Review: Vaspin (SERPINA12) Expression and Function in Endocrine Cells
Abstract
:1. Introduction
2. Vaspin Structure and Expression
3. Vaspin Receptor GRP78 and the Mechanism of Vaspin Action
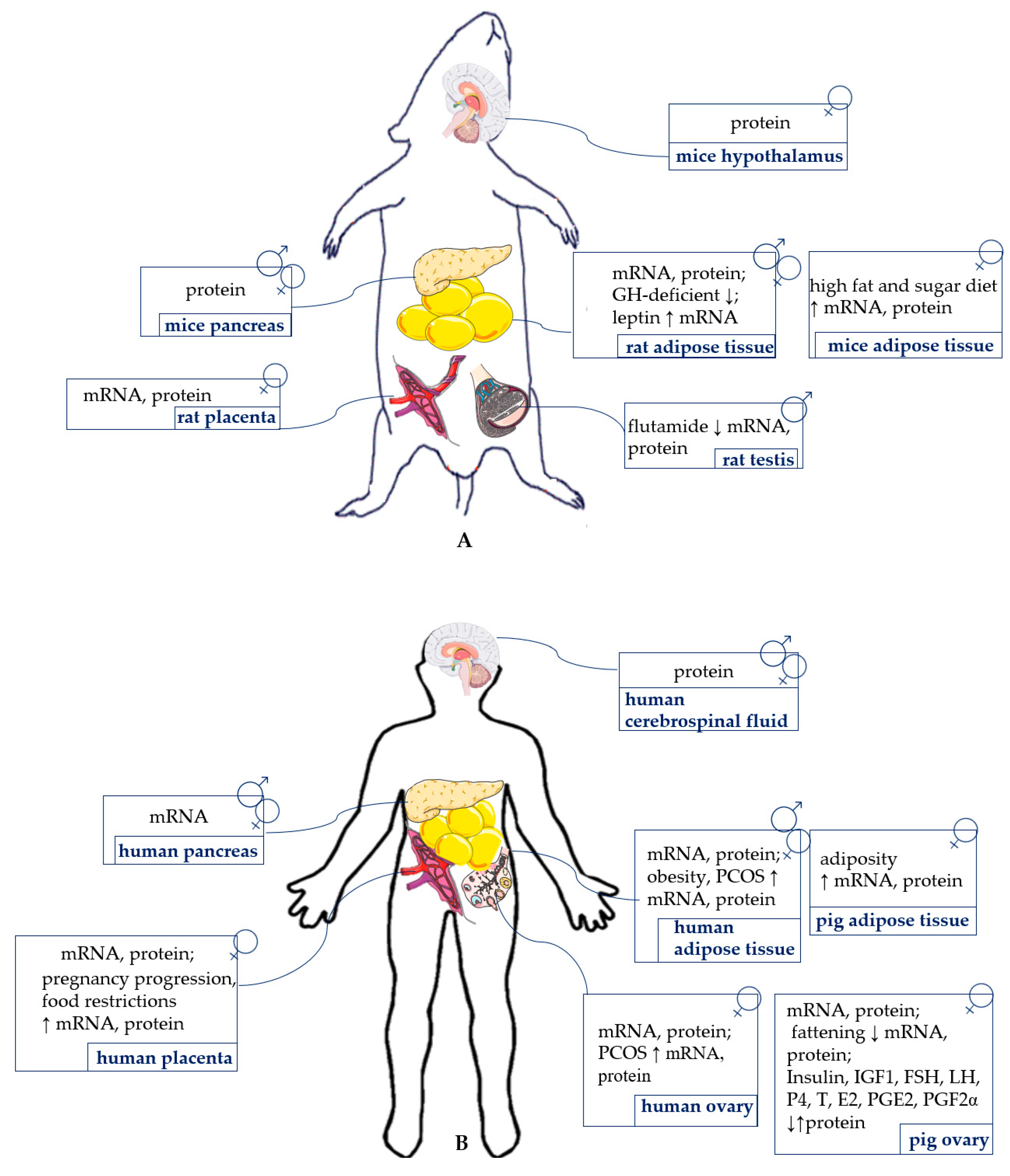
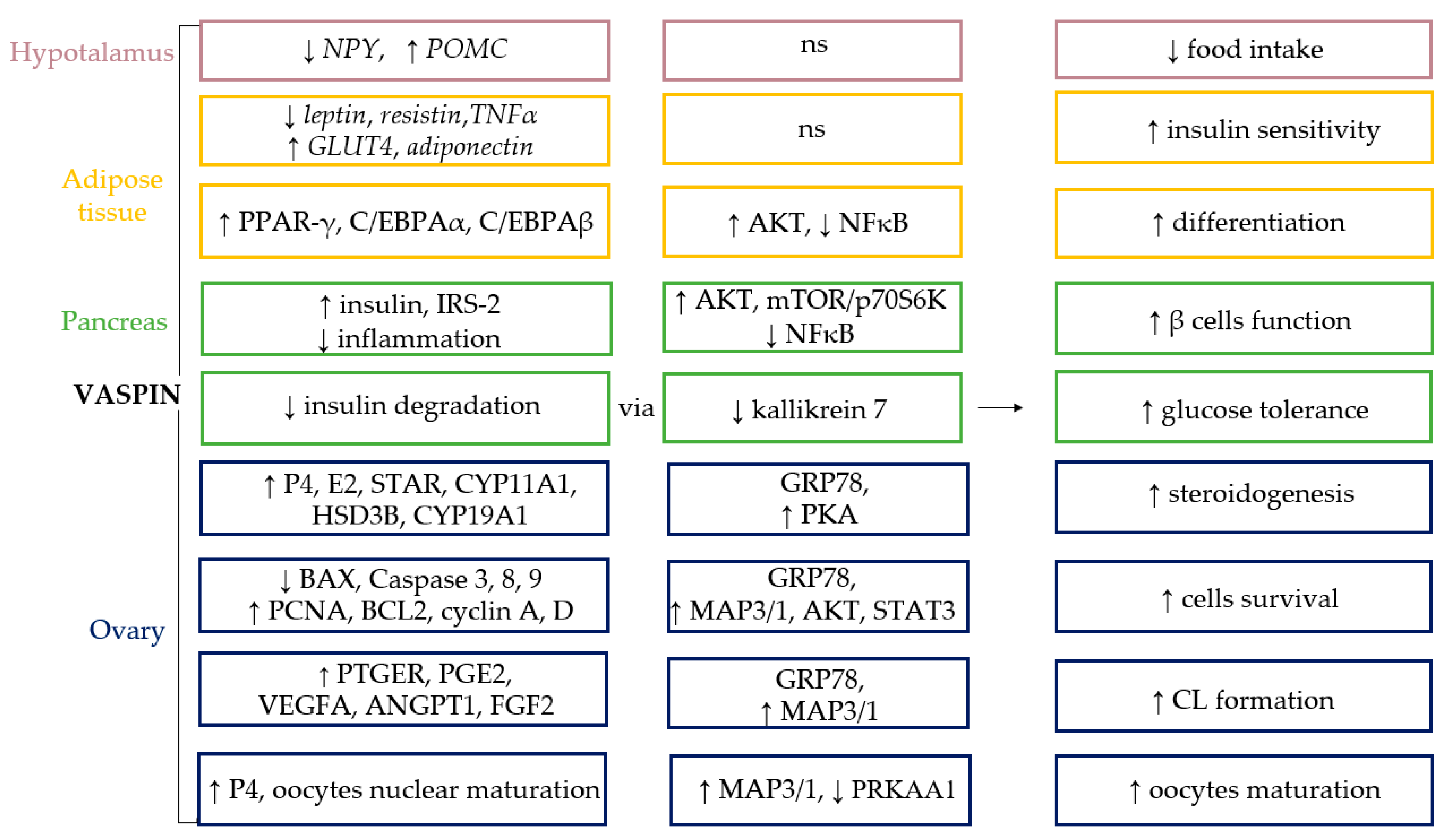
4. Vaspin Expression and Action in the Hypothalamus and Pituitary
5. Vaspin Expression and Action in Adipose Tissue
6. Vaspin Expression and Action in the Pancreas
7. Vaspin Expression and Action in the Thyroid Gland
8. Vaspin Expression and Action in the Ovaries
9. Vaspin Expression and Action in the Placenta
10. Vaspin Expression and Action in the Testes
11. Vaspin Connection with Endocrine Pathology
12. Perspectives
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parent, A.-S.; Naveau, E.; Gerard, A.; Bourguignon, J.-P.; Westbrook, G.L. Early Developmental Actions of Endocrine Disruptors on the Hypothalamus, Hippocampus, and Cerebral Cortex. J. Toxicol. Environ. Heal. Part B 2011, 14, 328–345. [Google Scholar] [CrossRef]
- Guillemin, R. Neuroendocrinology: A short historical review. Ann. N. Y. Acad. Sci. 2011, 1220, 1–5. [Google Scholar] [CrossRef]
- Kentner, A.C.; Pittman, Q.J. Minireview: Early-Life Programming by Inflammation of the Neuroendocrine System. Endocrinology 2010, 151, 4602–4606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nijs, J.; Meeus, M.; Van Oosterwijck, J.; Ickmans, K.; Moorkens, G.; Hans, G.; De Clerck, L.S. In the mind or in the brain? Scientific evidence for central sensitisation in chronic fatigue syndrome. Eur. J. Clin. Investig. 2011, 42, 203–212. [Google Scholar] [CrossRef]
- Brabant, G.; Cain, J.; Jackson, A.; Kreitschmann-Andermahr, I. Visualizing hormone actions in the brain. Trends Endocrinol. Metab. 2011, 22, 153–163. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Leonard, J.L.; Davis, P.J. Molecular Aspects of Thyroid Hormone Actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Obesity: Genes, brain, gut, and environment. Nutrition 2010, 26, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Arosh, J.A.; Banu, S.K.; Chapdelaine, P.; Madore, E.; Sirois, J.; Fortier, M.A. Prostaglandin Biosynthesis, Transport, and Signaling in Corpus Luteum: A Basis for Autoregulation of Luteal Function. Endocrinology 2004, 145, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Lehr, S.; Hartwig, S.; Sell, H. Adipokines: A treasure trove for the discovery of biomarkers for metabolic disorders. Proteom. Clin. Appl. 2012, 6, 91–101. [Google Scholar] [CrossRef]
- Dupont, J.; Pollet-Villard, X.; Reverchon, M.; Mellouk, N.; Levy, R. Adipokines in human reproduction. Horm. Mol. Biol. Clin. Investig. 2015, 24, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A.; Hashimoto, I.; Okada, T.; Yasuhara, A.; Nakatsuka, A.; et al. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615. [Google Scholar] [CrossRef] [Green Version]
- Klöting, N.; Kovacs, P.; Kern, M.; Heiker, J.T.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Beck-Sickinger, A.G.; Blüher, M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia 2011, 54, 1819–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.K.; Heutling, D.; Chen, J.; Farhatullah, S.; Adya, R.; Keay, S.D.; Kennedy, C.R.; Lehnert, H.; Randeva, H.S. Metformin Decreases the Adipokine Vaspin in Overweight Women with Polycystic Ovary Syndrome Concomitant With Improvement in Insulin Sensitivity and a Decrease in Insulin Resistance. Diabetes 2008, 57, 1501–1507. [Google Scholar] [CrossRef] [Green Version]
- Heiker, J.T.; Klöting, N.; Kovacs, P.; Kuettner, E.B.; Sträter, N.; Schultz, S.; Kern, M.; Stumvoll, M.; Blüher, M.; Beck-Sickinger, A.G. Vaspin inhibits kallikrein 7 by serpin mechanism. Cell. Mol. Life Sci. 2013, 70, 2569–2583. [Google Scholar] [CrossRef] [Green Version]
- Ulbricht, D.; Tindall, C.A.; Oertwig, K.; Hanke, S.; Sträter, N.; Heiker, J.T. Kallikrein-related peptidase 14 is the second KLK protease targeted by the serpin vaspin. Biol. Chem. 2018, 399, 1079–1084. [Google Scholar] [CrossRef]
- Ulbricht, D.; Oertwig, K.; Arnsburg, K.; Saalbach, A.; Pippel, J.; Sträter, N.; Heiker, J.T. Basic Residues of β-Sheet A Contribute to Heparin Binding and Activation of Vaspin (Serpin A12). J. Biol. Chem. 2017, 292, 994–1004. [Google Scholar] [CrossRef] [Green Version]
- Heiker, J.T. Vaspin (serpinA12) in obesity, insulin resistance, and inflammation. J. Pept. Sci. 2014, 20, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulbricht, D.; Pippel, J.; Schultz, S.; Meier, R.; Sträter, N.; Heiker, J.T. A unique serpin P1′ glutamate and a conserved β-sheet C arginine are key residues for activity, protease recognition and stability of serpinA12 (vaspin). Biochem. J. 2015, 470, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Oertwig, K.; Ulbricht, D.; Hanke, S.; Pippel, J.; Bellmann-Sickert, K.; Sträter, N.; Heiker, J.T. Glycosylation of human vaspin (SERPINA12) and its impact on serpin activity, heparin binding and thermal stability. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2017, 1865, 1188–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, J.; Zieger, K.; Pippel, J.; Heiker, J.T. Molecular Mechanisms of Vaspin Action-From Adipose Tissue to Skin and Bone, from Blood Vessels to the Brain. Adv. Exp. Med. Biol. 2019, 1111, 159–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tindall, C.A.; Erkner, E.; Stichel, J.; Beck-Sickinger, A.G.; Hoffmann, A.; Weiner, J.; Heiker, J.T. Cleavage of the vaspin N-terminus releases cell-penetrating peptides that affect early stages of adipogenesis and inhibit lipolysis in mature adipocytes. Adipocyte 2021, 10, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Breitfeld, J.; Heiker, J.T.; Böttcher, Y.; Schleinitz, D.; Tönjes, A.; Weidle, K.; Krause, K.; Kuettner, E.B.; Scholz, M.; Kiess, W.; et al. Analysis of a rare functional truncating mutation rs61757459 in vaspin (SERPINA12) on circulating vaspin levels. J. Mol. Med. 2013, 91, 1285–1292. [Google Scholar] [CrossRef]
- Teshigawara, S.; Wada, J.; Hida, K.; Nakatsuka, A.; Eguchi, J.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Serum Vaspin Concentrations Are Closely Related to Insulin Resistance, and rs77060950 atSERPINA12Genetically Defines Distinct Group with Higher Serum Levels in Japanese Population. J. Clin. Endocrinol. Metab. 2012, 97, E1202–E1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klöting, N.; Berndt, J.; Kralisch, S.; Kovacs, P.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M. Vaspin gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Biochem. Biophys. Res. Commun. 2006, 339, 430–436. [Google Scholar] [CrossRef]
- Saalbach, A.; Vester, K.; Rall, K.; Tremel, J.; Anderegg, U.; Beck-Sickinger, A.G.; Blüher, M.; Simon, J.C. Vaspin-a link of obesity and psoriasis? Exp. Dermatol. 2012, 21, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Körner, A.; Neef, M.; Friebe, D.; Erbs, S.; Kratzsch, J.; Dittrich, K.; Blüher, S.; Kapellen, T.M.; Kovacs, P.; Stumvoll, M.; et al. Vaspin is related to gender, puberty and deteriorating insulin sensitivity in children. Int. J. Obes. 2011, 35, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Caminos, J.E.; Bravo, S.B.; Garcés, M.F.; González, C.R.; Cepeda, L.A.; González, A.C.; Nogueiras, R.; Gallego, R.; García-Caballero, T.; Cordido, F.; et al. Vaspin and amylin are expressed in human and rat placenta and regulated by nutritional status. Histol. Histopathol. 2009, 24, 979–990. [Google Scholar]
- Kurowska, P.; Mlyczyńska, E.; Barbe, A.; Staub, C.; Gregoraszczuk, E.; Dupont, J.; Rak, A. Vaspin in the pig ovarian follicles: Expression and regulation by different hormones. Reproduction 2019, 158, 135–146. [Google Scholar] [CrossRef]
- Weiner, J.; Rohde, K.; Krause, K.; Zieger, K.; Klöting, N.; Kralisch, S.; Kovacs, P.; Stumvoll, M.; Blüher, M.; Böttcher, Y.; et al. Brown adipose tissue (BAT) specific vaspin expression is increased after obesogenic diets and cold exposure and linked to acute changes in DNA-methylation. Mol. Metab. 2017, 6, 482–493. [Google Scholar] [CrossRef]
- Feng, R.; Li, Y.; Wang, C.; Luo, C.; Liu, L.; Chuo, F.; Li, Q.; Sun, C. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pract. 2014, 106, 88–94. [Google Scholar] [CrossRef]
- Vehapoğlu, A.; Ustabas, F.; Ozgen, T.I.; Terzioglu, S.; Cermik, B.B.; Ozen, O.F. Role of circulating adipocytokines vaspin, apelin, and visfatin in the loss of appetite in underweight children: A pilot trial. J. Pediatr. Endocrinol. Metab. 2015, 28, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Golpaie, A.; Tajik, N.; Masoudkabir, F.; Karbaschian, Z.; Talebpour, M.; Hosseini, M.; Hosseinzadeh-Attar, M.J. Short-term effect of weight loss through restrictive bariatric surgery on serum levels of vaspin in morbidly obese subjects. Eur. Cytokine Netw. 2011, 22, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Shaker, O.; Sadik, N.A.H. Vaspin gene in rat adipose tissue: Relation to obesity-induced insulin resistance. Mol. Cell. Biochem. 2013, 373, 229–239. [Google Scholar] [CrossRef]
- Wada, J. Vaspin: A novel serpin with insulin-sensitizing effects. Expert Opin. Investig. Drugs 2008, 17, 327–333. [Google Scholar] [CrossRef] [PubMed]
- González, C.R.; Caminos, J.E.; Vázquez, M.J.; Garcés, M.F.; Cepeda, L.A.; Ángel, A.; González, A.C.; García-Rendueles, M.E.; Sangiao-Alvarellos, S.; López, M.; et al. Regulation of visceral adipose tissue-derived serine protease inhibitor by nutritional status, metformin, gender and pituitary factors in rat white adipose tissue. J. Physiol. 2009, 587, 3741–3750. [Google Scholar] [CrossRef] [PubMed]
- Von Loeffelholz, C.; Möhlig, M.; Arafat, A.; Isken, F.; Spranger, J.; Mai, K.; Randeva, H.S.; Pfeiffer, A.F.H.; O Weickert, M. Circulating vaspin is unrelated to insulin sensitivity in a cohort of nondiabetic humans. Eur. J. Endocrinol. 2010, 162, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, E.; Youn, B.-S.; Kim, D.W.; Kim, E.H.; Park, J.W.; Namkoong, C.; Jeong, J.Y.; Yoon, S.Y.; Park, J.Y.; Lee, K.-U.; et al. Circadian Rhythm of Serum Vaspin in Healthy Male Volunteers: Relation to Meals. J. Clin. Endocrinol. Metab. 2010, 95, 1869–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatsuka, A.; Wada, J.; Iseda, I.; Teshigawara, S.; Higashio, K.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Vaspin Is an Adipokine Ameliorating ER Stress in Obesity as a Ligand for Cell-Surface GRP78/MTJ-1 Complex. Diabetes 2012, 61, 2823–2832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevalier, M.; Rhee, H.; Elguindi, E.C.; Blond, S.Y. Interaction of Murine BiP/GRP78 with the DnaJ Homologue MTJ. J. Biol. Chem. 2000, 275, 19620–19627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brocchieri, L.; De Macario, E.C.; Macario, A.J.L. hsp70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol. Biol. 2008, 8, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lee, J.; Liem, D.; Ping, P. HSPA5 Gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene 2017, 618, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elfiky, A.A. GRP78: A cell’s response to stress. Life Sci. 2019, 226, 156–163. [Google Scholar] [CrossRef]
- Gonzalez–Gronow, M.; Selim, M.A.; Papalas, J.; Pizzo, S.V. GRP78: A Multifunctional Receptor on the Cell Surface. Antioxid. Redox Signal. 2009, 11, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, M.; Shan, Y.; Zheng, X.; Ma, H.; Ma, W.; Wang, Z.; Pei, X.; Wang, Y. Endoplasmic Reticulum Stress-Mediated Apoptotic Pathway Is Involved in Corpus Luteum Regression in Rats. Reprod. Sci. 2015, 22, 572–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cree, L.; Hammond, E.R.; Shelling, A.; Berg, M.C.; Peek, J.C.; Green, M.P. Maternal age and ovarian stimulation independently affect oocyte mtDNA copy number and cumulus cell gene expression in bovine clones. Hum. Reprod. 2015, 30, 1410–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thon, M.; Hosoi, T.; Yoshii, M.; Ozawa, K. Leptin induced GRP78 expression through the PI3K-mTOR pathway in neuronal cells. Sci. Rep. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.S. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem. Sci. 2001, 26, 504–510. [Google Scholar] [CrossRef]
- Zhang, C. Roles of Grp78 in Female Mammalian Reproduction. Adv. Anat. Embryol. Cell Biol. 2017, 222, 129–155. [Google Scholar] [CrossRef]
- Chinni, S.R.; Falchetto, R.; Gercel-Taylor, C.; Shabanowitz, J.; Hunt, D.F.; Taylor, D.D. Humoral immune responses to cathepsin D and glucose-regulated protein 78 in ovarian cancer patients. Clin. Cancer Res. 1997, 3, 1557–1564. [Google Scholar]
- Hardy, B.; Battler, A.; Weiss, C.; Kudasi, O.; Raiter, A. Therapeutic angiogenesis of mouse hind limb ischemia by novel peptide activating GRP78 receptor on endothelial cells. Biochem. Pharmacol. 2008, 75, 891–899. [Google Scholar] [CrossRef]
- Scheuner, D.; Mierde, D.V.; Song, B.; Flamez, D.; Creemers, J.W.M.; Tsukamoto, K.; Ribick, M.; Schuit, F.C.; Kaufman, R.J. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat. Med. 2005, 11, 757–764. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, M.; Zhou, L.; Yao, L.; Liao, Q.; Zhao, Y. Elevated GRP78 expression is associated with poor prognosis in patients with pancreatic cancer. Sci. Rep. 2015, 5, 16067. [Google Scholar] [CrossRef] [Green Version]
- Dores-Silva, P.R.; Cauvi, D.M.; Coto, A.L.S.; Kiraly, V.T.R.; Borges, J.C.; De Maio, A. Interaction of HSPA5 (Grp78, BIP) with negatively charged phospholipid membranes via oligomerization involving the N-terminal end domain. Cell Stress Chaperones 2020, 25, 979–991. [Google Scholar] [CrossRef]
- Tindall, C.A.; Dommel, S.; Riedl, V.; Ulbricht, D.; Hanke, S.; Sträter, N.; Heiker, J.T. Membrane Phospholipids and Polyphosphates as Cofactors and Binding Molecules of SERPINA12 (vaspin). Molecules 2020, 25, 1992. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Wu, Y.; Duan, R.; Zhang, J.; Du, F.; Zhang, Q.; Li, Y.; Li, N. Effects of vaspin on pancreatic β cell secretion via PI3K/Akt and NF-κB signaling pathways. PLoS ONE 2017, 12, e0189722. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.H.; Lee, M.J.; Kang, Y.M.; La Lee, Y.; Yoon, H.K.; Kang, S.-W.; Lee, W.J.; Park, J.-Y. Vaspin inhibits cytokine-induced nuclear factor-kappa B activation and adhesion molecule expression via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc. Diabetol. 2014, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.H.; Lee, W.J.; Hwang, J.Y.; Lee, M.J.; Seol, S.M.; Kim, Y.M.; La Lee, Y.; Kim, H.S.; Kim, M.-S.; Park, J.-Y. Vaspin Increases Nitric Oxide Bioavailability through the Reduction of Asymmetric Dimethylarginine in Vascular Endothelial Cells. PLoS ONE 2012, 7, e52346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Grzesiak, M.; Dupont, J.; Rak, A. The role of vaspin in porcine corpus luteum. J. Endocrinol. 2020, 247, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Opydo-Chanek, M.; Dupont, J.; Rak, A. In Vitro Effects of Vaspin on Porcine Granulosa Cell Proliferation, Cell Cycle Progression, and Apoptosis by Activation of GRP78 Receptor and Several Kinase Signaling Pathways Including MAP3/1, AKT, and STAT3. Int. J. Mol. Sci. 2019, 20, 5816. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Jiang, Y.; Shan, P.-F.; Shen, J.; Liang, Q.-H.; Cui, R.-R.; Liu, Y.; Liu, G.-Y.; Wu, S.-S.; Lu, Q.; et al. Vaspin attenuates the apoptosis of human osteoblasts through ERK signaling pathway. Amino Acids 2013, 44, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, K.; Zhang, C.; Yang, G.; Yang, M.; Jia, Y.; Zhang, L.; A Ma, Z.; Boden, G.; Li, L. Central administration of vaspin inhibits glucose production and augments hepatic insulin signaling in high-fat-diet-fed rat. Int. J. Obes. 2016, 40, 947–954. [Google Scholar] [CrossRef]
- Brunetti, L.; Di Nisio, C.; Recinella, L.; Chiavaroli, A.; Leone, S.; Ferrante, C.; Orlando, G.; Vacca, M. Effects of vaspin, chemerin and omentin-1 on feeding behavior and hypothalamic peptide gene expression in the rat. Peptides 2011, 32, 1866–1871. [Google Scholar] [CrossRef]
- Breitfeld, J.; Tonjes, A.; Gast, M.-T.; Schleinitz, R.; Blüher, M.; Stumvoll, M.; Kovacs, P.; Böttcher, Y. Role of Vaspin in Human Eating Behaviour. PLoS ONE 2013, 8, e54140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, C.; González-García, I.; Seoane-Collazo, P.; Martinez-Sanchez, N.; Liñares-Pose, L.; Rial-Pensado, E.; Fernø, J.; Tena-Sempere, M.; Casals, N.; Diéguez, C.; et al. Reduction of Hypothalamic Endoplasmic Reticulum Stress Activates Browning of White Fat and Ameliorates Obesity. Diabetes 2016, 66, 87–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liñares-Pose, L.; Rial-Pensado, E.; Estévez-Salguero, Á.; Milbank, E.; González-García, I.; Rodríguez, C.; Seoane-Collazo, P.; Martinez-Sánchez, N.; Nogueiras, R.; Prieto, D.; et al. Genetic Targeting of GRP78 in the VMH Improves Obesity Independently of Food Intake. Genes 2018, 9, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koiou, E.; Tziomalos, K.; Dinas, K.; Katsikis, I.; Kalaitzakis, E.; Delkos, D.; Kandaraki, E.A.; Panidis, D. The effect of weight loss and treatment with metformin on serum vaspin levels in women with polycystic ovary syndrome. Endocr. J. 2011, 58, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, T.; Church, C.; Tsintzas, K.; Jones, R.; Breen, L.; Davis, E.T.; Baker, D.J.; Jones, S.W. Vaspin promotes insulin sensitivity in elderly muscle and is upregulated in obesity. J. Endocrinol. 2019, 241, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.A.; Park, H.S.; Song, Y.S.; Jang, Y.J.; Kim, J.-H.; Lee, Y.J.; Heo, Y.-S. Relationship between vaspin gene expression and abdominal fat distribution of Korean women. Endocr. J. 2011, 58, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Barbe, A.; Kurowska, P.; Mlyczyńska, E.; Ramé, C.; Staub, C.; Venturi, E.; Billon, Y.; Rak, A.; Dupont, J. Adipokines expression profiles in both plasma and peri renal adipose tissue in Large White and Meishan sows: A possible involvement in the fattening and the onset of puberty. Gen. Comp. Endocrinol. 2020, 299, 113584. [Google Scholar] [CrossRef]
- Zieger, K.; Weiner, J.; Krause, K.; Schwarz, M.; Kohn, M.; Stumvoll, M.; Blüher, M.; Heiker, J.T. Vaspin suppresses cytokine-induced inflammation in 3T3-L1 adipocytes via inhibition of NFκB pathway. Mol. Cell. Endocrinol. 2018, 460, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Li, G.; Wu, J.; Zhou, X.; Wang, L.; Han, W.; Lv, Y.; Sun, C. Vaspin promotes 3T3-L1 preadipocyte differentiation. Exp. Biol. Med. 2015, 240, 1520–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, C.; Hu, W.; Wang, M.; Xiao, Y. The role of the adipocytokines vaspin and visfatin in vascular endothelial function and insulin resistance in obese children. BMC Endocr. Disord. 2019, 19, 127. [Google Scholar] [CrossRef]
- Liu, S.; Duan, R.; Wu, Y.; Du, F.; Zhang, J.; Li, X.; Guo, S.; Wang, M.; Zhang, Q.; Li, Y.; et al. Effects of Vaspin on Insulin Resistance in Rats and Underlying Mechanisms. Sci. Rep. 2018, 8, 13542. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Lee, W.J.; Hwang, J.Y.; Seol, S.M.; Kim, Y.M.; La Lee, Y.; Park, J.-Y. Vaspin protects vascular endothelial cells against free fatty acid-induced apoptosis through a phosphatidylinositol 3-kinase/Akt pathway. Biochem. Biophys. Res. Commun. 2011, 413, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Çinar, N.; Gülçelik, N.E.; Aydín, K.; Akín, Ş.; Usman, A.; Gürlek, A. Serum vaspin levels in hypothyroid patients. Eur. J. Endocrinol. 2011, 165, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Al-Jowari, S.A. Determination of the Level of some Adipokines in Hypo-and Hyperthyroids Patients in Baghdad City. Baghdad Sci. J. 2017, 14, 713–716. [Google Scholar] [CrossRef]
- Handisurya, A.; Riedl, M.; Vila, G.; Maier, C.; Clodi, M.; Prikoszovich, T.; Ludvik, B.; Prager, G.; Luger, A.; Kautzky-Willer, A. Serum Vaspin Concentrations in Relation to Insulin Sensitivity Following RYGB-Induced Weight Loss. Obes. Surg. 2010, 20, 198–203. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczyńska, E.; Estienne, A.; Barbe, A.; Rajska, I.; Soból, K.; Poniedziałek-Kempny, K.; Dupont, J.; Rak, A. Expression and Impact of Vaspin on In Vitro Oocyte Maturation through MAP3/1 and PRKAA1 Signalling Pathways. Int. J. Mol. Sci. 2020, 21, 9342. [Google Scholar] [CrossRef]
- Bongrani, A.; Mellouk, N.; Ramé, C.; Cornuau, M.; Guerif, F.; Froment, P.; Dupont, J. Vaspin, a novel adipokine in woman granulosa cells physiology and PCOS pathogenesis? J. Endocrinol. 2021, 249, 57–70. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Dupont, J.; Rak, A. Role of vaspin in porcine ovary: Effect on signaling pathways and steroid synthesis via GRP78 receptor and protein kinase A†. Biol. Reprod. 2020, 102, 1290–1305. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczyńska, E.; Dupont, J.; Rak, A. Novel Insights on the Corpus Luteum Function: Role of Vaspin on Porcine Luteal Cell Angiogenesis, Proliferation and Apoptosis by Activation of GRP78 Receptor and MAP3/1 Kinase Pathways. Int. J. Mol. Sci. 2020, 21, 6823. [Google Scholar] [CrossRef]
- Yuan, Y.; Krisher, R.L. In Vitro Maturation (IVM) of Porcine Oocytes. Adv. Struct. Saf. Stud. 2011, 825, 183–198. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, S.; Song, G.; Ren, L.; Wang, C.; Zhang, D. Plasma levels and placental expression of vaspin in pregnant women with diabetes mellitus. Braz. J. Med. Biol. Res. 2015, 48, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trojnar, M.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Czuba, M.; Mosiewicz, J.; Leszczyńska-Gorzelak, B. Vaspin in Serum and Urine of Post-Partum Women with Excessive Gestational Weight Gain. Medicina 2019, 55, 76. [Google Scholar] [CrossRef] [Green Version]
- Brzoskwinia, M.; Pardyak, L.; Rak, A.; Kaminska, A.; Hejmej, A.; Marek, S.; Kotula-Balak, M.; Bilinska, B. Flutamide Alters the Expression of Chemerin, Apelin, and Vaspin and Their Respective Receptors in the Testes of Adult Rats. Int. J. Mol. Sci. 2020, 21, 4439. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fadeil, M.R.; Allah, E.S.A.; Iraqy, H.; Elgamal, D.A.; Abdel-Ghani, M.A. Experimental obesity and diabetes reduce male fertility: Potential involvement of hypothalamic Kiss-1, pituitary nitric oxide, serum vaspin and visfatin. Pathophysiology 2019, 26, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ivars, J.; Butruille, L.; Knauf, C.; Bouckenooghe, T.; Mayeur, S.; Vieau, D.; Valet, P.; Deruelle, P.; Lesage, J. Maternal hypertension induces tissue-specific modulations of the apelinergic system in the fetoplacental unit in rat. Peptides 2012, 35, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Ginis, Z.; Ucar, F.; Erdogan, S.; Ozturk, G.; Akyol, S.; Erden, G.; Arslan, M.S.; Delibasi, T.; Erdoǧan, S. Serum vaspin and adiponectin levels in patients with prolactinoma. Scand. J. Clin. Lab. Investig. 2015, 76, 17–24. [Google Scholar] [CrossRef]
- Alnory, A.; Gad, H.; Hegazy, G.; Shaker, O. The association of vaspin rs2236242 and leptin rs7799039 polymorphism with metabolic syndrome in Egyptian women. Turk. J. Med. Sci. 2016, 46, 1335–1340. [Google Scholar] [CrossRef]
- Buyukinan, M.; Atar, M.; Can, Ü.; Pirgon, Ö.; Güzelant, A.; Deniz, I. The Association Between Serum Vaspin and Omentin-1 Levels in Obese Children with Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Esteghamati, A.; Noshad, S.; Mousavizadeh, K.; Zandieh, A.; Nakhjavani, M. Association of Vaspin with Metabolic Syndrome: The Pivotal Role of Insulin Resistance. Diabetes Metab. J. 2014, 38, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.H.; Kwak, S.H.; Lee, Y.; Moon, M.K.; Lim, S.; Park, Y.J.; Jang, H.C.; Kim, M.S. Plasma vaspin concentrations are elevated in metabolic syndrome in men and are correlated with coronary atherosclerosis in women. Clin. Endocrinol. 2011, 75, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, T.N.; Won, J.C. Association Between Serum Vaspin Level and Metabolic Syndrome in Healthy Korean Subjects. Metab. Syndr. Relat. Disord. 2013, 11, 385–391. [Google Scholar] [CrossRef]
- Chen, M.; Deng, D.; Fang, Z.; Xu, M.; Hu, H.; Luo, L.; Wang, Y. Fenofibrate increases serum vaspin by upregulating its expression in adipose tissue. Endocrine 2014, 31, 139–170. [Google Scholar] [CrossRef]
- Suliga, E.; Kozieł, D.; Cieśla, E.; Rębak, D.; Wawszczak, M.; Adamus-Białek, W.; Naszydłowska, E.; Piechowska, A.; Gluszek, S. Associations between vaspin rs2236242 gene polymorphism, walking time and the risk of metabolic syndrome. Balk. J. Med. Genet. 2019, 22, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Pendharkar, S.A.; Cervantes, A.; Cho, J.; Miranda-Soberanis, V.; Petrov, M.S. Abdominal obesity and insulin resistance after an episode of acute pancreatitis. Dig. Liver Dis. 2018, 50, 1081–1087. [Google Scholar] [CrossRef]
- De Castro, C.A.; Da Silva, K.A.; Buffo, M.M.; Pinto, K.N.Z.; Duarte, F.D.O.; Nonaka, K.O.; Aníbal, F.D.F.; Duarte, A.C.G.D.O. Experimental type 2 diabetes induction reduces serum vaspin, but not serum omentin, in Wistar rats. Int. J. Exp. Pathol. 2017, 98, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, S.; Yuan, G.; Wang, D.; Chen, J. Changes and clinical significance of serum vaspin levels in patients with type 2 diabetes. Genet. Mol. Res. 2015, 14, 11356–11361. [Google Scholar] [CrossRef]
- Hao, F.; Zhang, H.; Zhu, J.; Kuang, H.; Yu, Q.; Bai, M.; Mu, J. Association between vaspin level and coronary artery disease in patients with type 2 diabetes. Diabetes Res. Clin. Pr. 2016, 113, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Tomimatsu, T.; Mimura, K.; Matsuzaki, S.; Endo, M.; Kumasawa, K.; Kimura, T. Preeclampsia: Maternal Systemic Vascular Disorder Caused by Generalized Endothelial Dysfunction Due to Placental Antiangiogenic Factors. Int. J. Mol. Sci. 2019, 20, 4246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, Q.; Zhu, Y.; Wang, Y.; Gao, C.; Li, X.; Ji, T.; Bai, S. Association of vaspin rs2236242 gene variants with type 2 diabetes and obesity in a Chinese population: A prospective, single-center study. J. Cell. Physiol. 2019, 234, 16097–16101. [Google Scholar] [CrossRef]
- Jian, W.; Peng, W.; Xiao, S.; Li, H.; Jin, J.; Qin, L.; Dong, Y.; Su, Q. Role of Serum Vaspin in Progression of Type 2 Diabetes: A 2-Year Cohort Study. PLoS ONE 2014, 9, e94763. [Google Scholar] [CrossRef] [Green Version]
- Youn, B.-S.; Klöting, N.; Kratzsch, J.; Lee, N.; Park, J.W.; Song, E.-S.; Ruschke, K.; Oberbach, A.; Fasshauer, M.; Stumvoll, M.; et al. Serum Vaspin Concentrations in Human Obesity and Type 2 Diabetes. Diabetes 2007, 57, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rosa, G.; Franzetti, I.G.; Querci, F.; Carbone, A.; Ciccarelli, L.; Piccinni, M.N.; Fogari, E.; Maffioli, P. Variation in Inflammatory Markers and Glycemic Parameters After 12 Months of Exenatide Plus Metformin Treatment Compared with Metformin Alone: A Randomized Placebo-Controlled Trial. Pharmacotherapy 2013, 33, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Romano, D.; Maffioli, P. Effects of metformin extended release compared to immediate release formula on glycemic control and glycemic variability in patients with type 2 diabetes. Drug Des. Dev. Ther. 2017, 11, 1481–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbari, S.; Hedayati, M.; Yaghmaei, P.; Parivar, K. Medullary Thyroid Carcinoma-Circulating Status of Vaspin and Retinol Binding Protein-4 in Iranian Patients. Asian Pac. J. Cancer Prev. 2015, 16, 6507–6512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, I.F.; Leventhal, M.L. Amenorrhea associated with bilateral polycystic ovaries. Am. J. Obstet. Gynecol. 1935, 29, 181–191. [Google Scholar] [CrossRef]
- Cekmez, F.; Cekmez, Y.; Pirgon, Ö.; Canpolat, F.E.; Aydinöz, S.; Ipcioglu, O.M.; Karademir, F. Evaluation of new adipocytokines and insulin resistance in adolescents with polycystic ovary syndrome. Eur. Cytokine Netw. 2011, 22, 32–37. [Google Scholar] [CrossRef]
- Cakal, E.; Ustun, Y.; Engin-Ustun, Y.; Ozkaya, M.; Kilinç, M. Serum vaspin and C-reactive protein levels in women with polycystic ovaries and polycystic ovary syndrome. Gynecol. Endocrinol. 2010, 27, 491–495. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Ghasemi, S.; Kalantarhormozi, M.; Nabipour, I.; Abbasi, F.; Aminfar, A.; Jaffari, S.M.; Motamed, N.; Movahed, A.; Mirzaei, M.; et al. Relationship among plasma adipokines, insulin and androgens level as well as biochemical glycemic and lipidemic markers with incidence of PCOS in women with normal BMI. Gynecol. Endocrinol. 2012, 28, 521–524. [Google Scholar] [CrossRef]
- Guvenc, Y.; Var, A.; Goker, A.; Kuscu, N.K. Assessment of serum chemerin, vaspin and omentin-1 levels in patients with polycystic ovary syndrome. J. Int. Med. Res. 2016, 44, 796–805. [Google Scholar] [CrossRef] [Green Version]
- Dogan, K.; Ekin, M.; Helvacioğlu, Ç.; Yaşar, L. Can serum vaspin levels predict clomiphene resistance in infertile women with PCOS? Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 217, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, H.Ş.; Uzun, Y.E.; Kutlu, O.; Pençe, H.H.; Özçelik, F.; Çil, E.Ö.; Irak, L.; Altun, Ö.; Özcan, M.; Özsoy, N.; et al. The effects of high intensity-interval training on vaspin, adiponectin and leptin levels in women with polycystic ovary syndrome. Arch. Physiol. Biochem. 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mierzyński, R.; Poniedziałek-Czajkowska, E.; Dłuski, D.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Majsterek, M.; Leszczyńska-Gorzelak, B. Nesfatin-1 and Vaspin as Potential Novel Biomarkers for the Prediction and Early Diagnosis of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2019, 20, 159. [Google Scholar] [CrossRef] [Green Version]
- Mm, W.Q.; Fan, J.; Khor, S.; Song, M.; Hong, W.; Dai, X. Serum vaspin levels and vaspin mRNA expression in subcutaneous adipose tissue in women with gestational diabetes mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 98–101. [Google Scholar] [CrossRef]
- Tang, Y.; Qiao, P.; Qu, X.; Bao, Y.; Li, Y.; Liao, Y.; Ying, H. Comparison of serum vaspin levels and vaspin expression in adipose tissue and smooth muscle tissue in pregnant women with and without gestational diabetes. Clin. Endocrinol. 2017, 87, 344–349. [Google Scholar] [CrossRef]
- Stepan, H.; Kralisch, S.; Klostermann, K.; Schrey, S.; Reisenbüchler, C.; Verlohren, M.; Verlohren, H.-J.; Drynda, K.; Blüher, M.; Stumvoll, M.; et al. Preliminary report: Circulating levels of the adipokine vaspin in gestational diabetes mellitus and preeclampsia. Metabolism 2010, 59, 1054–1056. [Google Scholar] [CrossRef]
- Gkiomisi, A.; Makedou, K.; Anastasilakis, A.D.; Polyzos, S.; Kourtis, A.; Gerou, S.; Gavana, E.; Dagklis, T.; Rousso, D.; Giannoulis, C. Serum vaspin levels in women with and without gestational diabetes mellitus during pregnancy and postpartum. Cytokine 2013, 61, 127–132. [Google Scholar] [CrossRef]
- De Gennaro, G.; Palla, G.; Battini, L.; Simoncini, T.; Del Prato, S.; Bertolotto, A.; Bianchi, C. The role of adipokines in the pathogenesis of gestational diabetes mellitus. Gynecol. Endocrinol. 2019, 35, 737–751. [Google Scholar] [CrossRef]
- Rosenberg, A. The IUGR Newborn. Semin. Perinatol. 2008, 32, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Akcay, A.; Akar, M.; Demirel, G.; Canpolat, F.E.; Erdeve, O.; Dilmen, U. Umbilical cord and fifth-day serum vaspin concentrations in small-, appropriate-, and large-for-gestational age neonates. J. Pediatr. Endocrinol. Metab. 2013, 26, 635–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Pathology | Species | |
|---|---|---|
| Rat | Human | |
| Prolactinoma | ns | ↓ |
| Metabolic syndrome | ↑ | ↑; ↓ |
| Diabetes | ↓ | ↑; ↓ |
| Hypothyroidism | ns | ↑ |
| Hyperthyroidism | ns | ↑ |
| Medullary thyroid carcinoma | ns | ↑ |
| Polycystic ovarian syndrome | ns | ↑ |
| Gestational diabetes mellitus | ns | ↑; ↓ |
| Intrauterine growth restriction | ns | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Jurek, M.; Klimczyk, D.; Dupont, J.; Rak, A. Review: Vaspin (SERPINA12) Expression and Function in Endocrine Cells. Cells 2021, 10, 1710. https://doi.org/10.3390/cells10071710
Kurowska P, Mlyczyńska E, Dawid M, Jurek M, Klimczyk D, Dupont J, Rak A. Review: Vaspin (SERPINA12) Expression and Function in Endocrine Cells. Cells. 2021; 10(7):1710. https://doi.org/10.3390/cells10071710
Chicago/Turabian StyleKurowska, Patrycja, Ewa Mlyczyńska, Monika Dawid, Małgorzata Jurek, Dominika Klimczyk, Joelle Dupont, and Agnieszka Rak. 2021. "Review: Vaspin (SERPINA12) Expression and Function in Endocrine Cells" Cells 10, no. 7: 1710. https://doi.org/10.3390/cells10071710
APA StyleKurowska, P., Mlyczyńska, E., Dawid, M., Jurek, M., Klimczyk, D., Dupont, J., & Rak, A. (2021). Review: Vaspin (SERPINA12) Expression and Function in Endocrine Cells. Cells, 10(7), 1710. https://doi.org/10.3390/cells10071710








