Multifunctionality of the Telomere-Capping Shelterin Complex Explained by Variations in Its Protein Composition
Abstract
1. Foreword
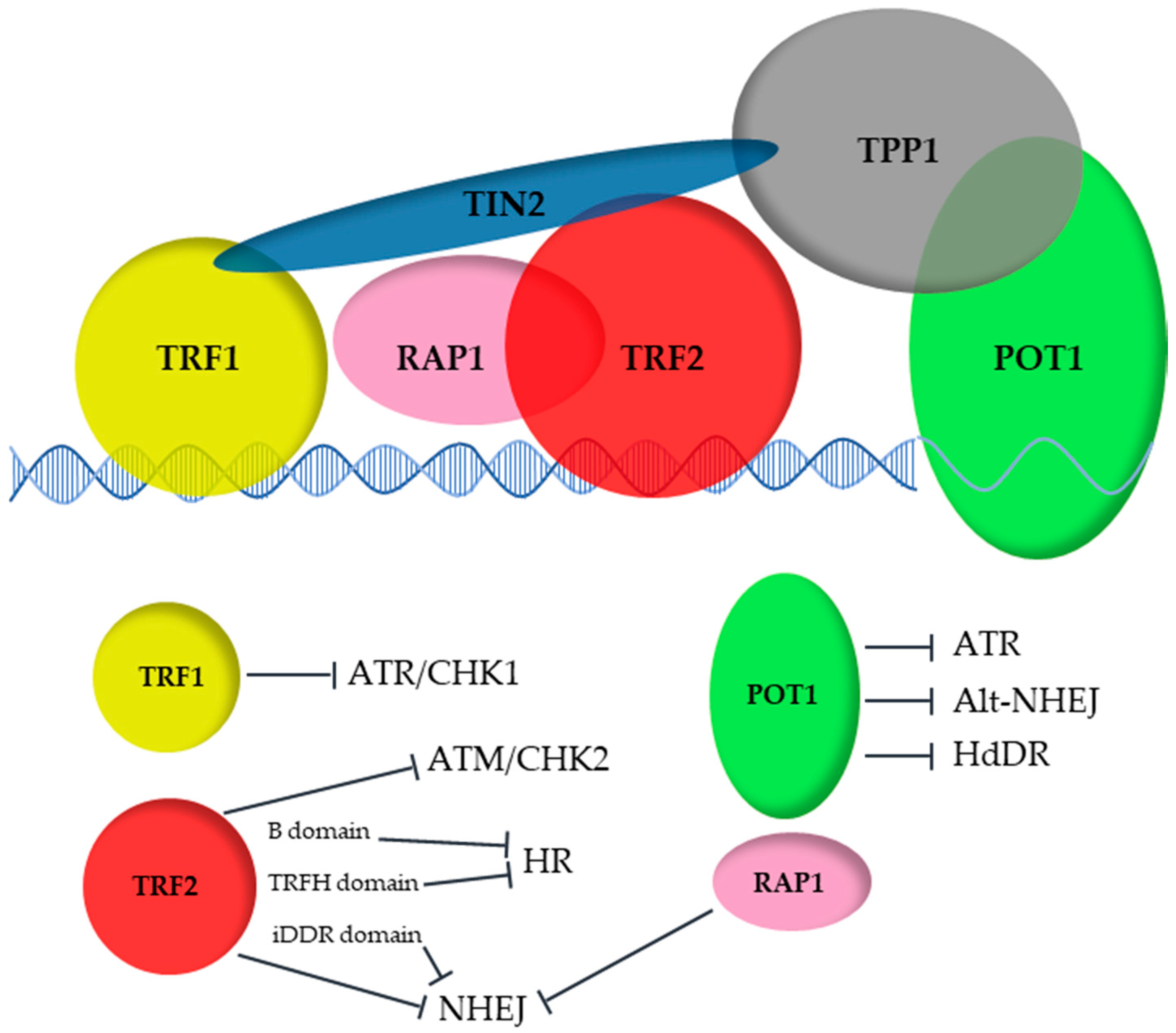
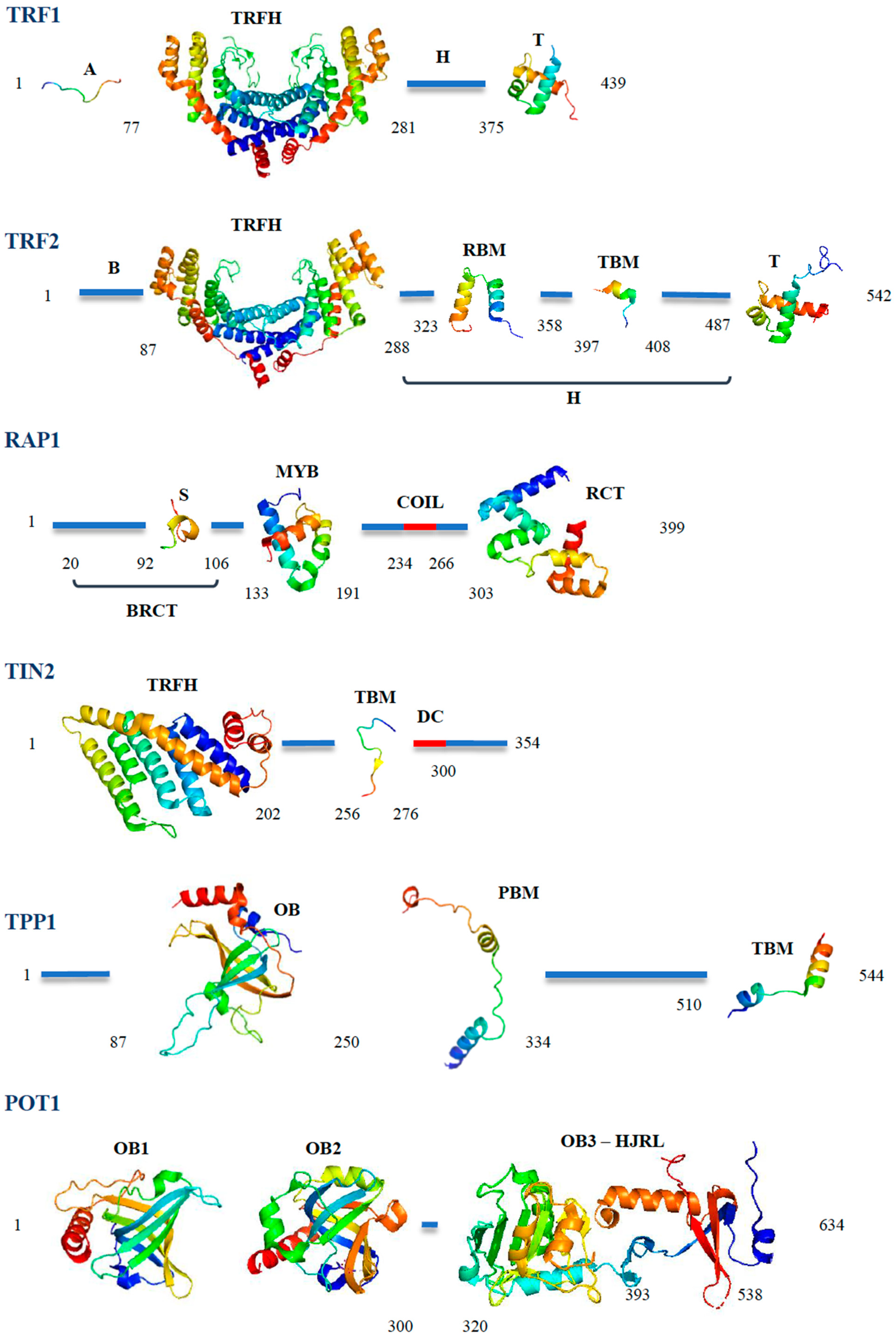
2. The Shelterin Complex: Composition and Biological Role
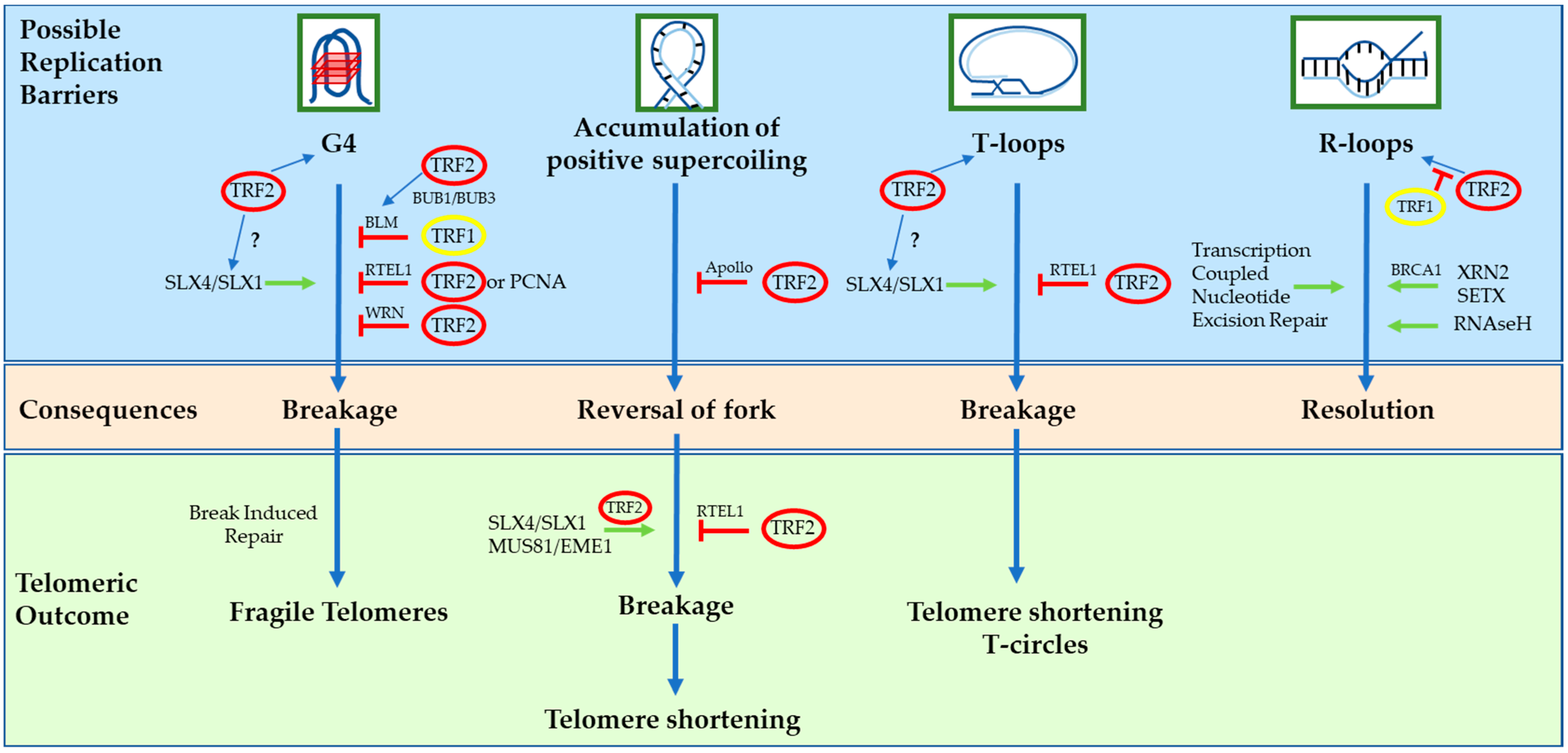
3. The DNA Binders: POT1, TRF1 and TRF2
4. The Distinct Biological Roles of TRF1 and TRF2
5. RAP1: An Accessory Protein for Telomere Protection
6. TIN2: The Bridge
7. Shelterin Quaternary Structure
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Gilson, E.; Géli, V. How telomeres are replicated. Nat. Rev. Mol. Cell Biol. 2007, 8, 825–838. [Google Scholar] [CrossRef]
- Ye, J.; Wu, Y.; Gilson, E. Dynamics of telomeric chromatin at the crossroads of aging and cancer. Essays Biochem. 2010, 48, 147–164. [Google Scholar] [CrossRef]
- Armando, R.G.; Gomez, D.L.M.; Maggio, J.; Sanmartin, M.C.; Gomez, D.E. Telomeropathies: Etiology, diagnosis, treatment and follow-up. Ethical and legal considerations. Clin. Genet. 2019, 96, 3–16. [Google Scholar] [CrossRef]
- Bhargava, R.; Fischer, M.; O’Sullivan, R.J. Genome rearrangements associated with aberrant telomere maintenance. Curr. Opin. Genet. Dev. 2020, 60, 31–40. [Google Scholar] [CrossRef] [PubMed]
- El Maï, M.; Hreich, S.J.D.; Gaggioli, C.; Roisin, A.; Wagner, N.; Ye, J.; Jalinot, P.; Cherfils-Vicini, J.; Gilson, E. A Novel Screen for Expression Regulators of the Telomeric Protein TRF2 Identified Small Molecules That Impair TRF2 Dependent Immunosuppression and Tumor Growth. Cancers 2021, 13, 2998. [Google Scholar] [CrossRef]
- Chong, L.; Van Steensel, B.; Broccoli, D.; Erdjument-Bromage, H.; Hanish, J.; Tempst, P.; De Lange, T. A Human Telomeric Protein. Science 1995, 270, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Shiue, L.; Kaplan, S.; De Lange, T. A mammalian factor that binds telomeric TTAGGG repeats in vitro. Mol. Cell. Biol. 1992, 12, 4834–4843. [Google Scholar] [CrossRef]
- Bilaud, T.; Koering, C.E.; Binet-Brasselet, E.; Ancelin, K.; Pollice, A.; Gasser, S.; Gilson, E. The Telobox, a Myb-Related Telomeric DNA Binding Motif Found in Proteins from Yeast, Plants and Human. Nucleic Acids Res. 1996, 24, 1294–1303. [Google Scholar] [CrossRef]
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef]
- Mendez-Bermudez, A.; Giraud-Panis, M.-J.; Ye, J.; Gilson, E. Heterochromatin replication goes hand in hand with telomere protection. Nat. Struct. Mol. Biol. 2020, 27, 313–318. [Google Scholar] [CrossRef]
- Cicconi, A.; Chang, S. Shelterin and the replisome: At the intersection of telomere repair and replication. Curr. Opin. Genet. Dev. 2020, 60, 77–84. [Google Scholar] [CrossRef]
- Sfeir, A.; Kosiyatrakul, S.T.; Hockemeyer, D.; MacRae, S.L.; Karlseder, J.; Schildkraut, C.L.; de Lange, T. Mammalian Telomeres Resemble Fragile Sites and Require TRF1 for Efficient Replication. Cell 2009, 138, 90–103. [Google Scholar] [CrossRef]
- Ye, J.; Lenain, C.; Bauwens, S.; Rizzo, A.; Saint-Léger, A.; Poulet, A.; Benarroch, D.; Magdinier, F.; Morere, J.; Amiard, S.; et al. TRF2 and Apollo Cooperate with Topoisomerase 2α to Protect Human Telomeres from Replicative Damage. Cell 2010, 142, 230–242. [Google Scholar] [CrossRef]
- De Lange, T. Shelterin-Mediated Telomere Protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef]
- Colgin, L.; Reddel, R. Telomere Biology: A New Player in the End Zone. Curr. Biol. 2004, 14, R901–R902. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bochkarev, A.; Bochkareva, E. From RPA to BRCA2: Lessons from single-stranded DNA binding by the OB-fold. Curr. Opin. Struct. Biol. 2004, 14, 36–42. [Google Scholar] [CrossRef]
- Chen, C.; Gu, P.; Wu, J.; Chen, X.; Niu, S.; Sun, H.; Wu, L.; Li, N.; Peng, J.; Shi, S.; et al. Structural insights into POT1-TPP1 interaction and POT1 C-terminal mutations in human cancer. Nat. Commun. 2017, 8, 14929. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.; Shastrula, P.K.; Kossenkov, A.V.; Hills, R.; Baird, D.; Showe, L.C.; Doukov, T.; Janicki, S.; Skordalakes, E. Structural and functional analysis of the human POT1-TPP1 telomeric complex. Nat. Commun. 2017, 8, 14928. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Podell, E.R.; Zaug, A.J.; Yang, Y.; Baciu, P.; Cech, T.R.; Lei, M. The POT1–TPP1 telomere complex is a Telomerase processivity factor. Nat. Cell Biol. 2007, 445, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Liu, D.; Wan, M.; Safari, A.; Kim, H.; Sun, W.; O’Connor, M.S.; Songyang, Z. TPP1 is a homologue of ciliate TEBP-β and interacts with POT1 to recruit Telomerase. Nat. Cell Biol. 2007, 445, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Rai, R.; Huang, C.; Broton, C.; Long, J.; Xu, Y.; Xue, J.; Lei, M.; Chang, S.; Chen, Y. Structural and functional analyses of the mammalian TIN2-TPP1-TRF2 telomeric complex. Cell Res. 2017, 27, 1485–1502. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.K.; Kibe, T.; Donigian, J.R.; Frescas, D.; de Lange, T. Telomere Protection by TPP1/POT1 Requires Tethering to TIN2. Mol. Cell 2011, 44, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Brambati, A.; Barry, R.M.; Sfeir, A. DNA polymerase theta (Polθ)—An error-prone polymerase necessary for genome stability. Curr. Opin. Genet. Dev. 2020, 60, 119–126. [Google Scholar] [CrossRef]
- Kratz, K.; de Lange, T. Protection of telomeres 1 proteins POT1a and POT1b can repress ATR signaling by RPA exclusion, but binding to CST limits ATR repression by POT1b. J. Biol. Chem. 2018, 293, 14384–14392. [Google Scholar] [CrossRef]
- Glousker, G.; Briod, A.; Quadroni, M.; Lingner, J. Human Shelterin protein POT 1 prevents severe telomere instability induced by homology-directed DNA repair. EMBO J. 2020, 39, e104500. [Google Scholar] [CrossRef]
- Wu, P.; Takai, H.; de Lange, T. Telomeric 3′ Overhangs Derive from Resection by Exo1 and Apollo and Fill-In by POT1b-Associated CST. Cell 2012, 150, 39–52. [Google Scholar] [CrossRef]
- Grill, S.; Tesmer, V.M.; Nandakumar, J. The N Terminus of the OB Domain of Telomere Protein TPP1 Is Critical for Telomerase Action. Cell Rep. 2018, 22, 1132–1140. [Google Scholar] [CrossRef]
- Nandakumar, J.; Bell, C.; Weidenfeld, I.; Zaug, A.J.; Leinwand, L.; Cech, T.R. The TEL patch of telomere protein TPP1 mediates Telomerase recruitment and processivity. Nat. Cell Biol. 2012, 492, 285–289. [Google Scholar] [CrossRef]
- Sexton, A.N.; Regalado, S.G.; Lai, C.S.; Cost, G.; O’Neil, C.M.; Urnov, F.D.; Gregory, P.; Jaenisch, R.; Collins, K.; Hockemeyer, D. Genetic and molecular identification of three human TPP1 functions in Telomerase action: Recruitment, activation, and homeostasis set point regulation. Genes Dev. 2014, 28, 1885–1899. [Google Scholar] [CrossRef]
- Laprade, H.; Querido, E.; Smith, M.J.; Guérit, D.; Crimmins, H.; Conomos, D.; Pourret, E.; Chartrand, P.; Sfeir, A. Single-Molecule Imaging of Telomerase RNA Reveals a Recruitment-Retention Model for Telomere Elongation. Mol. Cell 2020, 79, 115–126.e6. [Google Scholar] [CrossRef]
- Pike, A.M.; Strong, M.A.; Ouyang, J.P.T.; Greider, C.W. TIN2 Functions with TPP1/POT1 To Stimulate Telomerase Processivity. Mol. Cell. Biol. 2019, 39, e00593-18. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Stock, A.J.; Liu, Y. The enigma of excessively long telomeres in cancer: Lessons learned from rare human POT1 variants. Curr. Opin. Genet. Dev. 2020, 60, 48–55. [Google Scholar] [CrossRef]
- Tummala, H.; Collopy, L.C.; Walne, A.J.; Ellison, A.; Cardoso, S.; Aksu, T.; Yarali, N.; Aslan, D.; Akata, R.F.; Teo, J.; et al. Homozygous OB-fold variants in telomere protein TPP1 are associated with dyskeratosis congenita–like phenotypes. Blood 2018, 132, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Grill, S.; Bisht, K.; Tesmer, V.M.; Shami, A.N.; Hammoud, S.S.; Nandakumar, J. Two Separation-of-Function Isoforms of Human TPP1 Dictate Telomerase Regulation in Somatic and Germ Cells. Cell Rep. 2019, 27, 3511–3521.e7. [Google Scholar] [CrossRef]
- Poulet, A.; Pisano, S.; Faivre-Moskalenko, C.; Pei, B.; Tauran, Y.; Haftek-Terreau, Z.; Brunet, F.; Le Bihan, Y.-V.; Ledu, M.-H.; Montel, F.; et al. The N-terminal domains of TRF1 and TRF2 regulate their ability to condense telomeric DNA. Nucleic Acids Res. 2011, 40, 2566–2576. [Google Scholar] [CrossRef]
- Fairall, L.; Chapman, L.; Moss, H.; de Lange, T.; Rhodes, D. Structure of the TRFH Dimerization Domain of the Human Telomeric Proteins TRF1 and TRF2. Mol. Cell 2001, 8, 351–361. [Google Scholar] [CrossRef]
- Bilaud, T.; Brun, C.; Ancelin, K.; Koering, C.E.; Laroche, T.; Gilson, E. Telomeric localization of TRF2, a novel human telobox protein. Nat. Genet. 1997, 17, 236–239. [Google Scholar] [CrossRef]
- Broccoli, D.; Smogorzewska, A.; Chong, L.; de Lange, T. Human telomeres contain two distinct Myb–related proteins, TRF1 and TRF2. Nat. Genet. 1997, 17, 231–235. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Van Overbeek, M.; Donigian, J.R.; Baciu, P.; De Lange, T.; Lei, M. A Shared Docking Motif in TRF1 and TRF2 Used for Differential Recruitment of Telomeric Proteins. Science 2008, 319, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, O.-H.; Xin, H.; Chen, L.-Y.; Qin, J.; Chae, H.K.; Lin, S.-Y.; Safari, A.; Liu, D.; Songyang, Z. TRF2 functions as a protein hub and regulates telomere maintenance by recognizing specific peptide motifs. Nat. Struct. Mol. Biol. 2009, 16, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Guervilly, J.-H.; Gaillard, P.-H.L. SLX4: Multitasking to maintain genome stability. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 475–514. [Google Scholar] [CrossRef]
- Rai, R.; Hu, C.; Broton, C.; Chen, Y.; Lei, M.; Chang, S. NBS1 Phosphorylation Status Dictates Repair Choice of Dysfunctional Telomeres. Mol. Cell 2017, 65, 801–817.e4. [Google Scholar] [CrossRef]
- Cicconi, A.; Rai, R.; Xiong, X.; Broton, C.; Al-Hiyasat, A.; Hu, C.; Dong, S.; Sun, W.; Garbarino, J.; Bindra, R.S.; et al. Microcephalin 1/BRIT1-TRF2 interaction promotes telomere replication and repair, linking telomere dysfunction to primary microcephaly. Nat. Commun. 2020, 11, 5861. [Google Scholar] [CrossRef] [PubMed]
- Benarroch-Popivker, D.; Pisano, S.; Mendez-Bermudez, A.; Lototska, L.; Kaur, P.; Bauwens, S.; Djerbi, N.; Latrick, C.M.; Fraisier, V.; Pei, B.; et al. TRF2-Mediated Control of Telomere DNA Topology as a Mechanism for Chromosome-End Protection. Mol. Cell 2016, 61, 274–286. [Google Scholar] [CrossRef]
- Amiard, S.; Doudeau, M.; Pinte, S.; Poulet, A.; Lenain, C.; Faivre-Moskalenko, C.; Angelov, D.; Hug, N.; Vindigni, A.; Bouvet, P.; et al. A topological mechanism for TRF2-enhanced strand invasion. Nat. Struct. Mol. Biol. 2007, 14, 147–154. [Google Scholar] [CrossRef]
- Griffith, J.D.; Comeau, L.; Rosenfield, S.; Stansel, R.M.; Bianchi, A.; Moss, H.; de Lange, T. Mammalian Telomeres End in a Large Duplex Loop. Cell 1999, 97, 503–514. [Google Scholar] [CrossRef]
- Doksani, Y.; Wu, J.Y.; De Lange, T.; Zhuang, X. Super-Resolution Fluorescence Imaging of Telomeres Reveals TRF2-Dependent T-loop Formation. Cell 2013, 155, 345–356. [Google Scholar] [CrossRef]
- Stansel, R.M.; de Lange, T.; Griffith, J.D. T-loop assembly in vitro involves binding of TRF2 near the 3’ telomeric overhang. EMBO J. 2001, 20, 5532–5540. [Google Scholar] [CrossRef] [PubMed]
- Timashev, L.A.; De Lange, T. Characterization of t-loop formation by TRF2. Nucleus 2020, 11, 164–177. [Google Scholar] [CrossRef]
- Fouché, N.; Cesare, A.; Willcox, S.; Özgür, S.; Compton, S.A.; Griffith, J.D. The Basic Domain of TRF2 Directs Binding to DNA Junctions Irrespective of the Presence of TTAGGG Repeats. J. Biol. Chem. 2006, 281, 37486–37495. [Google Scholar] [CrossRef]
- Poulet, A.; Buisson, R.; Faivre-Moskalenko, C.; Koelblen, M.; Amiard, S.; Montel, F.; Cuesta-Lopez, S.; Bornet, O.; Guerlesquin, F.; Godet, T.; et al. TRF2 promotes, remodels and protects telomeric Holliday junctions. EMBO J. 2009, 28, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Saint-Léger, A.; Koelblen, M.; Civitelli, L.; Bah, A.; Djerbi, N.; Giraud-Panis, M.-J.; Londono-Vallejo, A.; Ascenzioni, F.; Gilson, E. The basic N-terminal domain of TRF2 limits recombination endonuclease action at human telomeres. Cell Cycle 2014, 13, 2469–2474. [Google Scholar] [CrossRef]
- Schmutz, I.; Timashev, L.; Xie, W.; Patel, D.J.; De Lange, T. TRF2 binds branched DNA to safeguard telomere integrity. Nat. Struct. Mol. Biol. 2017, 24, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, I.M.; Hayward, W.; Fletcher, T.M. The effect of the TRF2 N-terminal and TRFH regions on telomeric G-quadruplex structures. Nucleic Acids Res. 2009, 37, 1541–1554. [Google Scholar] [CrossRef]
- Neidle, S. Quadruplex Nucleic Acids as Novel Therapeutic Targets. J. Med. Chem. 2016, 59, 5987–6011. [Google Scholar] [CrossRef]
- Deng, Z.; Norseen, J.; Wiedmer, A.; Riethman, H.; Lieberman, P.M. TERRA RNA Binding to TRF2 Facilitates Heterochromatin Formation and ORC Recruitment at Telomeres. Mol. Cell 2009, 35, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Arora, R.; Wischnewski, H.; Azzalin, C.M. TRF1 participates in chromosome end protection by averting TRF2-dependent telomeric R loops. Nat. Struct. Mol. Biol. 2018, 25, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Muftuoglu, M.; Wong, H.K.; Imam, S.Z.; Wilson, D.M.; Bohr, V.A.; Opresko, P. Telomere Repeat Binding Factor 2 Interacts with Base Excision Repair Proteins and Stimulates DNA Synthesis by DNA Polymerase β. Cancer Res. 2006, 66, 113–124. [Google Scholar] [CrossRef]
- Atanasiu, C.; Deng, Z.; Wiedmer, A.; Norseen, J.; Lieberman, P.M. ORC binding to TRF2 stimulates OriP replication. EMBO Rep. 2006, 7, 716–721. [Google Scholar] [CrossRef]
- Li, B.; Qiao, R.; Wang, Z.; Zhou, W.; Li, X.; Xu, W.; Rao, Z. Crystal structure of a tankyrase 1–telomere repeat factor 1 complex. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2016, 72, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, L.; Teng, Y.; Chen, H.; Gao, Y.; Levine, A.S.; Nakajima, S.; Lan, L. Tankyrase1-mediated poly(ADP-ribosyl)ation of TRF1 maintains cell survival after telomeric DNA damage. Nucleic Acids Res. 2017, 45, 3906–3921. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Yang, Y.; Ueberheide, B.; Smith, S. Whole proteome analysis of human tankyrase knockout cells reveals targets of tankyrase-mediated degradation. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Dynek, J.N.; Smith, S. Resolution of Sister Telomere Association Is Required for Progression through Mitosis. Science 2004, 304, 97–100. [Google Scholar] [CrossRef]
- Zimmermann, M.; Kibe, T.; Kabir, S.; De Lange, T. TRF1 negotiates TTAGGG repeat-associated replication problems by recruiting the BLM helicase and the TPP1/POT1 repressor of ATR signaling. Genes Dev. 2014, 28, 2477–2491. [Google Scholar] [CrossRef]
- Okamoto, K.; Bartocci, C.; Ouzounov, I.; Diedrich, J.K.; Iii, J.R.Y.; Denchi, E.L. A two-step mechanism for TRF2-mediated chromosome-end protection. Nat. Cell Biol. 2013, 494, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; Thanasoula, M.; Muñoz, P.; Liao, C.; Tejera, A.M.; McNees, C.; Flores, J.M.; Fernandez-Capetillo, O.; Tarsounas, M.; Blasco, M.A. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009, 23, 2060–2075. [Google Scholar] [CrossRef]
- Yang, Z.; Takai, K.K.; Lovejoy, C.A.; de Lange, T. Break-induced replication promotes fragile telomere formation. Genes Dev. 2020, 34, 1392–1405. [Google Scholar] [CrossRef]
- Augereau, A.; Mariotti, M.; Pousse, M.; Filipponi, D.; Libert, F.; Beck, B.; Gorbunova, V.; Gilson, E.; Gladyshev, V.N. Naked mole rat TRF1 safeguards glycolytic capacity and telomere replication under low oxygen. Sci. Adv. 2021, 7, eabe0174. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Zhou, X.Z.; Kishi, S.; Kosugi, I.; Tsutsui, Y.; Lu, K.P. A specific interaction between the telomeric protein Pin2/TRF1 and the mitotic spindle. Curr. Biol. 2001, 11, 1512–1516. [Google Scholar] [CrossRef]
- Ohishi, T.; Muramatsu, Y.; Yoshida, H.; Seimiya, H. TRF1 Ensures the Centromeric Function of Aurora-B and Proper Chromosome Segregation. Mol. Cell. Biol. 2014, 34, 2464–2478. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tu, Z.; Liu, C.; Liu, H.; Kaldis, P.; Chen, Z.; Li, W. Dual roles of TRF1 in tethering telomeres to the nuclear envelope and protecting them from fusion during meiosis. Cell Death Differ. 2018, 25, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Marión, R.M.; Montero, J.J.; De Silanes, I.L.; Graña-Castro, O.; Martínez, P.; Schoeftner, S.; Palacios-Fábrega, J.A.; Blasco, M.A. TERRA regulate the transcriptional landscape of pluripotent cells through TRF1-dependent recruitment of PRC2. eLife 2019, 8, e44656. [Google Scholar] [CrossRef]
- Markiewicz-Potoczny, M.; Lobanova, A.; Loeb, A.M.; Kirak, O.; Olbrich, T.; Ruiz, S.; Denchi, E.L. TRF2-mediated telomere protection is dispensable in pluripotent stem cells. Nat. Cell Biol. 2021, 589, 110–115. [Google Scholar] [CrossRef]
- Ruis, P.; Van Ly, D.; Borel, V.; Kafer, G.R.; McCarthy, A.; Howell, S.; Blassberg, R.; Snijders, A.P.; Briscoe, J.; Niakan, K.K.; et al. TRF2-independent chromosome end protection during pluripotency. Nat. Cell Biol. 2021, 589, 103–109. [Google Scholar] [CrossRef]
- Buscemi, G.; Zannini, L.; Fontanella, E.; Lecis, D.; Lisanti, S.; Delia, D. The Shelterin Protein TRF2 Inhibits Chk2 Activity at Telomeres in the Absence of DNA Damage. Curr. Biol. 2009, 19, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Celli, G.B.; de Lange, T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat. Cell Biol. 2005, 7, 712–718. [Google Scholar] [CrossRef]
- Karlseder, J.; Broccoli, D.; Dai, Y.; Hardy, S.; de Lange, T. p53- and ATM-Dependent Apoptosis Induced by Telomeres Lacking TRF2. Science 1999, 283, 1321–1325. [Google Scholar] [CrossRef]
- Karlseder, J.; Hoke, K.; Mirzoeva, O.K.; Bakkenist, C.; Kastan, M.B.; Petrini, J.; De Lange, T. The Telomeric Protein TRF2 Binds the ATM Kinase and Can Inhibit the ATM-Dependent DNA Damage Response. PLoS Biol. 2004, 2, e240. [Google Scholar] [CrossRef]
- Takai, H.; Smogorzewska, A.; de Lange, T. DNA Damage Foci at Dysfunctional Telomeres. Curr. Biol. 2003, 13, 1549–1556. [Google Scholar] [CrossRef]
- Van Steensel, B.; Smogorzewska, A.; de Lange, T. TRF2 Protects Human Telomeres from End-to-End Fusions. Cell 1998, 92, 401–413. [Google Scholar] [CrossRef]
- Wang, R.C.; Smogorzewska, A.; de Lange, T. Homologous Recombination Generates T-Loop-Sized Deletions at Human Telomeres. Cell 2004, 119, 355–368. [Google Scholar] [CrossRef]
- Van Ly, D.; Low, R.R.J.; Frölich, S.; Bartolec, T.K.; Kafer, G.; Pickett, H.A.; Gaus, K.; Cesare, A.J. Telomere Loop Dynamics in Chromosome End Protection. Mol. Cell 2018, 71, 510–525.e6. [Google Scholar] [CrossRef] [PubMed]
- Lototska, L.; Yue, J.; Li, J.; Giraud-Panis, M.; Songyang, Z.; Royle, N.J.; Liti, G.; Ye, J.; Gilson, E.; Mendez-Bermudez, A. Human RAP 1 specifically protects telomeres of senescent cells from DNA damage. EMBO Rep. 2020, 21. [Google Scholar] [CrossRef]
- Ribes-Zamora, A.; Indiviglio, S.M.; Mihalek, I.; Williams, C.; Bertuch, A.A. TRF2 Interaction with Ku Heterotetramerization Interface Gives Insight into c-NHEJ Prevention at Human Telomeres. Cell Rep. 2013, 5, 194–206. [Google Scholar] [CrossRef]
- Nora, G.J.; Buncher, N.A.; Opresko, P. Telomeric protein TRF2 protects Holliday junctions with telomeric arms from displacement by the Werner syndrome helicase. Nucleic Acids Res. 2010, 38, 3984–3998. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Celli, G.B.; Denchi, E.L.; de Lange, T. Ku70 stimulates fusion of dysfunctional telomeres yet protects chromosome ends from homologous recombination. Nat. Cell Biol. 2006, 8, 885–890. [Google Scholar] [CrossRef]
- Mendez-Bermudez, A.; Lototska, L.; Bauwens, S.; Giraud-Panis, M.-J.; Croce, O.; Jamet, K.; Irizar, A.; Mowinckel, M.; Koundrioukoff, S.; Nottet, N.; et al. Genome-wide Control of Heterochromatin Replication by the Telomere Capping Protein TRF2. Mol. Cell 2018, 70, 449–461.e5. [Google Scholar] [CrossRef]
- Sarek, G.; Vannier, J.-B.; Panier, S.; Petrini, J.; Boulton, S.J. TRF2 Recruits RTEL1 to Telomeres in S Phase to Promote T-Loop Unwinding. Mol. Cell 2015, 57, 622–635. [Google Scholar] [CrossRef]
- Lenain, C.; Bauwens, S.; Amiard, S.; Brunori, M.; Giraud-Panis, M.-J.; Gilson, E. The Apollo 5′ Exonuclease Functions Together with TRF2 to Protect Telomeres from DNA Repair. Curr. Biol. 2006, 16, 1303–1310. [Google Scholar] [CrossRef]
- Van Overbeek, M.; de Lange, T. Apollo, an Artemis-Related Nuclease, Interacts with TRF2 and Protects Human Telomeres in S Phase. Curr. Biol. 2006, 16, 1295–1302. [Google Scholar] [CrossRef]
- Lam, Y.C.; Akhter, S.; Gu, P.; Ye, J.; Poulet, A.; Giraud-Panis, M.-J.; Bailey, S.M.; Gilson, E.; Legerski, R.J.; Chang, S. SNMIB/Apollo protects leading-strand telomeres against NHEJ-mediated repair. EMBO J. 2010, 29, 2230–2241. [Google Scholar] [CrossRef]
- Budhathoki, J.B.; Ray, S.; Urban, V.; Janscak, P.; Yodh, J.G.; Balci, H. RecQ-core of BLM unfolds telomeric G-quadruplex in the absence of ATP. Nucleic Acids Res. 2014, 42, 11528–11545. [Google Scholar] [CrossRef]
- Ding, H.; Schertzer, M.; Wu, X.; Gertsenstein, M.; Selig, S.; Kammori, M.; Pourvali, R.; Poon, S.; Vulto, I.; Chavez, E.; et al. Regulation of Murine Telomere Length by Rtel. Cell 2004, 117, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Opresko, P.; von Kobbe, C.; Laine, J.-P.; Harrigan, J.; Hickson, I.D.; Bohr, V.A. Telomere-binding Protein TRF2 Binds to and Stimulates the Werner and Bloom Syndrome Helicases. J. Biol. Chem. 2002, 277, 41110–41119. [Google Scholar] [CrossRef]
- Deng, Z.; Dheekollu, J.; Broccoli, D.; Dutta, A.; Lieberman, P.M. The Origin Recognition Complex Localizes to Telomere Repeats and Prevents Telomere-Circle Formation. Curr. Biol. 2007, 17, 1989–1995. [Google Scholar] [CrossRef]
- Drosopoulos, W.C.; Deng, Z.; Twayana, S.; Kosiyatrakul, S.T.; Vladimirova, O.; Lieberman, P.M.; Schildkraut, C.L. TRF2 Mediates Replication Initiation within Human Telomeres to Prevent Telomere Dysfunction. Cell Rep. 2020, 33, 108379. [Google Scholar] [CrossRef]
- Kurth, I.; Gautier, J. Origin-dependent initiation of DNA replication within telomeric sequences. Nucleic Acids Res. 2009, 38, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, Y.; Ezura, K.; Yoshida, K.; Yugawa, T.; Narisawa-Saito, M.; Kiyono, T.; Ohta, S.; Obuse, C.; Fujita, M. Involvement of human ORC and TRF2 in pre-replication complex assembly at telomeres. Genes Cells 2008, 13, 1045–1059. [Google Scholar] [CrossRef]
- Bauwens, S.; Lototska, L.; Koundrioukoff, S.; Debatisse, M.; Ye, J.; Gilson, E.; Mendez-Bermudez, A. The Telomeric Protein TRF2 Regulates Replication Origin Activity within Pericentromeric Heterochromatin. Life 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Renault, V.M.; Jamet, K.; Gilson, E. Transcriptional outcome of telomere signalling. Nat. Rev. Genet. 2014, 15, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Robin, J.D.; Burbano, M.J.; Peng, H.; Croce, O.; Thomas, J.L.; Laberthonniere, C.; Renault, V.; Lototska, L.; Pousse, M.; Tessier, F.; et al. Mitochondrial function in skeletal myofibers is controlled by a TRF2-SIRT3 axis over lifetime. Aging Cell 2020, 19, e13097. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Sharma, S.; Bagri, S.; Kutum, R.; Kumar, P.; Hussain, A.; Singh, P.; Saha, D.; Kar, A.; Dash, D.; et al. Telomere repeat–binding factor 2 binds extensively to extra-telomeric G-quadruplexes and regulates the epigenetic status of several gene promoters. J. Biol. Chem. 2019, 294, 17709–17722. [Google Scholar] [CrossRef] [PubMed]
- Biroccio, A.; Cherfils-Vicini, J.; Augereau, A.; Pinte, S.; Bauwens, S.; Ye, J.; Simonet, T.; Horard, B.; Jamet, K.; Cervera, L.; et al. TRF2 inhibits a cell-extrinsic pathway through which natural killer cells eliminate cancer cells. Nat. Cell Biol. 2013, 15, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Cherfils-Vicini, J.; Iltis, C.; Cervera, L.; Pisano, S.; Croce, O.; Sadouni, N.; Győrffy, B.; Collet, R.; Renault, V.M.; Rey-Millet, M.; et al. Cancer cells induce immune escape via glycocalyx changes controlled by the telomeric protein TRF 2. EMBO J. 2019, 38. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.; Thanasoula, M.; Carlos, A.R.; López, G.G.; Tejera, A.M.; Schoeftner, S.; Dominguez, O.; Pisano, D.; Tarsounas, M.; Blasco, M.A. Mammalian Rap1 controls telomere function and gene expression through binding to telomeric and extratelomeric sites. Nat. Cell Biol. 2010, 12, 768–780. [Google Scholar] [CrossRef]
- Martínez, P.; López, G.G.; García, F.; Mercken, E.; Mitchell, S.; Flores, J.M.; de Cabo, R.; Blasco, M.A. RAP1 Protects from Obesity through Its Extratelomeric Role Regulating Gene Expression. Cell Rep. 2013, 3, 2059–2074. [Google Scholar] [CrossRef]
- Bae, N.S.; Baumann, P. A RAP1/TRF2 Complex Inhibits Nonhomologous End-Joining at Human Telomeric DNA Ends. Mol. Cell 2007, 26, 323–334. [Google Scholar] [CrossRef]
- Bombarde, O.; Boby, C.; Gomez, D.; Frit, P.; Giraud-Panis, M.-J.; Gilson, E.; Salles, B.; Calsou, P. TRF2/RAP1 and DNA–PK mediate a double protection against joining at telomeric ends. EMBO J. 2010, 29, 1573–1584. [Google Scholar] [CrossRef]
- Sarthy, J.; Bae, N.S.; Scrafford, J.; Baumann, P. Human RAP1 inhibits non-homologous end joining at telomeres. EMBO J. 2009, 28, 3390–3399. [Google Scholar] [CrossRef]
- Jiang, W.-Q.; Zhong, Z.-H.; Henson, J.; Reddel, R. Identification of candidate alternative lengthening of telomeres genes by methionine restriction and RNA interference. Oncogene 2007, 26, 4635–4647. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Li, F.; He, Q.; Deng, T.; Xu, J.; Jin, F.; Coarfa, C.; Putluri, N.; Liu, D.; Songyang, Z. Systematic analysis of human telomeric dysfunction using inducible telosome/Shelterin CRISPR/Cas9 knockout cells. Cell Discov. 2017, 3, 17034. [Google Scholar] [CrossRef]
- Sfeir, A.; Kabir, S.; Van Overbeek, M.; Celli, G.B.; De Lange, T. Loss of Rap1 Induces Telomere Recombination in the Absence of NHEJ or a DNA Damage Signal. Science 2010, 327, 1657–1661. [Google Scholar] [CrossRef]
- Yeung, F.; Ramírez, C.; Mateos-Gomez, P.A.; Pinzaru, A.; Ceccarini, G.; Kabir, S.; Fernández-Hernando, C.; Sfeir, A. Nontelomeric Role for Rap1 in Regulating Metabolism and Protecting against Obesity. Cell Rep. 2013, 3, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Teo, H.; Ghosh, S.; Luesch, H.; Ghosh, A.; Wong, E.T.; Malik, N.; Orth, A.; De Jesus, P.; Perry, A.; Oliver, J.D.; et al. Telomere-independent Rap1 is an IKK adaptor and regulates NF-κB-dependent gene expression. Nat. Cell Biol. 2010, 12, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; López, G.G.; Pisano, D.G.; Flores, J.M.; Blasco, M.A. A genetic interaction between RAP1 and Telomerase reveals an unanticipated role for RAP1 in telomere maintenance. Aging Cell 2016, 15, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Oestreich, S.; de Lange, T. Identification of Human Rap1: Implications for Telomere Evolution. Cell 2000, 101, 471–483. [Google Scholar] [CrossRef]
- Chen, Y.; Rai, R.; Zhou, Z.-R.; Kanoh, J.; Ribeyre, C.; Yang, Y.; Zheng, H.; Damay, P.; Wang, F.; Tsujii, H.; et al. A conserved motif within RAP1 has diversified roles in telomere protection and regulation in different organisms. Nat. Struct. Mol. Biol. 2011, 18, 213–221. [Google Scholar] [CrossRef]
- Gaullier, G.; Miron, S.; Pisano, S.; Buisson, R.; Le Bihan, Y.-V.; Tellier-Lebègue, C.; Messaoud, W.; Roblin, P.; Guimarães, B.G.; Thai, R.; et al. A higher-order entity formed by the flexible assembly of RAP1 with TRF2. Nucleic Acids Res. 2016, 44, 1962–1976. [Google Scholar] [CrossRef]
- Kaminker, P.G.; Kim, S.H.; Desprez, P.Y.; Campisi, J. A novel form of the telomere-associated protein TIN2 localizes to the nuclear matrix. Cell Cycle 2009, 8, 931–939. [Google Scholar] [CrossRef]
- Nelson, N.D.; Dodson, L.; Escudero, L.; Sukumar, A.T.; Williams, C.; Mihalek, I.; Baldan, A.; Baird, D.; Bertuch, A.A. The C-Terminal Extension Unique to the Long Isoform of the Shelterin Component TIN2 Enhances Its Interaction with TRF2 in a Phosphorylation- and Dyskeratosis Congenita Cluster-Dependent Fashion. Mol. Cell. Biol. 2018, 38, e00025-18. [Google Scholar] [CrossRef]
- Ye, J.Z.-S.; De Lange, T. TIN2 is a tankyrase 1 PARP modulator in the TRF1 telomere length control complex. Nat. Genet. 2004, 36, 618–623. [Google Scholar] [CrossRef]
- Ye, J.Z.-S.; Donigian, J.R.; van Overbeek, M.; Loayza, D.; Luo, Y.; Krutchinsky, A.N.; Chait, B.T.; de Lange, T. TIN2 Binds TRF1 and TRF2 Simultaneously and Stabilizes the TRF2 Complex on Telomeres. J. Biol. Chem. 2004, 279, 47264–47271. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, W.; Yang, Y.; Chen, Y.; Yang, X.; Diehl, J.A.; Liu, X.; Lei, M. Structural Basis of Selective Ubiquitination of TRF1 by SCFFbx4. Dev. Cell 2010, 18, 214–225. [Google Scholar] [CrossRef]
- Canudas, S.; Houghtaling, B.R.; Bhanot, M.; Sasa, G.; Savage, S.; Bertuch, A.A.; Smith, S. A role for heterochromatin protein 1 at human telomeres. Genes Dev. 2011, 25, 1807–1819. [Google Scholar] [CrossRef]
- Abreu, E.; Aritonovska, E.; Reichenbach, P.; Cristofari, G.; Culp, B.; Terns, R.M.; Lingner, J.; Terns, M.P. TIN2-Tethered TPP1 Recruits Human Telomerase to Telomeres In Vivo. Mol. Cell. Biol. 2010, 30, 2971–2982. [Google Scholar] [CrossRef]
- Frescas, D.; De Lange, T. A TIN2 dyskeratosis congenita mutation causes Telomerase-independent telomere shortening in mice. Genes Dev. 2014, 28, 153–166. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Zhang, Y.; Zhang, Q.; Li, H.; Luo, Z.; Fang, H.; Kim, S.H.; Qin, L.; Yotnda, P.; Xu, J.; et al. Mitochondrial Localization of Telomeric Protein TIN2 Links Telomere Regulation to Metabolic Control. Mol. Cell 2012, 47, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Giannone, R.J.; McDonald, W.H.; Hurst, G.B.; Huang, Y.; Wu, J.; Liu, Y.; Wang, Y. Dual-tagging system for the affinity purification of mammalian protein complexes. Biotechniques 2007, 43, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Davalos, A.R.; Heo, S.-J.; Rodier, F.; Zou, Y.; Beausejour, C.; Kaminker, P.; Yannone, S.M.; Campisi, J. Telomere dysfunction and cell survival: Roles for distinct TIN2-containing complexes. J. Cell Biol. 2008, 181, 447–460. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.S.; Safari, A.; Xin, H.; Liu, D.; Songyang, Z. A critical role for TPP1 and TIN2 interaction in high-order telomeric complex assembly. Proc. Natl. Acad. Sci. 2006, 103, 11874–11879. [Google Scholar] [CrossRef]
- Erdel, F.; Kratz, K.; Willcox, S.; Griffith, J.D.; Greene, E.C.; de Lange, T. Telomere Recognition and Assembly Mechanism of Mammalian Shelterin. Cell Rep. 2017, 18, 41–53. [Google Scholar] [CrossRef]
- Lim, C.J.; Zaug, A.J.; Kim, H.J.; Cech, T.R. Reconstitution of human Shelterin complexes reveals unexpected stoichiometry and dual pathways to enhance Telomerase processivity. Nat. Commun. 2017, 8, 1075. [Google Scholar] [CrossRef]
- Frescas, D.; de Lange, T. TRF2-Tethered TIN2 Can Mediate Telomere Protection by TPP1/POT1. Mol. Cell. Biol. 2014, 34, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.K.; Hooper, S.; Blackwood, S.; Gandhi, R.; de Lange, T. In Vivo Stoichiometry of Shelterin Components. J. Biol. Chem. 2010, 285, 1457–1467. [Google Scholar] [CrossRef]
- Mattern, K.A.; Swiggers, S.J.J.; Nigg, A.L.; Löwenberg, B.; Houtsmuller, A.B.; Ziljmans, J.M.J.M. Dynamics of protein binding to telomeres in living cells: Implications for telomere structure and function. Mol. Cell. Biol. 2004, 12, 5587. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, I.; Fujita, K.; Harris, C.C. p53 governs telomere regulation feedback too, via TRF2. Aging 2011, 3, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Horikawa, I.; Mondal, A.M.; Jenkins, L.M.M.; Appella, E.; Vojtesek, B.; Bourdon, J.-C.; Lane, D.; Harris, C.C. Positive feedback between p53 and TRF2 during telomere-damage signalling and cellular senescence. Nat. Cell Biol. 2010, 12, 1205–1212. [Google Scholar] [CrossRef]
- Wagner, K.-D.; Ying, Y.; Leong, W.; Jiang, J.; Hu, X.; Chen, Y.; Michiels, J.-F.; Lu, Y.; Gilson, E.; Wagner, N.; et al. The differential spatiotemporal expression pattern of Shelterin genes throughout lifespan. Aging 2017, 9, 1219–1232. [Google Scholar] [CrossRef]
- Pirzada, R.H.; Orun, O.; Erzik, C.; Cagsin, H.; Serakinci, N. Role of TRF2 and TPP1 regulation in idiopathic recurrent pregnancy loss. Int. J. Biol. Macromol. 2019, 127, 306–310. [Google Scholar] [CrossRef]
- Frescas, D.; de Lange, T. Binding of TPP1 Protein to TIN2 Protein Is Required for POT1a,b Protein-mediated Telomere Protection. J. Biol. Chem. 2014, 289, 24180–24187. [Google Scholar] [CrossRef]
- Kim, J.-K.; Liu, J.; Hu, X.; Yu, C.; Roskamp, K.; Sankaran, B.; Huang, L.; Komives, E.A.; Qiao, F. Structural Basis for Shelterin Bridge Assembly. Mol. Cell 2017, 68, 698–714.e5. [Google Scholar] [CrossRef] [PubMed]
- Pisano, S.; Gilson, E.; Giraud-Panis, M.-J. Dynamics under the Telomeric Bridge. Mol. Cell 2017, 68, 643–644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Houghtaling, B.R.; Cuttonaro, L.; Chang, W.; Smith, S. A Dynamic Molecular Link between the Telomere Length Regulator TRF1 and the Chromosome End Protector TRF2. Curr. Biol. 2004, 14, 1621–1631. [Google Scholar] [CrossRef]
- Azarm, K.; Bhardwaj, A.; Kim, E.; Smith, S. Persistent telomere cohesion protects aged cells from premature senescence. Nat. Commun. 2020, 11, 3321. [Google Scholar] [CrossRef]
- Arat, N.Ö.; Griffith, J.D. Human Rap1 Interacts Directly with Telomeric DNA and Regulates TRF2 Localization at the Telomere. J. Biol. Chem. 2012, 287, 41583–41594. [Google Scholar] [CrossRef]
- Giannone, R.J.; McDonald, H.W.; Hurst, G.B.; Shen, R.-F.; Wang, Y.; Liu, Y. The Protein Network Surrounding the Human Telomere Repeat Binding Factors TRF1, TRF2, and POT1. PLoS ONE 2010, 5, e12407. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Kim, H.; Ji, Z.; Zhang, T.; Chen, B.; Ge, Y.; Hu, Y.; Feng, X.; Han, X.; Xu, H.; et al. The BUB3-BUB1 Complex Promotes Telomere DNA Replication. Mol. Cell 2018, 70, 395–407.e4. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghilain, C.; Gilson, E.; Giraud-Panis, M.-J. Multifunctionality of the Telomere-Capping Shelterin Complex Explained by Variations in Its Protein Composition. Cells 2021, 10, 1753. https://doi.org/10.3390/cells10071753
Ghilain C, Gilson E, Giraud-Panis M-J. Multifunctionality of the Telomere-Capping Shelterin Complex Explained by Variations in Its Protein Composition. Cells. 2021; 10(7):1753. https://doi.org/10.3390/cells10071753
Chicago/Turabian StyleGhilain, Claire, Eric Gilson, and Marie-Josèphe Giraud-Panis. 2021. "Multifunctionality of the Telomere-Capping Shelterin Complex Explained by Variations in Its Protein Composition" Cells 10, no. 7: 1753. https://doi.org/10.3390/cells10071753
APA StyleGhilain, C., Gilson, E., & Giraud-Panis, M.-J. (2021). Multifunctionality of the Telomere-Capping Shelterin Complex Explained by Variations in Its Protein Composition. Cells, 10(7), 1753. https://doi.org/10.3390/cells10071753




