Integrin-Linked Kinase (ILK) Plays an Important Role in the Laminin-Dependent Development of Dorsal Root Ganglia during Chicken Embryogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. RNA Isolation and Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
2.3. RNA Probes Preparation and In Situ Hybridization
2.4. Dorsal Root Ganglia Isolation, Dissociation, and Primary Culture
2.5. Immunocytochemistry and Confocal Microscopy
2.6. Western Blot Analysis
2.7. Antibodies-Mediated Disruption Of ILK Function
2.8. Neurite Outgrowth Assay
2.9. The Directionality of DRG Neurite Extension
2.10. Migration of SCPs and the Length of Axonal Halo
2.11. Analysis of Growth Cones’ Morphology
2.12. Experimental Design and Statistical Analysis
3. Results
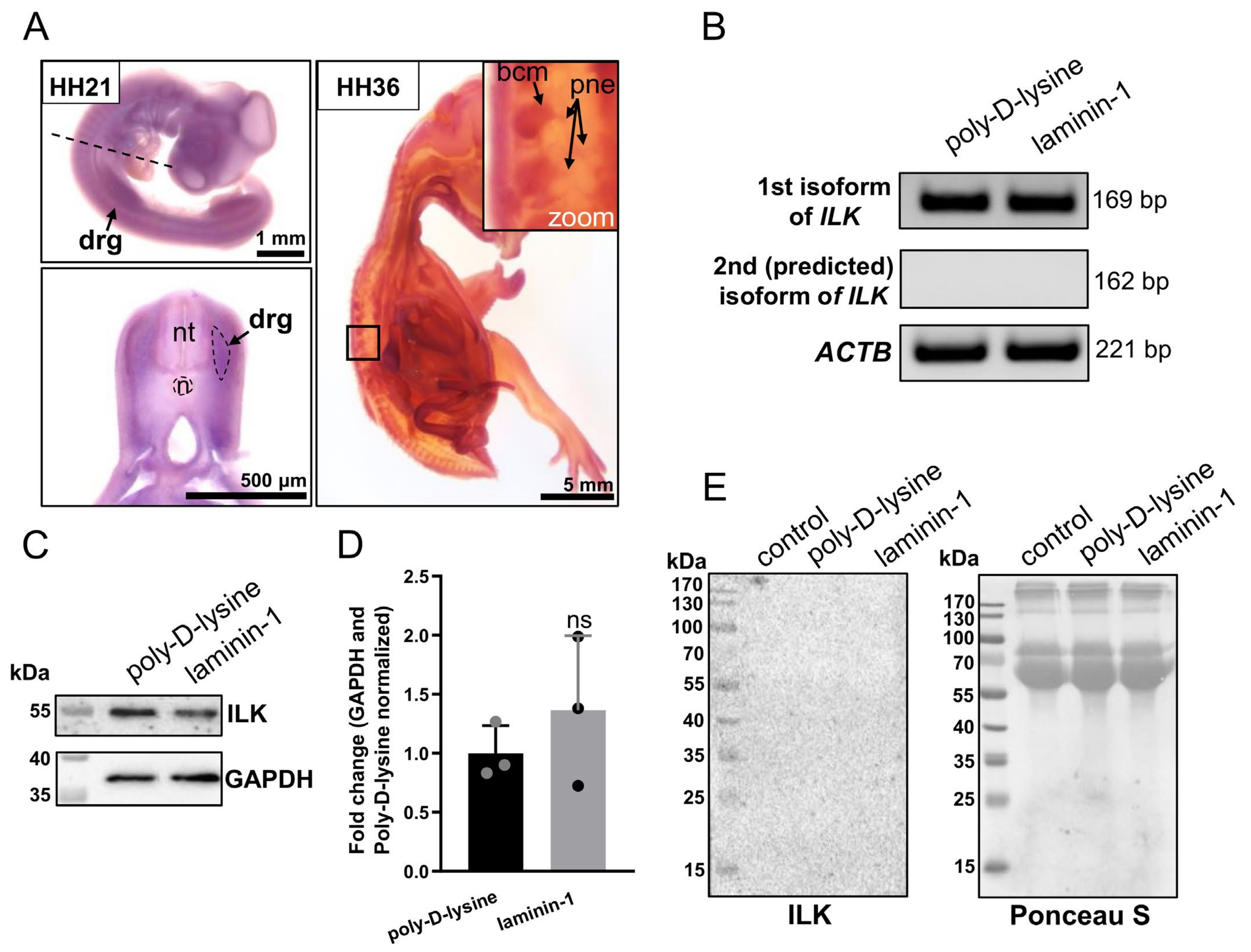
3.1. ILK Is Expressed in Chicken Embryo’s Dorsal Root Ganglia at mRNA and Protein Level, but It Is Not Secreted by DRG Cells
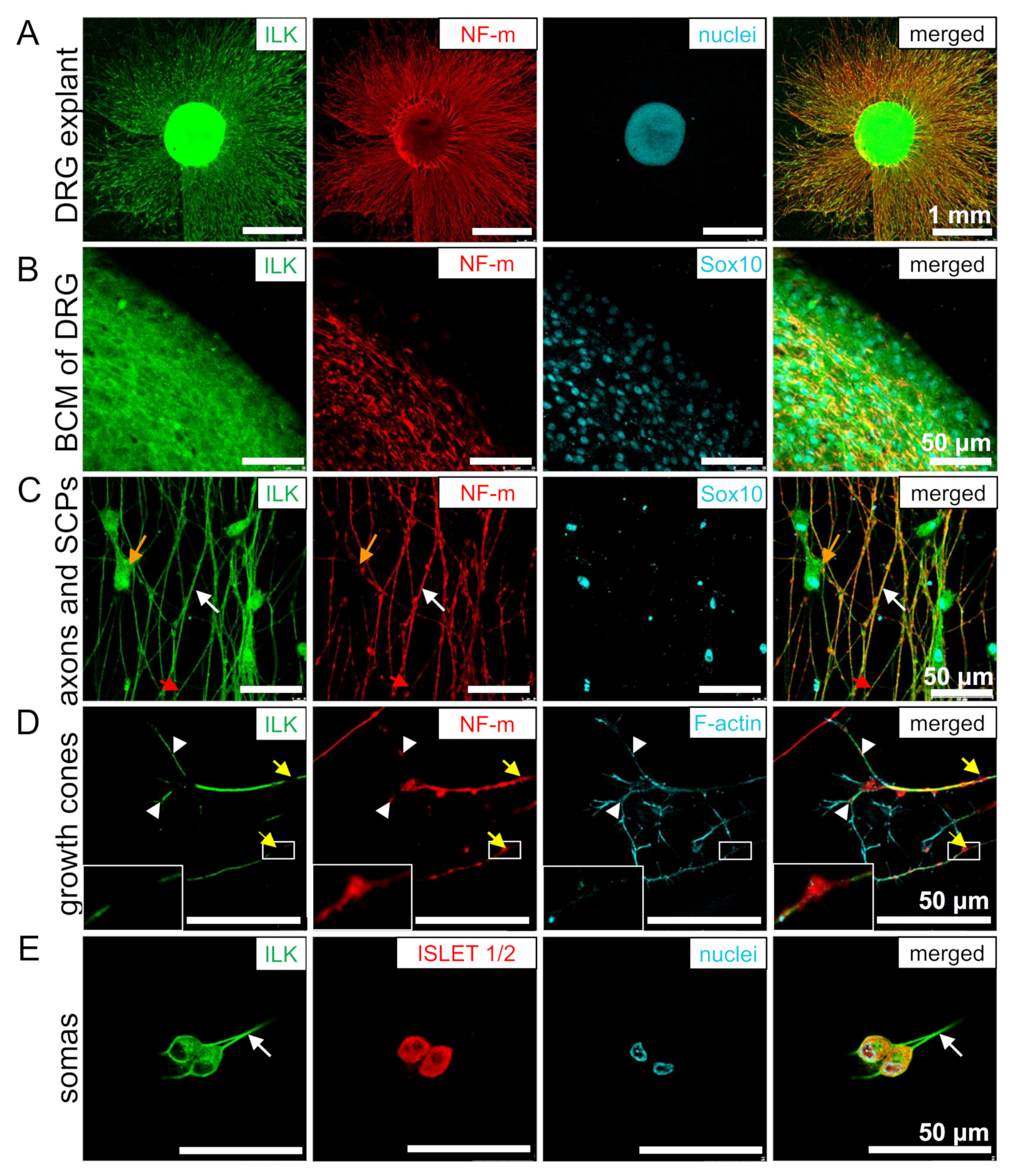
3.2. ILK Is Localized in the Soma and Neurites of DRG Neurons and SCPs
3.3. ILK Localizes as well on the Surface of Somas and Neurites of DRG Neurons as well as SCPs
3.4. Disruption of ILK’s Function Results in the Stimulation of Laminin-Mediated DRG Neurite Outgrowth In Vitro
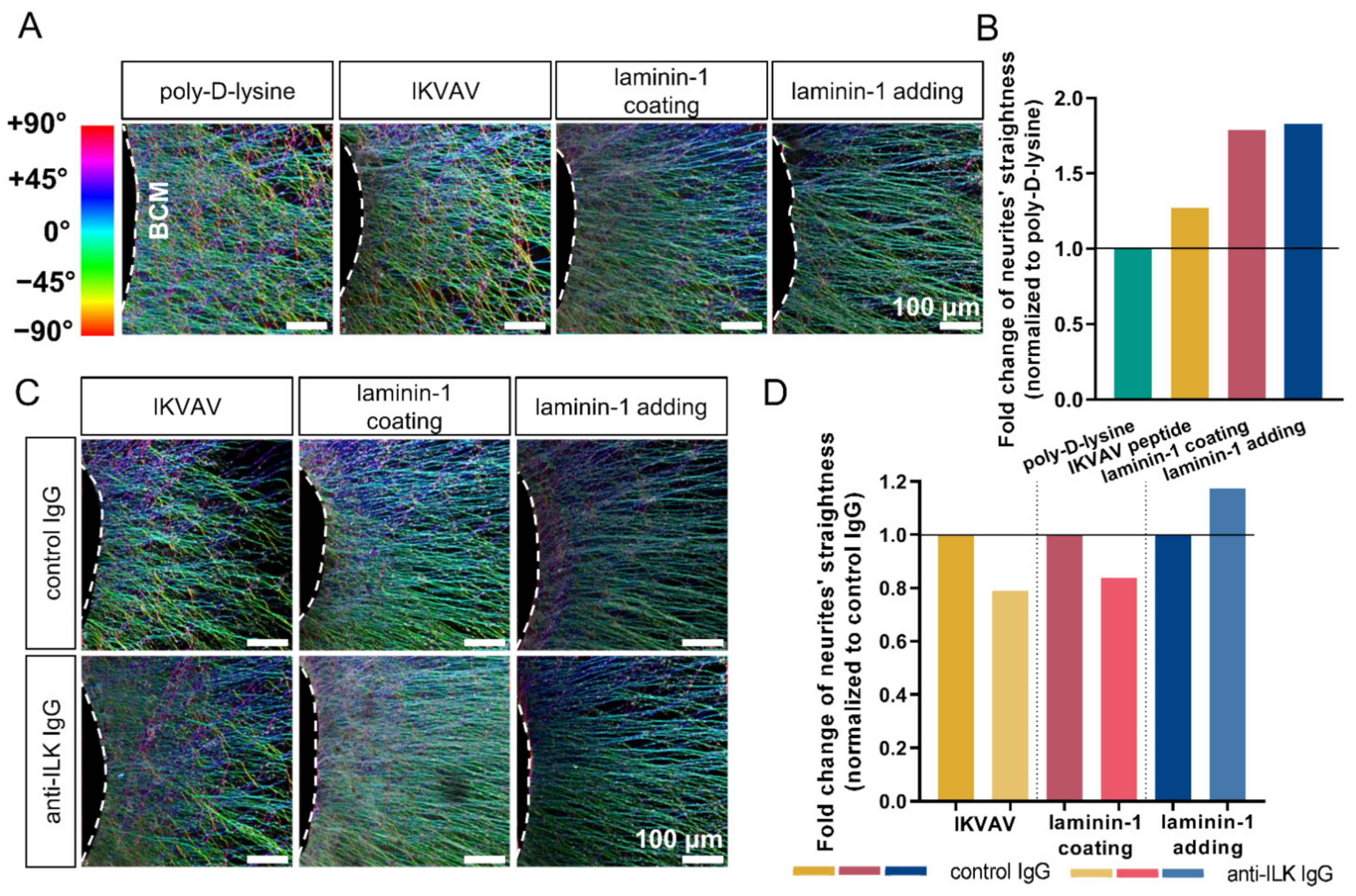
3.5. Anti-ILK Antibodies of High Specificity Impact the Directionality of Extending DRG Neurites In Vitro
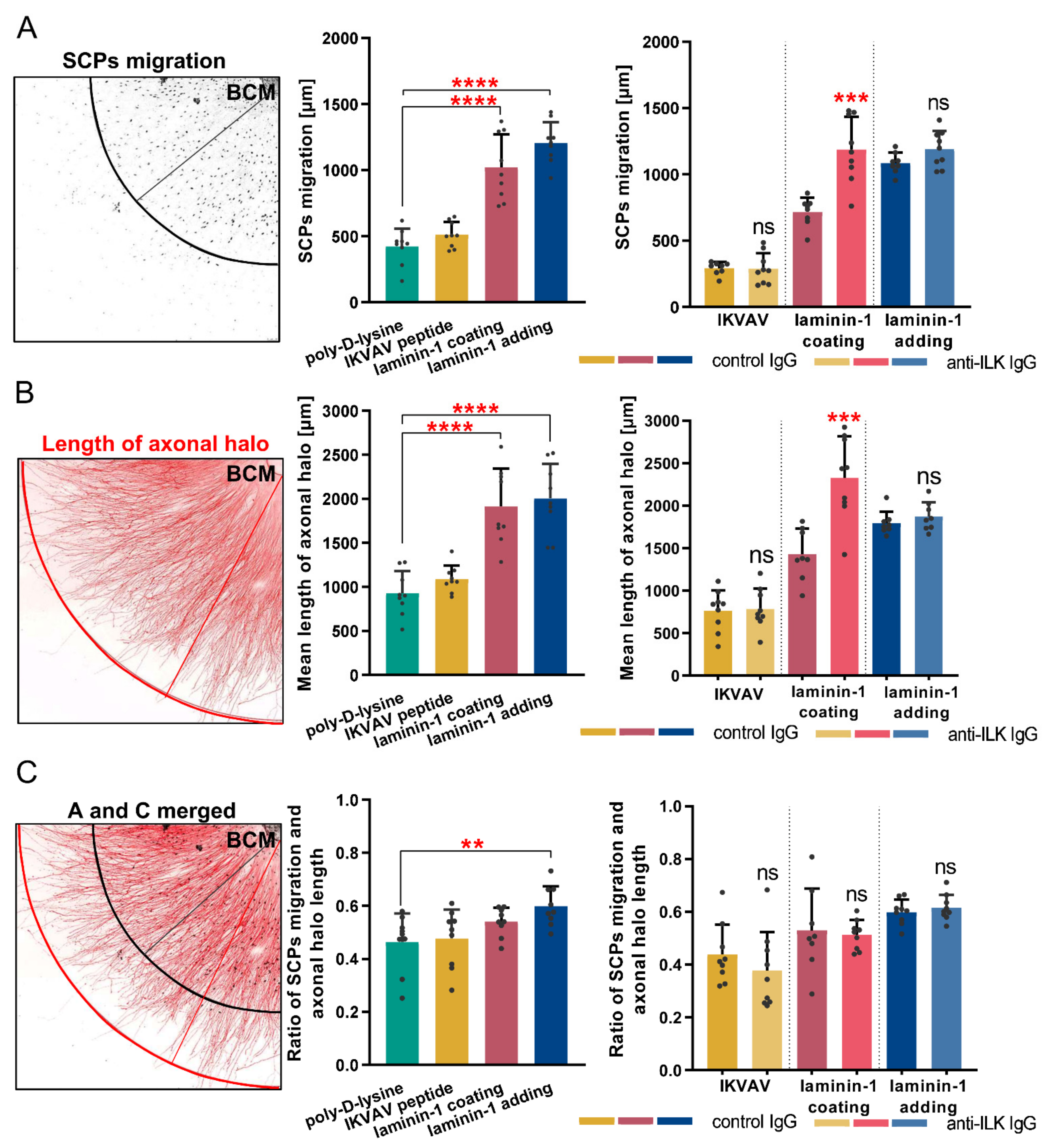
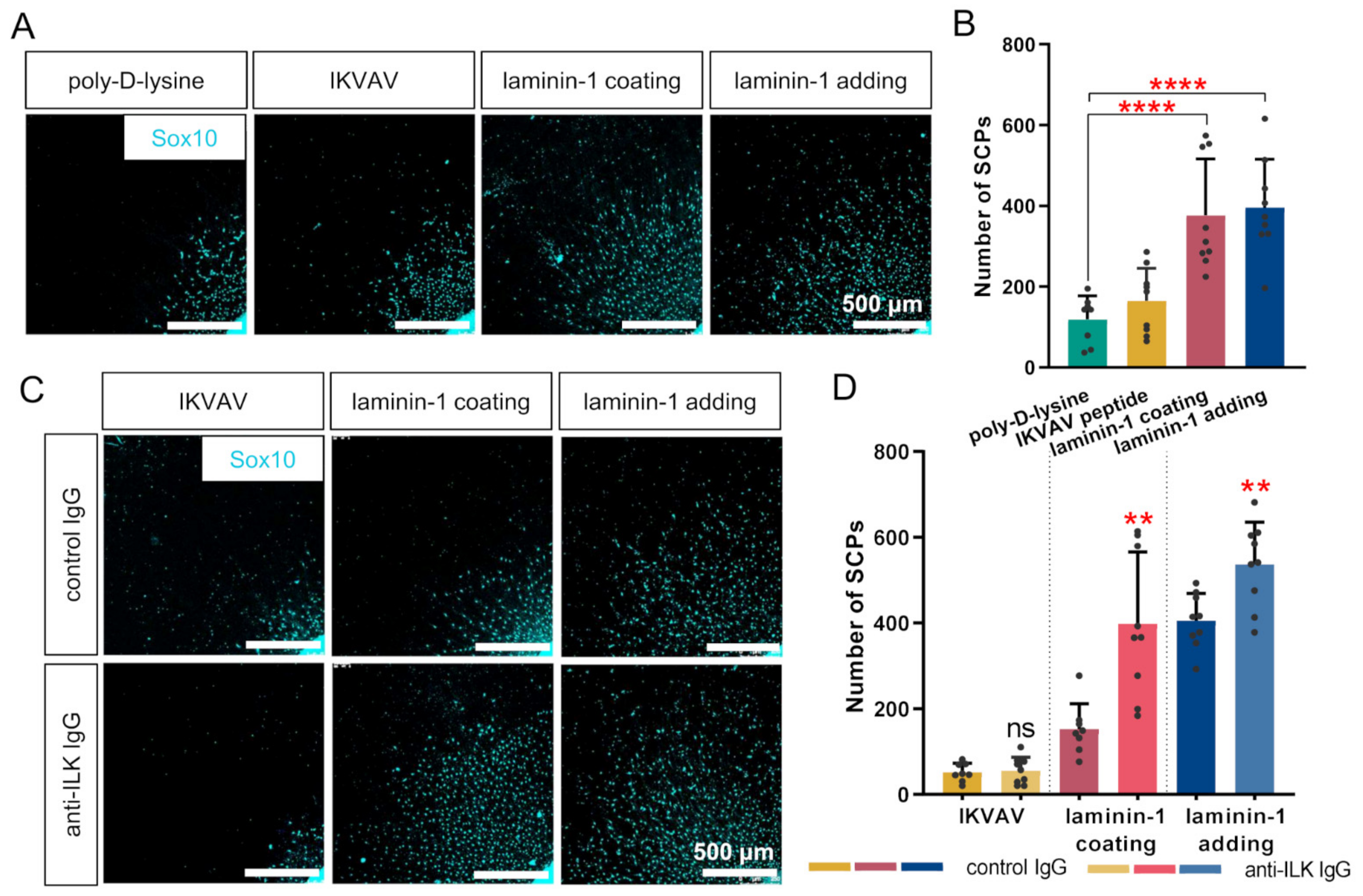
3.6. ILK Plays a Role in Laminin-Dependent Migration of Schwann Cell Precursors In Vitro, Increasing Their Number but Not Their Migration Distance
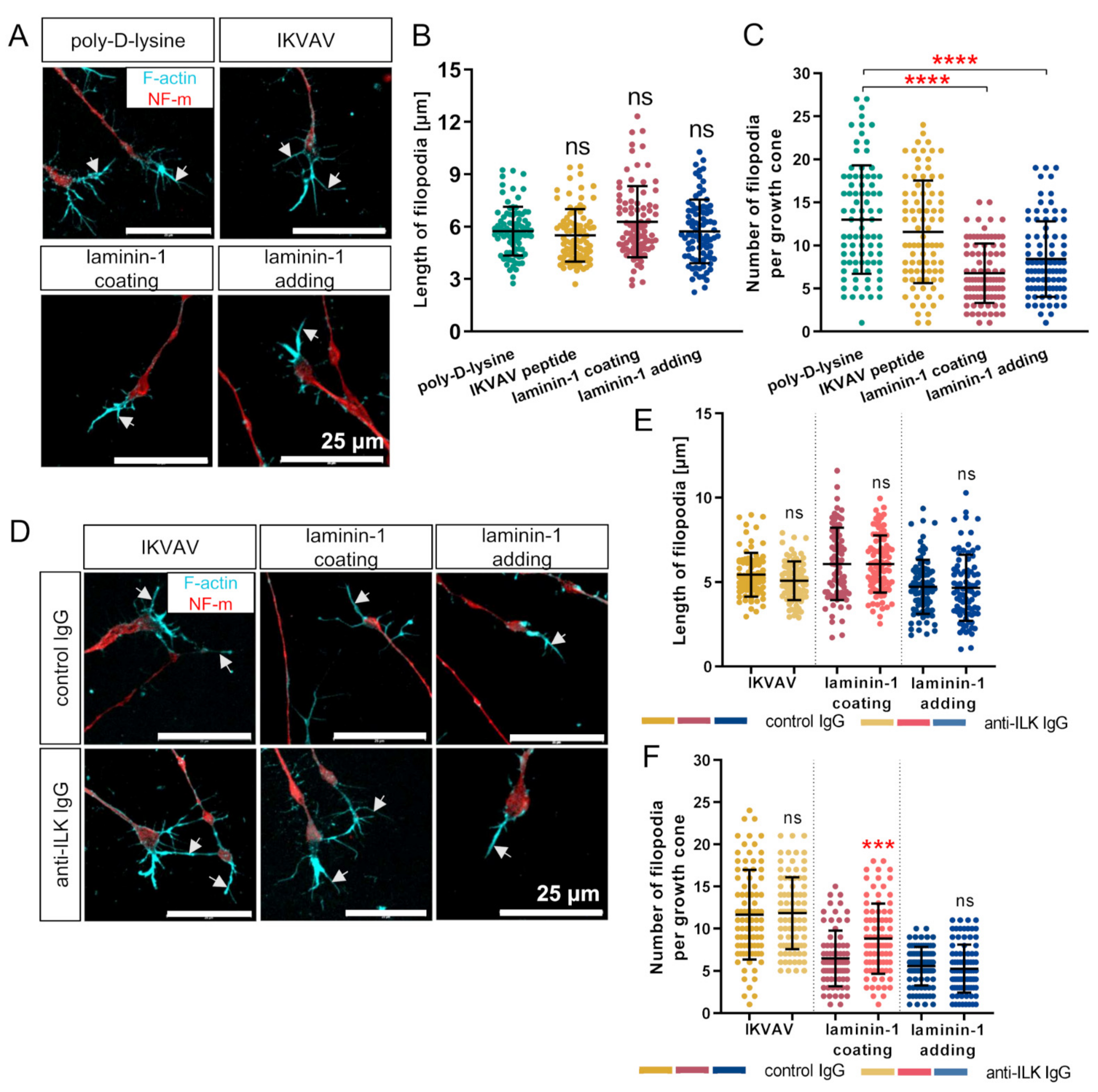
3.7. Affecting ILK’s Function Increases the Number but Not the Length of Filopodia of DRG Neuronal Growth Cones In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BCM | Body cell mass |
| DRG | Dorsal root ganglion/ganglia |
| ECM | Extracellular matrix |
| F-actin | Filamentous actin |
| HH stage | chicken developmental stage according to Hamburger and Hamilton |
| ILK | Integrin-linked kinase |
| IgG | Immunoglobulin G |
| NF-m | Mid-sized neurofilaments |
| NGF | Nerve growth factor |
| PNS | Peripheral nervous system |
| SCs | Schwann cells |
| SCPs | Schwann cell precursors |
References
- Zagorodnyuk, V.P.; Kyloh, M.; Nicholas, S.; Peiris, H.; Brookes, S.J.; Chen, B.N.; Spencer, N.J. Loss of visceral pain following colorectal distension in an endothelin-3 deficient mouse model of Hirschsprung’s disease. J. Physiol. 2011, 589, 1691–1706. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.I.; Mar, F.M.; Sousa, M.M. The intriguing nature of dorsal root ganglion neurons: Linking structure with polarity and function. Prog. Neurobiol. 2018, 168, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Adameyko, I.; Lallemend, F.; Aquino, J.B.; Pereira, J.A.; Topilko, P.; Müller, T.; Fritz, N.; Beljajeva, A.; Mochii, M.; Liste, I.; et al. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell 2009, 139, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L. Distribution of laminin in the developing peripheral nervous system of the chick. Dev. Biol. 1986, 435, 429–435. [Google Scholar] [CrossRef]
- Colognato, H.; Yurchenco, P.D. Form and function: The laminin family of heterotrimers. Dev. Dyn. 2000, 218, 213–234. [Google Scholar] [CrossRef]
- Rennard, I.; Martin, R. Laminin—A glycoprotein from basement membranes. J. Biol. Chem. 1979, 254, 9933–9937. [Google Scholar]
- Nomizu, M.; Kim, W.H.; Yamamura, K.; Utani, A.; Song, S.Y.; Otaka, A.; Roller, P.P.; Kleinman, H.K.; Yamada, Y. Identification of cell binding sites in the laminin α1 chain carboxyl-terminal globular domain by systematic screening of synthetic peptides. J. Biol. Chem. 1995, 270, 20583–20590. [Google Scholar] [CrossRef]
- Nomizu, M.; Kuratomi, Y.; Song, S.Y.; Ponce, M.L.; Hoffman, M.P.; Powell, S.K.; Miyoshi, K.; Otaka, A.; Kleinman, H.K.; Yamada, Y. Identification of cell binding sequences in mouse laminin chain by systematic peptide screening. J. Biol. Chem. 1997, 272, 32198–32205. [Google Scholar] [CrossRef]
- Tashiro, K.; Sephel, G.C.; Weeks, B.; Sasaki, M.; Martin, G.R.; Kleinman, H.K.; Yamada, Y. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J. Biol. Chem. 1989, 264, 16174–16182. [Google Scholar] [CrossRef]
- Desban, N.; Duband, J.L. Avian neural crest cell migration on laminin: Interaction of the a1β1 integrin with distinct laminin-1 domains mediates different adhesive responses. J. Cell Sci. 1997, 110, 2729–2744. [Google Scholar] [CrossRef]
- Plantman, S.; Patarroyo, M.; Fried, K.; Domogatskaya, A.; Tryggvason, K.; Hammarberg, H.; Cullheim, S. Integrin-laminin interactions controlling neurite outgrowth from adult DRG neurons In Vitro. Mol. Cell. Neurosci. 2008, 39, 50–62. [Google Scholar] [CrossRef]
- Manthorpe, M.; Engvall, E.; Ruoslahti, E.; Longo, F.M.; Davis, G.E.; Varon, S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J. Cell Biol. 1983, 97, 1882–1890. [Google Scholar] [CrossRef]
- Dhoot, N.O.; Tobias, C.A.; Fischer, I.; Wheatley, M.A. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J. Biomed. Mater. Res. Part A 2004, 71, 191–200. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Myelin Biol. Disord. 2002, 110, 673–687. [Google Scholar]
- Agius, E.; Sagot, Y.; Duprat, A.M.; Cochard, P. Antibodies directed against the β1-integrin subunit and peptides containing the IKVAV sequence of laminin perturb neurite outgrowth of peripheral neurons on immature spinal cord substrata. Neuroscience 1996, 71, 773–786. [Google Scholar] [CrossRef]
- Caniggia, I.; Liu, J.; Han, R.; Wang, J.; Tanswell, A.K.; Laurie, G.; Post, M. Identification of receptors binding fibronectin and laminin on fetal rat lung cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 1996, 270. [Google Scholar] [CrossRef]
- Hannigan, G.E.; Leung-Hagesteijn, C.; Fitz-Gibbon, L.; Coppolino, M.G.; Radeva, G.; Filmus, J.; Bell, J.C.; Dedhar, S. Regulation of cell adhesion and anchorage-dependent growth by a new β1-integrin-linked protein kinase. Nature 1996, 379, 91–96. [Google Scholar] [CrossRef]
- Edhar, S.H.D.; Delcommenne, M.; Tan, C.; Gray, V.; Rue, L.; Woodgett, J.; Dedhar, S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. USA 1998, 95, 11211–11216. [Google Scholar]
- Fletcher, L.; Dedhar, S.; Young, C.E.; Young, S.S.; Barr, A.M.; O’Connor, T.P.; Legg, A.T.; Digicaylioglu, M.; Mills, J. Role of integrin-linked kinase in nerve growth factor-stimulated neurite outgrowth. J. Neurosci. 2018, 23, 1638–1648. [Google Scholar]
- Sakai, T.; Li, S.; Docheva, D.; Grashoff, C.; Sakai, K.; Kostka, G.; Braun, A.; Pfeifer, A.; Yurchenco, P.D.; Fässler, R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2009, 17, 1–15. [Google Scholar] [CrossRef]
- Guo, W.; Jiang, H.; Gray, V.; Dedhar, S.; Rao, Y. Role of the integrin-linked kinase (ILK) in determining neuronal polarity. Dev. Biol. 2007, 306, 457–468. [Google Scholar] [CrossRef]
- Pereira, J.A.; Benninger, Y.; Baumann, R.; Gonçalves, A.F.; Özçelik, M.; Thurnherr, T.; Tricaud, N.; Meijer, D.; Fässler, R.; Suter, U.; et al. Integrin-linked kinase is required for radial sorting of axons and schwann cell remyelination in the peripheral nervous system. J. Cell Biol. 2009, 185, 147–161. [Google Scholar] [CrossRef]
- O’Meara, R.W.; Michalski, J.P.; Anderson, C.; Bhanot, K.; Rippstein, P.; Kothary, R. Integrin-linked kinase regulates process extension in oligodendrocytes via control of actin cytoskeletal dynamics. J. Neurosci. 2013, 33, 9781–9793. [Google Scholar] [CrossRef]
- Nakrieko, K.A.; Welch, I.; Dupuis, H.; Bryce, D.; Pajak, A.; Arnaud, R.; Dedhar, S.; D’Souza, S.J.A.; Dagnino, L. Impaired hair follicle morphogenesis and polarized keratinocyte movement upon conditional inactivation of integrin-linked kinase in the epidermis. Mol. Biol. Cell 2008, 19, 1462–1473. [Google Scholar] [CrossRef]
- Grashoff, C.; Aszódi, A.; Sakai, T.; Hunziker, E.B.; Fässler, R. Integrin-linked kinase regulates chondrocyte shape and proliferation. EMBO Rep. 2003, 4, 432–438. [Google Scholar] [CrossRef]
- Terpstra, L.; Prud’Homme, J.; Arabian, A.; Takeda, S.; Karsenty, G.; Dedhar, S.; Arnaud, R. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J. Cell Biol. 2003, 162, 139–148. [Google Scholar] [CrossRef]
- Bravou, V.; Klironomos, G.; Papadaki, E.; Stefanou, D.; Varakis, J. Integrin-linked kinase (ILK) expression in human colon cancer. Br. J. Cancer 2003, 89, 2340–2341. [Google Scholar] [CrossRef][Green Version]
- Ahmed, N.; Riley, C.; Oliva, K.; Stutt, E.; Rice, G.E.; Quinn, M.A. Integrin-linked kinase expression increases with ovarian tumour grade and is sustained by peritoneal tumour fluid. J. Pathol. 2003, 201, 229–237. [Google Scholar] [CrossRef]
- Ito, R.; Oue, N.; Zhu, X.; Yoshida, K.; Nakayama, H.; Yokozaki, H.; Yasui, W. Expression of integrin-linked kinase is closely correlated with invasion and metastasis of gastric carcinoma. Virchows Arch. 2003, 442, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.P.C.; Ng, P.; Dedhar, S.; Li, G. The role of integrin-linked kinase in melanoma cell migration, invasion, and tumor growth. Mol. Cancer Ther. 2007, 6, 1692–1700. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H. A series of normal stages in the development of the chick embryo. Dev. Dyn. 1993, 88, 231–272. [Google Scholar]
- Toni, L.S.; Garcia, A.M.; Jeffrey, D.A.; Jiang, X.; Stauffer, B.L.; Miyamoto, S.D.; Sucharov, C.C. Optimization of phenol-chloroform RNA extraction. MethodsX 2018, 5, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.J.; Morosan-Puopolo, G.; Makowiecka, A.; Malicka-Błaszkiewicz, M.; Nowak, D.; Brand-Saberi, B. Analysis of gelsolin expression pattern in developing chicken embryo reveals high GSN expression level in tissues of neural crest origin. Brain Struct. Funct. 2016, 221, 515–534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Acloque, H.; Wilkinson, D.G.; Nieto, M.A. In situ hybridization analysis of chick embryos in whole-mount and tissue sections. In Methods in Cell Biology; Bronner-Fraser, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Chapter 9; Volume 87, pp. 169–185. ISBN 9780125641746. [Google Scholar]
- Powell, S.; Vinod, A.; Lemons, M.L. Isolation and culture of dissociated sensory neurons from chick embryos. J. Vis. Exp. 2014, 91, e51991. [Google Scholar] [CrossRef]
- He, Y.; Baas, P.W. Growing and working with peripheral neurons. Methods Cell Biol. 2003, 2003, 17–35. [Google Scholar]
- Petrinovic, M.M.; Duncan, C.S.; Bourikas, D.; Weinman, O.; Montani, L.; Schroeter, A.; Maerki, D.; Sommer, L.; Stoeckli, E.T.; Schwab, M.E. Neuronal Nogo—A regulates neurite fasciculation, branching and extension in the developing nervous system. Development 2010, 137, 2539–2550. [Google Scholar] [CrossRef]
- Towbin, H.; Stehelin, T. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carrera, I.; Frise, E.; Verena, K.; Mark, L.; Tobias, P.; Stephan, P.; Curtis, R.; Stephan, S.; Benjamin, S.; et al. Fiji—An open platform for biological image analysis. Nat. Methods 2009, 9, 676–682. [Google Scholar] [CrossRef]
- Torres-Espín, A.; Santos, D.; González-Pérez, F.; del Valle, J.; Navarro, X. Neurite-J: An image-j plug-in for axonal growth analysis in organotypic cultures. J. Neurosci. Methods 2014, 236, 26–39. [Google Scholar] [CrossRef]
- Rezakhaniha, R.; Agianniotis, A.; Schrauwen, J.T.C.; Griffa, A.; Sage, D.; Bouten, C.V.C.; Van De Vosse, F.N.; Unser, M.; Stergiopulos, N. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech. Model. Mechanobiol. 2012, 11, 461–473. [Google Scholar] [CrossRef]
- Jayyosi, C.; Coret, M.; Bruyère-Garnier, K. Characterizing liver capsule microstructure via in situ bulge test coupled with multiphoton imaging. J. Mech. Behav. Biomed. Mater. 2016, 54, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ho, C.L.; Sun, D.; Liem, R.K.H.; Brown, A. Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nat. Cell Biol. 2000, 2, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Hammarstrom, S.; Hammarstrom, M.L.; Sundblad, G.; Arnarp, J.; Lonngren, J. Mitogenic leukoagglutinin from Phaseolus vulgaris binds to a pentasaccharide unit in N-acetyllactosamine-type glycoprotein glycans. Proc. Natl. Acad. Sci. USA 1982, 79, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sheng, Y.; Li, Q.; Ju, S.; Reyes, J.; Lebrilla, C.B. Determination of the glycoprotein specificity of lectins on cell membranes through oxidative proteomics. Chem. Sci. 2020, 11, 9501–9512. [Google Scholar] [CrossRef]
- Stanley, P.; Harry, S.; Taniguchi, N. N-glycans. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009; ISBN 9780879697709. [Google Scholar]
- Masuda, T.; Sakuma, C.; Kobayashi, K.; Kikuchi, K.; Soda, E.; Shiga, T.; Kobayashi, K.; Yaginuma, H. Laminin peptide YIGSR and its receptor regulate sensory axonal response to the chemoattractive guidance cue in the chick embryo. J. Neurosci. Res. 2009, 87, 353–359. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Pulai, J.; Loeser, R.F. Fibronectin fragments and blocking antibodies to α2β1 and α5β1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002, 46, 2368–2376. [Google Scholar] [CrossRef]
- Troussard, A.A.; Mawji, N.M.; Ong, C.; Mui, A.; Arnaud, R.; Dedhar, S. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J. Biol. Chem. 2003, 278, 22374–22378. [Google Scholar] [CrossRef]
- Persad, S.; Attwell, S.; Gray, V.; Mawji, N.; Deng, J.T.; Leung, D.; Yan, J.; Sanghera, J.; Walsh, M.P.; Dedhar, S. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: Critical roles for kinase activity and amino acids arginine 211 and serine 343. J. Biol. Chem. 2001, 276, 27462–27469. [Google Scholar] [CrossRef]
- Persad, S.; Troussard, A.; McPhee, T.R.; Mulholland, D.J.; Dedhar, S. Tumor suppressor PTEN inhibits nuclear accumulation of β-catenin and T cell/lymphoid enhancer factor 1—Mediated transcriptional activation. J. Cell Biol. 2001, 153, 1161–1174. [Google Scholar] [CrossRef]
- Peng, X.; Nelson, E.S.; Maiers, J.L.; DeMali, K.A. New Insights into vinculin function and regulation. Int. Rev. Cell Mol. Biol. 2011, 287, 191–231. [Google Scholar]
- Perris, R.; Perissinotto, D. Role of the extracellular matrix during neural crest cell migration. Mech. Dev. 2000, 95, 3–21. [Google Scholar] [CrossRef]
- Gary, D.S.; Milhavet, O.; Camandola, S.; Mattson, M.P. Essential role for integrin linked kinase in Akt-mediated integrin survival signaling in hippocampal neurons. J. Neurochem. 2003, 84, 878–890. [Google Scholar] [CrossRef]
- Hannigan, G.E.; McDonald, P.C.; Walsh, M.P.; Dedhar, S. Integrin-linked kinase: Not so pseudo after all. Oncogene 2011, 30, 4375–4385. [Google Scholar] [CrossRef]
- Melchior, C.; Kreis, S.; Janji, B.; Kieffer, N. Promoter characterization and genomic organization of the gene encoding integrin-linked kinase 1. Biochim. Biophys. Acta Gene Struct. Expr. 2002, 1575, 117–122. [Google Scholar] [CrossRef]
- Pasquali, C.; Bertschy-Meier, D.; Chabert, C.; Curchod, M.L.; Arod, C.; Booth, R.; Mechtler, K.; Vilbois, F.; Xenarios, I.; Ferguson, C.G.; et al. A chemical proteomics approach to phosphatidylinositol 3-kinase signaling in macrophages. Mol. Cell. Proteom. 2007, 6, 1829–1841. [Google Scholar] [CrossRef]
- Sastry, S.K.; Burridge, K. Focal adhesions: A nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell Res. 2000, 261, 25–36. [Google Scholar] [CrossRef]
- Yamaji, S.; Suzuki, A.; Sugiyama, Y.; Koide, Y.I.; Yoshida, M.; Kanamori, H.; Mohri, H.; Ohno, S.; Ishigatsubo, Y. A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell-substrate interaction. J. Cell Biol. 2001, 153, 1251–1264. [Google Scholar] [CrossRef]
- Acconcia, F.; Barnes, C.J.; Singh, R.R.; Talukder, A.H.; Kumar, R. Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase. Proc. Natl. Acad. Sci. USA 2007, 104, 6782–6787. [Google Scholar] [CrossRef]
- Jatiani, A.; Pannizzo, P.; Gualco, E.; Del-Valle, L.; Langford, D. Neuronal PINCH is regulated by TNF-α and is required for neurite extension. J. Neuroimmune Pharmacol. 2011, 6, 330–340. [Google Scholar] [CrossRef][Green Version]
- Koul, D.; Shen, R.; Bergh, S.; Lu, Y.; de Groot, J.F.; Liu, T.J.; Mills, G.B.; Yung, W.K.A. Targeting integrin-linked kinase inhibits Akt signaling pathways and decreases tumor progression of human glioblastoma. Mol. Cancer Ther. 2005, 4, 1681–1688. [Google Scholar] [CrossRef]
- Morris, E.J.; Assi, K.; Salh, B.; Dedhar, S. Integrin-linked kinase links dynactin-1/dynactin-2 with cortical Integrin receptors to orient the mitotic spindle relative to the substratum. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wang, C.; Benedict, C.; Huang, G.; Truongcao, M.; Roy, R.; Cimini, M.; Garikipati, V.N.S.; Cheng, Z.; Koch, W.J.; et al. Interleukin-10 deficiency alters endothelial progenitor cell-derived exosome reparative effect on myocardial repair via integrin-linked kinase enrichment. Circ. Res. 2020, 126, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Layseca, P.; Icha, J.; Hamidi, H.; Ivaska, J. Integrin trafficking in cells and tissues. Nat. Cell Biol. 2019, 21, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Venstrom, K.; Reichardt, L. B8 integrins mediate interactions of chick sensory neurons with laminin-1, collagen iv, and fibronectin. Mol. Biol. Cell 1995, 6, 419–431. [Google Scholar] [CrossRef]
- Powell, S.K.; Kleinman, H.K. Neuronal laminins and their cellular receptors. Int. J. Biochem. Cell Biol. 1997, 29, 401–414. [Google Scholar] [CrossRef]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef]
- Chernousov, M.A.; Carey, D.J. Schwann cell extracellular matrix molecules and their receptors. Histol. Histopathol. 2000, 15, 593–601. [Google Scholar]
- Malakpour-Permlid, A.; Buzzi, I.; Hegardt, C.; Johansson, F.; Oredsson, S. Identification of extracellular matrix proteins secreted by human dermal fibroblasts cultured in 3D electrospun scaffolds. Sci. Rep. 2021, 11, 6655. [Google Scholar] [CrossRef]
- White, D.E.; Cardiff, R.D.; Dedhar, S.; Muller, W.J. Mammary epithelial-specific expression of the integrin linked kinase (ILK) results in the induction of mammary gland hyperplasias and tumors in transgenic mice. Oncogene 2001, 20, 7064–7072. [Google Scholar] [CrossRef]
- Wang, S.; Basson, M.D. Integrin-linked kinase: A multi-functional regulator modulating extracellular pressure-stimulated cancer cell adhesion through focal adhesion kinase and AKT. Cell. Oncol. 2009, 31, 273–289. [Google Scholar] [CrossRef]
- Attwell, S.; Mills, J.; Troussard, A.; Wu, C.; Dedhar, S. Integration of cell attachment, cytoskeletal localization, and signaling by integrin-linked kinase (ILK), CH-ILKBP, and the tumor suppressor PTEN. Mol. Biol. Cell 2003, 14, 4813–4825. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haggerty, A.E.; Al-Ali, H.; Oudega, M. Soluble laminin polymers enhance axon growth of primary neurons in vitro. J. Biomed. Mater. Res. Part A 2018, 106, 2372–2381. [Google Scholar] [CrossRef] [PubMed]
- Freire, E.; Gomes, F.C.A.; Linden, R.; Neto, V.M.; Coelho-Sampaio, T. Structure of laminin substrate modulates cellular signaling for neuritogenesis. J. Cell Sci. 2002, 115, 4867–4876. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hammarback, J.A.; Letourneau, P.C. Neurite extension across regions of low cell-substratum adhesivity: Implications for the guidepost hypothesis of axonal pathfinding. Dev. Biol. 1986, 117, 655–662. [Google Scholar] [CrossRef]
- Gomez, T.M.; Letourneau, P.C. Filopodia initiate choices made by sensory neuron growth cones at laminin/fibronectin borders in vitro. J. Neurosci. 1994, 14, 5959–5972. [Google Scholar] [CrossRef]
- Tanaka, E.; Sabry, J. Making the connection: Cytoskeletal rearrangements during growth cone guidance. Cell 1995, 83, 171–176. [Google Scholar] [CrossRef]
- Ren, Y.; Suter, D.M. Increase in growth cone size correlates with decrease in neurite growth rate. Neural Plast. 2016, 2016, 20–22. [Google Scholar] [CrossRef]
- Chadborn, N.H. PTEN couples Sema3A signalling to growth cone collapse. J. Cell Sci. 2006, 119, 951–957. [Google Scholar] [CrossRef]
- Kapfhammer, J.P.; Raper, J.A. Collapse of growth cone structure on contact with specific neurites in culture. J. Neurosci. 1987, 7, 201–212. [Google Scholar] [CrossRef]
- Rosso, G.; Young, P.; Shahin, V. Mechanosensitivity of embryonic neurites promotes their directional extension and Schwann cells progenitors migration. Cell. Physiol. Biochem. 2017, 44, 1263–1270. [Google Scholar] [CrossRef]
- Rossi, F.; Gianola, S.; Corvetti, L. Regulation of intrinsic neuronal properties for axon growth and regeneration. Prog. Neurobiol. 2007, 81, 1–28. [Google Scholar] [CrossRef]
- Saxena, S.; Caroni, P. Mechanisms of axon degeneration: From development to disease. Prog. Neurobiol. 2007, 83, 174–191. [Google Scholar] [CrossRef]
- Garratt, A.N.; Britsch, S.; Birchmeier, C. Neuregulin, a factor with many functions in the life of a Schwann cell. Bioessays 2000, 22, 987–996. [Google Scholar] [CrossRef]
- Berti, C.; Nodari, A.; Wrabetz, L.; Feltri, M.L. Role of integrins in peripheral nerves and hereditary neuropathies. Neuromol. Med. 2006, 8, 191–204. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrówczyńska, E.; Mazur, A.J. Integrin-Linked Kinase (ILK) Plays an Important Role in the Laminin-Dependent Development of Dorsal Root Ganglia during Chicken Embryogenesis. Cells 2021, 10, 1666. https://doi.org/10.3390/cells10071666
Mrówczyńska E, Mazur AJ. Integrin-Linked Kinase (ILK) Plays an Important Role in the Laminin-Dependent Development of Dorsal Root Ganglia during Chicken Embryogenesis. Cells. 2021; 10(7):1666. https://doi.org/10.3390/cells10071666
Chicago/Turabian StyleMrówczyńska, Ewa, and Antonina Joanna Mazur. 2021. "Integrin-Linked Kinase (ILK) Plays an Important Role in the Laminin-Dependent Development of Dorsal Root Ganglia during Chicken Embryogenesis" Cells 10, no. 7: 1666. https://doi.org/10.3390/cells10071666
APA StyleMrówczyńska, E., & Mazur, A. J. (2021). Integrin-Linked Kinase (ILK) Plays an Important Role in the Laminin-Dependent Development of Dorsal Root Ganglia during Chicken Embryogenesis. Cells, 10(7), 1666. https://doi.org/10.3390/cells10071666





