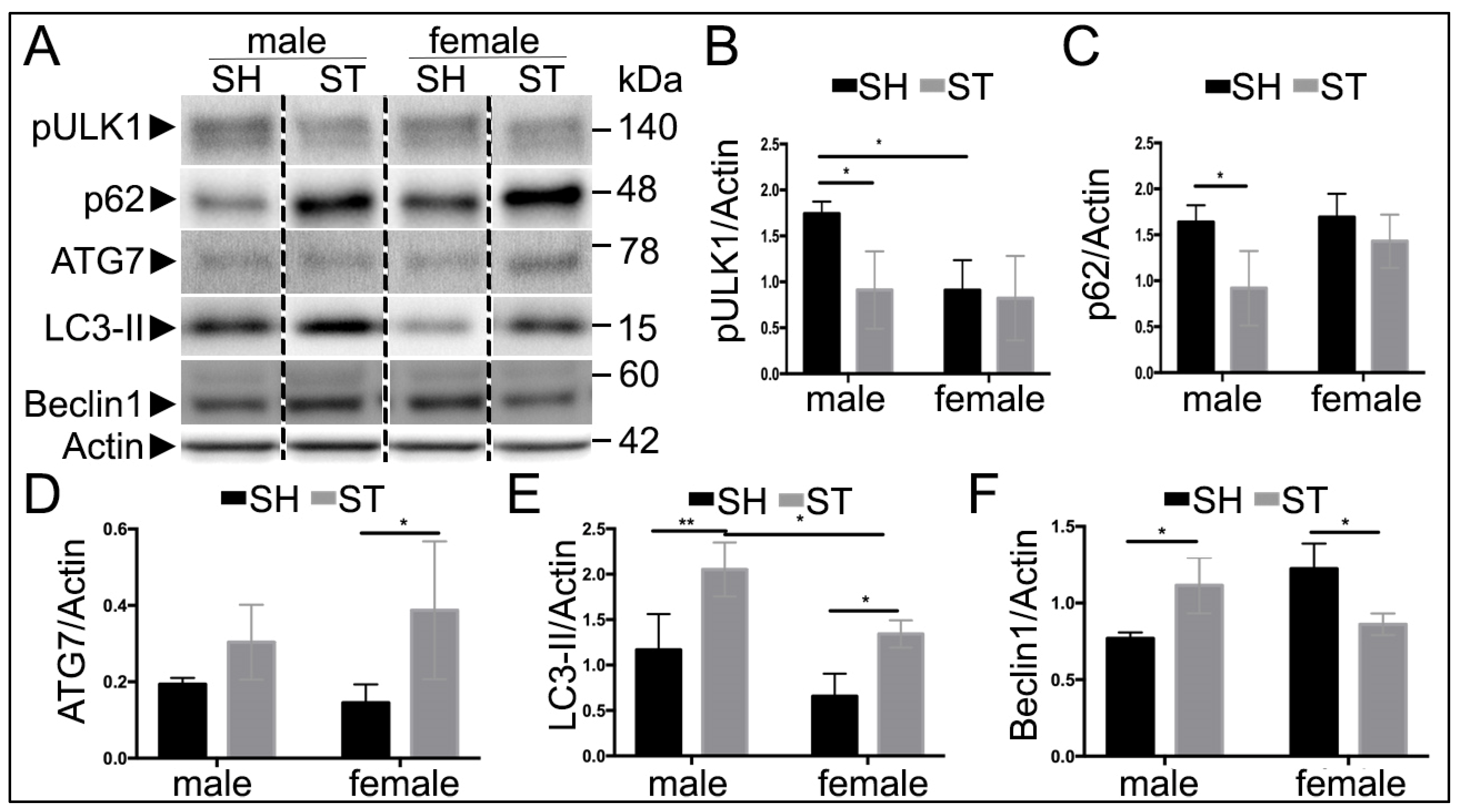
3.3. Sex Differences in Autophagy Protein Levels at 6 h
3.4. Sex Differences in Autophagy Protein Levels at 24 h
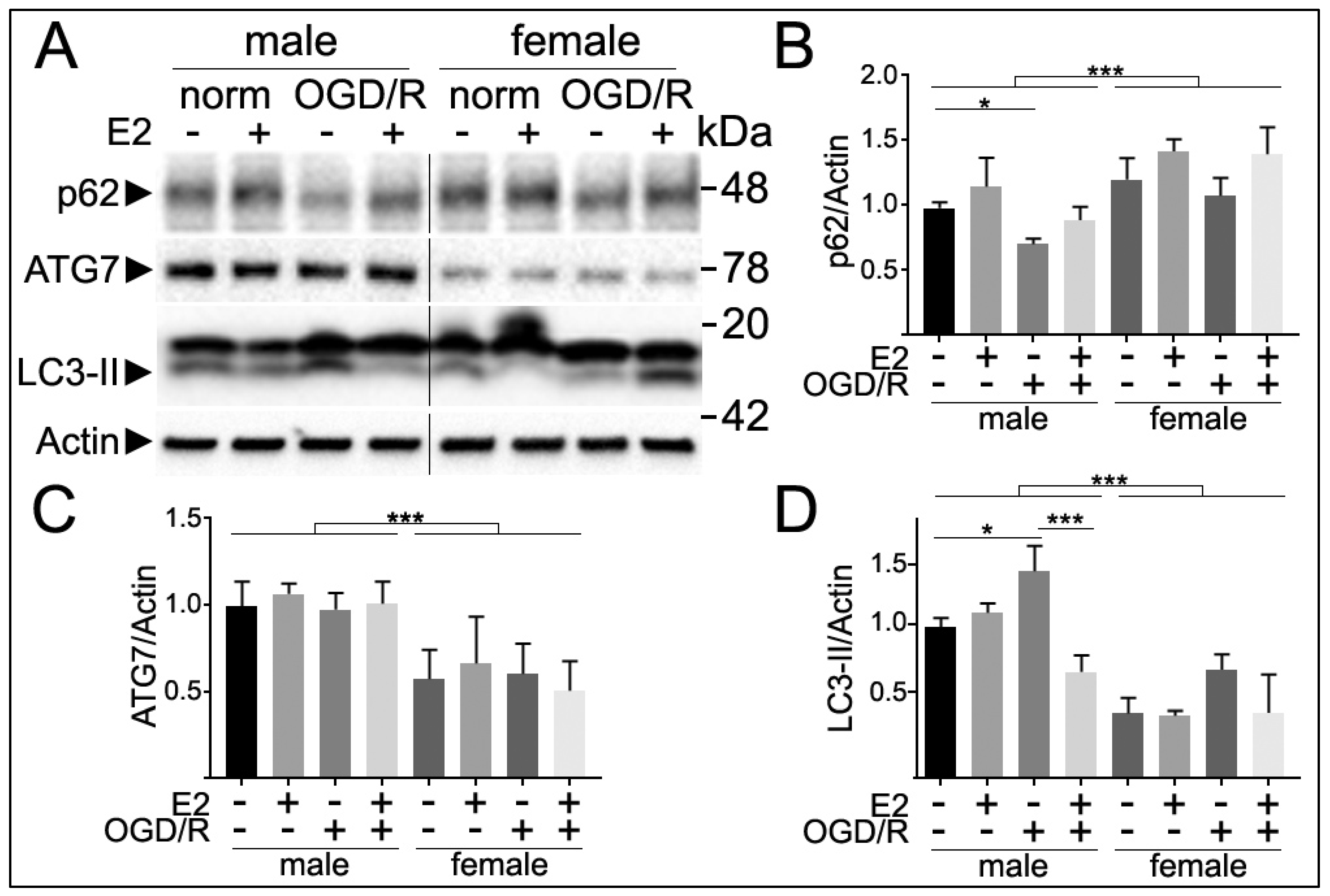
3.5. Sex Differences in Autophagy Markers Following E2 Treatment and Oxygen and Glucose Deprivation and Reperfusion (OGD/R)
3.6. Sex Differences in Neuronal Survival Following E2 Treatment and OGD/R
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CV | cresyl violet |
| DMSO | dimethyl sulfoxide |
| E2 | estradiol |
| MCAO | middle cerebral artery occlusion |
| PARP-1 | poly (ADP-ribose) polymerase |
| OVX | ovariectomy |
| 3MA | 3-methyladenine |
References
- Seshadri, S.; Beiser, A.; Kelly-Hayes, M.; Kase, C.S.; Au, R.; Kannel, W.B.; Wolf, P.A. The lifetime risk of stroke: Estimates from the Framingham Study. Stroke 2006, 37, 345–350. [Google Scholar] [CrossRef]
- Petrea, R.E.; Beiser, A.S.; Seshadri, S.; Kelly-Hayes, M.; Kase, C.S.; Wolf, P.A. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke 2009, 40, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012, 125, e2–e220. [Google Scholar] [CrossRef]
- Lofmark, U.; Hammarstrom, A. Evidence for age-dependent education-related differences in men and women with first-ever stroke. Results from a community-based incidence study in northern Sweden. Neuroepidemiology 2007, 28, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Renolleau, S.; Fau, S.; Goyenvalle, C.; Joly, L.M.; Chauvier, D.; Jacotot, E.; Mariani, J.; Charriaut-Marlangue, C. Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: A role for gender. J. Neurochem. 2007, 100, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.; Li, J.; Liu, F.; Benashski, S.E.; McCullough, L.D. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc. Natl. Acad. Sci. USA 2011, 108, 11662–11667. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, H.; Wilson, M.A.; Matsushita, H.; Zhu, C.; Lange, M.; Gustavsson, M.; Poitras, M.F.; Dawson, T.M.; Dawson, V.L.; Northington, F.; et al. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J. Neurochem. 2004, 90, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lang, J.; Li, J.; Benashski, S.E.; Siegel, M.; Xu, Y.; McCullough, L.D. Sex differences in the response to poly(ADP-ribose) polymerase-1 deletion and caspase inhibition after stroke. Stroke 2011, 42, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.L.; Boden-Albala, B.; Langa, K.M.; Lisabeth, L.D.; Fair, M.; Smith, M.A.; Sacco, R.L.; Morgenstern, L.B. Projected costs of ischemic stroke in the United States. Neurology 2006, 67, 1390–1395. [Google Scholar] [CrossRef]
- Ovbiagele, B.; Goldstein, L.B.; Higashida, R.T.; Howard, V.J.; Johnston, S.C.; Khavjou, O.A.; Lackland, D.T.; Lichtman, J.H.; Mohl, S.; Sacco, R.L.; et al. American Heart Association Advocacy Coordinating Committee and Stroke Council Forecasting the future of stroke in the United States: A policy statement from the American Heart Association and American Stroke Association. Stroke 2013, 44, 2361–2375. [Google Scholar] [CrossRef]
- Norrving, B.; Kissela, B. The global burden of stroke and need for a continuum of care. Neurology 2013, 80, S5–S12. [Google Scholar] [CrossRef]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Marino, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Beau, I.; Mehrpour, M.; Codogno, P. Autophagosomes and human diseases. Int. J. Biochem. Cell Biol. 2011, 43, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Lista, P.; Straface, E.; Brunelleschi, S.; Franconi, F.; Malorni, W. On the role of autophagy in human diseases: A gender perspective. J. Cell Mol. Med. 2011, 15, 1443–1457. [Google Scholar] [CrossRef]
- Wen, Y.D.; Sheng, R.; Zhang, L.S.; Han, R.; Zhang, X.; Zhang, X.D.; Han, F.; Fukunaga, K.; Qin, Z.H. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy 2008, 4, 762–769. [Google Scholar] [CrossRef]
- Puyal, J.; Vaslin, A.; Mottier, V.; Clarke, P.G. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann. Neurol. 2009, 66, 378–389. [Google Scholar] [CrossRef]
- Sheng, R.; Zhang, L.S.; Han, R.; Liu, X.Q.; Gao, B.; Qin, Z.H. Autophagy activation is associated with neuroprotection in a rat model of focal cerebral ischemic preconditioning. Autophagy 2010, 6, 482–494. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, H.; Bai, X.; Lu, Y.; Dong, H.; Xiong, L. Autophagy activation is involved in neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats. Brain Res. 2011, 1402, 109–121. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, X.Y.; Yin, J.; Zuo, G.; Zhang, J.; Chen, G. Role of autophagy in early brain injury after experimental subarachnoid hemorrhage. J. Mol. Neurosci. 2012, 46, 192–202. [Google Scholar] [CrossRef]
- Noh, H.S.; Shin, I.W.; Ha, J.H.; Hah, Y.S.; Baek, S.M.; Kim, D.R. Propofol protects the autophagic cell death induced by the ischemia/reperfusion injury in rats. Mol. Cells 2010, 30, 455–460. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xia, Q.; Chu, K.T.; Pan, J.; Sun, L.N.; Zeng, B.; Zhu, Y.J.; Wang, Q.; Wang, K.; Luo, B.Y. Severe global cerebral ischemia-induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3-methyladenine: A widely used inhibitor of autophagy. J. Neuropathol. Exp. Neurol. 2011, 70, 314–322. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, J.; Wu, L.; Xu, G.; Dai, J.; Liu, X. Tetracycline inhibits local inflammation induced by cerebral ischemia via modulating autophagy. PLoS ONE 2012, 7, e48672. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.R.; Wang, L.; Jiang, W.; Qi, A.H.; Zhou, Q.H.; Zhang, X.L. Propofol prevents cerebral ischemia-triggered autophagy activation and cell death in the rat hippocampus through the NF-kappaB/p53 signaling pathway. Neuroscience 2013, 246, 117–132. [Google Scholar] [CrossRef]
- Weis, S.N.; Toniazzo, A.P.; Ander, B.P.; Zhan, X.; Careaga, M.; Ashwood, P.; Wyse, A.T.; Netto, C.A.; Sharp, F.R. Autophagy in the brain of neonates following hypoxia-ischemia shows sex- and region-specific effects. Neuroscience 2014, 256, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kilic, E.; Dietz, G.P.; Hermann, D.M.; Bahr, M. Intravenous TAT-Bcl-Xl is protective after middle cerebral artery occlusion in mice. Ann. Neurol. 2002, 52, 617–622. [Google Scholar] [CrossRef]
- White, B.J.; Tarabishy, S.; Venna, V.R.; Manwani, B.; Benashski, S.; McCullough, L.D.; Li, J. Protection from cerebral ischemia by inhibition of TGFbeta-activated kinase. Exp. Neurol. 2012, 237, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Blizzard, K.K.; Zeng, Z.; DeVries, A.C.; Hurn, P.D.; McCullough, L.D. Chronic behavioral testing after focal ischemia in the mouse: Functional recovery and the effects of gender. Exp. Neurol. 2004, 187, 94–104. [Google Scholar] [CrossRef]
- Li, J.; Lang, J.; Zeng, Z.; McCullough, L.D. Akt1 gene deletion and stroke. J. Neurol. Sci. 2008, 269, 105–112. [Google Scholar] [CrossRef]
- Chevallier, F.; Serougne, C.; Champarnaud, G. Effect upon brain weight and cholesterol content of maintaining rats of various ages at constant weight. J. Nutr. 1975, 105, 1003–1011. [Google Scholar] [CrossRef]
- Wahlsten, D.; Bachmanov, A.; Finn, D.A.; Crabbe, J.C. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc. Natl. Acad. Sci. USA 2006, 103, 16364–16369. [Google Scholar] [CrossRef]
- McCullough, L.D.; Blizzard, K.; Simpson, E.R.; Oz, O.K.; Hurn, P.D. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J. Neurosci. 2003, 23, 8701–8705. [Google Scholar] [CrossRef]
- Liu, F.; Benashski, S.E.; Xu, Y.; Siegel, M.; McCullough, L.D. Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. J. Neuroendocrinol. 2012, 24, 319–330. [Google Scholar] [CrossRef]
- McCullough, L.D.; Zeng, Z.; Li, H.; Landree, L.E.; McFadden, J.; Ronnett, G.V. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J. Biol. Chem. 2005, 280, 20493–20502. [Google Scholar] [CrossRef] [PubMed]
- Moruno Manchon, J.F.; Uzor, N.E.; Dabaghian, Y.; Furr-Stimming, E.E.; Finkbeiner, S.; Tsvetkov, A.S. Cytoplasmic sphingosine-1-phosphate pathway modulates neuronal autophagy. Sci. Rep. 2015, 5, 15213. [Google Scholar] [CrossRef]
- Moruno-Manchon, J.F.; Uzor, N.E.; Blasco-Conesa, M.P.; Mannuru, S.; Putluri, N.; Furr-Stimming, E.E.; Tsvetkov, A.S. Inhibiting sphingosine kinase 2 mitigates mutant Huntingtin-induced neurodegeneration in neuron models of Huntington disease. Hum. Mol. Genet. 2017, 26, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Moruno-Manchon, J.F.; Koellhoffer, E.C.; Gopakumar, J.; Hambarde, S.; Kim, N.; McCullough, L.D.; Tsvetkov, A.S. The G-quadruplex DNA stabilizing drug pyridostatin promotes DNA damage and downregulates transcription of Brca1 in neurons. Aging 2017, 9, 1957–1970. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Li, Z.; Wan, Q.; Chen, Q.; Liu, M.; Jiang, X.; Xie, L. A qPCR method to characterize the sex type of the cell strains from rats. Biosci. Biotechnol. Biochem. 2016, 80, 1917–1924. [Google Scholar] [CrossRef][Green Version]
- Moruno-Manchon, J.F.; Uzor, N.E.; Kesler, S.R.; Wefel, J.S.; Townley, D.M.; Nagaraja, A.S.; Pradeep, S.; Mangala, L.S.; Sood, A.K.; Tsvetkov, A.S. TFEB ameliorates the impairment of the autophagy-lysosome pathway in neurons induced by doxorubicin. Aging 2016, 8, 3507–3519. [Google Scholar] [CrossRef]
- Moruno-Manchon, J.F.; Uzor, N.E.; Kesler, S.R.; Wefel, J.S.; Townley, D.M.; Nagaraja, A.S.; Pradeep, S.; Mangala, L.S.; Sood, A.K.; Tsvetkov, A.S. Peroxisomes contribute to oxidative stress in neurons during doxorubicin-based chemotherapy. Mol. Cell Neurosci. 2018, 86, 65–71. [Google Scholar] [CrossRef]
- Moruno-Manchon, J.F.; Uzor, N.E.; Ambati, C.R.; Shetty, V.; Putluri, N.; Jagannath, C.; McCullough, L.D.; Tsvetkov, A.S. Sphingosine kinase 1-associated autophagy differs between neurons and astrocytes. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef]
- Liu, F.; Yuan, R.; Benashski, S.E.; McCullough, L.D. Changes in experimental stroke outcome across the life span. J. Cereb. Blood Flow Metab. 2009, 29, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Manwani, B.; Bentivegna, K.; Benashski, S.E.; Venna, V.R.; Xu, Y.; Arnold, A.P.; McCullough, L.D. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J. Cereb. Blood Flow Metab. 2015, 35, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, F.A.; Carvalho, L.R.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.; Leite, R.E.; Jacob Filho, W.; Lent, R.; Herculano-Houzel, S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Chen, J.; Maday, S. Neuronal autophagy and intercellular regulation of homeostasis in the brain. Curr. Opin. Neurobiol. 2018, 51, 29–36. [Google Scholar] [CrossRef]
- Sanchez-Martin, P.; Komatsu, M. p62/SQSTM1—Steering the cell through health and disease. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, A.; Spiezia, M.C.; Boggio, R.; Cariulo, C.; Nordheim, A.; Altobelli, R.; Kuhlbrodt, K.; Dominguez, C.; Munoz-Sanjuan, I.; Wityak, J.; et al. Quantifying autophagy using novel LC3B and p62 TR-FRET assays. PLoS ONE 2018, 13, e0194423. [Google Scholar] [CrossRef]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Mizushima, N.; Sugita, H.; Yoshimori, T.; Ohsumi, Y. A new protein conjugation system in human. The counterpart of the yeast Apg12p conjugation system essential for autophagy. J. Biol. Chem. 1998, 273, 33889–33892. [Google Scholar] [CrossRef]
- Mizushima, N.; Noda, T.; Yoshimori, T.; Tanaka, Y.; Ishii, T.; George, M.D.; Klionsky, D.J.; Ohsumi, M.; Ohsumi, Y. A protein conjugation system essential for autophagy. Nature 1998, 395, 395–398. [Google Scholar] [CrossRef]
- Moruno-Manchon, J.F.; Lejault, P.; Wang, Y.; McCauley, B.; Honarpisheh, P.; Morales Scheihing, D.A.; Singh, S.; Dang, W.; Kim, N.; Urayama, A.; et al. Small-molecule G-quadruplex stabilizers reveal a novel pathway of autophagy regulation in neurons. eLife 2020, 9. [Google Scholar] [CrossRef]
- Deng, L.; Feng, J.; Broaddus, R.R. The novel estrogen-induced gene EIG121 regulates autophagy and promotes cell survival under stress. Cell Death Dis. 2010, 1, e32. [Google Scholar] [CrossRef]
- Felzen, V.; Hiebel, C.; Koziollek-Drechsler, I.; Reissig, S.; Wolfrum, U.; Kogel, D.; Brandts, C.; Behl, C.; Morawe, T. Estrogen receptor alpha regulates non-canonical autophagy that provides stress resistance to neuroblastoma and breast cancer cells and involves BAG3 function. Cell Death Dis. 2015, 6, e1812. [Google Scholar] [CrossRef] [PubMed]
- Mazure, N.M.; Pouyssegur, J. Hypoxia-induced autophagy: Cell death or cell survival? Curr. Opin. Cell Biol. 2010, 22, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L. Time is brain—Quantified. Stroke 2006, 37, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Rami, A.; Kogel, D. Apoptosis meets autophagy-like cell death in the ischemic penumbra: Two sides of the same coin? Autophagy 2008, 4, 422–426. [Google Scholar] [CrossRef]
- Wirth, M.; Joachim, J.; Tooze, S.A. Autophagosome formation--the role of ULK1 and Beclin1-PI3KC3 complexes in setting the stage. Semin. Cancer Biol. 2013, 23, 301–309. [Google Scholar] [CrossRef]
- Zhang, A.; Song, Y.; Zhang, Z.; Jiang, S.; Chang, S.; Cai, Z.; Liu, F.; Zhang, X.; Ni, G. Effects of autophagy inhibitor 3-Methyladenine on ischemic stroke: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e23873. [Google Scholar] [CrossRef]
- Wang, M.; Liang, X.; Cheng, M.; Yang, L.; Liu, H.; Wang, X.; Sai, N.; Zhang, X. Homocysteine enhances neural stem cell autophagy in in vivo and in vitro model of ischemic stroke. Cell Death Dis. 2019, 10. [Google Scholar] [CrossRef]
- Shi, R.; Weng, J.; Zhao, L.; Li, X.M.; Gao, T.M.; Kong, J. Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci. Ther. 2012, 18, 250–260. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, T.; Zhang, Y.; Li, J.; Jin, L.; Sun, Y.; Shi, N.; Liu, K.; Sun, X. Ischemic Postconditioning Alleviates Cerebral Ischemia-Reperfusion Injury Through Activating Autophagy During Early Reperfusion in Rats. Neurochem. Res. 2018, 43, 1826–1840. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Shang, L.; Chen, S.; Du, F.; Li, S.; Zhao, L.; Wang, X. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc. Natl. Acad. Sci. USA 2011, 108, 4788–4793. [Google Scholar] [CrossRef]
- Acaz-Fonseca, E.; Castelló-Ruiz, M.; Burguete, M.C.; Aliena-Valero, A.; Salom, J.B.; Torregrosa, G.; García-Segura, L.M. Insight into the molecular sex dimorphism of ischaemic stroke in rat cerebral cortex: Focus on neuroglobin, sex steroids and autophagy. Eur. J. Neurosci. 2020, 52, 2756–2770. [Google Scholar] [CrossRef]
- Grishchuk, Y.; Ginet, V.; Truttmann, A.C.; Clarke, P.G.; Puyal, J. Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Autophagy 2011, 7, 1115–1131. [Google Scholar] [CrossRef]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef]
- Lin, C.W.; Chen, B.; Huang, K.L.; Dai, Y.S.; Teng, H.L. Inhibition of Autophagy by Estradiol Promotes Locomotor Recovery after Spinal Cord Injury in Rats. Neurosci. Bull. 2016, 32, 137–144. [Google Scholar] [CrossRef]
- Du, L.; Hickey, R.W.; Bayir, H.; Watkins, S.C.; Tyurin, V.A.; Guo, F.; Kochanek, P.M.; Jenkins, L.W.; Ren, J.; Gibson, G.; et al. Starving neurons show sex difference in autophagy. J. Biol. Chem. 2009, 284, 2383–2396. [Google Scholar] [CrossRef]
- Shen, H.M.; Codogno, P. Autophagic cell death: Loch Ness monster or endangered species? Autophagy 2011, 7, 457–465. [Google Scholar] [CrossRef]
- Liu, F.; Li, Z.; Li, J.; Siegel, C.; Yuan, R.; McCullough, L.D. Sex differences in caspase activation after stroke. Stroke 2009, 40, 1842–1848. [Google Scholar] [CrossRef]
- Huang, Q.; Shen, H.M. To die or to live: The dual role of poly(ADP-ribose) polymerase-1 in autophagy and necrosis under oxidative stress and DNA damage. Autophagy 2009, 5, 273–276. [Google Scholar] [CrossRef]
- Kulkarni, A.; Dong, A.; Kulkarni, V.V.; Chen, J.; Laxton, O.; Anand, A.; Maday, S. Differential regulation of autophagy during metabolic stress in astrocytes and neurons. Autophagy 2019, 16, 1651–1667. [Google Scholar] [CrossRef]
- Moruno Manchon, J.F.; Uzor, N.E.; Finkbeiner, S.; Tsvetkov, A.S. SPHK1/sphingosine kinase 1-mediated autophagy differs between neurons and SH-SY5Y neuroblastoma cells. Autophagy 2016, 12, 1418–1424. [Google Scholar] [CrossRef][Green Version]
- Sharma, J.; Nelluru, G.; Wilson, M.A.; Johnston, M.V.; Hossain, M.A. Sex-specific activation of cell death signalling pathways in cerebellar granule neurons exposed to oxygen glucose deprivation followed by reoxygenation. ASN Neuro 2011, 3. [Google Scholar] [CrossRef]
- Fairbanks, S.L.; Young, J.M.; Nelson, J.W.; Davis, C.M.; Koerner, I.P.; Alkayed, N.J. Mechanism of the sex difference in neuronal ischemic cell death. Neuroscience 2012, 219, 183–191. [Google Scholar] [CrossRef]
- Johnsen, D.; Murphy, S.J. Isoflurane preconditioning protects neurons from male and female mice against oxygen and glucose deprivation and is modulated by estradiol only in neurons from female mice. Neuroscience 2011, 199, 368–374. [Google Scholar] [CrossRef]
- Heiss, W.D. The ischemic penumbra: How does tissue injury evolve? Ann. N. Y. Acad. Sci. 2012, 1268, 26–34. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Marino, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef]
- Del Roso, A.; Vittorini, S.; Cavallini, G.; Donati, A.; Gori, Z.; Masini, M.; Pollera, M.; Bergamini, E. Ageing-related changes in the in vivo function of rat liver macroautophagy and proteolysis. Exp. Gerontol. 2003, 38, 519–527. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, M.; Fan, J.; Yan, W.; Zha, X.; Song, H.; Wan, R.; Yin, Y.; Wang, W. Ischemia-induced upregulation of autophagy preludes dysfunctional lysosomal storage and associated synaptic impairments in neurons. Autophagy 2021, 17, 1519–1542. [Google Scholar] [CrossRef]
- Shang, D.; Wang, L.; Klionsky, D.J.; Cheng, H.; Zhou, R. Sex differences in autophagy-mediated diseases: Toward precision medicine. Autophagy 2021, 17, 1065–1076. [Google Scholar] [CrossRef]
- Deng, X.; Berletch, J.B.; Nguyen, D.K.; Disteche, C.M. X chromosome regulation: Diverse patterns in development, tissues and disease. Nat. Rev. Genet. 2014, 15, 367–378. [Google Scholar] [CrossRef]
- Merkatz, R.B.; Temple, R.; Subel, S.; Feiden, K.; Kessler, D.A. Women in clinical trials of new drugs. A change in Food and Drug Administration policy. The Working Group on Women in Clinical Trials. N. Engl. J. Med. 1993, 329, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrizz, A.N.; Moruno-Manchon, J.F.; O’Keefe, L.M.; Doran, S.J.; Patel, A.R.; Venna, V.R.; Tsvetkov, A.S.; Li, J.; McCullough, L.D. Sex-Specific Differences in Autophagic Responses to Experimental Ischemic Stroke. Cells 2021, 10, 1825. https://doi.org/10.3390/cells10071825
Patrizz AN, Moruno-Manchon JF, O’Keefe LM, Doran SJ, Patel AR, Venna VR, Tsvetkov AS, Li J, McCullough LD. Sex-Specific Differences in Autophagic Responses to Experimental Ischemic Stroke. Cells. 2021; 10(7):1825. https://doi.org/10.3390/cells10071825
Chicago/Turabian StylePatrizz, Anthony N., Jose F. Moruno-Manchon, Lena M. O’Keefe, Sarah J. Doran, Anita R. Patel, Venugopal R. Venna, Andrey S. Tsvetkov, Jun Li, and Louise D. McCullough. 2021. "Sex-Specific Differences in Autophagic Responses to Experimental Ischemic Stroke" Cells 10, no. 7: 1825. https://doi.org/10.3390/cells10071825
APA StylePatrizz, A. N., Moruno-Manchon, J. F., O’Keefe, L. M., Doran, S. J., Patel, A. R., Venna, V. R., Tsvetkov, A. S., Li, J., & McCullough, L. D. (2021). Sex-Specific Differences in Autophagic Responses to Experimental Ischemic Stroke. Cells, 10(7), 1825. https://doi.org/10.3390/cells10071825






