Cytokinin Modulates Cellular Trafficking and the Cytoskeleton, Enhancing Defense Responses
Abstract
1. Introduction
2. Results
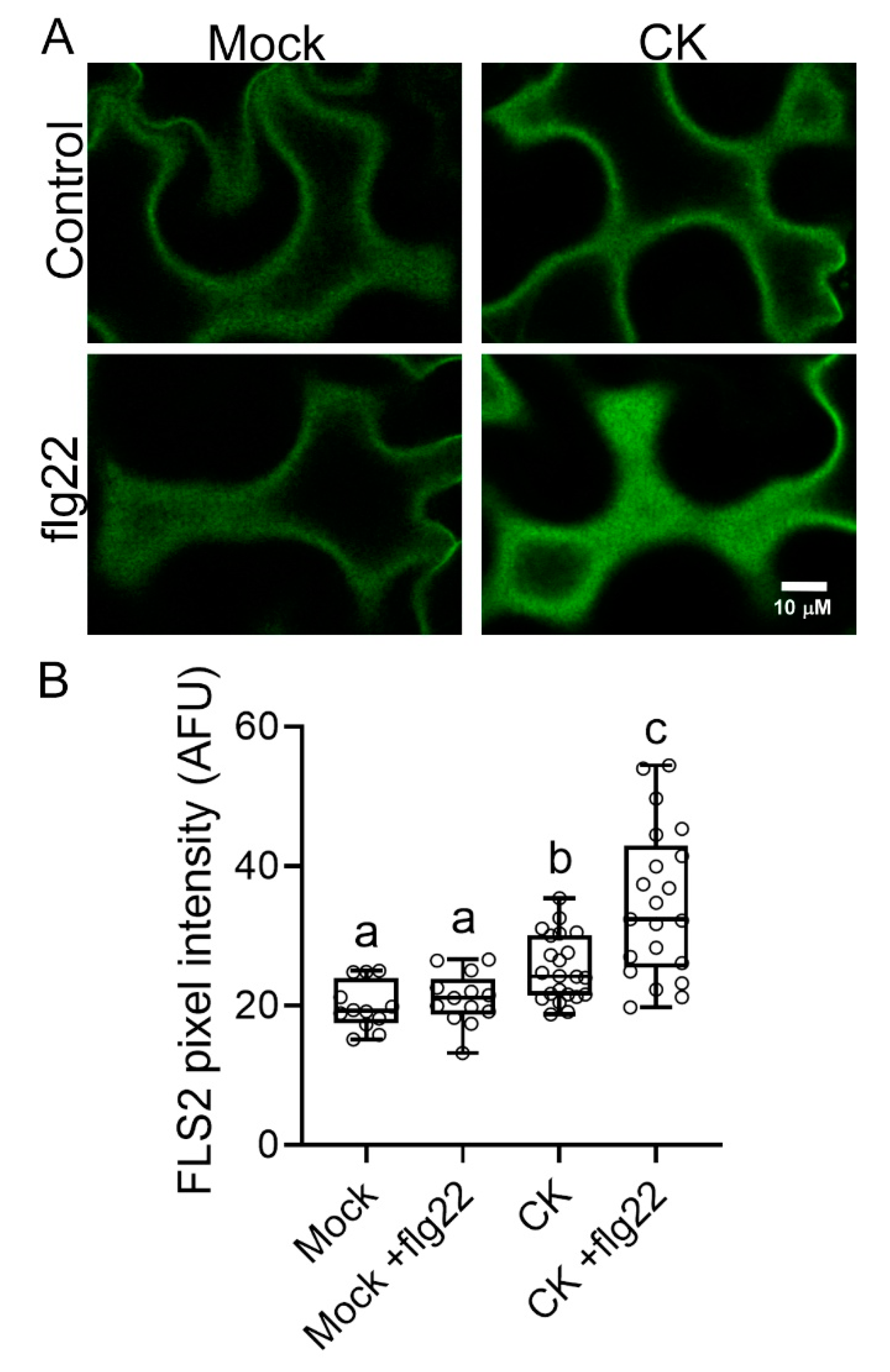
2.1. CK Affects PRR Trafficking
2.2. CK Response Is Activated during Immunity Elicitation
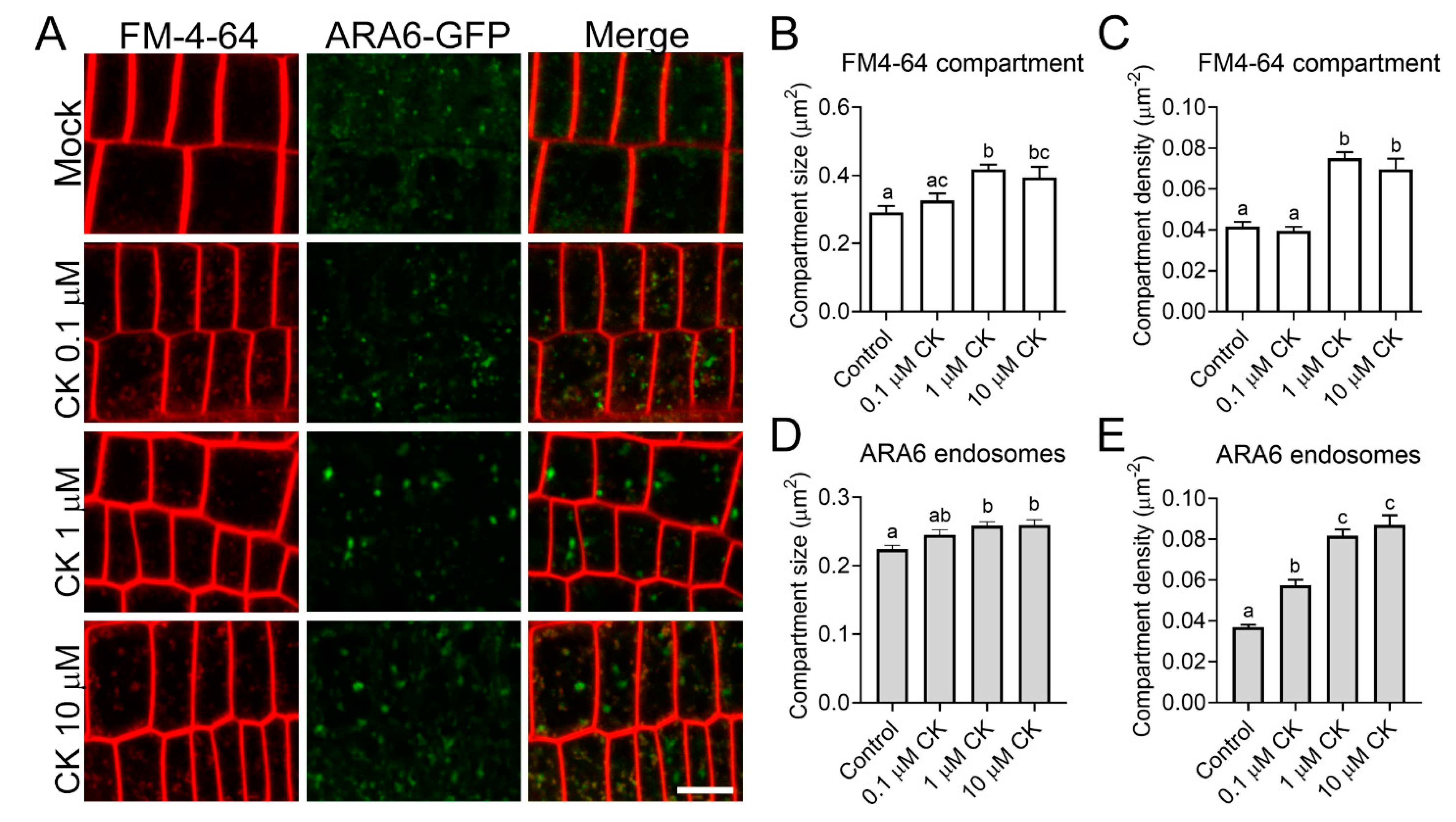
2.3. CK Treatment Modulates Cellular Trafficking
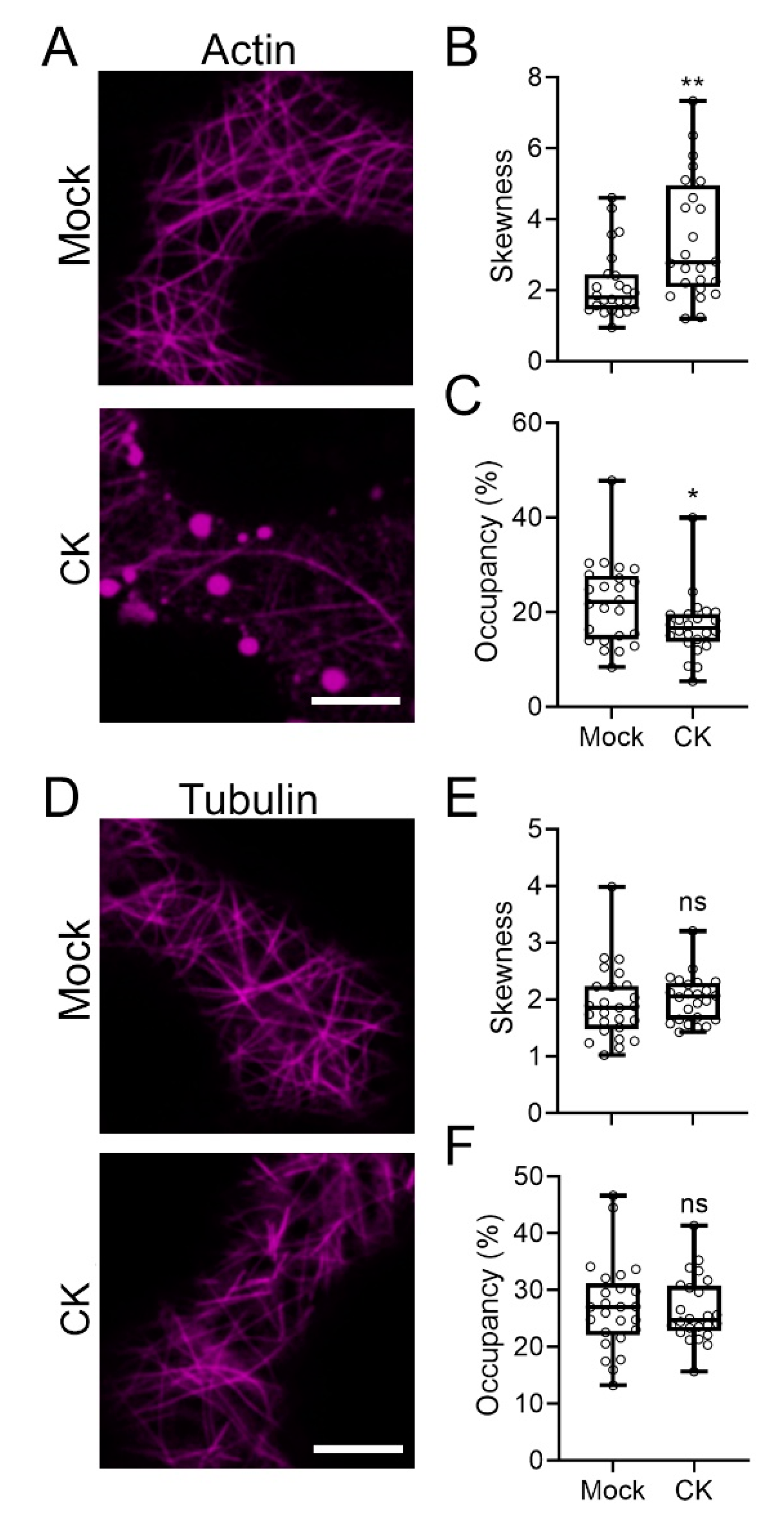
2.4. CK Treatment Affects the Cellular Cytoskeleton
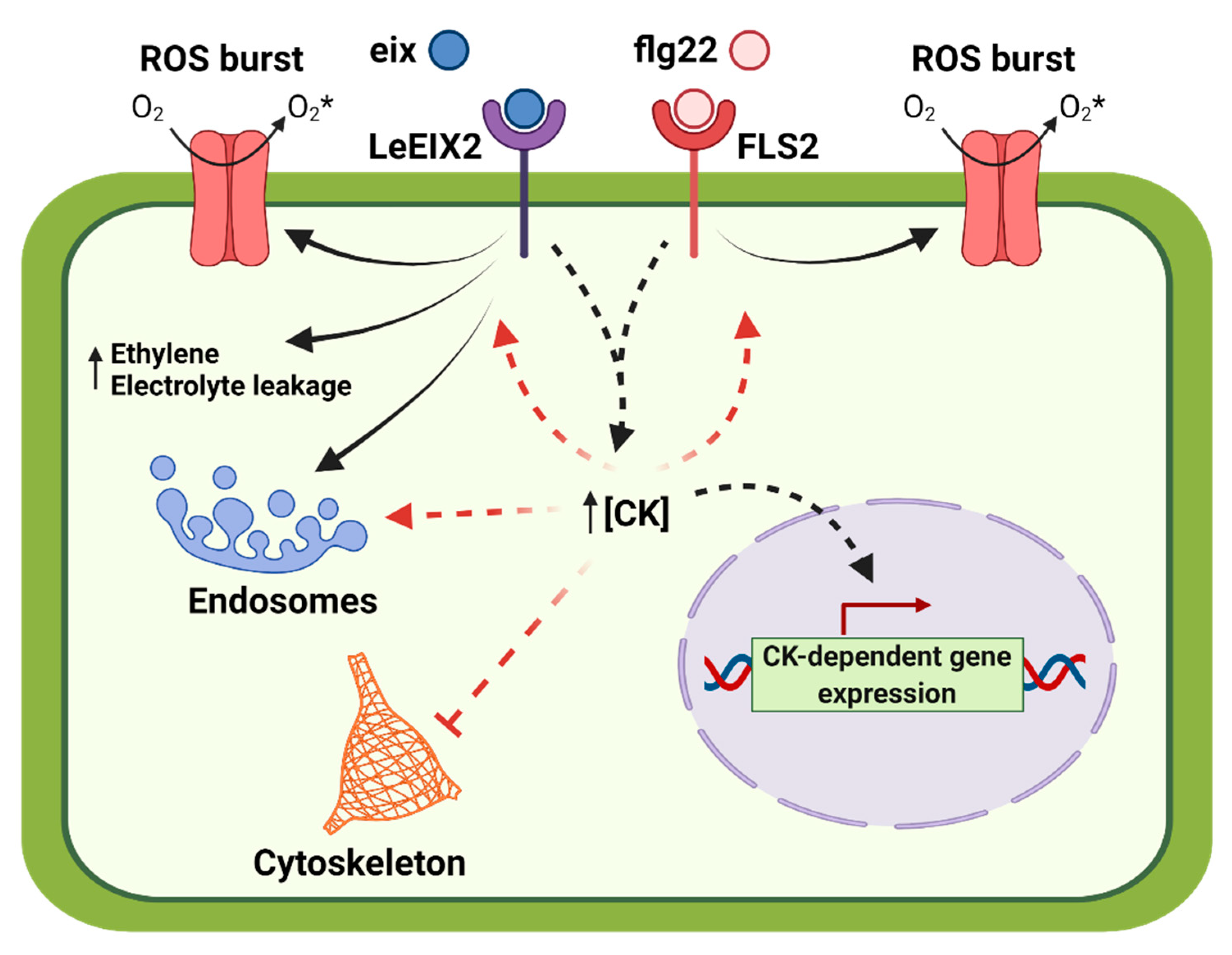
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. Transient Expression
4.3. Chemical Treatments
4.4. Confocal Microscopy
4.5. Reactive Oxygen Species Burst Measurement
4.6. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kieber, J.J.; Schaller, G.E. Cytokinins. Arab. Book 2014, 12, e0168. [Google Scholar] [CrossRef]
- D’Agostino, I.B.; Deruère, J.; Kieber, J.J. Characterization of the Response of the Arabidopsis Response Regulator Gene Family to Cytokinin. Plant Physiol. 2000, 124, 1706–1717. [Google Scholar] [CrossRef]
- Hutchison, C.E.; Li, J.; Argueso, C.; Gonzalez, M.; Lee, E.; Lewis, M.W.; Maxwell, B.B.; Perdue, T.D.; Schaller, G.E.; Alonso, J.; et al. The Arabidopsis Histidine Phosphotransfer Proteins Are Redundant Positive Regulators of Cytokinin Signaling. Plant Cell 2006, 18, 3073–3087. [Google Scholar] [CrossRef]
- Wulfetange, K.; Lomin, S.N.; Romanov, G.A.; Stolz, A.; Heyl, A.; Schmülling, T. The Cytokinin Receptors of Arabidopsis Are Located Mainly to the Endoplasmic Reticulum. Plant Physiol. 2011, 156, 1808–1818. [Google Scholar] [CrossRef]
- Romanov, G.A.; Schmülling, T. Opening Doors for Cytokinin Trafficking at the ER Membrane. Trends Plant Sci. 2021, 26, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Sheen, J. Arabidopsis Cytokinin Signaling Pathway. Sci. STKE 2007, 2007, cm5. [Google Scholar] [CrossRef] [PubMed]
- Zürcher, E.; Müller, B. Cytokinin Synthesis, Signaling, and Function—Advances and New Insights. Int. Rev. Cell Mol. Biol. 2016, 324, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Antoniadi, I.; Novák, O.; Gelová, Z.; Johnson, A.; Plíhal, O.; Simerský, R.; Mik, V.; Vain, T.; Mateo-Bonmatí, E.; Karady, M.; et al. Cell-surface receptors enable perception of extracellular cytokinins. Nat. Commun. 2020, 11, 4284. [Google Scholar] [CrossRef] [PubMed]
- Kubiasová, K.; Montesinos, J.C.; Šamajová, O.; Nisler, J.; Mik, V.; Semerádová, H.; Plíhalová, L.; Novák, O.; Marhavý, P.; Cavallari, N.; et al. Cytokinin fluoroprobe reveals multiple sites of cytokinin perception at plasma membrane and endoplasmic reticulum. Nat. Commun. 2020, 11, 4285. [Google Scholar] [CrossRef]
- Müller, B.; Sheen, J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 2008, 453, 1094–1097. [Google Scholar] [CrossRef]
- Ioio, R.D.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef]
- Zhao, Z.; Andersen, S.U.; Ljung, K.; Dolezal, K.; Miotk, A.; Schultheiss, S.J.; Lohmann, J.U. Hormonal control of the shoot stem-cell niche. Nature 2010, 465, 1089–1092. [Google Scholar] [CrossRef]
- Marhavy, P.; Bielach, A.; Abas, L.; Abuzeineh, A.; Duclercq, J.; Tanaka, H.; Pařezová, M.; Petrášek, J.; Friml, J.; Kleine-Vehn, J.; et al. Cytokinin Modulates Endocytic Trafficking of PIN1 Auxin Efflux Carrier to Control Plant Organogenesis. Dev. Cell 2011, 21, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Huh, S.U.; Kojima, M.; Sakakibara, H.; Paek, K.-H.H.; Hwang, I. The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in arabidopsis. Dev. Cell 2010, 19, 284–295. [Google Scholar] [CrossRef]
- Argueso, C.T.; Ferreira, F.J.; Epple, P.; To, J.P.C.; Hutchison, C.E.; Schaller, G.E.; Dangl, J.L.; Kieber, J.J. Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity. PLoS Genet. 2012, 8, e1002448. [Google Scholar] [CrossRef]
- Grosskinsky, D.K.; Naseem, M.; Abdelmohsen, U.R.; Plickert, N.; Engelke, T.; Griebel, T.; Zeier, J.; Novák, O.; Strnad, M.; Pfeifhofer, H.; et al. Cytokinins Mediate Resistance against Pseudomonas syringae in Tobacco through Increased Antimicrobial Phytoalexin Synthesis Independent of Salicylic Acid Signaling. Plant Physiol. 2011, 157, 815–830. [Google Scholar] [CrossRef]
- Naseem, M.; Philippi, N.; Hussain, A.; Wangorsch, G.; Ahmed, N.; Dandekar, T. Integrated Systems View on Networking by Hormones in Arabidopsis Immunity Reveals Multiple Crosstalk for Cytokinin. Plant Cell 2012, 24, 1793–1814. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, R.; Pizarro, L.; Leibman-Markus, M.; Marash, I.; Bar, M. Cytokinin response induces immunity and fungal pathogen resistance, and modulates trafficking of the PRR LeEIX2 in tomato. Mol. Plant Pathol. 2020, 21, 1287–1306. [Google Scholar] [CrossRef]
- Gupta, R.; Leibman-Markus, M.; Pizarro, L.; Bar, M. Cytokinin induces bacterial pathogen resistance in tomato. Plant Pathol. 2021, 70, 318–325. [Google Scholar] [CrossRef]
- Pizarro, L.; Leibman-Markus, M.; Schuster, S.; Bar, M.; Avni, A. SlPRA1A/RAB attenuate EIX immune responses via degradation of LeEIX2 pattern recognition receptor. Plant Signal. Behav. 2018, 13, e1467689. [Google Scholar] [CrossRef]
- Gupta, R.; Anand, G.; Pizarro, L.; Laor, D.; Kovetz, N.; Sela, N.; Yehuda, T.; Gazit, E.; Bar, M. Cytokinin inhibits fungal development and virulence by targeting the cytoskeleton and cellular trafficking. bioRxiv 2020. [Google Scholar] [CrossRef]
- Chinchilla, D.; Zipfel, C.; Robatzek, S.; Kemmerling, B.; Nürnberger, T.; Jones, J.D.G.; Felix, G.; Boller, T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 2007, 448, 497–500. [Google Scholar] [CrossRef]
- Roberts, R.; Liu, A.E.; Wan, L.; Geiger, A.M.; Hind, S.R.; Rosli, H.G.; Martin, G.B. Molecular characterization of differences between the tomato immune receptors flagellin sensing 3 and flagellin sensing 2. Plant Physiol. 2020, 183, 1825–1837. [Google Scholar] [CrossRef]
- Wahlig, T.A.; Bixler, B.J.; Valdés-López, O.; Mysore, K.S.; Wen, J.; Ané, J.M.; Kaspar, C.W. Salmonella enterica serovar Typhimurium ATCC 14028S is tolerant to plant defenses triggered by the flagellin receptor FLS2. FEMS Microbiol. Lett. 2019, 366, fny296. [Google Scholar] [CrossRef]
- Shi, Q.; Febres, V.J.; Jones, J.B.; Moore, G.A. A survey of FLS2 genes from multiple citrus species identifies candidates for enhancing disease resistance to Xanthomonas citri ssp. citri. Hortic. Res. 2016, 3, 16022. [Google Scholar] [CrossRef]
- Arnaud, D.; Lee, S.; Takebayashi, Y.; Choi, D.; Choi, J.; Sakakibara, H.; Hwang, I. Cytokinin-mediated regulation of reactive oxygen species homeostasis modulates stomatal immunity in Arabidopsis. Plant Cell 2017, 29, 543–559. [Google Scholar] [CrossRef]
- Ebine, K.; Fujimoto, M.; Okatani, Y.; Nishiyama, T.; Goh, T.; Ito, E.; Dainobu, T.; Nishitani, A.; Uemura, T.; Sato, M.H.; et al. A membrane trafficking pathway regulated by the plant-specific RAB GTPase ARA6. Nat. Cell Biol. 2011, 13, 853–859. [Google Scholar] [CrossRef]
- Rigal, A.; Doyle, S.M.; Robert, S. Live Cell Imaging of FM4-64, a Tool for Tracing the Endocytic Pathways in Arabidopsis Root Cells. In Plant Cell Expansion; Estevez, J.M., Ed.; Methods in Molecular Biology; Springer Nature: Basingstoke, UK, 2015; Volume 1242, pp. 93–103. [Google Scholar]
- Ketelaar, T.; Emons, A.M.C. The cytoskeleton in plant cell growth: Lessons from root hairs. New Phytol. 2001, 152, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Šamaj, J.; Baluška, F.; Voigt, B.; Schlicht, M.; Volkmann, D.; Menzel, D. Endocytosis, Actin Cytoskeleton, and Signaling. Plant Physiol. 2004, 135, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Kurakawa, T.; Ueda, N.; Maekawa, M.; Kobayashi, K.; Kojima, M.; Nagato, Y.; Sakakibara, H.; Kyozuka, J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 2007, 445, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.P.; Chickarmane, V.S.; Ohno, C.; Meyerowitz, E.M. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc. Natl. Acad. Sci. USA 2009, 106, 16529–16534. [Google Scholar] [CrossRef]
- Smith, J.M.; Salamango, D.J.; Leslie, M.E.; Collins, C.A.; Heese, A. Sensitivity to Flg22 Is Modulated by Ligand-Induced Degradation and de Novo Synthesis of the Endogenous Flagellin-Receptor FLAGELLIN-SENSING2. Plant Physiol. 2014, 164, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, J.; Robatzek, S. Detection and Analyses of Endocytosis of Plant Receptor Kinases. In Plant Receptor Kinases; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1621, pp. 177–189. [Google Scholar]
- Shoresh, M.; Yedidia, I.; Chet, I. Involvement of Jasmonic Acid/Ethylene Signaling Pathway in the Systemic Resistance Induced in Cucumber by Trichoderma asperellum T203. Phytopathology 2005, 95, 76–84. [Google Scholar] [CrossRef]
- Tateda, C.; Zhang, Z.; Shrestha, J.; Jelenska, J.; Chinchilla, D.; Greenberg, J.T. Salicylic Acid Regulates Arabidopsis Microbial Pattern Receptor Kinase Levels and Signaling. Plant Cell 2014, 26, 4171–4187. [Google Scholar] [CrossRef]
- Beck, M.; Zhou, J.; Faulkner, C.; MacLean, D.; Robatzek, S. Spatio-Temporal Cellular Dynamics of the Arabidopsis Flagellin Receptor Reveal Activation Status-Dependent Endosomal Sorting. Plant Cell 2012, 24, 4205–4219. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef] [PubMed]
- Steiner, E.; Israeli, A.; Gupta, R.; Shwartz, I.; Nir, I.; Leibman-Markus, M.; Tal, L.; Farber, M.; Amsalem, Z.; Ori, N.; et al. Characterization of the cytokinin sensor TCSv2 in arabidopsis and tomato. Plant Methods 2020, 16, 152. [Google Scholar] [CrossRef]
- Robatzek, S.; Chinchilla, D.; Boller, T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006, 20, 537–542. [Google Scholar] [CrossRef]
- Ivanov, S.; Harrison, M.J. A set of fluorescent protein-based markers expressed from constitutive and arbuscular mycorrhiza-inducible promoters to label organelles, membranes and cytoskeletal elements in Medicago truncatula. Plant J. 2014, 80, 1151–1163. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Leibman-Markus, M.; Schuster, S.; Avni, A. LeEIX2 Interactors’ Analysis and EIX-Mediated Responses Measurement. In Plant Pattern Recognition Receptors; Shan, L., He, P., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1578, pp. 167–172. [Google Scholar]


| Line/Construct | Source | Organism/Use | Fig# |
|---|---|---|---|
| pAtFLS2::AtFLS2−3xmyc-GFP [40] | Addgene plasmid#86157; http://n2t.net/addgene:86157, accessed on 31 May 2021; RRID:Addgene_86157 | Transient expression in N. benthamiana | 1 |
| pTCS::3XVENUS [39] | David Weiss, Faculty of Agriculture, HUJI. | Stable transgenic A. thaliana, col. background | 2 |
| pARA6:ARA6-Venus [27] | Takashi Ueda. | Stable transgenic A. thaliana, col. background | 4 |
| pCMU-ACTLr [41] | Addgene plasmid #61193; http://n2t.net/addgene:61193, accessed on 31 May 2021; | Transient expression in N. benthamiana | 5 |
| pCMU-MTUBr [41] | Addgene plasmid #61196; http://n2t.net/addgene:, accessed on 31 May 2021; RRID:Addgene_61196 | Transient expression in N. benthamiana | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizarro, L.; Munoz, D.; Marash, I.; Gupta, R.; Anand, G.; Leibman-Markus, M.; Bar, M. Cytokinin Modulates Cellular Trafficking and the Cytoskeleton, Enhancing Defense Responses. Cells 2021, 10, 1634. https://doi.org/10.3390/cells10071634
Pizarro L, Munoz D, Marash I, Gupta R, Anand G, Leibman-Markus M, Bar M. Cytokinin Modulates Cellular Trafficking and the Cytoskeleton, Enhancing Defense Responses. Cells. 2021; 10(7):1634. https://doi.org/10.3390/cells10071634
Chicago/Turabian StylePizarro, Lorena, Daniela Munoz, Iftah Marash, Rupali Gupta, Gautam Anand, Meirav Leibman-Markus, and Maya Bar. 2021. "Cytokinin Modulates Cellular Trafficking and the Cytoskeleton, Enhancing Defense Responses" Cells 10, no. 7: 1634. https://doi.org/10.3390/cells10071634
APA StylePizarro, L., Munoz, D., Marash, I., Gupta, R., Anand, G., Leibman-Markus, M., & Bar, M. (2021). Cytokinin Modulates Cellular Trafficking and the Cytoskeleton, Enhancing Defense Responses. Cells, 10(7), 1634. https://doi.org/10.3390/cells10071634






