Photobiomodulation with a 660-Nanometer Light-Emitting Diode Promotes Cell Proliferation in Astrocyte Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
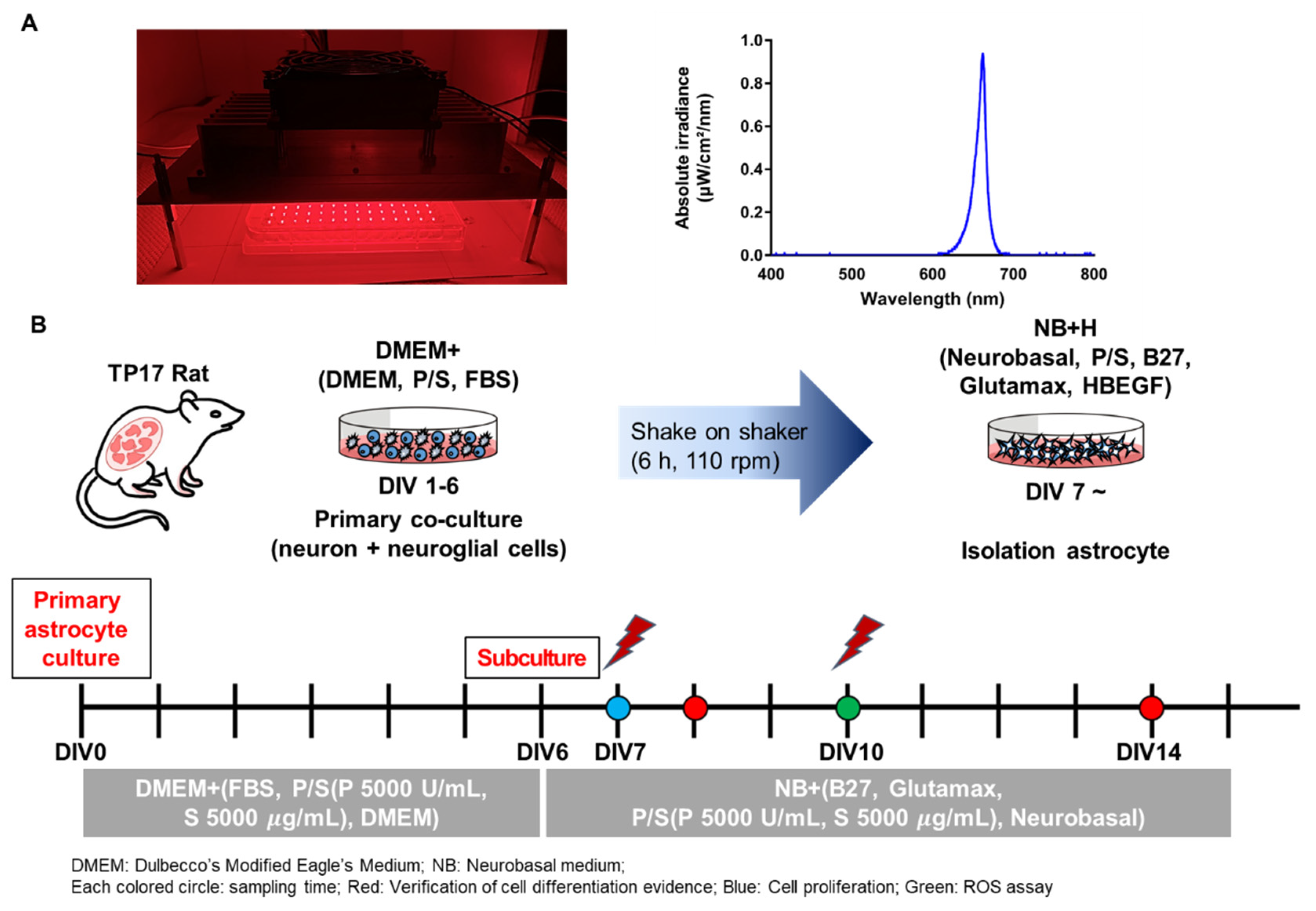
2.2. LED Irradiation (PBM)
2.3. Cell Culture
2.4. Cell Viability Assay
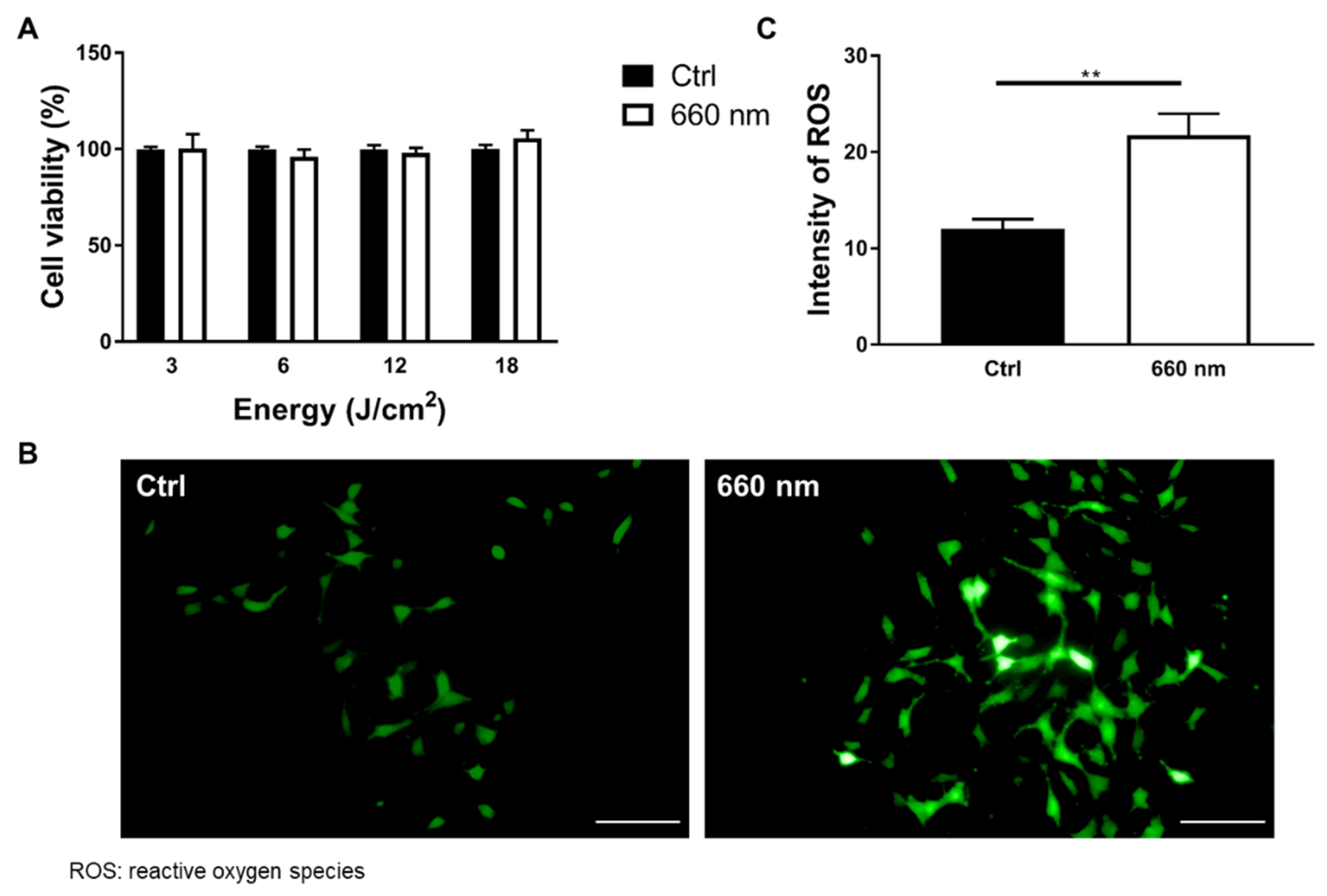
2.5. ROS Assay
2.6. Immunocytochemistry
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
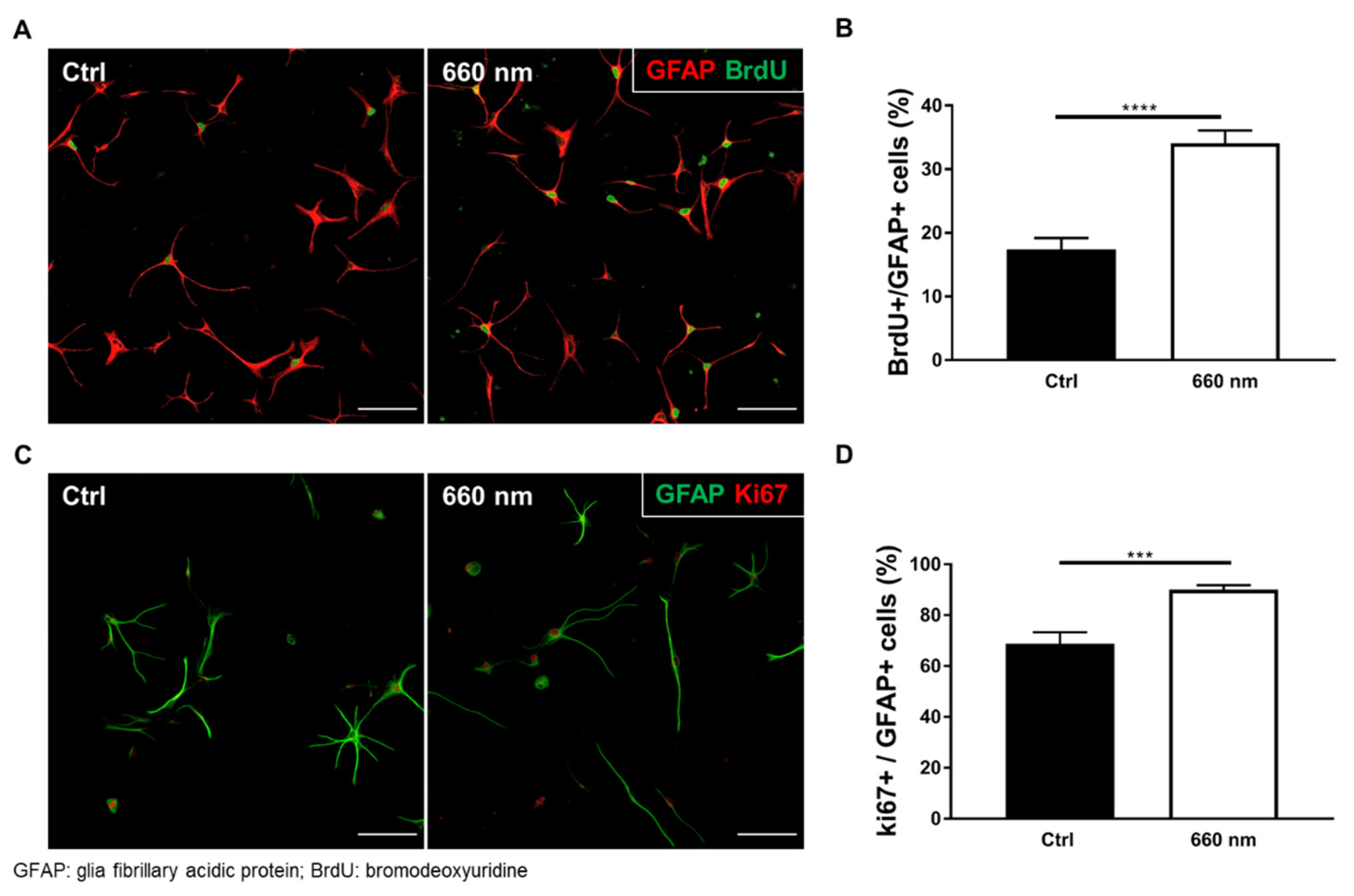
3.1. Verification of the Astrocyte Culture
3.2. Astrocyte Proliferation was Exclusively Enhanced by 660-nm LED Irradiation
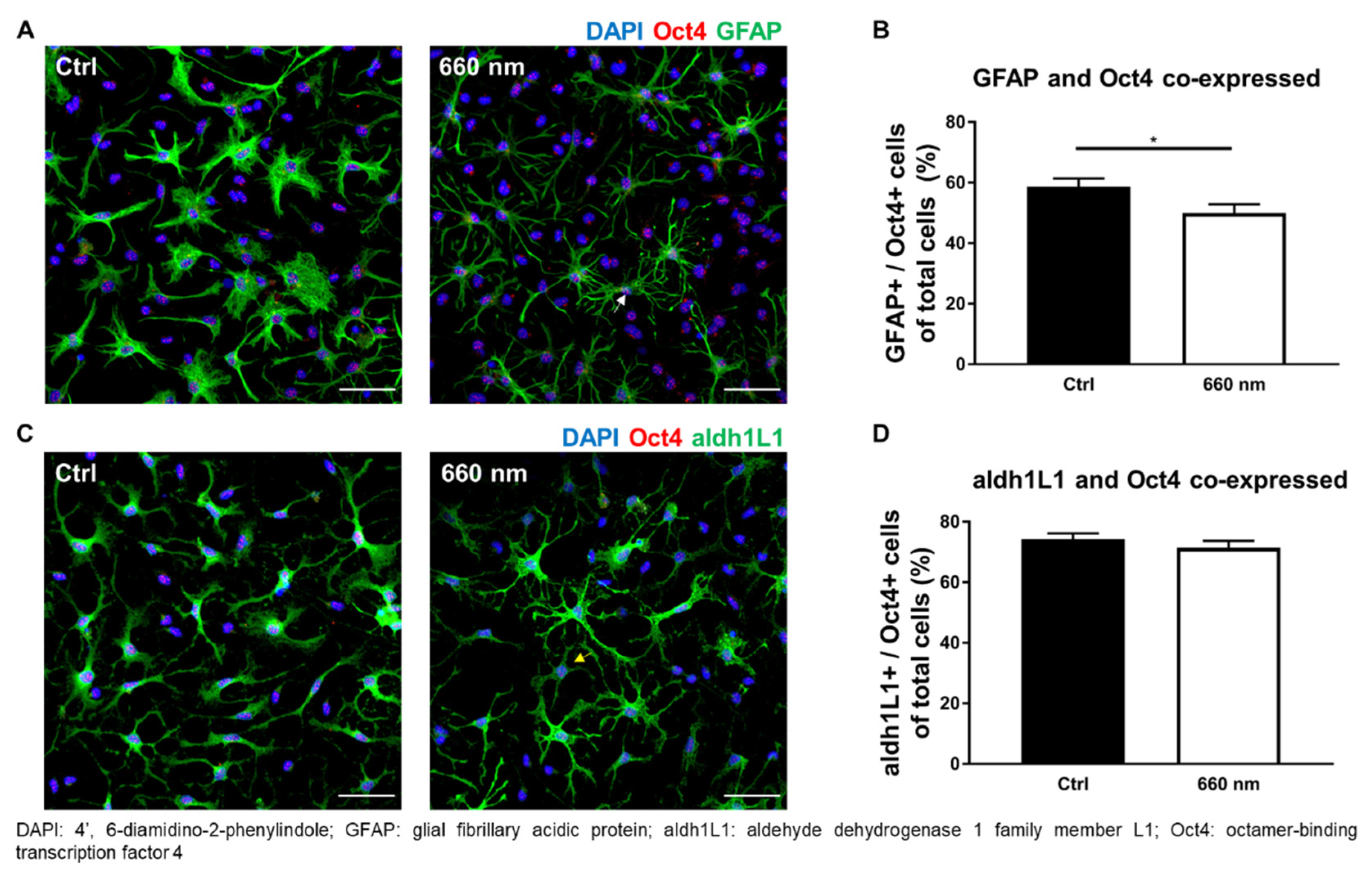
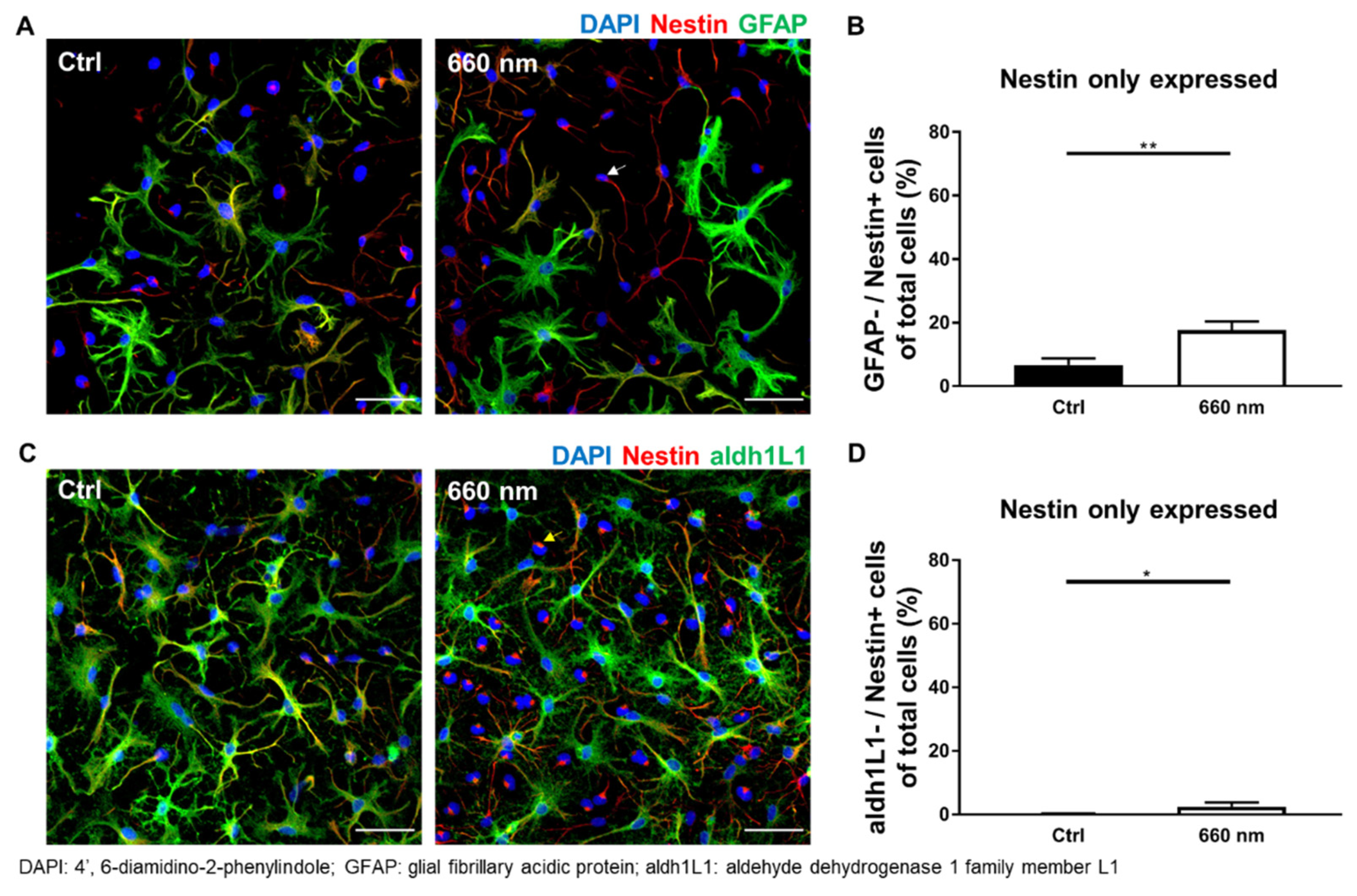
3.3. Possibility of Astrocyte Differentiation by 660-nm LED Irradiation
3.4. Alteration of Neurotrophic Factors in Astrocyte Culture by 660-nm LED Irradiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engel, M.; Do-Ha, D.; Muñoz, S.S.; Ooi, L. Common pitfalls of stem cell differentiation: A guide to improving protocols for neurodegenerative disease models and research. Cell. Mol. Life Sci. 2016, 73, 3693–3709. [Google Scholar] [CrossRef] [Green Version]
- Bernal, A.; Arranz, L. Nestin-expressing progenitor cells: Function, identity and therapeutic implications. Cell. Mol. Life Sci. 2018, 75, 2177–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, M.; Messing, A. Regulation of GFAP expression. ASN Neuro 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Khankan, R.R.; Caneda, C.; Godoy, M.I.; Haney, M.S.; Krawczyk, M.C.; Bassik, M.C.; Sloan, S.A.; Zhang, Y. Astrocyte-to-astrocyte contact and a positive feedback loop of growth factor signaling regulate astrocyte maturation. Glia 2019, 67, 1571–1597. [Google Scholar] [CrossRef] [PubMed]
- Kugler, P.; Schleyer, V. Developmental expression of glutamate transporters and glutamate dehydrogenase in astrocytes of the postnatal rat hippocampus. Hippocampus 2004, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Regan, M.R.; Huang, Y.H.; Kim, Y.S.; Dykes-Hoberg, M.I.; Jin, L.; Watkins, A.M.; Bergles, D.E.; Rothstein, J.D. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J. Neurosci. 2007, 27, 6607–6619. [Google Scholar] [CrossRef] [Green Version]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Vidensky, S.; Jin, L.; Jie, C.; Lorenzini, I.; Frankl, M.; Rothstein, J.D. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia 2011, 59, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef]
- Santello, M.; Toni, N.; Volterra, A. Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 2019, 22, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Lobsiger, C.S.; Cleveland, D.W. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat. Neurosci. 2007, 10, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, E.; Griffin, É.W.; Cunningham, C. Astrocytes are primed by chronic neurodegeneration to produce exaggerated chemokine and cell infiltration responses to acute stimulation with the cytokines IL-1β and TNF-α. J. Neurosci. 2015, 35, 8411–8422. [Google Scholar] [CrossRef]
- Tuchin, V. Tissue optics and photonics: Light-tissue interaction II. J. Biomed. Photonics Eng. 2016, 2, 030201. [Google Scholar] [CrossRef] [Green Version]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Shen, Q.; Wu, X.; Zhang, D.; Xing, D. Activation of PKA/SIRT1 signaling pathway by photobiomodulation therapy reduces Aβ levels in Alzheimer’s disease models. Aging Cell 2020, 19, e13054. [Google Scholar] [CrossRef]
- Meng, C.; He, Z.; Xing, D. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: Implications for Alzheimer’s disease. J. Neurosci. 2013, 33, 13505–13517. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.-C.; Park, J.-A.; Kim, D.-K.; Lee, J.-H. Photobiomodulation (660 nm) therapy reduces oxidative stress and induces BDNF expression in the hippocampus. Sci. Rep. 2019, 9, 10114. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Zhou, F.; Chen, W.R. Low-level laser therapy regulates microglial function through Src-mediated signaling pathways: Implications for neurodegenerative diseases. J. Neuroinflamm. 2012, 9, 219. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Shen, Q.; Wu, X.; Xing, D. Photobiomodulation therapy ameliorates glutamatergic dysfunction in mice with chronic unpredictable mild stress-induced depression. Oxid. Med. Cell. Longev. 2021, 2021, 6678276. [Google Scholar] [CrossRef]
- Ruan, Y.; Kato, H.; Taguchi, Y.; Yamauchi, N.; Umeda, M. Irradiation by high-intensity red light-emitting diode enhances human bone marrow mesenchymal stem cells osteogenic differentiation and mineralization through Wnt/β-catenin signaling pathway. Lasers Med. Sci. 2021, 36, 55–65. [Google Scholar] [CrossRef]
- Andreo, L.; Mesquita-Ferrari, R.A.; Grenho, L.; Gomes, P.S.; Bussadori, S.K.; Fernandes, K.P.S.; Fernandes, M.H. Effects of 660-nm and 780-nm laser therapy on ST88-14 Schwann cells. Photochem. Photobiol. 2021, 97, 198–204. [Google Scholar] [CrossRef]
- Jere, S.W.; Houreld, N.N.; Abrahamse, H. Photobiomodulation at 660nm stimulates proliferation and migration of diabetic wounded cells via the expression of epidermal growth factor and the JAK/STAT pathway. J. Photochem. Photobiol. B 2018, 179, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Wolfes, A.C.; Ahmed, S.; Awasthi, A.; Stahlberg, M.A.; Rajput, A.; Magruder, D.S.; Bonn, S.; Dean, C. A novel method for culturing stellate astrocytes reveals spatially distinct Ca2+ signaling and vesicle recycling in astrocytic processes. J. Gen. Physiol. 2017, 149, 149–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfes, A.C.; Dean, C. Culturing In vivo-like murine astrocytes using the fast, simple, and inexpensive AWESAM protocol. J. Vis. Exp. 2018. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.O.; Marques, D.P.; Venega, R.A.G.; Chacur, M. Photobiomodulation and B vitamins administration produces antinociception in an orofacial pain model through the modulation of glial cells and cytokines expression. Brain Behav. Immun. Health 2020, 2, 100040. [Google Scholar] [CrossRef]
- Lu, Y.-Z.; Fernando, N.; Natoli, R.; Madigan, M.; Valter, K. 670 nm light treatment following retinal injury modulates Müller cell gliosis: Evidence from in vivo and in vitro stress models. Exp. Eye Res. 2018, 169, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vinck, E.M.; Cagnie, B.J.; Cornelissen, M.J.; Declercq, H.A.; Cambier, D.C. Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Lasers Med. Sci. 2003, 18, 95–99. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.-Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci. Rep. 2017, 7, 7781. [Google Scholar] [CrossRef]
- Bergamo, M.T.; Vitor, L.L.R.; Dionísio, T.J.; Marques, N.C.T.; Oliveira, R.C.; Ambrosio, E.C.P.; Sakai, V.T.; Santos, C.F.; Lourenço Neto, N.; Machado, M.; et al. Could the photobiomodulation therapy induce angiogenic growth factors expression from dental pulp cells? Lasers Med. Sci. 2021. [Google Scholar] [CrossRef]
- Aaron Chi-Hao, C.; Ying-Ying, H.; Praveen, R.A.; Michael, R.H. Role of reactive oxygen species in low level light therapy. In Proceedings of SPIE; SPIE: Bellingham, WA, USA, 2009. [Google Scholar]
- Lavi, R.; Shainberg, A.; Shneyvays, V.; Hochauser, E.; Isaac, A.; Zinman, T.; Friedmann, H.; Lubart, R. Detailed analysis of reactive oxygen species induced by visible light in various cell types. Lasers Surg. Med. 2010, 42, 473–480. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Huang, Y.-Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: Role of intracellular calcium and light-gated ion channels. Sci. Rep. 2016, 6, 33719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamani, A.R.N.; Saberianpour, S.; Geranmayeh, M.H.; Bani, F.; Haghighi, L.; Rahbarghazi, R. Modulatory effect of photobiomodulation on stem cell epigenetic memory: A highlight on differentiation capacity. Lasers Med. Sci. 2020, 35, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, M.J.; Crous, A.; Abrahamse, H. Potential of photobiomodulation to induce differentiation of adipose- derived mesenchymal stem cells into neural cells. Curr. Stem Cell Res. Ther. 2021, 16, 307–322. [Google Scholar] [CrossRef]
- George, S.; Hamblin, M.R.; Abrahamse, H. Photobiomodulation-induced differentiation of immortalized adipose stem cells to neuronal cells. Lasers Surg. Med. 2020, 52, 1032–1040. [Google Scholar] [CrossRef]
- Mirhosseini, M.; Shiari, R.; Esmaeili Motlagh, P.; Farivar, S. Cerebrospinal fluid and photobiomodulation effects on neural gene expression in dental pulp stem cells. J. Lasers Med. Sci. 2019, 10, S30–S36. [Google Scholar] [CrossRef] [Green Version]
- Mokoena, D.R.; Houreld, N.N.; Dhilip Kumar, S.S.; Abrahamse, H. Photobiomodulation at 660 nm stimulates fibroblast differentiation. Lasers Surg. Med. 2020, 52, 671–681. [Google Scholar] [CrossRef]
- Parpura, V.; Basarsky, T.A.; Liu, F.; Jeftinija, K.; Jeftinija, S.; Haydon, P.G. Glutamate-mediated astrocyte-neuron signalling. Nature 1994, 369, 744–747. [Google Scholar] [CrossRef]
- Vesce, S.; Bezzi, P.; Volterra, A. The active role of astrocytes in synaptic transmission. Cell. Mol. Life Sci. 1999, 56, 991–1000. [Google Scholar] [CrossRef]
- Durkee, C.A.; Araque, A. Diversity and specificity of astrocyte-neuron communication. Neuroscience 2019, 396, 73–78. [Google Scholar] [CrossRef]
- Zhou, B.; Zuo, Y.X.; Jiang, R.T. Astrocyte morphology: Diversity, plasticity, and role in neurological diseases. CNS Neurosci. Ther. 2019, 25, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Calderhead, R.G. Is light-emitting diode phototherapy (LED-LLLT) really effective? Laser Ther. 2011, 20, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, S.J.; Dawbarn, D. Clinical relevance of the neurotrophins and their receptors. Clin. Sci. 2006, 110, 175–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, M.H.; Nichols, T.W. Treatment of neurodegeneration: Integrating photobiomodulation and neurofeedback in Alzheimer’s dementia and Parkinson’s: A review. Photobiomodul. Photomed. Laser Surg. 2019, 37, 623–634. [Google Scholar] [CrossRef]
- Hashmi, J.T.; Huang, Y.-Y.; Osmani, B.Z.; Sharma, S.K.; Naeser, M.A.; Hamblin, M.R. Role of low-level laser therapy in neurorehabilitation. Phys. Med. Rehabil. 2010, 2, S292–S305. [Google Scholar] [CrossRef] [Green Version]
- Bouvet-Gerbettaz, S.; Merigo, E.; Rocca, J.P.; Carle, G.F.; Rochet, N. Effects of low-level laser therapy on proliferation and differentiation of murine bone marrow cells into osteoblasts and osteoclasts. Lasers Surg. Med. 2009, 41, 291–297. [Google Scholar] [CrossRef]
- Yang, L.; Tucker, D.; Dong, Y.; Wu, C.; Lu, Y.; Li, Y.; Zhang, J.; Liu, T.C.; Zhang, Q. Photobiomodulation therapy promotes neurogenesis by improving post-stroke local microenvironment and stimulating neuroprogenitor cells. Exp. Neurol. 2018, 299, 86–96. [Google Scholar] [CrossRef]
- Argibay, B.; Campos, F.; Perez-Mato, M.; Vieites-Prado, A.; Correa-Paz, C.; López-Arias, E.; da Silva-Candal, A.; Moreno, V.; Montero, C.; Sobrino, T.; et al. Light-emitting diode photobiomodulation after cerebral ischemia. Front. Neurol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Mason, M.G.; Nicholls, P.; Cooper, C.E. Re-evaluation of the near infrared spectra of mitochondrial cytochrome c oxidase: Implications for non invasive in vivo monitoring of tissues. BBA Bioenerg. 2014, 1837, 1882–1891. [Google Scholar] [CrossRef] [Green Version]
- Lima, P.L.V.; Pereira, C.V.; Nissanka, N.; Arguello, T.; Gavini, G.; Maranduba, C.; Diaz, F.; Moraes, C.T. Photobiomodulation enhancement of cell proliferation at 660 nm does not require cytochrome c oxidase. J. Photochem. Photobiol. B Biol. 2019, 194, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, S.; Fong, C.C.; Tsang, C.H.; Yang, Z.; Yang, M. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J. Investig. Dermatol. 2003, 120, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, G.E. Photobiomodulation: A review of the molecular evidence for low level light therapy. J. Plastic Reconstr. Aesthet. Surg. 2021, 74, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdani, S.O.; Golestaneh, A.F.; Shafiee, A.; Hafizi, M.; Omrani, H.A.; Soleimani, M. Effects of low level laser therapy on proliferation and neurotrophic factor gene expression of human schwann cells in vitro. J. Photochem. Photobiol. B Biol. 2012, 107, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Black, I.B.; Dreyfus, C.F. NGF increases brain astrocyte number in culture. Exp. Neurol. 1993, 124, 377–380. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Wavelength (nm) | 660 |
| Operating Mode | Continuous wave |
| Voltage Value (V) | 25.2 |
| Distance of LED from Cell Culture Plate (cm) | 4 |
| Irradiance (SUM) (mW/cm2) | 10 |
| Exposure Duration (min) | 10 |
| Radiant Energy (J/cm2) | 6 |
| Cell Type | Protein Expression | Ctrl vs. 660 nm | ||
|---|---|---|---|---|
| Mean ± SEM (# of Cells) | p-Value | U or t | ||
| Pluripotent stem cell | Oct4+/GFAP− | 16.36 ± 3.026 vs. 22.67 ± 2.826 (35) | 0.0595 | 452 |
| Oct4+/aldh1L1− | 2.166 ± 0.6865 vs. 2.735 ± 0.6442 (36) | 0.1542 | 531.5 | |
| Neural stem cell | Nestin+/GFAP− | 6.725 ± 2.072 vs. 17.7 ± 2.648 (38) | 0.0057 ** | 462.5 |
| Nestin+/aldh1L1− | 0.1517 ± 0.1425 vs. 2.446 ± 1.3 (39) | 0.0335 * | 640.5 | |
| Developmental astrocytes | GFAP+/Oct4− | 2.5 ± 0.6376 vs. 3.343 ± 0.5668 (35) | 0.1681 | 495 |
| GFAP+/Nestin− | 1.782 ± 0.2909 vs. 1.497 ± 0.2142 (39) | 0.8988 | 709.5 | |
| Maturity astrocytes | aldh1L1+/Oct4− | 8.484 ± 1.474 vs. 9.874 ± 1.694 (36) | 0.1609 | 523 |
| aldh1L1+/Nestin− | 5.219 ± 0.9469 vs. 9.785 ± 1.786 (39) | 0.0617 | 573.5 | |
| Immature astrocytes with ability to differentiate to other specific cell types | GFAP+/Oct4+ | 58.78 ± 2.59 vs. 49.98 ± 2.88 (35) | 0.0240 * | 421 |
| GFAP+/Nestin+ | 56.76 ± 2.474 vs. 52.57 ± 3.059 (38) | 0.2901 | 1.065 | |
| Mature astrocytes with ability to differentiate to other specific cell types | aldh1L1+/Oct4+ | 74.23 ± 1.921 vs. 71.37 ± 2.345 (36) | 0.5177 | 590 |
| aldh1L1+/Nestin+ | 70.99 ± 1.686 vs. 66.17 ± 2.435 (39) | 0.1907 | 629 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.-R.; Hong, N.; Lee, M.-Y.; Ahn, J.-C. Photobiomodulation with a 660-Nanometer Light-Emitting Diode Promotes Cell Proliferation in Astrocyte Culture. Cells 2021, 10, 1664. https://doi.org/10.3390/cells10071664
Yoon S-R, Hong N, Lee M-Y, Ahn J-C. Photobiomodulation with a 660-Nanometer Light-Emitting Diode Promotes Cell Proliferation in Astrocyte Culture. Cells. 2021; 10(7):1664. https://doi.org/10.3390/cells10071664
Chicago/Turabian StyleYoon, Sung-Ryeong, Namgue Hong, Min-Young Lee, and Jin-Chul Ahn. 2021. "Photobiomodulation with a 660-Nanometer Light-Emitting Diode Promotes Cell Proliferation in Astrocyte Culture" Cells 10, no. 7: 1664. https://doi.org/10.3390/cells10071664
APA StyleYoon, S.-R., Hong, N., Lee, M.-Y., & Ahn, J.-C. (2021). Photobiomodulation with a 660-Nanometer Light-Emitting Diode Promotes Cell Proliferation in Astrocyte Culture. Cells, 10(7), 1664. https://doi.org/10.3390/cells10071664






