Regenerative Strategy for Persistent Periprosthetic Leakage around Tracheoesophageal Puncture: Is It an Effective Long-Term Solution?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population
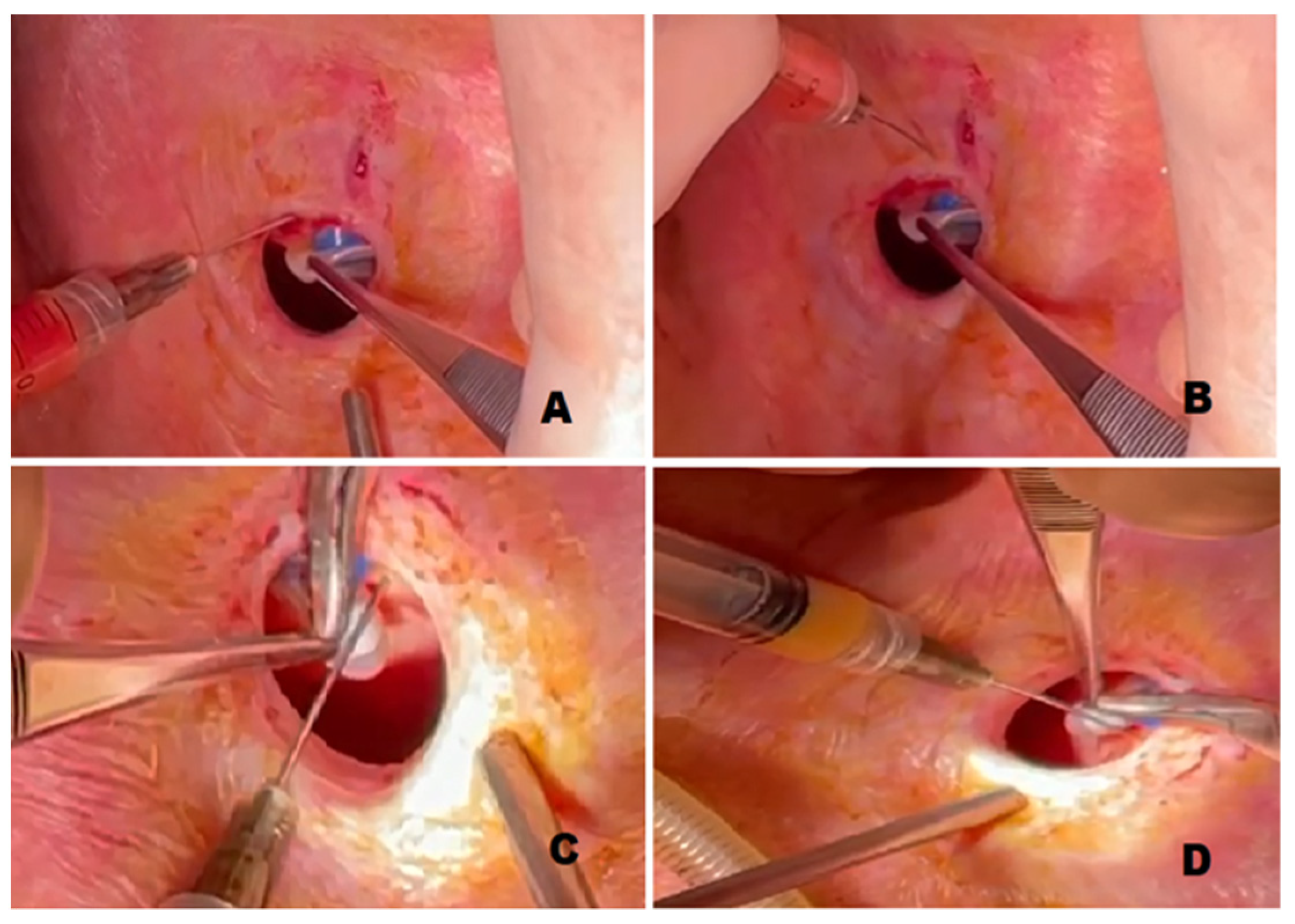
2.3. Surgical Procedure
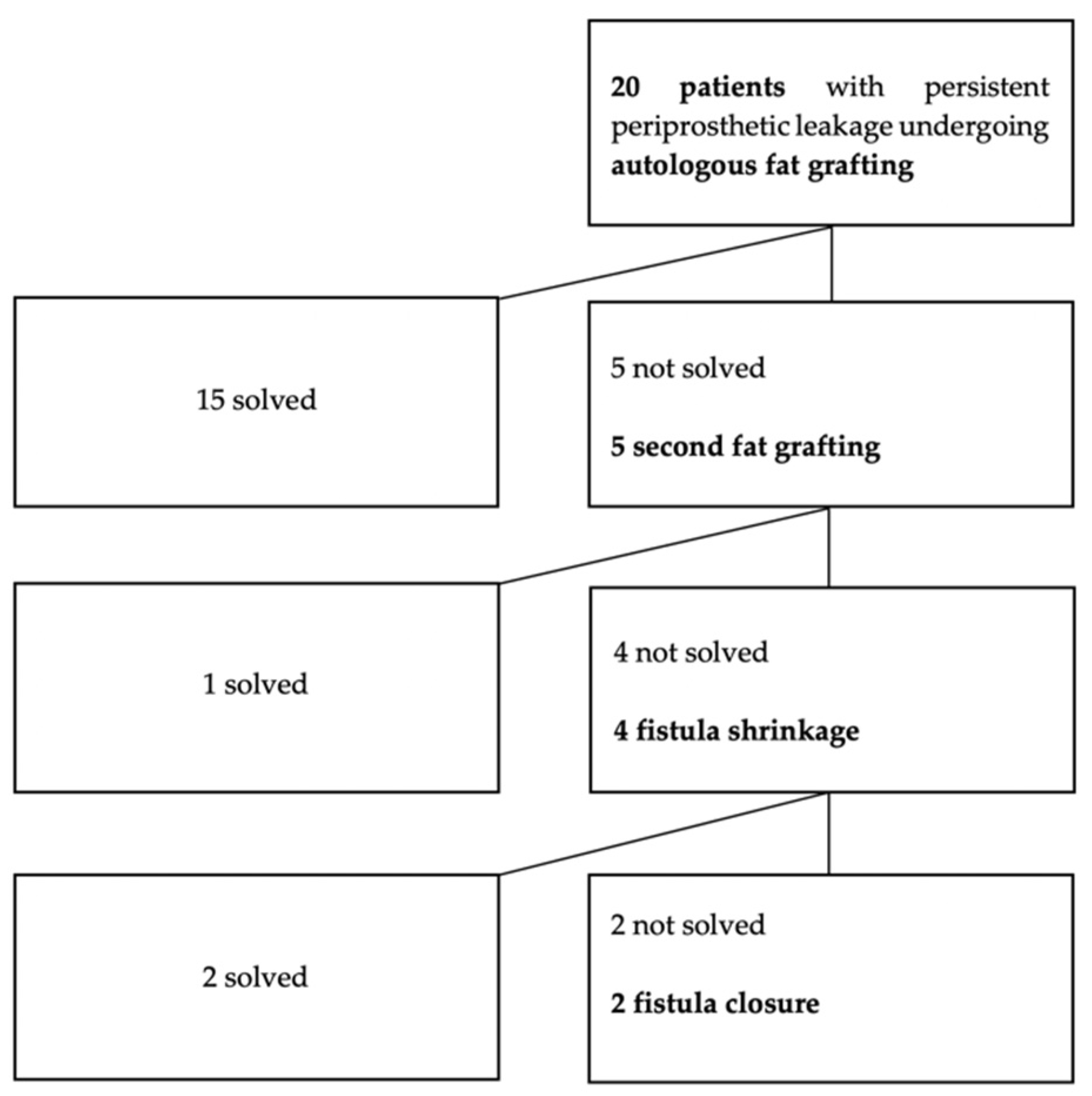
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Fang, J.; Chen, F.; Liu, D.; Gu, F.; Wang, Y. Adipose tissue-derived stem cells in breast reconstruction: A brief review on biology and translation. Stem Cell Res. Ther. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- James, I.; Bourne, D.; Silva, M.; Havis, E.; Albright, K.; Zhang, L.; Kostereva, N.; Wang, S.; DiBernardo, G.; Guest, R.; et al. Adipose stem cells enhance excisional wound healing in a porcine model. J. Surg. Res. 2018, 229, 243–253. [Google Scholar] [CrossRef]
- Parrilla, C.; Saulnier, N.; Bernardini, C.; Patti, R.; Tartaglione, T.; Fetoni, A.R.; Pola, E.; Paludetti, G.; Michetti, F.; Lattanzi, W. Undifferentiated human adipose tissue–derived stromal cells induce mandibular bone healing in rats. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 463–470. [Google Scholar] [CrossRef][Green Version]
- Parrilla, C.; Lattanzi, W.; Fetoni, A.R.; Bussu, F.; Pola, E.; Paludetti, G. Ex vivo gene therapy using autologous dermal fibroblasts expressing hLMP3 for rat mandibular bone regeneration. Head Neck 2009, 32, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Prantl, L.; Brix, E.; Kempa, S.; Felthaus, O.; Eigenberger, A.; Brébant, V.; Anker, A.; Strauss, C. Facial rejuvenation with concentrated lipograft—A 12-month follow-up study. Cells 2021, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Almadori, A.; Griffin, M.; Ryan, C.M.; Hunt, D.F.; Hansen, E.; Kumar, R.; Abraham, D.J.; Denton, C.P.; Butler, P.E.M. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS ONE 2019, 14, e0218068. [Google Scholar] [CrossRef] [PubMed]
- Langridge, B.; Jeon, F.H.K.; Almadori, A.; Griffin, M.F.; Denton, C.P.; Butler, P.E.M. Autologous lipotransfer can improve the outcomes of localised scleroderma. Clin. Exp. Rheumatol. 2021. [Google Scholar]
- Parrilla, C.; Longobardi, Y.; Paludetti, G.; Marenda, M.E.; D’Alatri, L.; Bussu, F.; Scarano, E.; Galli, J. A one-year time frame for voice prosthesis management. What should the physician expect? Is it an overrated job? Acta Otorhinolaryngol. Ital. 2020, 40, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Macri, G.; Bogaardt, H.; Parrilla, C.; Minni, A.; D’Alatri, L.; De Vincentiis, M.; Greco, A.; Paludetti, G. Patients’ experiences with HMEs and attachments after total laryngectomy. Clin. Otolaryngol. 2015, 41, 652–659. [Google Scholar] [CrossRef]
- Parrilla, C.; Minni, A.; Bogaardt, H.; Macri, G.F.; Battista, M.; Roukos, R.; Pandolfini, M.; Ruoppolo, G.; Paludetti, G.; D’Alatri, L.; et al. Pulmonary rehabilitation after total laryngectomy: A Multicenter time-series clinical trial evaluating the provox XtraHME in HME-naïve patients. Ann. Otol. Rhinol. Laryngol. 2015, 124, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Danker, H.; Wollbrück, D.; Singer, S.; Fuchs, M.; Brähler, E.; Meyer, A. Social withdrawal after laryngectomy. Head Neck 2010, 267, 593–600. [Google Scholar] [CrossRef]
- Longobardi, Y.; Savoia, V.; Bussu, F.; Morra, L.; Mari, G.; Nesci, D.A.; Parrilla, C.; D’Alatri, L. Integrated rehabilitation after total laryngectomy: A pilot trial study. Support. Care Cancer 2019, 27, 3537–3544. [Google Scholar] [CrossRef]
- Parrilla, C.; Longobardi, Y.; Galli, J.; Rigante, M.; Paludetti, G.; Bussu, F.; Scarano, E. Periprosthetic leakage in tracheoesophageal prosthesis: Proposal of a standardized therapeutic algorithm. Otolaryngol. Head Neck Surg. 2021, 5. [Google Scholar] [CrossRef]
- Hutcheson, K.A.; Lewin, J.S.; Sturgis, E.M.; Kapadia, A.; Risser, J. Enlarged tracheoesophageal puncture after total laryngectomy: A systematic review and meta-analysis. Head Neck. 2011, 33, 20–30. [Google Scholar] [CrossRef]
- Lorenz, K.J. The development and treatment of periprosthetic leakage after prosthetic voice restoration. A literature review and personal experience part I: The development of periprosthetic leakage. Eur. Arch. Otorhinolaryngol. 2014, 272, 641–659. [Google Scholar] [CrossRef]
- Labruna, A.; Huo, J.; Klatsky, I.; Weiss, M.H. Tracheoesophageal puncture in irradiated patients. Ann. Otol. Rhinol. Laryngol. 1995, 104, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, S.J.; Johns, M.E.; Baker, S.R. The Singer-Blom voice restoration procedure. Arch. Otolaryngol. Head Neck Surg. 1981, 107, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Twomey, K.; Lehn, B.; Vasani, S. Management of leaking tracheoesophageal puncture with hyaluronic acid injection. Head Neck 2018, 40, 1573–1576. [Google Scholar] [CrossRef]
- Almadori, A.; Hansen, E.; Boyle, D.; Zenner, N.; Swale, V.; Reid, W.; MacLane, A.; Butler, P.E. Fat grafting improves fibrosis and scarring in vulvar lichen sclerosus: Results from a prospective cohort study. J. Low. Genit. Tract Dis. 2020, 24, 305–310. [Google Scholar] [CrossRef]
- Trivisonno, A.; Nachira, D.; Boškoski, I.; Porziella, V.; Di Rocco, G.; Baldari, S.; Toietta, G. Regenerative medicine approaches for the management of respiratory tract fistulas. Stem Cell Res. Ther. 2020, 11, 451. [Google Scholar] [CrossRef]
- Panés, J.; Olmo, D.G.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Philandrianos, C.; Serrero, M.; Grimaud, F.; Magalon, J.; Visée, C.; Velier, M.; Francois, P.; Orsoni, P.; Magalon, G.; Grimaud, J.-C.; et al. First clinical case report of local microinjection of autologous fat and adipose-derived stromal vascular fraction for perianal fistula in Crohn’s disease. Stem Cell Res. Ther. 2018, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Laccourreye, O.; Papon, J.F.; Brasnu, D.; Hans, S. Autogenous fat injection for the incontinent tracheoesophageal puncture site. Laryngoscope 2002, 112, 1512–1514. [Google Scholar] [CrossRef] [PubMed]
- Perie, S.; Ming, X.; DeWolf, E.; Guily, J.L.S. Autologous fat injection to treat leakage around tracheoesophageal puncture. Am. J. Otolaryngol. 2002, 23, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Komatsubara, S.; Hanamure, Y.; Suda, Y.; Haruta, A.; Kasano, F.; Kashima, N. Fat injection to treat widening of a tracheoesophageal fistula with a voice prosthesis. Nippon. Jibiinkoka Gakkai Kaiho 2008, 111, 412–415. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hilgers, F.J.M.; Soolsma, J.; Ackerstaff, A.H.; Balm, F.J.M.; Tan, I.B.; Brekel, M.W.M.V.D. A thin tracheal silicone washer to solve periprosthetic leakage in laryngectomies: Direct results and long-term clinical effects. Laryngoscope 2008, 118, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Kress, P.; Schafer, P.; Schwerdtfeger, F.P. The custom-fit voice prosthesis, for treatment of periprosthetic leakage after tracheoesophageal voice restoration. Laryngorhinootologie 2006, 85, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Rokade, A.V.; Mathews, J.; Reddy, K.T.V. Tissue augmentation using Bioplastique® as a treatment of leakage around a Provox® 2 voice prosthesis. J. Laryngol. Otol. 2003, 117, 80–82. [Google Scholar] [CrossRef]
- Di Taranto, G.; Cicione, C.; Visconti, G.; Isgrò, M.A.; Barba, M.; Di Stasio, E.; Stigliano, E.; Bernardini, C.; Michetti, F.; Salgarello, M.; et al. Qualitative and quantitative differences of adipose-derived stromal cells from superficial and deep subcutaneous lipoaspirates: A matter of fat. Cytotherapy 2015, 17, 1076–1089. [Google Scholar] [CrossRef]
- Rigotti, G.; Marchi, A.; Galie’, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A.; Rubin, J.P.; Marra, K.G. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg. 2007, 119, 1409–1422. [Google Scholar] [CrossRef]
- Sultan, S.M.; Stern, C.S.; Allen, R.J.; Thanik, V.D.; Chang, C.C.; Nguyen, P.D.; Canizares, O.; Szpalski, C.; Saadeh, P.; Warren, S.M.; et al. Human Fat grafting alleviates radiation skin damage in a murine model. Plast. Reconstr. Surg. 2011, 128, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.F.; Drago, J.; Almadori, A.; Kalavrezos, N.; Butler, P.E. Evaluation of the efficacy of lipotransfer to manage radiation-induced fibrosis and volume defects in head and neck oncology. Head Neck 2019, 10, 3647–3655. [Google Scholar] [CrossRef] [PubMed]
- Salgarello, M.; Visconti, G.; Farallo, E. Autologous fat graft in radiated tissue prior to alloplastic reconstruction of the breast: Report of two cases. Aesthetic Plast Surg. 2009, 34, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Sharath, S.S.; Ramu, J.; Nair, S.V.; Iyer, S.; Mony, U.; Rangasamy, J. Human adipose tissue derivatives as a potent native biomaterial for tissue regenerative therapies. Tissue Eng. Regen. Med. 2020, 17, 123–140. [Google Scholar] [CrossRef]
- Pallua, N.; Pulsfort, A.K.; Suschek, C.; Wolter, T.P. Content of the growth factors bFGF, IGF-1, VEGF, and PDGF-BB in freshly harvested lipoaspirate after centrifugation and incubation. Plast. Reconstr. Surg. 2009, 123, 826–833. [Google Scholar] [CrossRef]
- Kurita, M.; Matsumoto, D.; Shigeura, T.; Sato, K.; Gonda, K.; Harii, K.; Yoshimura, K. Influences of centrifugation on cells and tissues in liposuction aspirates: Optimized centrifugation for lipotransfer and cell isolation. Plast. Reconstr. Surg. 2008, 121, 1033–1041. [Google Scholar] [CrossRef]
- Gir, P.; Brown, S.A.; Oni, G.; Kashefi, N.; Mojallal, A.; Rohrich, R.J. Fat grafting: Evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast. Reconstr. Surg. 2012, 130, 249–258. [Google Scholar] [CrossRef]
- Serratrice, N.; Bruzzese, L.; Magalon, J.; Véran, J.; Giraudo, L.; Aboudou, H.; Ould-Ali, D.; Nguyen, P.S.; Bausset, O.; Daumas, A.; et al. New fat-derived products for treating skin-induced lesions of scleroderma in nude mice. Stem Cell Res. Ther. 2014, 5, 138. [Google Scholar] [CrossRef] [PubMed]
- Daumas, A.; Magalon, J.; Delaunay, F.; Abellan, M.; Philandrianos, C.; Sabatier, F.; Granel, B.; Magalon, G. Fat grafting for treatment of facial scleroderma. Clin. Plast. Surg. 2020, 47, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Magalon, J.; Daumas, A.; Veran, J.; Rossi, P.; Granel, B.; Sabatier, F. Autologous adipose tissue-derived cells: Are we talking about adipose derived stem cells, stromal vascular fraction, or coleman fat grafting? Cell Transplant. 2015, 24, 2667–2668. [Google Scholar] [CrossRef] [PubMed]
- Eto, H.; Kato, H.; Suga, H.; Aoi, N.; Doi, K.; Kuno, S.; Yoshimura, K. The fate of adipocytes after nonvascularized fat grafting: Evidence of early death and replacement of adipocytes. Plast. Reconstr. Surg. 2012, 129, 1081–1092. [Google Scholar] [CrossRef]
- Kato, H.; Mineda, K.; Eto, H.; Doi, K.; Kuno, S.; Kinoshita, K.; Kanayama, K.; Yoshimura, K. Degeneration, regeneration, and cicatrization after fat grafting. Plast. Reconstr. Surg. 2014, 133, 303e–313e. [Google Scholar] [CrossRef]
- Matsumoto, D.; Sato, K.; Gonda, K.; Takaki, Y.; Shigeura, T.; Sato, T.; Aiba-Kojima, E.; Iizuka, F.; Inoue, K.; Suga, H.; et al. Cell-assisted lipotransfer: Supportive Use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006, 12, 3375–3382. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast. Surg. 2020, 44, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Inoue, K.; Suga, H.; Eto, H.; Kato, H.; Hirohi, T.; Harii, K. Cell-assisted lipotransfer for facial lipoatrophy: Efficacy of clinical use of adipose-derived stem cells. Dermatol. Surg. 2008, 34, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Laloze, J.; Varin, A.; Gilhodes, J.; Bertheuil, N.; Grolleau, J.L.; Brie, J.; Usseglio, J.; Sensebe, L.; Filleron, T.; Chaput, B. Cell-assisted lipotransfer: Friend or foe in fat grafting? Systematic review and meta-analysis. J. Tissue Eng. Regen. Med. 2018, 12, e1237–e1250. [Google Scholar] [CrossRef]
- Kølle, S.T.; Duscher, D.; Taudorf, M.; Fischer-Nielsen, A.; Svalgaard, J.D.; Munthe-Fog, L.; Jønsson, B.; Selvig, P.B.; Mamsen, F.P.; Katz, A.J. Ex vivo-expanded autologous adipose tissue-derived stromal cells ensure enhanced fat graft retention in breast augmentation: A randomized controlled clinical trial. STEM CELLS Transl. Med. 2020, 9, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Waldner, M.; Zhang, W.; James, I.B.; Allbright, K.; Havis, E.; Bliley, J.M.; Almadori, A.; Schweizer, R.; Plock, J.A.; Washington, K.M.; et al. Characteristics and immunomodulating functions of adipose-derived and bone marrow-derived mesenchymal Stem cells across defined human leukocyte antigen barriers. Front. Immunol. 2018, 9, 1642. [Google Scholar] [CrossRef]
- Serra-Mestre, J.M.; Serra-Renom, J.M.; Martinez, L.; Almadori, A.; D’Andrea, F. Platelet-rich plasma mixed-fat grafting: A reasonable prosurvival strategy for fat grafts? Aesthetic Plast. Surg. 2014, 38, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Visconti, G.; Salgarello, M. Magnetic resonance imaging and ultrasound evaluation after breast autologous fat grafting combined with platelet-rich plasma. Plast. Reconstr. Surg. 2014, 133, 593e–594e. [Google Scholar] [CrossRef] [PubMed]
- Salgarello, M.; Visconti, G.; Rusciani, A. Breast fat grafting with platelet-rich plasma: A comparative clinical study and current state of the art. Plast. Reconstr. Surg. 2011, 127, 2176–2185. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Patients Overall (n = 164) | Patients Undergoing Fat Grafting (n = 20) |
| Age | ||
| Mean ± SD | 68.6 ± 7.03 | 70.4 ± 4.54 |
| Range | 30–81 | 56–72 |
| Sex—n (%) | ||
| Male | 156/164 (95.12%) | 20/20 (100%) |
| Female | 8/164 (4.87%) | 0/20 (0%) |
| Body Mass Index (BMI) | ||
| Mean ± SD | 24 ± 4.4 | 23 ± 4.1 |
| Pharynx closure—no (%) | ||
| Primary | 146/164 (89.02%) | 19/20 (95%) |
| Non-tubed free flap (anterolateral thigh) | 14/164 (8.53%) | 1/20 (5%) |
| Non-tubed free flap (forearm) | 4/164 (2.43%) | 0/20 (0%) |
| Primary vs. Rescue Surgery—n (%) | ||
| Primary total laryngectomy | 120/164 (73.17%) | 8/20 (40%) |
| Rescue total laryngectomy after surgical organ preservation attempt | 22/164 (13.41%) | 2/20 (10%) |
| Rescue total laryngectomy after non-surgical organ preservation attempt | 22/164 (13.41%) | 10/20 (50%) |
| Radiotherapy—no (%) | ||
| Before surgery | 22/164 (13.41%) | 10/20 (50%) |
| Adjuvant | 94/164 (57.31%) | 8/20 (40%) |
| No treatment | 48/164 (29.26%) | 2/20 (10%) |
| Tracheoesophageal Puncture Time—no (%) | ||
| Primary | 104/164 (63.41%) | 13/20 (65%) |
| Secondary | 60/164 (36.58%) | 7/20 (35%) |
| Author, Year | Processing Method | Injection | Amount Injected (mL) | N of Injection | Sample SIZE | Age (Year) Mean ± SD | RT, Dose | Follow-Up (Months) Mean ± SD | Outcome Assessment | Efficacy | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Laccourreye, 2002 [25] | Harvesting with automated aspirator system (n 2); surgical fat dissection and mincing (n 5). | 19-gauge needle in 4 entry points around the puncture. VP removed during injection. | Range 0.8 to 1.5 | 1.4 (±0.8) | 7 | 63.3 ± 16.4 (range 41–82) | 28.57% (n 2), 50 Gy 60 Gy | 19.4 ± 23.7 (range 10–36) | Physician-based observation | 57.14% (n 4/7) | 14.29% (n 1/7) fat extrusion |
| Perie, 2002 [26] | Harvesting with suction machine (n 6); surgical microdissection (n 4). | Needle in 2 or 3 entry points around TEP. VP kept in place during injection. | Range 3 to 4 | 1.1 (±0.4) | 10 | 64.4 (range 56–73) | 90% (n 9), NR | 39.3 (range 10–61) | Methylene blue liquid swallowing | 60% (n 6/10) | - |
| Komatsubara, 2008 [27] | Harvesting with manual aspiration; processing with filtration and washing. | 16-gauge needle in 3 entry points (2, 6, 10 o’clock). VP removed during injection. | 1.2 | 2 | 1 | 68 | 100% (n 1) 41.4 Gy | NR | Physician-based observation | 100% (n 1/1) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parrilla, C.; Almadori, A.; Longobardi, Y.; Lattanzi, W.; Salgarello, M.; Almadori, G. Regenerative Strategy for Persistent Periprosthetic Leakage around Tracheoesophageal Puncture: Is It an Effective Long-Term Solution? Cells 2021, 10, 1695. https://doi.org/10.3390/cells10071695
Parrilla C, Almadori A, Longobardi Y, Lattanzi W, Salgarello M, Almadori G. Regenerative Strategy for Persistent Periprosthetic Leakage around Tracheoesophageal Puncture: Is It an Effective Long-Term Solution? Cells. 2021; 10(7):1695. https://doi.org/10.3390/cells10071695
Chicago/Turabian StyleParrilla, Claudio, Aurora Almadori, Ylenia Longobardi, Wanda Lattanzi, Marzia Salgarello, and Giovanni Almadori. 2021. "Regenerative Strategy for Persistent Periprosthetic Leakage around Tracheoesophageal Puncture: Is It an Effective Long-Term Solution?" Cells 10, no. 7: 1695. https://doi.org/10.3390/cells10071695
APA StyleParrilla, C., Almadori, A., Longobardi, Y., Lattanzi, W., Salgarello, M., & Almadori, G. (2021). Regenerative Strategy for Persistent Periprosthetic Leakage around Tracheoesophageal Puncture: Is It an Effective Long-Term Solution? Cells, 10(7), 1695. https://doi.org/10.3390/cells10071695







