RGS5 Attenuates Baseline Activity of ERK1/2 and Promotes Growth Arrest of Vascular Smooth Muscle Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Generation of Inducible SMC-Specific Rgs5-Knockout Mice
2.3. Telemetric Blood Pressure Measurement
2.4. Cell Culture
2.5. siRNA-Based Gene Silencing
2.6. Adenoviral Transfection
2.7. Generation of 3D Spheroids
2.8. 3D Migration Assay
2.9. Microarray-Based Transcriptome Analysis
2.10. Quantitative Real Time RT-PCR (qPCR) Analysis
2.11. Electrophoresis-Based Protein Analyses
2.12. Human Phosphokinase Array
2.13. Whole Mount Immunofluorescence Analysis
2.14. Measurement of Intracellular Calcium Mobilization
2.15. RhoA Activity Assay
2.16. Immunofluorescence-Based Detection of Ki67
2.17. Statistical Analysis
3. Results
3.1. Regulatory Features of RGS5
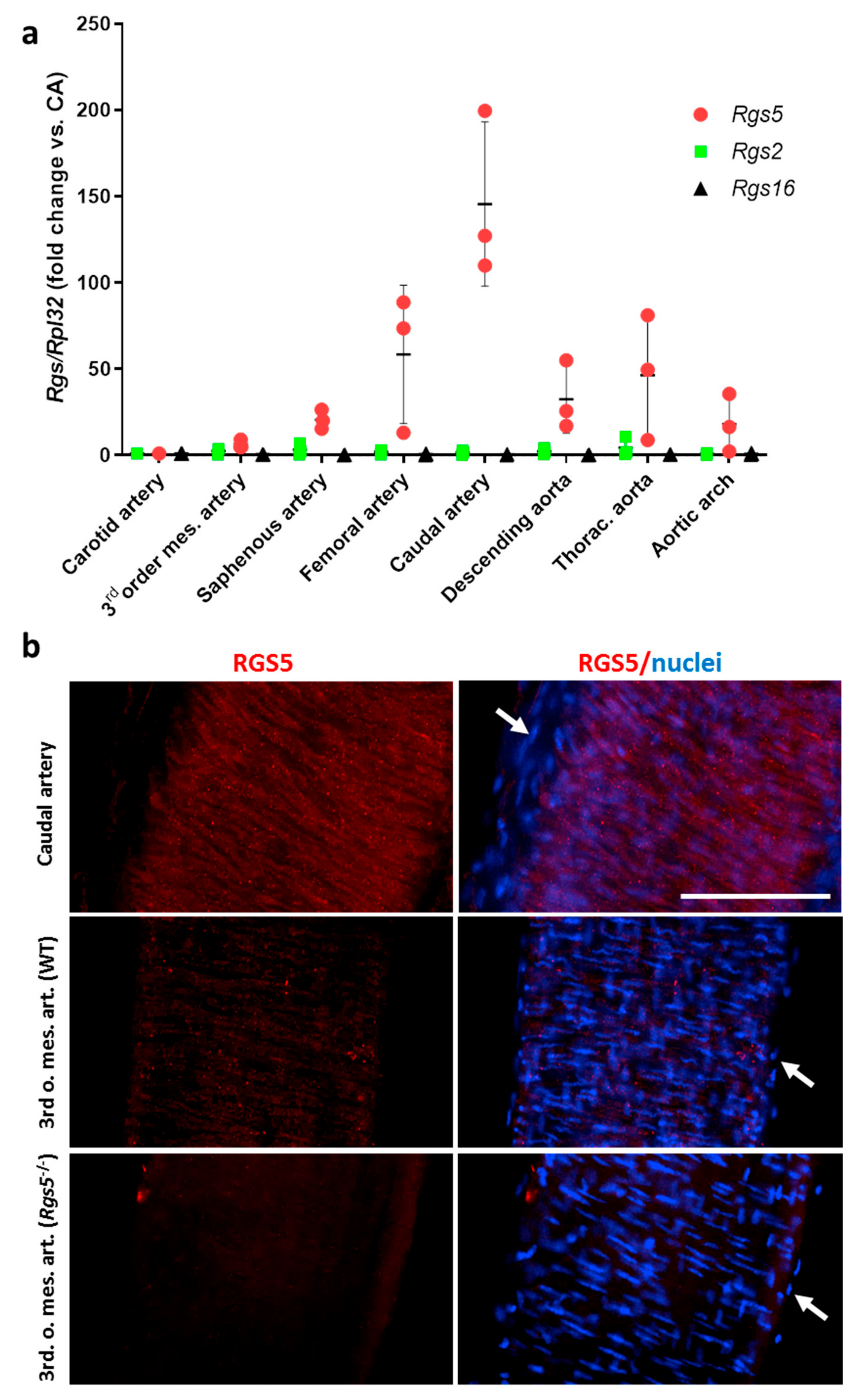
3.1.1. Heterogeneous Artery Type-Specific Expression of Rgs5 in Mice
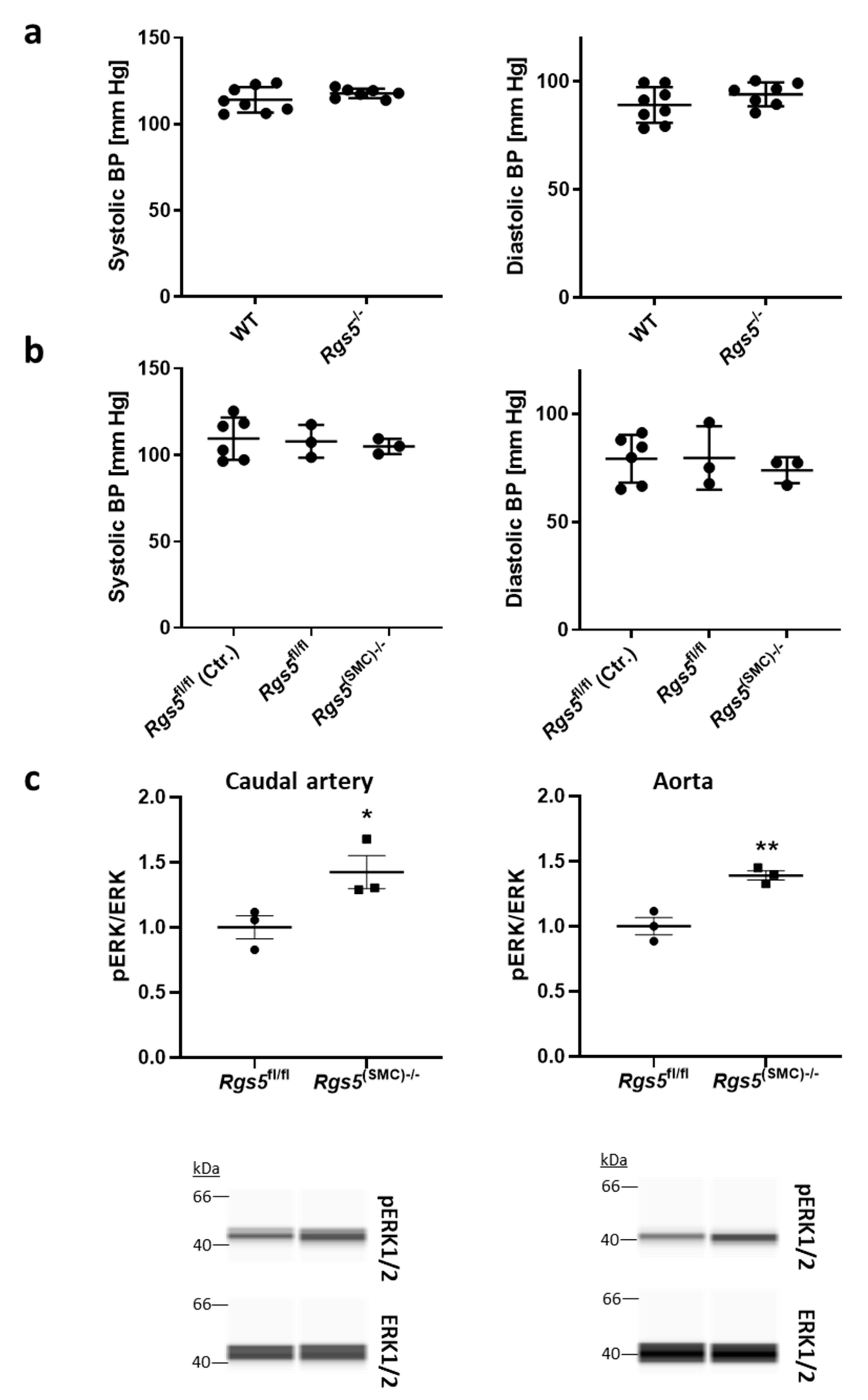
3.1.2. Ablation of Rgs5 Does Not Alter the Systolic and Diastolic Blood Pressure Values but Stimulates ERK1/2 Phosphorylation
3.1.3. High Levels of RGS5 Are Maintained in Mouse and Human VSMCs under 3D Culture Conditions In Vitro
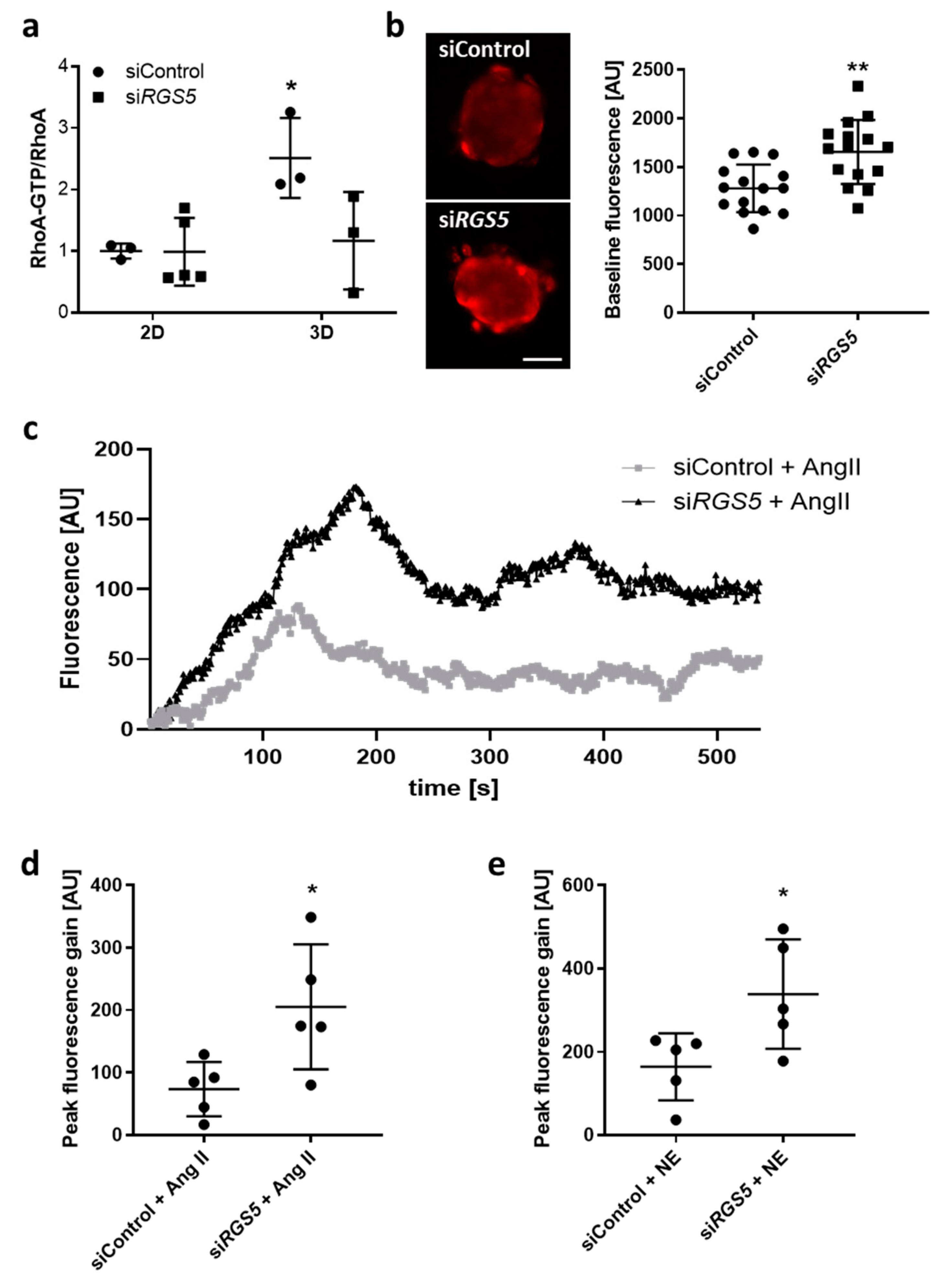
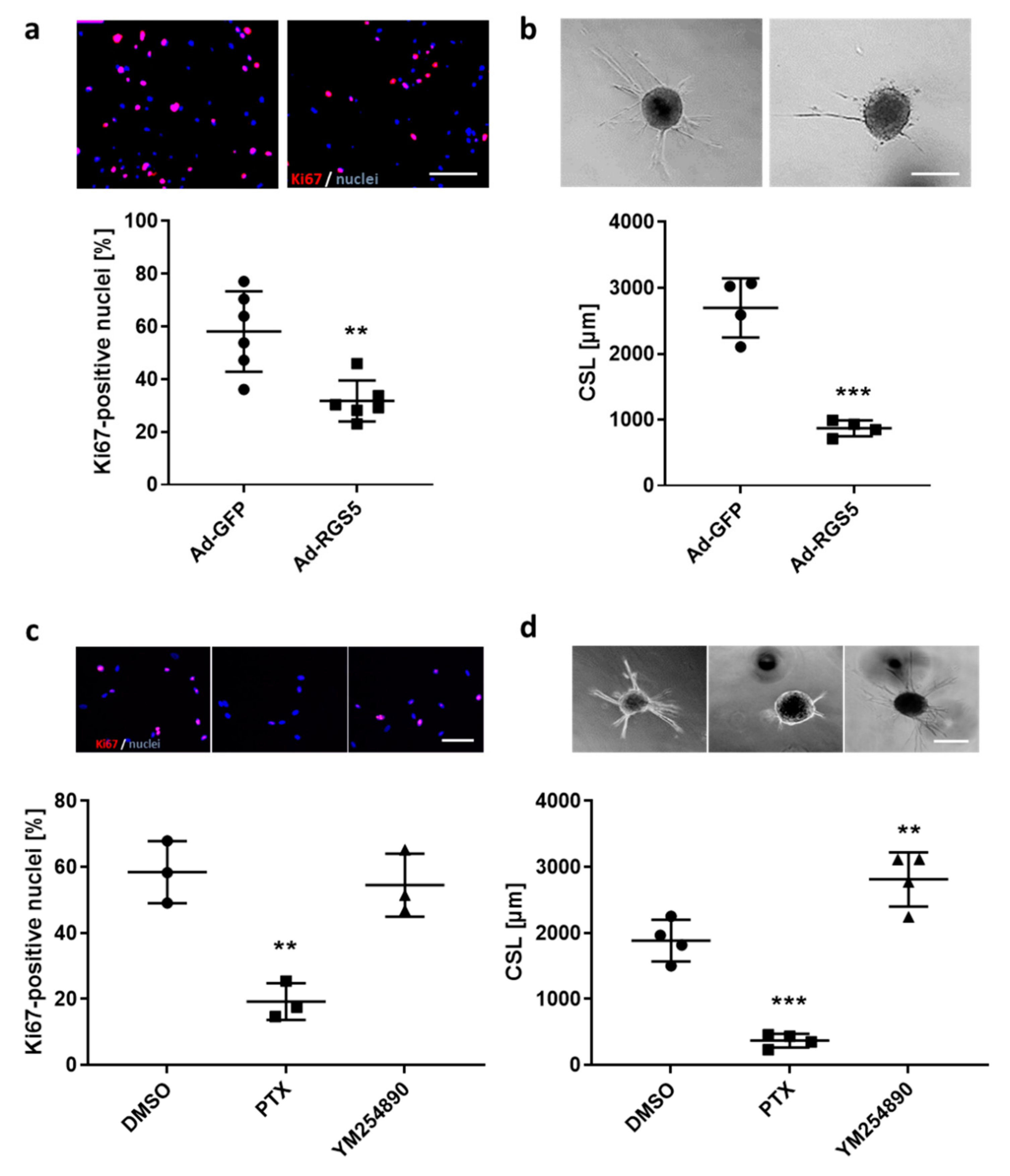
3.1.4. Knockdown of RGS5 in Human VSMC Spheroids Inhibits RhoA Activity and Augments Agonist-Induced Calcium Release
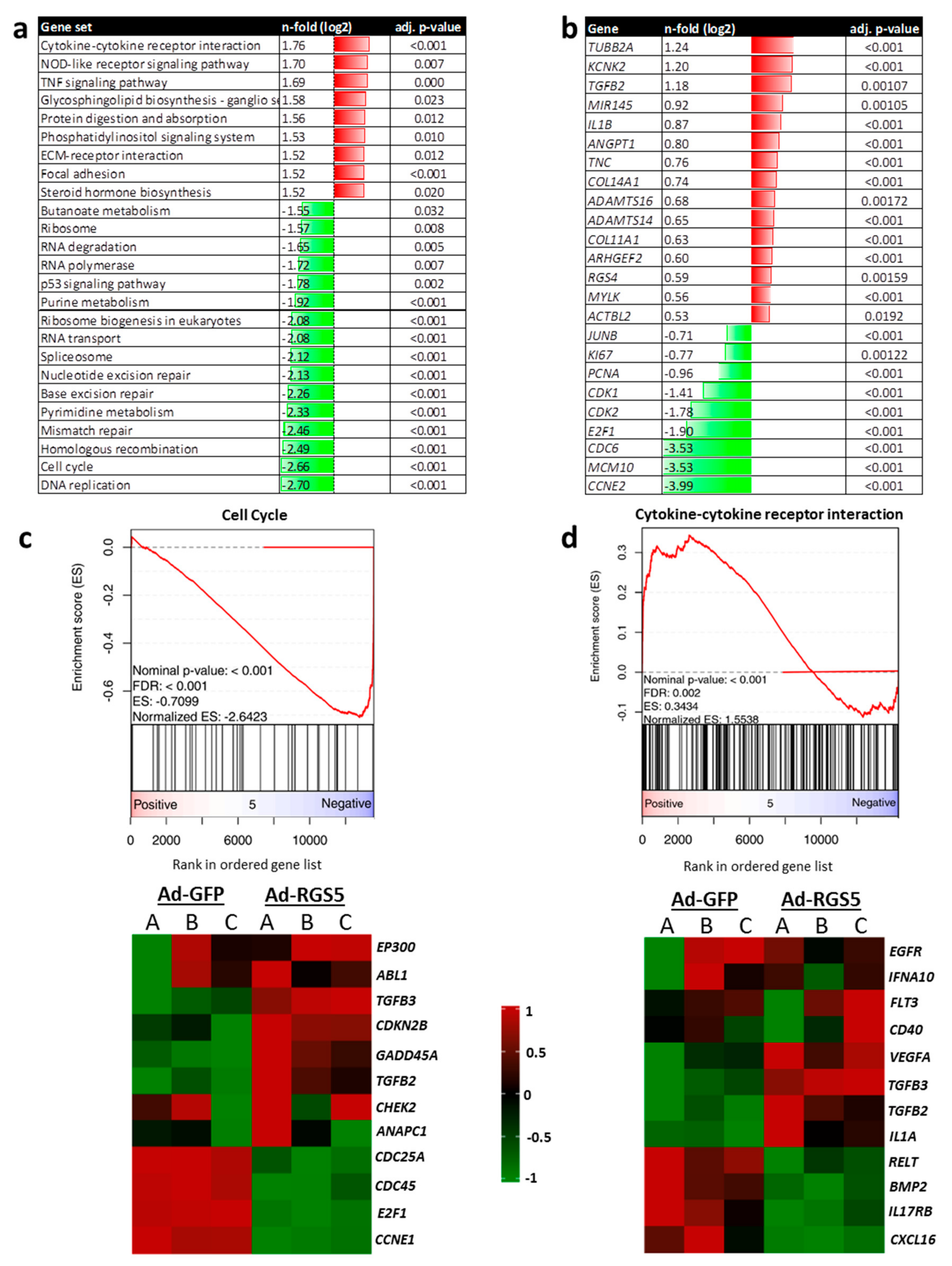
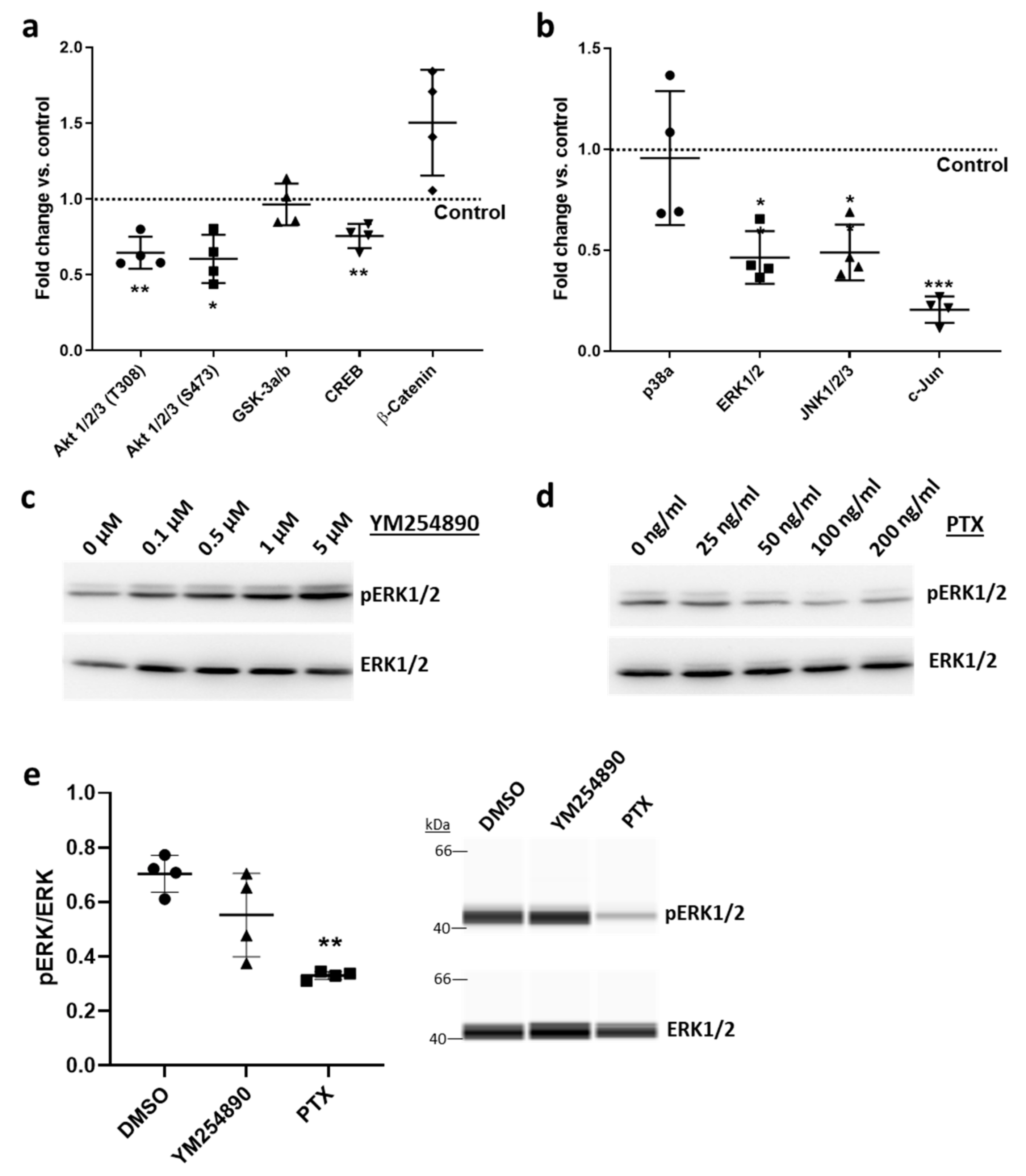
3.1.5. Overexpression of Rgs5 and Inhibition of Gαi/o Signaling Inhibits ERK1/2 Activity, Proliferation and Migration of Human VSMCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Adenylate cyclase |
| AngII | Angiotensin II |
| cAMP | Cyclic adenosine monophosphate |
| DOCA | Deoxycorticosterone acetate |
| ERK | Extracellular-signal regulated kinase |
| FSK | Forskolin |
| GAP | GTPase activating protein |
| GDP | Guanosine-5′-diphosphate |
| GPCR | G protein coupled receptor |
| GSE(A) | Gene set enrichment analysis |
| GTP | Guanosine-5′-triphosphate |
| HUASMC | Human umbilical artery smooth muscle cell |
| MAP | Mitogen activated kinase |
| NE | Norepinephrine |
| PCNA | Proliferating-Cell-Nuclear-Antigen |
| PKC/A | Protein kinase C/A |
| PLC | Phospholipase C |
| PTX | Pertussis Toxin |
| RGS | Regulator of G-protein signaling |
| Rpl32 | Ribosomal Protein L32 |
| SM-MHC | Smooth muscle-myosin heavy chain |
| VCP | Valosin containing protein |
| VSMC | Vascular smooth muscle cell |
| WT | Wild type |
| αSMA | alpha smooth muscle actin |
References
- Althoff, T.F.; Juarez, J.A.; Troidl, K.; Tang, C.; Wang, S.; Wirth, A.; Takefuji, M.; Wettschureck, N.; Offermanns, S. Procontractile G protein-mediated signaling pathways antagonistically regulate smooth muscle differentiation in vascular remodeling. J. Exp. Med. 2012, 209, 2277–2290. [Google Scholar] [CrossRef] [Green Version]
- Offermanns, S. G-proteins as transducers in transmembrane signalling. Prog. Biophys. Mol. Biol. 2003, 83, 101–130. [Google Scholar] [CrossRef]
- Gutkind, J.S. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J. Biol. Chem. 1998, 273, 1839–1842. [Google Scholar] [CrossRef] [Green Version]
- Grundmann, M.; Merten, N.; Malfacini, D.; Inoue, A.; Preis, P.; Simon, K.; Ruttiger, N.; Ziegler, N.; Benkel, T.; Schmitt, N.K.; et al. Lack of beta-arrestin signaling in the absence of active G proteins. Nat. Commun. 2018, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Khokhlatchev, A.V.; Canagarajah, B.; Wilsbacher, J.; Robinson, M.; Atkinson, M.; Goldsmith, E.; Cobb, M.H. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 1998, 93, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Brunet, A.; Roux, D.; Lenormand, P.; Dowd, S.; Keyse, S.; Pouyssegur, J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999, 18, 664–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespo, P.; Xu, N.; Simonds, W.F.; Gutkind, J.S. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature 1994, 369, 418–420. [Google Scholar] [CrossRef]
- Goldsmith, Z.G.; Dhanasekaran, D.N. G protein regulation of MAPK networks. Oncogene 2007, 26, 3122–3142. [Google Scholar] [CrossRef] [Green Version]
- Wieland, T.; Mittmann, C. Regulators of G-protein signalling: Multifunctional proteins with impact on signalling in the cardiovascular system. Pharmacol. Ther. 2003, 97, 95–115. [Google Scholar] [CrossRef]
- Wieland, T.; Lutz, S.; Chidiac, P. Regulators of G protein signalling: A spotlight on emerging functions in the cardiovascular system. Curr. Opin. Pharmacol. 2007, 7, 201–207. [Google Scholar] [CrossRef]
- Ross, E.M.; Wilkie, T.M. GTPase-activating proteins for heterotrimeric G proteins: Regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 2000, 69, 795–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, D.M.; Wilkie, T.M.; Gilman, A.G. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell 1996, 86, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Cooper, P.R.; Damera, G.; Mukhopadhyay, I.; Cho, H.; Kehrl, J.H.; Panettieri, R.A., Jr.; Druey, K.M. Beta-agonist-associated reduction in RGS5 expression promotes airway smooth muscle hyper-responsiveness. J. Biol. Chem. 2011, 286, 11444–11455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Huang, X.; Zhong, H.; Mortensen, R.M.; D’Alecy, L.G.; Neubig, R.R. Endogenous RGS proteins and Galpha subtypes differentially control muscarinic and adenosine-mediated chronotropic effects. Circ. Res. 2006, 98, 659–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heximer, S.P.; Knutsen, R.H.; Sun, X.; Kaltenbronn, K.M.; Rhee, M.H.; Peng, N.; Oliveira-dos-Santos, A.; Penninger, J.M.; Muslin, A.J.; Steinberg, T.H.; et al. Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J. Clin. Investig. 2003, 111, 1259. [Google Scholar]
- Cho, H.; Park, C.; Hwang, I.Y.; Han, S.B.; Schimel, D.; Despres, D.; Kehrl, J.H. Rgs5 targeting leads to chronic low blood pressure and a lean body habitus. Mol. Cell Biol. 2008, 28, 2590–2597. [Google Scholar] [CrossRef] [Green Version]
- Arnold, C.; Feldner, A.; Pfisterer, L.; Hodebeck, M.; Troidl, K.; Genove, G.; Wieland, T.; Hecker, M.; Korff, T. RGS5 promotes arterial growth during arteriogenesis. EMBO Mol. Med. 2014, 6, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Holobotovskyy, V.; Manzur, M.; Tare, M.; Burchell, J.; Bolitho, E.; Viola, H.; Hool, L.C.; Arnolda, L.F.; McKitrick, D.J.; Ganss, R. Regulator of G-protein signaling 5 controls blood pressure homeostasis and vessel wall remodeling. Circ. Res. 2013, 112, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Holobotovskyy, V.; Chong, Y.S.; Burchell, J.; He, B.; Phillips, M.; Leader, L.; Murphy, T.V.; Sandow, S.L.; McKitrick, D.J.; Charles, A.K.; et al. Regulator of G protein signaling 5 is a determinant of gestational hypertension and preeclampsia. Sci. Transl. Med. 2015, 7, 290ra288. [Google Scholar] [CrossRef] [Green Version]
- Daniel, J.M.; Prock, A.; Dutzmann, J.; Sonnenschein, K.; Thum, T.; Bauersachs, J.; Sedding, D.G. Regulator of G-Protein Signaling 5 Prevents Smooth Muscle Cell Proliferation and Attenuates Neointima Formation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Arnold, C.; Demirel, E.; Feldner, A.; Genove, G.; Zhang, H.; Sticht, C.; Wieland, T.; Hecker, M.; Heximer, S.; Korff, T. Hypertension-evoked RhoA activity in vascular smooth muscle cells requires RGS5. FASEB J. 2018, 32, 2021–2035. [Google Scholar] [CrossRef] [Green Version]
- Mills, P.A.; Huetteman, D.A.; Brockway, B.P.; Zwiers, L.M.; Gelsema, A.J.; Schwartz, R.S.; Kramer, K. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J. Appl. Physiol. 2000, 88, 1537–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, M.; Wang, P.; Boyd, A.D.; Kostov, G.; Athey, B.; Jones, E.G.; Bunney, W.E.; Myers, R.M.; Speed, T.P.; Akil, H.; et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 2005, 33, e175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Gu, S.; Al-Sabeq, B.; Wang, S.; He, J.; Tam, A.; Cifelli, C.; Mathalone, N.; Tirgari, S.; Boyd, S.; et al. Origin-specific epigenetic program correlates with vascular bed-specific differences in Rgs5 expression. FASEB J. 2012, 26, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, C.; Feng, J.; Zhang, Y.; Tang, Q.; Bian, Z.; Bai, X.; Zhou, H.; Jiang, H.; Heximer, S.P.; et al. Regulator of G protein signaling 5 protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Proc. Natl. Acad. Sci. USA 2010, 107, 13818–13823. [Google Scholar] [CrossRef] [Green Version]
- Jager, M.A.; De La Torre, C.; Arnold, C.; Kohlhaas, J.; Kappert, L.; Hecker, M.; Feldner, A.; Korff, T. Assembly of vascular smooth muscle cells in 3D aggregates provokes cellular quiescence. Exp. Cell Res. 2020, 388, 111782. [Google Scholar] [CrossRef] [PubMed]
- Mack, C.P.; Somlyo, A.V.; Hautmann, M.; Somlyo, A.P.; Owens, G.K. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J. Biol. Chem. 2001, 276, 341–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiedmann, F.; Rinne, S.; Donner, B.; Decher, N.; Katus, H.A.; Schmidt, C. Mechanosensitive TREK-1 two-pore-domain potassium (K2P) channels in the cardiovascular system. Prog. Biophys. Mol. Biol. 2021, 159, 126–135. [Google Scholar] [CrossRef]
- Cordes, K.R.; Sheehy, N.T.; White, M.P.; Berry, E.C.; Morton, S.U.; Muth, A.N.; Lee, T.H.; Miano, J.M.; Ivey, K.N.; Srivastava, D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009, 460, 705–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, H.; Carvalho, J.; Looso, M.; Singh, P.; Chennupati, R.; Preussner, J.; Gunther, S.; Albarran-Juarez, J.; Tischner, D.; Classen, S.; et al. Single-cell profiling reveals heterogeneity and functional patterning of GPCR expression in the vascular system. Nat. Commun. 2017, 8, 15700. [Google Scholar] [CrossRef] [Green Version]
- Consortium, G.T. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Vanlandewijck, M.; Mae, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Lavina, B.; Gouveia, L.; et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci. Data 2018, 5, 180160. [Google Scholar] [CrossRef] [Green Version]
- Gunaje, J.J.; Bahrami, A.J.; Schwartz, S.M.; Daum, G.; Mahoney, W.M., Jr. PDGF-dependent regulation of regulator of G protein signaling-5 expression and vascular smooth muscle cell functionality. Am. J. Physiol. Cell Physiol. 2011, 301, C478–C489. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Crawford, M.; Day, R.M.; Briones, V.R.; Leader, J.E.; Jose, P.A.; Lechleider, R.J. RhoA modulates Smad signaling during transforming growth factor-beta-induced smooth muscle differentiation. J. Biol. Chem. 2006, 281, 1765–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsapara, A.; Luthert, P.; Greenwood, J.; Hill, C.S.; Matter, K.; Balda, M.S. The RhoA activator GEF-H1/Lfc is a transforming growth factor-beta target gene and effector that regulates alpha-smooth muscle actin expression and cell migration. Mol. Biol. Cell 2010, 21, 860–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeil, E.M.; Brands, J.; Merten, N.; Vogtle, T.; Vescovo, M.; Rick, U.; Albrecht, I.M.; Heycke, N.; Kawakami, K.; Ono, Y.; et al. Heterotrimeric G Protein Subunit Galphaq Is a Master Switch for Gbetagamma-Mediated Calcium Mobilization by Gi-Coupled GPCRs. Mol. Cell 2020, 80, 940–954.e6. [Google Scholar] [CrossRef]
- Chen, Q.W.; Edvinsson, L.; Xu, C.B. Role of ERK/MAPK in endothelin receptor signaling in human aortic smooth muscle cells. BMC Cell Biol. 2009, 10, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, D.F.; Monia, B.; Dean, N.; Berk, B.C. Protein kinase C-zeta mediates angiotensin II activation of ERK1/2 in vascular smooth muscle cells. J. Biol. Chem. 1997, 272, 6146–6150. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Zhou, H.; Mahavadi, S.; Sriwai, W.; Murthy, K.S. Inhibition of Galphaq-dependent PLC-beta1 activity by PKG and PKA is mediated by phosphorylation of RGS4 and GRK2. Am. J. Physiol. Cell Physiol. 2007, 292, C200–C208. [Google Scholar] [CrossRef]
- Wen, Y.; Anwer, K.; Singh, S.P.; Sanborn, B.M. Protein kinase-A inhibits phospholipase-C activity and alters protein phosphorylation in rat myometrial plasma membranes. Endocrinology 1992, 131, 1377–1382. [Google Scholar] [CrossRef]
- Nalli, A.D.; Kumar, D.P.; Al-Shboul, O.; Mahavadi, S.; Kuemmerle, J.F.; Grider, J.R.; Murthy, K.S. Regulation of Gbetagammai-dependent PLC-beta3 activity in smooth muscle: Inhibitory phosphorylation of PLC-beta3 by PKA and PKG and stimulatory phosphorylation of Galphai-GTPase-activating protein RGS2 by PKG. Cell Biochem. Biophys. 2014, 70, 867–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene Name | Sequence | Annealing Temperature |
|---|---|---|
| mRgs5 | 5′-GCGGAGAAGGCAAAGCAA-3′ 5′-GTGGTCAATGTTCACCTCTTTAGG-3′ | 60 °C |
| mRgs2 | 5′-ATCAAGCCTTCTCCTGAGGAA-3′ 5′-GCCAGCAGTTCATCAAATGC-3′ | |
| mRgs16 | 5′-CCTGGTACTTGCTACTCGCTTTT-3′ 5′-AGCACGTCGTGGAGAGGAT-3′ | |
| mRpl32 | 5′-GGGAGCAACAAGAAAACCAA-3′ 5′-ATTGTGGACCAGGAACTTGC-3′ | |
| hRGS5 | 5′-GGTGGAACCTTCCCTGAGCAGC-3′ 5′-AGAGCGCACAAAGCGAGGCA-3′ | |
| hRPL32 | 5′-GTTCATCCGGCACCAGTCAG-3′ 5′-ACGTGCACATGAGCTGCCTAC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirel, E.; Arnold, C.; Garg, J.; Jäger, M.A.; Sticht, C.; Li, R.; Kuk, H.; Wettschureck, N.; Hecker, M.; Korff, T. RGS5 Attenuates Baseline Activity of ERK1/2 and Promotes Growth Arrest of Vascular Smooth Muscle Cells. Cells 2021, 10, 1748. https://doi.org/10.3390/cells10071748
Demirel E, Arnold C, Garg J, Jäger MA, Sticht C, Li R, Kuk H, Wettschureck N, Hecker M, Korff T. RGS5 Attenuates Baseline Activity of ERK1/2 and Promotes Growth Arrest of Vascular Smooth Muscle Cells. Cells. 2021; 10(7):1748. https://doi.org/10.3390/cells10071748
Chicago/Turabian StyleDemirel, Eda, Caroline Arnold, Jaspal Garg, Marius Andreas Jäger, Carsten Sticht, Rui Li, Hanna Kuk, Nina Wettschureck, Markus Hecker, and Thomas Korff. 2021. "RGS5 Attenuates Baseline Activity of ERK1/2 and Promotes Growth Arrest of Vascular Smooth Muscle Cells" Cells 10, no. 7: 1748. https://doi.org/10.3390/cells10071748
APA StyleDemirel, E., Arnold, C., Garg, J., Jäger, M. A., Sticht, C., Li, R., Kuk, H., Wettschureck, N., Hecker, M., & Korff, T. (2021). RGS5 Attenuates Baseline Activity of ERK1/2 and Promotes Growth Arrest of Vascular Smooth Muscle Cells. Cells, 10(7), 1748. https://doi.org/10.3390/cells10071748






