Abstract
The scaffolding protein family Fe65, composed of Fe65, Fe65L1, and Fe65L2, was identified as an interaction partner of the amyloid precursor protein (APP), which plays a key function in Alzheimer’s disease. All three Fe65 family members possess three highly conserved interaction domains, forming complexes with diverse binding partners that can be assigned to different cellular functions, such as transactivation of genes in the nucleus, modulation of calcium homeostasis and lipid metabolism, and regulation of the actin cytoskeleton. In this article, we rule out putative new intracellular signaling mechanisms of the APP-interacting protein Fe65 in the regulation of actin cytoskeleton dynamics in the context of various neuronal functions, such as cell migration, neurite outgrowth, and synaptic plasticity.
Keywords:
Mena; ELMO; Tip60; cortactin; DOCK; Arf6; Rac; Arp2/3; neurite outgrowth; structural synaptic plasticity 1. The Fe65 Protein Family
In mammals, the scaffolding protein family Fe65 is composed of Fe65 itself and two Fe65-like proteins, Fe65L1 and Fe65L2. They are all encoded by single genes called APBB1, APBB2, and APBB3, respectively [1,2,3]. APBB stands for APP-binding family B and refers to the observation that all three Fe65 proteins bind to the amyloid precursor protein (APP; Box 1) involved in the pathogenesis of Alzheimer’s disease (AD) [1,4,5,6,7,8,9]. Initially, the cDNA of Fe65 was cloned from rat brain and later described as a putative transcriptionally active protein with similarity to retroviral integrases [10,11]. Later, the predominant expression of Fe65 in the brain was confirmed by different studies, whereas, both Fe65L1 and Fe65L2 mRNAs were more widely expressed in non-neuronal tissues [1,2,3,7,12]. Thus far, due to limitations of available antibodies, only Fe65 distribution in brain tissue has been studied in detail [13]. Here, Fe65 shows broad expression throughout the brain, increasing from birth to adulthood.
All members of the Fe65 family have a conserved domain structure, with a WW domain and two C-terminal phosphotyrosine-binding domains (PTB1 and PTB2), whereas human Fe65 and Fe65L1 have a long N-terminal domain with a length of 258 and 290 amino acids, respectively. The corresponding region is missing in Fe65L2. In addition, there are several splice variants of all members of the Fe65 family. At least six different isoforms have been reported for Fe65: Isoform 1, also called p97Fe65, is the longest isoform with 710 amino acids; isoforms 2 and 3 lack two amino acids, E462 and R463, resulting from a deletion of mini-exon 9 [14]; isoforms 3, 5 and 6 have an N-terminal deletion of 240 amino acids and instead carrying a short N-terminal sequences of 6–20 amino acids derived from alternative start positions; isoform 4, also called p60Fe65, is N-terminally truncated by 259 amino acids. Remarkably, splice variants 2 and 3, which lack exon 9, are predominantly expressed in non-neuronal cells [14], while p60Fe65 is expressed in neurons but absent from some brain regions, such as the cerebellum [15]. Interestingly, Fe65 is also a subject of proteolytic cleavage, resulting in the product p65Fe65, which has an increased affinity for APP compared to the full-length p97Fe65 [16,17]. For Fe65L1, at least four isoforms are annotated in the database (UniProt), but to our knowledge only three isoforms have been yet experimentally validated [2,12,18]. For Fe65L2, at least six different isoforms are annotated in the database (UniProt), which show variability in the PTB1 domain, but have not yet been experimentally validated.
Fe65 homologs have been reported for many different vertebrates, including humans, mice, and fish. Furthermore, in non-vertebrates, a homolog protein of Fe65, Feh-1 from Caenorhabditis elegans, was reported that also has a conserved domain structure [19,20]. However, the closest homolog to Feh-1 in Drosophila, showing high homology in the PTB domain, is Numb1. This indicates that the gene of Fe65 with a WW and two PTB domains was lost in the group of arthropods. However, more detailed studies will be required to clarify the phylogenetic development of the Fe65 gene family.
Recent X-ray crystallography and NMR measurements suggest a homotypic dimerization of Fe65 via the PTB2 domain, involving unwinding of a C-terminal α-helix at the end of one PTB2 domain, binding to the PTB2 domain of a second Fe65 molecule [21]. This intermolecular PTB2–PTB2 binding might occur simultaneously with the predicted intramolecular WW–PTB2 interaction, which involves the PTB1–PTB2 boundary [22]. Likely, the dimerization property is unique to Fe65, as the structural essential Aspartate 662 and Arginine 665, forming a salt bridge in the dimerization pocket, are not conserved in Fe65L1 and Fe65L2. However, currently it is unclear to what extent Fe65 dimerization might affect the binding of interaction partners and its physiological function.
Box 1. The Amyloid Precursor Protein and its Physiological Function.
The amyloid precursor protein (APP) is a type I transmembrane protein with a large extracellular and a short intracellular domain. It undergoes a complex proteolytic processing by sequential cleavage of different sheddases that cleave extracellular portions of transmembrane proteins, releasing the soluble ectodomains, followed by γ-secretase cleavage of the residual membrane tethered stub. Best investigated sheddases are the α- and β-secretase, releasing the soluble fragment sAPPα or sAPPβ, respectively, with only 13 amino acids difference in length but severe differences in function. While sAPPα has clear neuroprotective properties, these were mostly observed in lower activity or not at all in comparative studies for sAPPβ. Depending on the sheddase cleavage, also the fragments released after intramembranous cleavage by γ-secretase differ dramatically. In the so-called amyloidogenic pathway after sAPPβ was cleaved off, two fragments are generated. The APP intracellular domain that gets released in the cytoplasm and the extracellular released Aβ peptide that forms oligomers and large aggregates that accumulate in form of β-amyloid plaques in Alzheimer’s disease. In contrast, in the non-amyloidogenic pathway after sAPPα generation, a non-toxic instable P3 fragment and AICD get released. Notably, the AICD is proposed to be involved in regulation of transcriptional activity together with Fe65. However, extensive research revealed more complex APP processing, involving a large variety of different secreted factors (>10) with diverse neuroprotective or pathogenic properties. Supplementary to the function of the secreted fragments, additional functions of full-length membrane-bound APP have been proposed as co-receptors for very different signaling pathways, involved in neurite outgrowth and synaptic plasticity. Interestingly, a function of APP forming transcellular dimers as a synaptic adhesion molecule has also been suggested in this context. However, the molecular signaling of APP is not yet understood, genetic studies, particularly considering the overlapping function of the two APP homologous proteins, APLP1 and APLP2, clearly showed an essential contribution in diverse cellular functions such as neuronal outgrowth, synaptic plasticity, and vesicular trafficking [23,24,25,26].
2. Fe65 Interaction Partners
Important insights into the putative function of the Fe65 family come from the analysis of interaction partners. Most attention has been paid to the Fe65-PTB2 domain as it directly interacts with the APP C-terminus that links Fe65 to a central protein of AD [6]. Notably, the mode of interaction observed for Fe65 homo-dimerization mimics the interaction of the APP-C-terminus with the Fe65-PTB2 domain, indicating that Fe65 dimerization might prevent low-affinity interactions. The binding to APP might cause a switch to an active monomeric Fe65 state [21].
In addition to APP, more than 20 different Fe65 interaction partners have been reported [27] that can be clustered into different functional groups. A central protein in one of these clusters is the histone acetyltransferase Tip60, possibly forming a transcriptional active ternary complex with the liberated APP intracellular domain (AICD), AICD/Fe65/Tip60, allowing transition of Fe65 from a closed to an open active conformation [28,29]. Interestingly, solely Fe65 in complex with APP is capable of regulating gene expression, while co-expression of APP together with Fe65L1 or Fe65L2 did not mediate transcriptional activity and did not cause an AICD translocation to nuclear spots [28,30,31,32,33]. In line with the assumption that the AICD/Fe65 complex is involved in transcriptional regulation, Fe65-PTB1 binding to a transcription factor, CP2/LSF/LBP1, was reported [34]. Moreover, the Fe65 WW domain was found to interact with Abl tyrosine kinase and the nucleosome assembly factor SET, which plays an intriguing role in nuclear signaling and transcriptional activation [30,35,36]. Different target genes activated by the AICD/Fe65 complex were proposed, including KAI1, APP, and also actin cytoskeleton regulators, such as alpha-actin2 and transgelin [33,37,38,39]. Despite the strength of data clearly pointing to a role of AICD and Fe65 in transcriptional regulation [40,41], the topic is still controversially discussed [41,42,43], mainly because the precise mode of action and the relevant target genes have yet to be defined.
A second group of interaction partners links Fe65 function to lipid metabolism. For instance, the family of low-density lipoprotein receptors (LDLR), including the low-density lipoprotein receptor-related protein 1 (LRP1), very low-density lipoprotein receptor (VLDLR), Megalin/LRP2, and ApoEr2 were shown to bind to the Fe65 PTB1 domain [44,45,46,47,48]. This putative link of Fe65 to ApoE is of particular interest, as ApoE4 has been reported as a major risk factor for AD [49].
A third group of interaction partners was identified in a mass spectrometry-based analysis, reporting more than a hundred Fe65 interaction partners, including some involved in calcium regulation [50]. In this context, it is worth mentioning that Fe65 was reported to bind to P2X2 receptors, involved in synaptic plasticity of excitatory synapses [51]. Together, these data clearly indicate that the adaptor protein Fe65 is functionally involved in distinct cellular processes. However, how these competing interactions of different binding partners get regulated is not clear yet and will require further in vivo analysis to enlighten the interplay of the multiple at least partially competing interaction partners in a physiological context.
3. Fe65 Associated Actin Cytoskeleton Regulators
Another major group of Fe65 interacting proteins is involved in actin regulation, encompassing Mena (mammalian ENA), cortactin, and ELMO1/DOCK1 (Table 1) [52,53,54,55,56]. In the following, we will discuss the role of these proteins in cellular functions to unravel putative mechanistic links of Fe65 to the actin cytoskeleton.

Table 1.
Fe65 interaction partners involved in actin dynamics.
3.1. Mena
Mena, the mammalian homolog of Drosophila enabled, is a member of the Mena/VASP protein family, consisting of vasodilator-stimulated phosphoprotein (VASP), Ena-VASP-like protein (EVL), and Mena. All members are composed of two Ena/VASP homology domains (EVH1 + EVH2) and a proline-rich core region [59,60,61,62,63].
Mena/VASP members are highly abundant in the brain and concentrated in regions of high actin dynamics such as focal adhesions, stress fibers, lamellipodia, filopodia, ruffles, and growth cones [59,64,65,66,67,68,69,70,71,72,73]. In these processes, they are supposed to have an important physiological function in neurite positioning, outgrowth, axon guidance, and cell movement [71,72,74,75,76,77,78,79,80].
Mena and its homologs act as anti-capping proteins and support filamentous actin polymerization [63,81,82,83]. The association of G-actin to Mena/VASP proteins was found to be tenfold higher in the presence of profilin, and the loading efficiency of profilin with G-actin was increased in the presence of the Mena/VASP family [84,85]. Thus, Mena/VASP proteins are assumed to stabilize and recruit the polymerization competent profilin/G-actin complex to the elongation site of filopodia. Profilin accelerates the exchange of ADP to ATP in G-actin leading to the replenishment of activated ATP-actin pool subsequently favoring actin polymerization [85]. In addition, the Mena/VASP family was shown to reduce the frequency of actin filament branching, mainly provided by the Arp2/3 complex [81,86,87,88]. However, it is unclear whether the reduced actin branching is an active inhibitory process of Mena/VASP or a consequence of competition with monomeric actin. Although, Skruber et al. demonstrated a concentration-dependent profilin manner of actin regulation inducing filopodia formation at low profilin concentrations and additional Arp2/3-dependent lamellipodia formation at higher profilin concentrations [89]. Furthermore, Mena protein family activity can be modulated by posttranslational modifications like phosphorylation and ubiquitination [67,78,90]. Thus, fast and refined remodeling and adaptation during neuronal development can be achieved.
Initial evidence that Fe65 may be involved in actin cytoskeleton regulation comes from a biochemical study showing that Mena preferentially interacts with the WW domain of Fe65 via two central PPxPP motifs, analyzed in detail by crystallography [20,57]. Consequently, the connection of Fe65 with the Mena/VASP family may allow to couple external stimuli to changes in actin cytoskeleton dynamics, similarly as shown for vinculin, zyxin, Robo and semaphorin 6A-1 [64,77,91,92,93], which link the Mena function directly to signals of the extracellular matrix. Although not shown directly, APP interacting with Slit, a repulsive cue and ligand of Robo that promotes filopodia formation while leading to the collapse of lamellipodia structures for the right pathfinding, might involve Fe65 scaffolding activity [55,94]. Thus, Fe65 binding to cell surface proteins, such as APP, ApoEr2 or LDL receptors, may recruit Mena to the plasma membrane, promoting actin polymerization. Consistently, P. Greengard’s lab reported binding and co-localization of Mena with Fe65, APP, and β1-integrin in mobile lamellipodia and focal complexes [52].
3.2. Cortactin
Cortactin, a class II nucleation promoting factor, recruits N-WASP (neural Wiskott–Aldrich syndrome protein), which in turn activates the Arp 2/3 complex by changing the position of Arp2 forming an Arp2–Arp3 short-pitch dimer and generating a new daughter actin filament [95,96]. After initiation, N-WASP dissociates and cortactin stays to further stabilize newly formed filament branching points [97,98,99,100,101]. Cortactin, like Mena, is highly abundant at the leading edge of cells, regulating cytoskeletal remodeling [56,97,102,103,104,105,106,107,108,109,110]. While Mena increases the filamentous polymerization of actin, cortactin promotes the formation of meshwork structures by activating the Arp2/3 complex.
Cortactin undergoes different posttranslational modifications, regulating its activity. Phosphorylation by distinct kinases, including Src, Abl, Arg, Erk, PAK, and PKD [102,105,107,111,112,113,114,115], was shown to promote interaction with actin-binding proteins (ABPs), such as N-WASP and Arp2/3 complex [116], stimulating cortactin activity. Acetylation, in contrast to phosphorylation, lowers the association of cortactin with actin and inhibits its Arp2/3-dependent polymerization function [102,103,116,117,118,119,120].
As Fe65 binds to the histone acetylase Tip60 that is capable of increasing cortactin acetylation, it inhibits the association of cortactin with actin [56,116]. In line with this, it was confirmed by using an inducible knockdown of Fe65 in HEK 293T cells that expression of Tip60 increases acetylation of cortactin in the presence but not the absence of Fe65 [56]. As cortactin was shown to have an important role in dendritic spine plasticity [121,122,123], it is tempting to speculate that inhibition of cortactin activity by Fe65/Tip60 mediated acetylation is involved in structural synaptic plasticity and possibly also in cell migration.
In addition to the regulation of actin dynamics, the interaction of cortactin and Fe65 might also play a role in the nucleus, as acetylated cortactin gets translocated to the nucleus, similarly as shown for the Fe65-APP-Tip60 tripartite complex [28,30,32,33,124]. However, the nuclear function of cortactin and its interplay with Fe65 is still unclear.
3.3. ELMO1/DOCK1/Arf6/Rac
Rac (Ras-related C3 botulinum toxin substrate 1) is a member of the Rho family of small GTPases that regulates axon and dendrite differentiation, prolongation, and arborization while it is antagonized by RhoA activity [125,126,127]. Rac proteins, like any other small GTPases, cycle between GDP- and GTP-bound (inactive/active) states. Active GTP-bound Rac proteins activate the Arp2/3 complex via the WASP, N-WASP, and WAVE proteins that, in turn, promotes actin polymerization.
Rac proteins get activated by guanine exchange factors (GEFs) that promote nucleotide exchange from GDP to GTP [128]. The GEFs for Rho/Rac GTPases are divided into the Dbl and DOCK (dictator of cytokinesis) families. Dbl family members can activate all members of the Rho family, whereas DOCK GEFs specifically activate Rac and/or Cdc42. In total, more than 80 GEFs are known, of which only 11 belong to the DOCK family that is subdivided into four groups (DOCK A to D), based on sequence similarity and domain organization. DOCK1, also called DOCK180, belongs to the DOCK A family that activates only Rac1. It has an N-terminal SH3 domain that binds the engulfment and cell motility proteins ELMO1–3 and activates RhoG at the plasma membrane. As DOCK1 binds to PI(3,4,5)P3, this might help to localize the complex to the membrane. However, more recent data suggest that the PIP3 binding site binds preferentially to phosphatidic acid (PA). In response to growth factors, PA is partly generated through hydrolysis of phosphatidylcholine by phospholipase D involved in signaling [129]. The DOCK1/ELMO complex mediates the activation of Rac at the leading edge or focal adhesion sites to form lamellipodia, which further promote cell spreading and migration [130]. Notably, the direction of migration further relies on microtubule stabilization, mediated by ACF7, a partner of ELMO [131].
ELMO proteins were first discovered in a genetic screen to identify components required for engulfment of dead cells and cell motility in C. elegans [132]. ELMO and DOCK often form a complex, which exists in an active and inactive state. Activation of cell surface receptors typically stimulates the GEF activity of ELMO/DOCK complexes, which in turn activate Rac proteins for cell migration. However, the mechanism regulating the ELMO/DOCK complex activity has not been fully understood. It can associate with ARNO/Arf family GTPases at the plasma membrane [133,134] where Arf6 controls endocytosis, actin dynamics, and lipid modifications [135,136]. Interestingly, ELMO/DOCK1 and Rac are also involved in the initiation and maintenance of dendritic spines. Along this line, a knockdown of either ELMO or DOCK1 reduces the formation of spines [137] and overexpression of constitutive active Rac1 induces the transition from filopodia to spine formation, increasing the spine density and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) clustering but simultaneously reduces spine head size. In line with this, the dominant negative form of the Rac1 downstream partner PAK leads to a decrease in spine density and an enlargement of synapses [138]. PAK further phosphorylates the LIM-kinase (LIMK) that deactivates cofilin, an important actin depolymerization factor and thereby promotes profilin-actin polymerization pathways [139,140,141,142,143,144]. Additionally, PAK can also activate Arp2/3 complex-dependent actin meshwork formation.
The interaction of Fe65 and ELMO1 was identified by the group of Dr. Lau [54,58,145,146], showing the binding of ELMO1 to the most N-terminal domain of Fe65, not present in Fe65L2. Interestingly, Fe65 binding releases the intramolecular autoinhibition of ELMO1, which in turn recruits DOCK1 [54,58,146,147]. Furthermore, Fe65 was shown to form presumably a quadripartite complex with Arf6 and ELMO1/DOCK1 facilitating retargeting of ELMO1 to the plasma membrane via involvement of Arf6 [58]. The ELMO1/Fe65/DOCK1/Arf6 complex subsequently activates Rac by GTP loading.
4. Scaffolding Protein as Actin Regulators
The basic idea of scaffolding protein functions is that they bring components of a signaling cascade together into spatial proximity. This can increase the efficiency of signal transduction as well as signal specificity. This function appears crucial as modeling has shown that kinases in a cascade without scaffold proteins have a higher probability of being dephosphorylated by phosphatases before they are even able to phosphorylate downstream targets [148]. In this scenario, scaffold proteins protect active signaling molecules from inactivation or in similar constellations from degradation. They may also act as molecular switches, as interaction with signaling proteins can cause allosteric changes, resulting in signaling activation/inactivation [149].
One well-studied example of a scaffolding protein involved in actin cytoskeleton regulation is β-catenin, which binds to the cytoplasmic end of E-cadherin and to α-catenin, which interacts with the underlying actin cytoskeleton [150]. Cadherin-mediated cell–cell adhesion is thought to also couple the subcellular actin cytoskeleton mechanically between two cells in this way. Such a function implies a stable complex between E-cadherins and the actin cytoskeleton. Remarkably, it has been shown that monomeric α-catenin cannot bind to F-actin and β-catenin simultaneously [151]. Moreover, the monomeric α-catenin preferentially binds β-catenin, whereas the dimeric form competes with Arp2/3 for binding to F-actin, suppressing Arp2/3 activity and favoring actin fibril bundling [152]. In line with this, FRAP analyses at epithelial cell junctions showed a threefold higher dynamic for actin than for E-cadherin, β- and α-catenin, showing similar dynamics [151,152]. Together, these data indicate a dynamic rather than a stable link between the adhesion complex and the actin cytoskeleton [153].
Although not yet shown experimentally, it appears well feasible that Fe65 also functions as a highly dynamic adaptor protein. First, Fe65 was shown to form dimers via the PTB2 domain using the interaction site of the APP-C-terminus [21], indicating that Fe65 might switch between a monomeric membrane-bound and dimeric-free cytosolic state with different interaction partners and functions. Second, the diverse set of above discussed Fe65 interaction partners, might not bind simultaneously, but instead the complex formation is most likely regulated in a dynamic, spatial, and temporal manner by intra- and extracellular signaling events.
Since Fe65 is a phosphoprotein, it seems quite conceivable that phosphorylation is involved in regulating spatial and temporal Fe65 dynamics. Fe65 can be phosphorylated by various protein kinases, including extracellular signal-regulated kinase (ERK1/2), serum- and glucocorticoid-regulated kinase (SGK1), Abelson tyrosine kinase (c-Abl), ataxia telangiectasia mutated/ataxia-telangiectasia- and Rad3-related protein (ATM/ATR) kinase, and glycogen synthase kinase 3β (GSK3β) [36,154,155,156,157]. Interestingly, Fe65 phosphorylation by GSK3β or SGK1 was shown to affect APP processing [155,156], possibly caused by impacts on Fe65 homotypic dimerization [156]. Moreover, phosphorylation of Fe65 at S228, T547, and S566 by ATM/ATR, c-Abl, and SGK1, respectively, affects its nuclear activity [36,154,158,159]. Most interestingly, kinases like ERK1/2 and c-Abl were also shown to be important regulators for actin polymerization [74,160,161], supporting the hypothesis that at least a part of the impact of those kinases on actin cytoskeleton depends on altered regulation of the Fe65 function. However, different kinases also target Fe65 binding partners, such as APP or LRP, which in turn influence the binding affinity to Fe65 [162,163,164,165]. The current understanding of these processes is very much in the beginning and the exact regulatory mechanisms, in particular in highly dynamic processes, such as actin dynamics, need further detailed investigations.
5. Genetic Evidence for Fe65 Function in Actin Cytoskeleton Regulation
Important insights into the Fe65 function were gained by analyses of genetically modified mice [15,166,167,168,169]. In addition to the Fe65 family KO mice, mice overexpressing Fe65 together with APP or APP fragments, such as AICD, were also analyzed [170,171]. Those studies showed an impact of Fe65 on APP processing, not observed in Fe65 KO mice, and highlight a function of Fe65 and AICD in neuronal survival and synaptic plasticity, possibly caused by upregulation of GSK3β activity that in turn affects actin polymerization. However, based on these combined overexpression studies, it is challenging to assign specific functions to either Fe65 or APP. To decipher the phenotypes more clearly, genetic studies of Fe65 transgenic animals with APP KO mice might be beneficial. Therefore, here we like to focus on loss of function studies of APP, Fe65, and interacting actin regulators of the Mena/VASP family. Interestingly, some key features, observed in Fe65 KO mice were also found in mice lacking the APP or Mena/VASP family (Table 2). Thus, Fe65/Fe65L1 DKO as well as APP and Mena/VASP TKO mice all exhibit abnormal ectopic accumulations of neuroblasts, migrating through the basal lamina and pial membrane during brain development [76,167,172], resembling a cobblestone or type II lissencephaly [173,174,175]. Additionally, they all represent failures in axon tract formation and reduction or displacement of Cajal Retzius (CR) cells, resulting in disruption of cortical/meningeal layering. Mena/VASP TKO mice exhibit exencephaly that is also found in two out of 31 APP TKO mice [76,172]. The cause for cortical malformation in APP and Fe65 KO mice is not yet understood but could be well explained by defects in actin cytoskeleton regulation, possibly causing problems in glial endfoot formation, lamination, neuronal migration, or defective recognition of stop signals [75,76,167,172]. Fe65 and its interacting ABPs were also shown to positively influence dendritic and axonal outgrowth by elevation of actin polymerization [54,58,72,75,76,145,167,176,177,178]. Misregulation of these pathways arise in neuronal brain malformations, like impaired decussation of the corpus callosum and hippocampal fiber structures, as observed for Fe65/Fe65L1 DKO, APP, and Mena/VASP TKO mice (Table 2).

Table 2.
Systematic summary of phenotypes in Fe65, APP, and Mena/VASP family KOs.
Additionally, Fe65 and APP family KO mice show severe learning and memory deficits, resulting likely from impairments of synaptic plasticity (Table 2). Some of these phenotypes might be due to alterations in actin cytoskeleton regulation. However, so far there is very limited information on behavior defects of Mena, ELMO/DOCK1, and cortactin KO mice, making it difficult to draw clear conclusions. Notably, Fe65 and APP family KO mice were reported to exhibit deficits at the neuromuscular junction (NMJ) followed by muscle weakness. Changes in NMJ formation in Mena TKO mice were not yet investigated, but studies of Drosophila NMJs revealed a pre- and postsynaptic abundance and function of Ena [179,180,181]. However, to gain further insights, future genetic studies will be required.
Fe65 and APP can regulate cell motility. Therefore, overexpression of APP or Fe65 in MDCK cells increased the cell migration velocity in a wound-healing assay and co-expression of APP and Fe65 further accelerates cell movement [52]. This suggests that Fe65 might function as a downstream signaling factor of APP in this process. In contrast, Fe65 was demonstrated to inhibit cell motility in MDA-MB-231 breast cancer cells [56]. The reason for these contradictious results could be due to differences of the used cell lines and/or target protein expression levels. Overexpression of Ena/VASP proteins in fibroblasts also decreases cell motility in a dose-dependent manner and in line with this, deletion of Ena/VASP family increased fibroblast motility [71]. However, in contrast to these studies, experimental data from Listeria monocytes, Drosophila hemocytes, mouse fibroblasts, B16-F1 mouse melanoma cells, and MTLn3 cells demonstrated a positive regulation of actin-dependent cell movement by the Ena/VASP family [182,183,184,185,186]. Although the impact of Fe65, APP, and Mena on cell motility is quite obvious, the results from the different cell lines cannot be easily compared.
In line with changes of the motility and of the plasma membrane traction, Fe65 was also shown to suppress invasion by binding to Tip60 and cortactin [56]. Actin-dependent invasive capabilities are often related to cancer. Hence, it is not surprising that Fe65, APP, Mena, cortactin, and ELMO/DOCK180/Rac are involved in suppressing or supporting different kinds of cancerous signaling cascades, for example, in breast, thyroid, colon, lung, and pancreas cancer [56,128,185,187,188,189,190,191,192,193,194,195]. It was astonishing that especially breast cancer was highly investigated in association with Fe65 interaction partners (APP, Mena, cortactin, ELMO/DOCK180/Rac) separately but not in a common pathway [56,187,188,189,190,191,196,197,198,199,200,201,202,203,204].
To unravel the functional interdependence of Fe65 and its interaction partners in cell motility, additional genetic studies using the same cell lines and comparable mouse models will be necessary.
6. Future Perspectives of Fe65 in Actin Dynamics
Overall, there is clear evidence for a central function of the APP-binding protein family Fe65 in actin regulation. Fe65 likely supports the formation of branched and unbranched actin polymerization, affecting multiple cellular functions, including axonal/dendritic outgrowth, cell migration, structural synaptic plasticity, and intracellular vesicular trafficking. Those diverse functions could be mainly explained by Fe65 interaction with central regulators of actin dynamics. First, Fe65 binds to Mena, which promotes actin elongation by enhancing profilin function. Increased actin polymerization then favors the growth of unbranched actin filaments, leading to filopodia formation, important for environment scanning and positioning as well as for dendritic spine initiation. However, depending on the availability of Arp2/3 complexes, Mena/profilin may also contribute to increasing actin meshwork formation (Figure 1). Furthermore, Fe65 can form complexes with ELMO1, DOCK1, and Arf6 that favor plasma membrane targeting and activation of Rac1. This small Rho GTPase leads to the inhibition of actin severing by phosphorylation of cofilin and the induction of Arp2/3-dependent branched actin polymerization, promoting the formation of lamellipodia and the maintenance and maturation of dendritic spines (Figure 1). Notably, Fe65 interacts with cortactin and might facilitate its acetylation by recruitment of Tip60, which negatively influence Arp2/3-dependent actin formation. However, to fully understand the relationship between cell shape and cell function, we must better understand the transitions between different types of actin networks, important for many cellular processes like cell outgrowth and locomotion, spine plasticity, and intracellular vesicular motility. Here, it will be fundamental to unravel the dynamics and the regulation of the distinct Fe65 complexes.
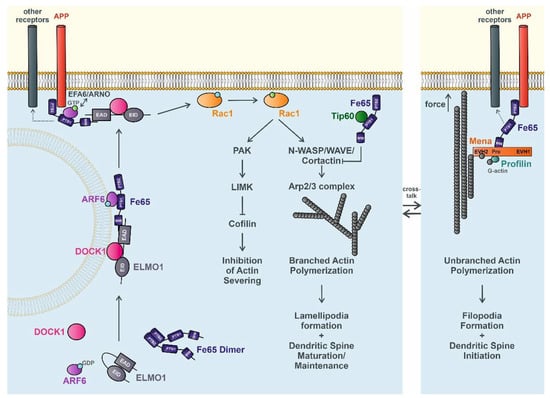
Figure 1.
Putative new role of Fe65 in regulating actin dynamics. Fe65 associates with ELMO1/DOCK1 and Arf6 to form a functional complex that is translocated to the plasma membrane and trapped by APP or other potential receptors, such as the ApoE receptor. Arf6-bound GDP gets replaced with GTP via EFA6/ARNO. Subsequent activation of Rac1 induces a cascade inhibiting the severing activity of cofilin and promoting the polymerization of branched actin, which results in the formation of lamellipodia and the induction of dendritic spine plasticity. The Fe65–Tip60 complex may additionally adjust the association of cortactin to actin by acetylation. In a second potential pathway membrane, receptor-bound Fe65 bind to the polymerization-competent complex of Mena, profilin, and monomeric G-actin that supports the elongation of unbranched actin, leading to filopodia formation and dendritic spine initiation. During migration and outgrowth, it is very likely that these processes are regulated dynamically in a homeostasis. ELMO1, engulfment and cell motility protein 1; EAD, ELMO autoregulatory domain; EID, ELMO inhibitory domain; Arf6, ADP-ribosylation factor 6; GDP, guanosine diphosphate; GTP, guanosine triphosphate; WW, tryptophan-tryptophan domain; PTB1/2, phosphotyrosine binding domain 1/2; DOCK1, dictator of cytokinesis 1; APP, amyloid precursor protein; EFA6, exchange factor for Arf6; ARNO, ADP-ribosylation factor nucleotide-binding site opener; Rac1, ras-related C3 botulinum toxin substrate 1; PAK, p21-activated kinase; LIMK, LIM kinase; Tip60, Tat-interacting protein 60 kDa; N-WASP, neural Wiskott–Aldrich syndrome protein; WAVE, WASP family verprolin homologous protein; Arp2/3, actin related protein 2/3; Mena, mammalian enabled; EVH1/2, Ena/VASP homology domain 1/2; Pro, proline-rich region; G-actin, globular actin.
In addition to the question of how the scaffolding protein Fe65 helps to orchestrate the reorganization of the actin cytoskeleton, it will be central to understand what the upstream regulators of Fe65 are. Based on the high-affinity binding of the Fe65 family members to the APP family, and due to widely overlapping phenotypes in APP/APLP1/APLP2 TKO and Fe65/Fe65L1 DKO mice, it appears very likely that APP/APLPs are important upstream regulators of Fe65 family members (Table 2) [1,4,5,6,7,8,9,23,234]. Consistently, Fe65 and APP are highly abundant in growth cones, are present at the pre- and postsynapse, and were both shown to affect synaptic plasticity [52,53,235,236,237,238,239,240,241,242,243]. The different molecular signaling pathways clearly require more than the formation of one stable complex, but how the functions of Fe65, APP, and their family members are regulated is still not understood. This is further complicated by the fact that Fe65 can functionally link APP with VLDL, ApoEr2, and LRP modulating both the ApoE receptor and APP trafficking as well as processing [45]. Furthermore, APP was described to interact and to increase NMDA receptor surface localization [242,244,245,246,247] and ApoE receptors were shown to regulate NMDA receptor activity that involves alterations in APP endocytosis and interactions with Fe65 [44,47,48,248,249,250,251,252]. Thus, APP-bound Fe65 might link the NMDA and ApoE receptor activity to the actin cytoskeleton, which in turn translates the extracellular signals to alterations in cell shape and function.
The pathological role of the Fe65 family in AD is extensively investigated, but so far, only minor changes in APP processing and amyloid pathology were reported [14,15,16,47,165,167,170,253,254,255,256,257]. However, the link of Fe65 to ApoE, which is a major risk factor of AD, is very obvious [49]. Thus, a better understanding of the molecular signaling of ApoE receptors to Fe65 might unravel novel potential therapeutic strategies. Another central pathological feature of AD is the early loss of synapses [258,259,260,261,262]. As actin dynamics play a key role in this process, Fe65 might also be involved in AD-associated changes in spine regulation by affecting different pathways [263,264,265,266,267,268,269,270,271,272,273,274].
In summary, we found convincing evidence for an important role of Fe65 in the regulation of the actin cytoskeleton in neuronal development and synaptic plasticity. To better understand the pathophysiological role of the Fe65 family in the different actin-dependent processes and the transitions between the different types of actin networks, it will be important to study the control and dynamics of the different Fe65 complexes in more detail.
Author Contributions
Conceptualization, S.K. and V.A.; Validation, S.K. and V.A.; Writing—Original Draft Preparation, S.K. and V.A.; Writing—Review & Editing, S.K. and V.A.; Visualization, V.A.; Supervision, S.K.; Project Administration, S.K.; Funding Acquisition, S.K. Both authors have read and agreed to the published version of the manuscript.
Funding
Funding by the DFG—German Research Foundation to S.K.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bressler, S.L.; Gray, M.D.; Sopher, B.L.; Hu, Q.; Hearn, M.G.; Pham, D.G.; Dinulos, M.B.; Fukuchi, K.; Sisodia, S.S.; Miller, M.A.; et al. cDNA cloning and chromosome mapping of the human Fe65 gene: Interaction of the conserved cytoplasmic domains of the human beta-amyloid precursor protein and its homologues with the mouse Fe65 protein. Hum. Mol. Genet. 1996, 5, 1589–1598. [Google Scholar] [CrossRef]
- Blanco, G.; Irving, N.G.; Brown, S.D.; Miller, C.C.; McLoughlin, D.M. Mapping of the human and murine X11-like genes (APBA2 and apba2), the murine Fe65 gene (Apbb1), and the human Fe65-like gene (APBB2): Genes encoding phosphotyrosine-binding domain proteins that interact with the Alzheimer’s disease amyloid precursor protein. Mamm. Genome 1998, 9, 473–475. [Google Scholar] [CrossRef]
- Tanahashi, H.; Tabira, T. Genome structure and chromosomal mapping of the gene for Fe65L2 interacting with Alzheimer’s beta-amyloid precursor protein. Biochem. Biophys. Res. Commun. 1999, 258, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Fiore, F.; Zambrano, N.; Minopoli, G.; Donini, V.; Duilio, A.; Russo, T. The regions of the Fe65 protein homologous to the phosphotyrosine interaction/phosphotyrosine binding domain of Shc bind the intracellular domain of the Alzheimer’s amyloid precursor protein. J. Biol. Chem. 1995, 270, 30853–30856. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, D.M.; Miller, C.C.J. The intracellular cytoplasmic domain of the Alzheimer’s disease amyloid precursor protein interacts with phosphotyrosine-binding domain proteins in the yeast two-hybrid system. FEBS Lett. 1996, 397, 197–200. [Google Scholar] [CrossRef]
- Borg, J.P.; Ooi, J.; Levy, E.; Margolis, B. The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Mol. Cell. Biol. 1996, 16, 6229–6241. [Google Scholar] [CrossRef] [PubMed]
- Guénette, S.Y.; Chen, J.; Jondro, P.D.; Tanzi, R.E. Association of a novel human FE65-like protein with the cytoplasmic domain of the beta-amyloid precursor protein. Proc. Natl. Acad. Sci. USA 1996, 93, 10832–10837. [Google Scholar] [CrossRef]
- Duilio, A.; Faraonio, R.; Minopoli, G.; Zambrano, N.; Russo, T. Fe65L2: A new member of the Fe65 protein family interacting with the intracellular domain of the Alzheimer’s beta-amyloid precursor protein. Biochem. J. 1998, 330 (Pt 1), 513–519. [Google Scholar] [CrossRef]
- Tanahashi, H.; Tabira, T. Molecular cloning of human Fe65L2 and its interaction with the Alzheimer’s β-amyloid precursor protein. Neurosci. Lett. 1999, 261, 143–146. [Google Scholar] [CrossRef]
- Esposito, F.; Ammendola, R.; Duilio, A.; Costanzo, F.; Giordano, M.; Zambrano, N.; D’Agostino, P.; Russo, T.; Cimino, F. Isolation of cDNA fragments hybridizing to rat brain-specific mRNAs. Dev. Neurosci. 1990, 12, 373–381. [Google Scholar] [CrossRef]
- Duilio, A.; Zambrano, N.; Mogavero, A.R.; Ammendola, R.; Cimino, F.; Russo, T. A rat brain mRNA encoding a transcriptional activator homologous to the DNA binding domain of retroviral integrases. Nucleic Acids Res. 1991, 19, 5269–5274. [Google Scholar] [CrossRef]
- Tanahashi, H.; Tabira, T. Characterization of an amyloid precursor protein-binding protein Fe65L2 and its novel isoforms lacking phosphotyrosine-interaction domains. Biochem. J. 2002, 367, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Kesavapany, S.; Banner, S.; Lau, K.-F.; Shaw, C.; Miller, C.; Cooper, J.; McLoughlin, D. Expression of the Fe65 adapter protein in adult and developing mouse brain. Neuroscience 2002, 115, 951–960. [Google Scholar] [CrossRef]
- Hu, Q.; Jin, L.W.; Starbuck, M.Y.; Martin, G.M. Broadly altered expression of the mRNA isoforms of FE65, a facilitator of beta amyloidogenesis, in Alzheimer cerebellum and other brain regions. J. Neurosci. Res. 2000, 60, 73–86. [Google Scholar] [CrossRef]
- Wang, B.; Hu, Q.; Hearn, M.G.; Shimizu, K.; Ware, C.B.; Liggitt, D.H.; Jin, L.-W.; Cool, B.H.; Storm, D.R.; Martin, G.M. Isoform-specific knockout ofFE65 leads to impaired learning and memory. J. Neurosci. Res. 2004, 75, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, L.; Yang, Z.; Cool, B.H.; Zitnik, G.; Martin, G.M. Endoproteolytic cleavage of FE65 converts the adaptor protein to a potent suppressor of the sAPPalpha pathway in primates. J. Biol. Chem. 2005, 280, 12548–12558. [Google Scholar] [CrossRef]
- Saeki, K.; Nose, Y.; Hirao, N.; Takasawa, R.; Tanuma, S.-I. Amyloid precursor protein binding protein Fe65 is cleaved by caspases during DNA damage-induced apoptosis. Biol. Pharm. Bull. 2011, 34, 290–294. [Google Scholar] [CrossRef]
- Penna, I.; Vassallo, I.; Nizzari, M.; Russo, D.; Costa, D.; Menichini, P.; Poggi, A.; Russo, C.; Dieci, G.; Florio, T.; et al. A novel snRNA-like transcript affects amyloidogenesis and cell cycle progression through perturbation of Fe65L1 (APBB2) alternative splicing. Biochim. Biophys. Acta 2013, 1833, 1511–1526. [Google Scholar] [CrossRef]
- Zambrano, N.; Bimonte, M.; Arbucci, S.; Gianni, D.; Russo, T.; Bazzicalupo, P. feh-1 and apl-1, the Caenorhabditis elegans orthologues of mammalian Fe65 and beta-amyloid precursor protein genes, are involved in the same pathway that controls nematode pharyngeal pumping. J. Cell Sci. 2002, 115, 1411–1422. [Google Scholar] [CrossRef]
- Meiyappan, M.; Birrane, G.; Ladias, J.A.A. Structural basis for polyproline recognition by the FE65 WW domain. J. Mol. Biol. 2007, 372, 970–980. [Google Scholar] [CrossRef][Green Version]
- Feilen, L.P.; Haubrich, K.; Strecker, P.; Probst, S.; Eggert, S.; Stier, G.; Sinning, I.; Konietzko, U.; Kins, S.; Simon, B.; et al. Fe65-PTB2 Dimerization Mimics Fe65-APP Interaction. Front. Mol. Neurosci. 2017, 10, 140. [Google Scholar] [CrossRef]
- Cao, X.; Südhof, T.C. Dissection of amyloid-beta precursor protein-dependent transcriptional transactivation. J. Biol. Chem. 2004, 279, 24601–24611. [Google Scholar] [CrossRef]
- Guénette, S.; Strecker, P.; Kins, S. APP Protein Family Signaling at the Synapse: Insights from Intracellular APP-Binding Proteins. Front. Mol. Neurosci. 2017, 10, 87. [Google Scholar] [CrossRef]
- Müller, U.C.; Deller, T.; Korte, M. Not just amyloid: Physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 2017, 18, 281–298. [Google Scholar] [CrossRef]
- Zheng, H.; Koo, E.H. The amyloid precursor protein: Beyond amyloid. Mol. Neurodegener. 2006, 1, 5. [Google Scholar] [CrossRef][Green Version]
- Baumkötter, F.; Wagner, K.; Eggert, S.; Wild, K.; Kins, S. Structural aspects and physiological consequences of APP/APLP trans-dimerization. Exp. Brain Res. 2012, 217, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.N.V.; Cheung, H.N.M.; Li, W.; Lau, K.-F. FE65: Roles beyond amyloid precursor protein processing. Cell. Mol. Biol. Lett. 2015, 20, 272. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Südhof, T.C. A transcriptionally correction of transcriptively active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 2001, 293, 115–120. [Google Scholar] [CrossRef]
- Yang, Z.; Cool, B.H.; Martin, G.M.; Hu, Q. A dominant role for FE65 (APBB1) in nuclear signaling. J. Biol. Chem. 2006, 281, 4207–4214. [Google Scholar] [CrossRef]
- Telese, F.; Bruni, P.; Donizetti, A.; Gianni, D.; D’Ambrosio, C.; Scaloni, A.; Zambrano, N.; Rosenfeld, M.G.; Russo, T. Transcription regulation by the adaptor protein Fe65 and the nucleosome assembly factor SET. EMBO Rep. 2005, 6, 77–82. [Google Scholar] [CrossRef]
- Bruni, P.; Minopoli, G.; Brancaccio, T.; Napolitano, M.; Faraonio, R.; Zambrano, N.; Hansen, U.; Russo, T. Fe65, a ligand of the Alzheimer’s beta-amyloid precursor protein, blocks cell cycle progression by down-regulating thymidylate synthase expression. J. Biol. Chem. 2002, 277, 35481–35488. [Google Scholar] [CrossRef] [PubMed]
- Konietzko, U.; Gersbacher, M.T.; Streuli, J.; Krüger, M.; Thöni, S.; Kins, S.; Nitsch, R.M. A fluorescent protein-readout for transcriptional activity reveals regulation of APP nuclear signaling by phosphorylation sites. Biol. Chem. 2019, 400, 1191–1203. [Google Scholar] [CrossRef]
- Probst, S.; Krüger, M.; Kägi, L.; Thöni, S.; Schuppli, D.; Nitsch, R.M.; Konietzko, U. Fe65 is the sole member of its family that mediates transcription regulated by the amyloid precursor protein. J. Cell. Sci. 2020, 133. [Google Scholar] [CrossRef]
- Zambrano, N.; Minopoli, G.; de Candia, P.; Russo, T. The Fe65 Adaptor Protein Interacts through Its PID1 Domain with the Transcription Factor CP2/LSF/LBP1. J. Biol. Chem. 1998, 273, 20128–20133. [Google Scholar] [CrossRef]
- Zambrano, N.; Bruni, P.; Minopoli, G.; Mosca, R.; Molino, D.; Russo, C.; Schettini, G.; Sudol, M.; Russo, T. The beta-amyloid precursor protein APP is tyrosine-phosphorylated in cells expressing a constitutively active form of the Abl protoncogene. J. Biol. Chem. 2001, 276, 19787–19792. [Google Scholar] [CrossRef] [PubMed]
- Perkinton, M.S.; Standen, C.L.; Lau, K.-F.; Kesavapany, S.; Byers, H.L.; Ward, M.; McLoughlin, D.M.; Miller, C.C.J. The c-Abl tyrosine kinase phosphorylates the Fe65 adaptor protein to stimulate Fe65/amyloid precursor protein nuclear signaling. J. Biol. Chem. 2004, 279, 22084–22091. [Google Scholar] [CrossRef]
- Konietzko, U.; Goodger, Z.V.; Meyer, M.; Kohli, B.M.; Bosset, J.; Lahiri, D.K.; Nitsch, R.M. Co-localization of the amyloid precursor protein and Notch intracellular domains in nuclear transcription factories. Neurobiol. Aging 2010, 31, 58–73. [Google Scholar] [CrossRef]
- Müller, T.; Concannon, C.G.; Ward, M.W.; Walsh, C.M.; Tirniceriu, A.L.; Tribl, F.; Kögel, D.; Prehn, J.H.; Egensperger, R.; Forscher, P. Modulation of Gene Expression and Cytoskeletal Dynamics by the Amyloid Precursor Protein Intracellular Domain (AICD). MBoC 2007, 18, 201–210. [Google Scholar] [CrossRef]
- Ward, M.W.; Concannon, C.G.; Whyte, J.; Walsh, C.M.; Corley, B.; Prehn, J.H.M. The amyloid precursor protein intracellular domain (AICD) disrupts actin dynamics and mitochondrial bioenergetics. J. Neurochem. 2010, 113, 275–284. [Google Scholar] [CrossRef]
- Bukhari, H.; Glotzbach, A.; Kolbe, K.; Leonhardt, G.; Loosse, C.; Müller, T. Small things matter: Implications of APP intracellular domain AICD nuclear signaling in the progression and pathogenesis of Alzheimer’s disease. Prog. Neurobiol. 2017, 156, 189–213. [Google Scholar] [CrossRef]
- McLoughlin, D.M.; Miller, C.C.J. The FE65 proteins and Alzheimer’s disease. J. Neurosci. Res. 2008, 86, 744–754. [Google Scholar] [CrossRef]
- Hébert, S.S.; Serneels, L.; Tolia, A.; Craessaerts, K.; Derks, C.; Filippov, M.A.; Müller, U.; de Strooper, B. Regulated intramembrane proteolysis of amyloid precursor protein and regulation of expression of putative target genes. EMBO Rep. 2006, 7, 739–745. [Google Scholar] [CrossRef]
- Giliberto, L.; Zhou, D.; Weldon, R.; Tamagno, E.; de Luca, P.; Tabaton, M.; D’Adamio, L. Evidence that the Amyloid beta Precursor Protein-intracellular domain lowers the stress threshold of neurons and has a “regulated” transcriptional role. Mol. Neurodegener. 2008, 3, 12. [Google Scholar] [CrossRef]
- Trommsdorff, M.; Borg, J.-P.; Margolis, B.; Herz, J. Interaction of Cytosolic Adaptor Proteins with Neuronal Apolipoprotein E Receptors and the Amyloid Precursor Protein. J. Biol. Chem. 1998, 273, 33556–33560. [Google Scholar] [CrossRef]
- Dumanis, S.B.; Chamberlain, K.A.; Jin Sohn, Y.; Jin Lee, Y.; Guénette, S.Y.; Suzuki, T.; Mathews, P.M.; Pak, D.T.; Rebeck, G.W.; Suh, Y.-H.; et al. FE65 as a link between VLDLR and APP to regulate their trafficking and processing. Mol. Neurodegener. 2012, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Alvira-Botero, X.; Pérez-Gonzalez, R.; Spuch, C.; Vargas, T.; Antequera, D.; Garzón, M.; Bermejo-Pareja, F.; Carro, E. Megalin interacts with APP and the intracellular adapter protein FE65 in neurons. Mol. Cell. Neurosci. 2010, 45, 306–315. [Google Scholar] [CrossRef]
- Hoe, H.-S.; Magill, L.A.; Guenette, S.; Fu, Z.; Vicini, S.; Rebeck, G.W. FE65 interaction with the ApoE receptor ApoEr2. J. Biol. Chem. 2006, 281, 24521–24530. [Google Scholar] [CrossRef]
- Pohlkamp, T.; Wasser, C.R.; Herz, J. Functional Roles of the Interaction of APP and Lipoprotein Receptors. Front. Mol. Neurosci. 2017, 10, 54. [Google Scholar] [CrossRef]
- Bertram, L.; Tanzi, R.E. Thirty years of Alzheimer’s disease genetics: The implications of systematic meta-analyses. Nat. Rev. Neurosci. 2008, 9, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Nensa, F.M.; Neumann, M.H.D.; Schrötter, A.; Przyborski, A.; Mastalski, T.; Susdalzew, S.; Looβe, C.; Helling, S.; El Magraoui, F.; Erdmann, R.; et al. Amyloid beta a4 precursor protein-binding family B member 1 (FE65) interactomics revealed synaptic vesicle glycoprotein 2A (SV2A) and sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2) as new binding proteins in the human brain. Mol. Cell. Proteom. 2014, 13, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Masin, M.; Kerschensteiner, D.; Dümke, K.; Rubio, M.E.; Soto, F. Fe65 interacts with P2X2 subunits at excitatory synapses and modulates receptor function. J. Biol. Chem. 2006, 281, 4100–4108. [Google Scholar] [CrossRef]
- Sabo, S.L.; Ikin, A.F.; Buxbaum, J.D.; Greengard, P. The Alzheimer amyloid precursor protein (APP) and FE65, an APP-binding protein, regulate cell movement. J. Cell Biol. 2001, 153, 1403–1414. [Google Scholar] [CrossRef]
- Sabo, S.L.; Ikin, A.F.; Buxbaum, J.D.; Greengard, P. The Amyloid Precursor Protein and Its Regulatory Protein, FE65, in Growth Cones and Synapses In Vitro and In Vivo. J. Neurosci. 2003, 23, 5407–5415. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tam, K.M.V.; Chan, W.W.R.; Koon, A.C.; Ngo, J.C.K.; Chan, H.Y.E.; Lau, K.-F. Neuronal adaptor FE65 stimulates Rac1-mediated neurite outgrowth by recruiting and activating ELMO1. J. Biol. Chem. 2018, 293, 7674–7688. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, H.; Mutlu, S.A.; Bowser, D.A.; Moore, M.J.; Wang, M.C.; Zheng, H. The Amyloid Precursor Protein Is a Conserved Receptor for Slit to Mediate Axon Guidance. eNeuro 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, J.; Lungchukiet, P.; Quarni, W.; Yang, S.; Zhang, X.; Bai, W. Fe65 Suppresses Breast Cancer Cell Migration and Invasion through Tip60 Mediated Cortactin Acetylation. Sci. Rep. 2015, 5, 11529. [Google Scholar] [CrossRef]
- Ermekova, K.S.; Zambrano, N.; Linn, H.; Minopoli, G.; Gertler, F.; Russo, T.; Sudol, M. The WW domain of neural protein FE65 interacts with proline-rich motifs in Mena, the mammalian homolog of Drosophila enabled. J. Biol. Chem. 1997, 272, 32869–32877. [Google Scholar] [CrossRef]
- Chan, W.W.R.; Li, W.; Chang, R.C.C.; Lau, K.-F. ARF6-Rac1 signaling-mediated neurite outgrowth is potentiated by the neuronal adaptor FE65 through orchestrating ARF6 and ELMO1. FASEB J. 2020, 34, 16397–16413. [Google Scholar] [CrossRef]
- Gertler, F.B.; Niebuhr, K.; Reinhard, M.; Wehland, J.; Soriano, P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell 1996, 87, 227–239. [Google Scholar] [CrossRef]
- Haffner, C.; Jarchau, T.; Reinhard, M.; Hoppe, J.; Lohmann, S.M.; Walter, U. Molecular cloning, structural analysis and functional expression of the proline-rich focal adhesion and microfilament-associated protein VASP. EMBO J. 1995, 14, 19–27. [Google Scholar] [CrossRef]
- Bachmann, C.; Fischer, L.; Walter, U.; Reinhard, M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J. Biol. Chem. 1999, 274, 23549–23557. [Google Scholar] [CrossRef]
- Reinhard, M.; Jarchau, T.; Walter, U. Actin-based motility: Stop and go with Ena/VASP proteins. Trends Biochem. Sci. 2001, 26, 243–249. [Google Scholar] [CrossRef]
- Bear, J.E.; Gertler, F.B. Ena/VASP: Towards resolving a pointed controversy at the barbed end. J. Cell. Sci. 2009, 122, 1947–1953. [Google Scholar] [CrossRef]
- Niebuhr, K.; Ebel, F.; Frank, R.; Reinhard, M.; Domann, E.; Carl, U.D.; Walter, U.; Gertler, F.B.; Wehland, J.; Chakraborty, T. A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J. 1997, 16, 5433–5444. [Google Scholar] [CrossRef]
- Reinhard, M.; Halbrügge, M.; Scheer, U.; Wiegand, C.; Jockusch, B.M.; Walter, U. The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. EMBO J. 1992, 11, 2063–2070. [Google Scholar] [CrossRef]
- Reinhard, M.; Jouvenal, K.; Tripier, D.; Walter, U. Identification, purification, and characterization of a zyxin-related protein that binds the focal adhesion and microfilament protein VASP (vasodilator-stimulated phosphoprotein). Proc. Natl. Acad. Sci. USA 1995, 92, 7956–7960. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, A.; Kwiatkowski, A.V.; Lanier, L.M.; Bear, J.E.; Vandekerckhove, J.; Ampe, C.; Gertler, F.B. cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J. Biol. Chem. 2000, 275, 36143–36151. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Sechi, A.S.; Konradt, M.; Monner, D.; Gertler, F.B.; Wehland, J. Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/3 complex link T cell receptor (TCR) signaling to the actin cytoskeleton. J. Cell Biol. 2000, 149, 181–194. [Google Scholar] [CrossRef]
- Ahern-Djamali, S.M.; Comer, A.R.; Bachmann, C.; Kastenmeier, A.S.; Reddy, S.K.; Beckerle, M.C.; Walter, U.; Hoffmann, F.M. Mutations in Drosophila enabled and rescue by human vasodilator-stimulated phosphoprotein (VASP) indicate important functional roles for Ena/VASP homology domain 1 (EVH1) and EVH2 domains. MBoC 1998, 9, 2157–2171. [Google Scholar] [CrossRef]
- Rottner, K.; Behrendt, B.; Small, J.V.; Wehland, J. VASP dynamics during lamellipodia protrusion. Nat. Cell Biol. 1999, 1, 321–322. [Google Scholar] [CrossRef]
- Bear, J.E.; Loureiro, J.J.; Libova, I.; Fässler, R.; Wehland, J.; Gertler, F.B. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell 2000, 101, 717–728. [Google Scholar] [CrossRef]
- Lanier, L.M.; Gates, M.A.; Witke, W.; Menzies, A.; Wehman, A.M.; Macklis, J.D.; Kwiatkowski, D.; Soriano, P.; Gertler, F.B. Mena Is Required for Neurulation and Commissure Formation. Neuron 1999, 22, 313–325. [Google Scholar] [CrossRef]
- Vasioukhin, V.; Bauer, C.; Yin, M.; Fuchs, E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 2000, 100, 209–219. [Google Scholar] [CrossRef]
- Lanier, L.M.; Gertler, F.B. From Abl to actin: Abl tyrosine kinase and associated proteins in growth cone motility. Curr. Opin. Neurobiol. 2000, 10, 80–87. [Google Scholar] [CrossRef]
- Dent, E.W.; Kwiatkowski, A.V.; Mebane, L.M.; Philippar, U.; Barzik, M.; Rubinson, D.A.; Gupton, S.; van Veen, J.E.; Furman, C.; Zhang, J.; et al. Filopodia are required for cortical neurite initiation. Nat. Cell Biol. 2007, 9, 1347–1359. [Google Scholar] [CrossRef]
- Kwiatkowski, A.V.; Rubinson, D.A.; Dent, E.W.; van Edward Veen, J.; Leslie, J.D.; Zhang, J.; Mebane, L.M.; Philippar, U.; Pinheiro, E.M.; Burds, A.A.; et al. Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron 2007, 56, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Bashaw, G.J.; Kidd, T.; Murray, D.; Pawson, T.; Goodman, C.S. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell 2000, 101, 703–715. [Google Scholar] [CrossRef]
- Lebrand, C.; Dent, E.W.; Strasser, G.A.; Lanier, L.M.; Krause, M.; Svitkina, T.M.; Borisy, G.G.; Gertler, F.B. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron 2004, 42, 37–49. [Google Scholar] [CrossRef]
- Wills, Z.; Bateman, J.; Korey, C.A.; Comer, A.; van Vactor, D. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron 1999, 22, 301–312. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Gao, F.-B. Abelson, enabled, and p120 catenin exert distinct effects on dendritic morphogenesis in Drosophila. Dev. Dyn. 2005, 234, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Bear, J.E.; Svitkina, T.M.; Krause, M.; Schafer, D.A.; Loureiro, J.J.; Strasser, G.A.; Maly, I.V.; Chaga, O.Y.; Cooper, J.A.; Borisy, G.G.; et al. Antagonism between Ena/VASP Proteins and Actin Filament Capping Regulates Fibroblast Motility. Cell 2002, 109, 509–521. [Google Scholar] [CrossRef]
- Barzik, M.; Kotova, T.I.; Higgs, H.N.; Hazelwood, L.; Hanein, D.; Gertler, F.B.; Schafer, D.A. Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins. J. Biol. Chem. 2005, 280, 28653–28662. [Google Scholar] [CrossRef] [PubMed]
- Breitsprecher, D.; Kiesewetter, A.K.; Linkner, J.; Urbanke, C.; Resch, G.P.; Small, J.V.; Faix, J. Clustering of VASP actively drives processive, WH2 domain-mediated actin filament elongation. EMBO J. 2008, 27, 2943–2954. [Google Scholar] [CrossRef] [PubMed]
- Chereau, D.; Dominguez, R. Understanding the role of the G-actin-binding domain of Ena/VASP in actin assembly. J. Struct. Biol. 2006, 155, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ferron, F.; Rebowski, G.; Lee, S.H.; Dominguez, R. Structural basis for the recruitment of profilin-actin complexes during filament elongation by Ena/VASP. EMBO J. 2007, 26, 4597–4606. [Google Scholar] [CrossRef]
- Plastino, J.; Olivier, S.; Sykes, C. Actin filaments align into hollow comets for rapid VASP-mediated propulsion. Curr. Biol. 2004, 14, 1766–1771. [Google Scholar] [CrossRef]
- Samarin, S.; Romero, S.; Kocks, C.; Didry, D.; Pantaloni, D.; Carlier, M.-F. How VASP enhances actin-based motility. J. Cell Biol. 2003, 163, 131–142. [Google Scholar] [CrossRef]
- Skoble, J.; Auerbuch, V.; Goley, E.D.; Welch, M.D.; Portnoy, D.A. Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation, and Listeria monocytogenes motility. J. Cell Biol. 2001, 155, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Skruber, K.; Warp, P.V.; Shklyarov, R.; Thomas, J.D.; Swanson, M.S.; Henty-Ridilla, J.L.; Read, T.-A.; Vitriol, E.A. Arp2/3 and Mena/VASP Require Profilin 1 for Actin Network Assembly at the Leading Edge. Curr. Biol. 2020, 30, 2651–2664. [Google Scholar] [CrossRef]
- Menon, S.; Boyer, N.P.; Winkle, C.C.; McClain, L.M.; Hanlin, C.C.; Pandey, D.; Rothenfußer, S.; Taylor, A.M.; Gupton, S.L. The E3 Ubiquitin Ligase TRIM9 Is a Filopodia Off Switch Required for Netrin-Dependent Axon Guidance. Dev. Cell 2015, 35, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.J.; Kühne, R.; Hoffmann, B.; Häfner, A.; Schmieder, P.; Volkmer-Engert, R.; Hof, M.; Wahl, M.; Schneider-Mergener, J.; Walter, U.; et al. Dual epitope recognition by the VASP EVH1 domain modulates polyproline ligand specificity and binding affinity. EMBO J. 2000, 19, 4903–4914. [Google Scholar] [CrossRef]
- Drees, B.; Friederich, E.; Fradelizi, J.; Louvard, D.; Beckerle, M.C.; Golsteyn, R.M. Characterization of the interaction between zyxin and members of the Ena/vasodilator-stimulated phosphoprotein family of proteins. J. Biol. Chem. 2000, 275, 22503–22511. [Google Scholar] [CrossRef]
- Klostermann, A.; Lutz, B.; Gertler, F.; Behl, C. The orthologous human and murine semaphorin 6A-1 proteins (SEMA6A-1/Sema6A-1) bind to the enabled/vasodilator-stimulated phosphoprotein-like protein (EVL) via a novel carboxyl-terminal zyxin-like domain. J. Biol. Chem. 2000, 275, 39647–39653. [Google Scholar] [CrossRef]
- McConnell, R.E.; van Edward Veen, J.; Vidaki, M.; Kwiatkowski, A.V.; Meyer, A.S.; Gertler, F.B. A requirement for filopodia extension toward Slit during Robo-mediated axon repulsion. J. Cell Biol. 2016, 213, 261–274. [Google Scholar] [CrossRef]
- Rodnick-Smith, M.; Luan, Q.; Liu, S.-L.; Nolen, B.J. Role and structural mechanism of WASP-triggered conformational changes in branched actin filament nucleation by Arp2/3 complex. Proc. Natl. Acad. Sci. USA 2016, 113, E3834–E3843. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.R.; Egile, C.; Gil, S.; Snapper, S.B.; Li, R.; Thomas, S.M. Cortactin regulates cell migration through activation of N-WASP. J. Cell. Sci. 2005, 118, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Weed, S.A.; Karginov, A.V.; Schafer, D.A.; Weaver, A.M.; Kinley, A.W.; Cooper, J.A.; Parsons, J.T. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol. 2000, 151, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Uruno, T.; Liu, J.; Zhang, P.; Fan, Y.; Egile, C.; Li, R.; Mueller, S.C.; Zhan, X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 2001, 3, 259–266. [Google Scholar] [CrossRef]
- Weaver, A.M.; Karginov, A.V.; Kinley, A.W.; Weed, S.A.; Li, Y.; Parsons, J.T.; Cooper, J.A. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 2001, 11, 370–374. [Google Scholar] [CrossRef]
- Egile, C.; Rouiller, I.; Xu, X.-P.; Volkmann, N.; Li, R.; Hanein, D. Mechanism of filament nucleation and branch stability revealed by the structure of the Arp2/3 complex at actin branch junctions. PLoS Biol. 2005, 3, e383. [Google Scholar] [CrossRef] [PubMed]
- Goley, E.D.; Welch, M.D. The ARP2/3 complex: An actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 2006, 7, 713–726. [Google Scholar] [CrossRef]
- Huang, C.; Liu, J.; Haudenschild, C.C.; Zhan, X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J. Biol. Chem. 1998, 273, 25770–25776. [Google Scholar] [CrossRef]
- Oser, M.; Mader, C.C.; Gil-Henn, H.; Magalhaes, M.; Bravo-Cordero, J.J.; Koleske, A.J.; Condeelis, J. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J. Cell. Sci. 2010, 123, 3662–3673. [Google Scholar] [CrossRef]
- Rosenberg, B.J.; Gil-Henn, H.; Mader, C.C.; Halo, T.; Yin, T.; Condeelis, J.; Machida, K.; Wu, Y.I.; Koleske, A.J. Phosphorylated cortactin recruits Vav2 guanine nucleotide exchange factor to activate Rac3 and promote invadopodial function in invasive breast cancer cells. Mol. Biol. Cell 2017, 28, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Lua, B.L.; Low, B.C. Cortactin phosphorylation as a switch for actin cytoskeletal network and cell dynamics control. FEBS Lett. 2005, 579, 577–585. [Google Scholar] [CrossRef]
- Vuori, K.; Ruoslahti, E. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J. Biol. Chem. 1995, 270, 22259–22262. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Quiles, N.; Ho, H.-Y.H.; Kirschner, M.W.; Ramesh, N.; Geha, R.S. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol. Cell. Biol. 2004, 24, 5269–5280. [Google Scholar] [CrossRef]
- Wu, H.; Parsons, J.T. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J. Cell Biol. 1993, 120, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Peng, H.B.; Rauvala, H. Association of cortactin with dynamic actin in lamellipodia and on endosomal vesicles. J. Cell Sci. 2000, 113 Pt 24, 4421–4426. [Google Scholar] [CrossRef]
- Bryce, N.S.; Clark, E.S.; Leysath, J.L.; Currie, J.D.; Webb, D.J.; Weaver, A.M. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr. Biol. 2005, 15, 1276–1285. [Google Scholar] [CrossRef]
- Wu, H.; Reynolds, A.B.; Kanner, S.B.; Vines, R.R.; Parsons, J.T. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Cell. Biol. 1991, 11, 5113–5124. [Google Scholar] [CrossRef]
- Boyle, S.N.; Michaud, G.A.; Schweitzer, B.; Predki, P.F.; Koleske, A.J. A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr. Biol. 2007, 17, 445–451. [Google Scholar] [CrossRef]
- Campbell, D.H.; Sutherland, R.L.; Daly, R.J. Signaling pathways and structural domains required for phosphorylation of EMS1/cortactin. Cancer Res. 1999, 59, 5376–5385. [Google Scholar] [PubMed]
- Vidal, C.; Geny, B.; Melle, J.; Jandrot-Perrus, M.; Fontenay-Roupie, M. Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: Implication of the cortical-actin binding protein cortactin. Blood 2002, 100, 4462–4469. [Google Scholar] [CrossRef] [PubMed]
- Eiseler, T.; Hausser, A.; de Kimpe, L.; van Lint, J.; Pfizenmaier, K. Protein kinase D controls actin polymerization and cell motility through phosphorylation of cortactin. J. Biol. Chem. 2010, 285, 18672–18683. [Google Scholar] [CrossRef]
- Schnoor, M.; Stradal, T.E.; Rottner, K. Cortactin: Cell Functions of A Multifaceted Actin-Binding Protein. Trends Cell Biol. 2018, 28, 79–98. [Google Scholar] [CrossRef]
- Weed, S.A.; Du, Y.; Parsons, J.T. Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J. Cell Sci. 1998, 111 (Pt 16), 2433–2443. [Google Scholar] [CrossRef]
- Head, J.A.; Jiang, D.; Li, M.; Zorn, L.J.; Schaefer, E.M.; Parsons, J.T.; Weed, S.A. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. MBoC 2003, 14, 3216–3229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, Z.; Zhang, Y.; Yong, S.; Salas-Burgos, A.; Koomen, J.; Olashaw, N.; Parsons, J.T.; Yang, X.-J.; Dent, S.R.; et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol. Cell 2007, 27, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Kozyreva, V.K.; McLaughlin, S.L.; Livengood, R.H.; Calkins, R.A.; Kelley, L.C.; Rajulapati, A.; Ice, R.J.; Smolkin, M.B.; Weed, S.A.; Pugacheva, E.N. NEDD9 regulates actin dynamics through cortactin deacetylation in an AURKA/HDAC6-dependent manner. Mol. Cancer Res. 2014, 12, 681–693. [Google Scholar] [CrossRef]
- Tanaka, S.; Masuda, Y.; Harada, A.; Okabe, S. Impaired actin dynamics and suppression of Shank2-mediated spine enlargement in cortactin knockout mice. Microscopy 2020, 69, 44–52. [Google Scholar] [CrossRef]
- Hering, H.; Sheng, M. Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J. Neurosci. 2003, 23, 11759–11769. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Negishi, M.; Katoh, H. Rac GEF Dock4 interacts with cortactin to regulate dendritic spine formation. Mol. Biol. Cell. 2013, 24, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Shimazu, T.; Maeda, S.; Shah, A.A.; Tsunoda, T.; Iemura, S.-I.; Natsume, T.; Suzuki, T.; Motohashi, H.; Yamamoto, M.; et al. The subcellular localization and activity of cortactin is regulated by acetylation and interaction with Keap1. Sci. Signal. 2015, 8, ra120. [Google Scholar] [CrossRef] [PubMed]
- Newey, S.E.; Velamoor, V.; Govek, E.-E.; van Aelst, L. Rho GTPases, dendritic structure, and mental retardation. J. Neurobiol. 2005, 64, 58–74. [Google Scholar] [CrossRef]
- Duman, J.G.; Mulherkar, S.; Tu, Y.-K.; Cheng, X.J.; Tolias, K.F. Mechanisms for spatiotemporal regulation of Rho-GTPase signaling at synapses. Neurosci. Lett. 2015, 601, 4–10. [Google Scholar] [CrossRef]
- Nakayama, A.Y.; Harms, M.B.; Luo, L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J. Neurosci. 2000, 20, 5329–5338. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jin, T. ELMO proteins transduce G protein-coupled receptor signal to control reorganization of actin cytoskeleton in chemotaxis of eukaryotic cells. Small GTPases 2019, 10, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Sanematsu, F.; Nishikimi, A.; Watanabe, M.; Hongu, T.; Tanaka, Y.; Kanaho, Y.; Côté, J.-F.; Fukui, Y. Phosphatidic acid-dependent recruitment and function of the Rac activator DOCK1 during dorsal ruffle formation. J. Biol. Chem. 2013, 288, 8092–8100. [Google Scholar] [CrossRef]
- Gadea, G.; Blangy, A. Dock-family exchange factors in cell migration and disease. Eur. J. Cell Biol. 2014, 93, 466–477. [Google Scholar] [CrossRef]
- Margaron, Y.; Fradet, N.; Côté, J.-F. ELMO recruits actin cross-linking family 7 (ACF7) at the cell membrane for microtubule capture and stabilization of cellular protrusions. J. Biol. Chem. 2013, 288, 1184–1199. [Google Scholar] [CrossRef]
- Reddien, P.W.; Horvitz, H.R. The engulfment process of programmed cell death in caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 2004, 20, 193–221. [Google Scholar] [CrossRef]
- Santy, L.C.; Ravichandran, K.S.; Casanova, J.E. The DOCK180/Elmo complex couples ARNO-mediated Arf6 activation to the downstream activation of Rac1. Curr. Biol. 2005, 15, 1749–1754. [Google Scholar] [CrossRef]
- Patel, M.; Chiang, T.-C.; Tran, V.; Lee, F.-J.S.; Côté, J.-F. The Arf family GTPase Arl4A complexes with ELMO proteins to promote actin cytoskeleton remodeling and reveals a versatile Ras-binding domain in the ELMO proteins family. J. Biol. Chem. 2011, 286, 38969–38979. [Google Scholar] [CrossRef]
- Jaworski, J. ARF6 in the nervous system. Eur. J. Cell Biol. 2007, 86, 513–524. [Google Scholar] [CrossRef]
- Song, J.; Khachikian, Z.; Radhakrishna, H.; Donaldson, J.G. Localization of endogenous ARF6 to sites of cortical actin rearrangement and involvement of ARF6 in cell spreading. J. Cell Sci. 1998, 111 (Pt 15), 2257–2267. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Oh, M.H.; Bernard, L.P.; Macara, I.G.; Zhang, H. The RhoG/ELMO1/Dock180 signaling module is required for spine morphogenesis in hippocampal neurons. J. Biol. Chem. 2011, 286, 37615–37624. [Google Scholar] [CrossRef]
- Hayashi, M.L.; Choi, S.-Y.; Rao, B.S.S.; Jung, H.-Y.; Lee, H.-K.; Zhang, D.; Chattarji, S.; Kirkwood, A.; Tonegawa, S. Altered cortical synaptic morphology and impaired memory consolidation in forebrain- specific dominant-negative PAK transgenic mice. Neuron 2004, 42, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.C.; Sanders, L.C.; Bokoch, G.M.; Gill, G.N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1999, 1, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Arber, S.; Barbayannis, F.A.; Hanser, H.; Schneider, C.; Stanyon, C.A.; Bernard, O.; Caroni, P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 1998, 393, 805–809. [Google Scholar] [CrossRef]
- Bamburg, J.R.; Bernstein, B.W.; Davis, R.C.; Flynn, K.C.; Goldsbury, C.; Jensen, J.R.; Maloney, M.T.; Marsden, I.T.; Minamide, L.S.; Pak, C.W.; et al. ADF/Cofilin-actin rods in neurodegenerative diseases. Curr. Alzheimer Res. 2010, 7, 241–250. [Google Scholar] [CrossRef]
- Ohashi, K. Roles of cofilin in development and its mechanisms of regulation. Dev. Growth Differ. 2015, 57, 275–290. [Google Scholar] [CrossRef]
- Wioland, H.; Guichard, B.; Senju, Y.; Myram, S.; Lappalainen, P.; Jégou, A.; Romet-Lemonne, G. ADF/Cofilin Accelerates Actin Dynamics by Severing Filaments and Promoting Their Depolymerization at Both Ends. Curr. Biol. 2017, 27, 1956–1967. [Google Scholar] [CrossRef]
- Kanellos, G.; Frame, M.C. Cellular functions of the ADF/cofilin family at a glance. J. Cell Sci. 2016, 129, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.N.M.; Dunbar, C.; Mórotz, G.M.; Cheng, W.H.; Chan, H.Y.E.; Miller, C.C.J.; Lau, K.-F. FE65 interacts with ADP-ribosylation factor 6 to promote neurite outgrowth. FASEB J. 2014, 28, 337–349. [Google Scholar] [CrossRef]
- Li, W.; Chan, W.R.; Ngo, J.K.; Lau, K.-F. Emerging roles of the neural adaptor FE65 in neurite outgrowth. Neural Regen Res 2018, 13, 2085. [Google Scholar] [CrossRef]
- Patel, M.; Margaron, Y.; Fradet, N.; Yang, Q.; Wilkes, B.; Bouvier, M.; Hofmann, K.; Côté, J.-F. An evolutionarily conserved autoinhibitory molecular switch in ELMO proteins regulates Rac signaling. Curr. Biol. 2010, 20, 2021–2027. [Google Scholar] [CrossRef]
- Locasale, J.W.; Shaw, A.S.; Chakraborty, A.K. Scaffold proteins confer diverse regulatory properties to protein kinase cascades. Proc. Natl. Acad. Sci. USA 2007, 104, 13307–13312. [Google Scholar] [CrossRef]
- Burack, W.R.; Shaw, A.S. Signal transduction: Hanging on a scaffold. Curr. Opin. Cell Biol. 2000, 12, 211–216. [Google Scholar] [CrossRef]
- Jamora, C.; Fuchs, E. Intercellular adhesion, signalling and the cytoskeleton. Nat. Cell Biol. 2002, 4, E101–E108. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Pokutta, S.; Drees, F.; Weis, W.I.; Nelson, W.J. Deconstructing the cadherin-catenin-actin complex. Cell 2005, 123, 889–901. [Google Scholar] [CrossRef]
- Drees, F.; Pokutta, S.; Yamada, S.; Nelson, W.J.; Weis, W.I. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 2005, 123, 903–915. [Google Scholar] [CrossRef]
- Garbett, D.; Bretscher, A. The surprising dynamics of scaffolding proteins. Mol. Biol. Cell. 2014, 25, 2315–2319. [Google Scholar] [CrossRef]
- Jowsey, P.A.; Blain, P.G. Fe65 Ser228 is phosphorylated by ATM/ATR and inhibits Fe65-APP-mediated gene transcription. Biochem. J. 2015, 465, 413–421. [Google Scholar] [CrossRef]
- Chow, W.N.V.; Ngo, J.C.K.; Li, W.; Chen, Y.W.; Tam, K.M.V.; Chan, H.Y.E.; Miller, C.C.J.; Lau, K.-F. Phosphorylation of FE65 Ser610 by serum- and glucocorticoid-induced kinase 1 modulates Alzheimer’s disease amyloid precursor protein processing. Biochem. J. 2015, 470, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Chow, W.N.V.; Lau, K.-F. Phosphorylation of FE65 at threonine 579 by GSK3β stimulates amyloid precursor protein processing. Sci. Rep. 2017, 7, 12456. [Google Scholar] [CrossRef] [PubMed]
- Standen, C.L.; Perkinton, M.S.; Byers, H.L.; Kesavapany, S.; Lau, K.-F.; Ward, M.; McLoughlin, D.; Miller, C.C.J. The neuronal adaptor protein Fe65 is phosphorylated by mitogen-activated protein kinase (ERK1/2). Mol. Cell. Neurosci. 2003, 24, 851–857. [Google Scholar] [CrossRef]
- Lee, E.J.; Chun, J.; Hyun, S.; Ahn, H.R.; Jeong, J.M.; Hong, S.-K.; Hong, J.T.; Chang, I.K.; Jeon, H.Y.; Han, Y.S.; et al. Regulation Fe65 localization to the nucleus by SGK1 phosphorylation of its Ser566 residue. BMB Rep. 2008, 41, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.-F.; Chan, W.-M.; Perkinton, M.S.; Tudor, E.L.; Chang, R.C.C.; Chan, H.-Y.E.; McLoughlin, D.M.; Miller, C.C.J. Dexras1 interacts with FE65 to regulate FE65-amyloid precursor protein-dependent transcription. J. Biol. Chem. 2008, 283, 34728–34737. [Google Scholar] [CrossRef]
- Tanimura, S.; Takeda, K. ERK signalling as a regulator of cell motility. J. Biochem. 2017, 162, 145–154. [Google Scholar] [CrossRef]
- Mendoza, M.C.; Vilela, M.; Juarez, J.E.; Blenis, J.; Danuser, G. ERK reinforces actin polymerization to power persistent edge protrusion during motility. Sci. Signal. 2015, 8, ra47. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-A.; Kim, H.-S.; Ha, T.-Y.; Ha, J.-W.; Shin, K.Y.; Jeong, Y.H.; Lee, J.-P.; Park, C.-H.; Kim, S.; Baik, T.-K.; et al. Phosphorylation of amyloid precursor protein (APP) at Thr668 regulates the nuclear translocation of the APP intracellular domain and induces neurodegeneration. Mol. Cell. Biol. 2006, 26, 4327–4338. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, T.; Suzuki, T. Role of APP phosphorylation in FE65-dependent gene transactivation mediated by AICD. Genes Cells 2006, 11, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Klug, W.; Dietl, A.; Simon, B.; Sinning, I.; Wild, K. Phosphorylation of LRP1 regulates the interaction with Fe65. FEBS Lett. 2011, 585, 3229–3235. [Google Scholar] [CrossRef][Green Version]
- Ando, K.; Iijima, K.I.; Elliott, J.I.; Kirino, Y.; Suzuki, T. Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J. Biol. Chem. 2001, 276, 40353–40361. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Moon, C.; Hu, Q.; Wang, B.; Martin, G.; Sun, Z.; Wang, H. The APP-interacting protein FE65 is required for hippocampus-dependent learning and long-term potentiation. Learn. Mem. 2009, 16, 537–544. [Google Scholar] [CrossRef]
- Guénette, S.; Chang, Y.; Hiesberger, T.; Richardson, J.A.; Eckman, C.B.; Eckman, E.A.; Hammer, R.E.; Herz, J. Essential roles for the FE65 amyloid precursor protein-interacting proteins in brain development. EMBO J. 2006, 25, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Moncaster, J.A.; Wang, L.; Hafeez, I.; Herz, J.; Tanzi, R.E.; Goldstein, L.E.; Guénette, S.Y. FE65 and FE65L1 amyloid precursor protein-binding protein compound null mice display adult-onset cataract and muscle weakness. FASEB J. 2015, 29, 2628–2639. [Google Scholar] [CrossRef]
- Strecker, P.; Ludewig, S.; Rust, M.; Mundinger, T.A.; Görlich, A.; Krächan, E.G.; Mehrfeld, C.; Herz, J.; Korte, M.; Guénette, S.Y.; et al. FE65 and FE65L1 share common synaptic functions and genetically interact with the APP family in neuromuscular junction formation. Sci. Rep. 2016, 6, 25652. [Google Scholar] [CrossRef]
- Santiard-Baron, D.; Langui, D.; Delehedde, M.; Delatour, B.; Schombert, B.; Touchet, N.; Tremp, G.; Paul, M.-F.; Blanchard, V.; Sergeant, N.; et al. Expression of human FE65 in amyloid precursor protein transgenic mice is associated with a reduction in beta-amyloid load. J. Neurochem. 2005, 93, 330–338. [Google Scholar] [CrossRef]
- Ghosal, K.; Vogt, D.L.; Liang, M.; Shen, Y.; Lamb, B.T.; Pimplikar, S.W. Alzheimer’s disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proc. Natl. Acad. Sci. USA 2009, 106, 18367–18372. [Google Scholar] [CrossRef]
- Herms, J.; Anliker, B.; Heber, S.; Ring, S.; Fuhrmann, M.; Kretzschmar, H.; Sisodia, S.; Müller, U. Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members. EMBO J. 2004, 23, 4106–4115. [Google Scholar] [CrossRef]
- Ross, M.E.; Walsh, C.A. Human brain malformations and their lessons for neuronal migration. Annu. Rev. Neurosci. 2001, 24, 1041–1070. [Google Scholar] [CrossRef]
- Olson, E.C.; Walsh, C.A. Smooth, rough and upside-down neocortical development. Curr. Opin. Genet. Dev. 2002, 12, 320–327. [Google Scholar] [CrossRef]
- Buchsbaum, I.Y.; Cappello, S. Neuronal migration in the CNS during development and disease: Insights from in vivo and in vitro models. Development 2019, 146. [Google Scholar] [CrossRef]
- Kwiatkowski, A.V.; Garner, C.C.; Nelson, W.J.; Gertler, F.B. Cell autonomous defects in cortical development revealed by two-color chimera analysis. Mol. Cell. Neurosci. 2009, 41, 44–50. [Google Scholar] [CrossRef][Green Version]
- Kurklinsky, S.; Chen, J.; McNiven, M.A. Growth cone morphology and spreading are regulated by a dynamin-cortactin complex at point contacts in hippocampal neurons. J. Neurochem. 2011, 117, 48–60. [Google Scholar] [CrossRef]
- He, Y.; Ren, Y.; Wu, B.; Decourt, B.; Lee, A.C.; Taylor, A.; Suter, D.M. Src and cortactin promote lamellipodia protrusion and filopodia formation and stability in growth cones. Mol. Biol. Cell 2015, 26, 3229–3244. [Google Scholar] [CrossRef] [PubMed]
- McNeill, E.M.; Thompson, C.; Berke, B.; Chou, V.T.; Rusch, J.; Duckworth, A.; DeProto, J.; Taylor, A.; Gates, J.; Gertler, F.; et al. Drosophila enabled promotes synapse morphogenesis and regulates active zone form and function. Neural Dev. 2020, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, C.; Saito, Y.; Umehara, T.; Kamimura, K.; Maeda, N.; Mosca, T.J.; Miura, M.; Chihara, T. The Strip-Hippo Pathway Regulates Synaptic Terminal Formation by Modulating Actin Organization at the Drosophila Neuromuscular Synapses. Cell Rep. 2016, 16, 2289–2297. [Google Scholar] [CrossRef]
- Loya, C.M.; McNeill, E.M.; Bao, H.; Zhang, B.; van Vactor, D. miR-8 controls synapse structure by repression of the actin regulator enabled. Development 2014, 141, 1864–1874. [Google Scholar] [CrossRef][Green Version]
- Laurent, V.; Loisel, T.P.; Harbeck, B.; Wehman, A.; Gröbe, L.; Jockusch, B.M.; Wehland, J.; Gertler, F.B.; Carlier, M.F. Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes. J. Cell Biol. 1999, 144, 1245–1258. [Google Scholar] [CrossRef]
- Loisel, T.P.; Boujemaa, R.; Pantaloni, D.; Carlier, M.F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 1999, 401, 613–616. [Google Scholar] [CrossRef]
- Tucker, P.K.; Evans, I.R.; Wood, W. Ena drives invasive macrophage migration in Drosophila embryos. Dis. Model. Mech. 2011, 4, 126–134. [Google Scholar] [CrossRef]
- Philippar, U.; Roussos, E.T.; Oser, M.; Yamaguchi, H.; Kim, H.-D.; Giampieri, S.; Wang, Y.; Goswami, S.; Wyckoff, J.B.; Lauffenburger, D.A.; et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev. Cell 2008, 15, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Damiano-Guercio, J.; Kurzawa, L.; Mueller, J.; Dimchev, G.; Schaks, M.; Nemethova, M.; Pokrant, T.; Brühmann, S.; Linkner, J.; Blanchoin, L.; et al. Loss of Ena/VASP interferes with lamellipodium architecture, motility and integrin-dependent adhesion. Elife 2020, 9. [Google Scholar] [CrossRef]
- Sun, Y.; Kasiappan, R.; Tang, J.; Webb, P.L.; Quarni, W.; Zhang, X.; Bai, W. A novel function of the Fe65 neuronal adaptor in estrogen receptor action in breast cancer cells. J. Biol. Chem. 2014, 289, 12217–12231. [Google Scholar] [CrossRef] [PubMed]
- Danish Rizvi, S.M.; Hussain, T.; Subaiea, G.M.; Shakil, S.; Ahmad, A. Therapeutic Targeting of Amyloid Precursor Protein and its Processing Enzymes for Breast Cancer Treatment. Curr. Protein Pept. Sci. 2018, 19, 841–849. [Google Scholar] [CrossRef]
- Pandey, P.; Sliker, B.; Peters, H.L.; Tuli, A.; Herskovitz, J.; Smits, K.; Purohit, A.; Singh, R.K.; Dong, J.; Batra, S.K.; et al. Amyloid precursor protein and amyloid precursor-like protein 2 in cancer. Oncotarget 2016, 7, 19430–19444. [Google Scholar] [CrossRef]
- Lee, H.N.; Jeong, M.S.; Jang, S.B. Molecular Characteristics of Amyloid Precursor Protein (APP) and Its Effects in Cancer. Int. J. Mol. Sci. 2021, 22, 4999. [Google Scholar] [CrossRef]
- Carmona, G.; Perera, U.; Gillett, C.; Naba, A.; Law, A.-L.; Sharma, V.P.; Wang, J.; Wyckoff, J.; Balsamo, M.; Mosis, F.; et al. Lamellipodin promotes invasive 3D cancer cell migration via regulated interactions with Ena/VASP and SCAR/WAVE. Oncogene 2016, 35, 5155–5169. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Li, F. ELMO2 association with Gαi2 regulates pancreatic cancer cell chemotaxis and metastasis. PeerJ 2020, 8, e8910. [Google Scholar] [CrossRef] [PubMed]
- MacGrath, S.M.; Koleske, A.J. Cortactin in cell migration and cancer at a glance. J. Cell Sci. 2012, 125, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiong, X.; Abdalla, A.; Alejo, S.; Zhu, L.; Lu, F.; Sun, H. HGF-induced formation of the MET-AXL-ELMO2-DOCK180 complex promotes RAC1 activation, receptor clustering, and cancer cell migration and invasion. J. Biol. Chem. 2018, 293, 15397–15418. [Google Scholar] [CrossRef]
- Yin, M.; Ma, W.; An, L. Cortactin in cancer cell migration and invasion. Oncotarget 2017, 8, 88232–88243. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Müller, M.; Chiha, S.; Ren, J.; Albat, D.; Soicke, A.; Dohmen, S.; Klein, M.; Bruns, J.; van Dinther, M.; et al. Designed nanomolar small-molecule inhibitors of Ena/VASP EVH1 interaction impair invasion and extravasation of breast cancer cells. Proc. Natl. Acad. Sci. USA 2020, 117, 29684–29690. [Google Scholar] [CrossRef]
- Di Modugno, F.; DeMonte, L.; Balsamo, M.; Bronzi, G.; Nicotra, M.R.; Alessio, M.; Jager, E.; Condeelis, J.S.; Santoni, A.; Natali, P.G.; et al. Molecular cloning of hMena (ENAH) and its splice variant hMena+11a: Epidermal growth factor increases their expression and stimulates hMena+11a phosphorylation in breast cancer cell lines. Cancer Res. 2007, 67, 2657–2665. [Google Scholar] [CrossRef]
- Di Modugno, F.; Iapicca, P.; Boudreau, A.; Mottolese, M.; Terrenato, I.; Perracchio, L.; Carstens, R.P.; Santoni, A.; Bissell, M.J.; Nisticò, P. Splicing program of human MENA produces a previously undescribed isoform associated with invasive, mesenchymal-like breast tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 19280–19285. [Google Scholar] [CrossRef]
- Trono, P.; Di Modugno, F.; Circo, R.; Spada, S.; Di Benedetto, A.; Melchionna, R.; Palermo, B.; Matteoni, S.; Soddu, S.; Mottolese, M.; et al. hMENA(11a) contributes to HER3-mediated resistance to PI3K inhibitors in HER2-overexpressing breast cancer cells. Oncogene 2016, 35, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Gertler, F.B.; Balsamo, M.; Condeelis, J.S.; Camp, R.L.; Xue, X.; Lin, J.; Rohan, T.E.; Rimm, D.L. Quantitative assessment of invasive mena isoforms (Menacalc) as an independent prognostic marker in breast cancer. Breast Cancer Res. 2012, 14, R124. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, L.; Fu, H.; Yan, J.; Wang, Y.; Guo, H.; Hao, X.; Xu, X.; Jin, T.; Zhang, N. Association between Gαi2 and ELMO1/Dock180 connects chemokine signalling with Rac activation and metastasis. Nat. Commun. 2013, 4, 1706. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, S.; Zhang, Y. Downregulation of Dock1 and Elmo1 suppresses the migration and invasion of triple-negative breast cancer epithelial cells through the RhoA/Rac1 pathway. Oncol. Lett. 2018, 16, 3481–3488. [Google Scholar] [CrossRef]
- Abu-Thuraia, A.; Gauthier, R.; Chidiac, R.; Fukui, Y.; Screaton, R.A.; Gratton, J.-P.; Côté, J.-F. Axl phosphorylates Elmo scaffold proteins to promote Rac activation and cell invasion. Mol. Cell. Biol. 2015, 35, 76–87. [Google Scholar] [CrossRef]
- Hasan, M.K.; Widhopf, G.F.; Zhang, S.; Lam, S.M.; Shen, Z.; Briggs, S.P.; Parker, B.A.; Kipps, T.J. Wnt5a induces ROR1 to recruit cortactin to promote breast-cancer migration and metastasis. NPJ Breast Cancer 2019, 5, 35. [Google Scholar] [CrossRef]
- Soba, P.; Eggert, S.; Wagner, K.; Zentgraf, H.; Siehl, K.; Kreger, S.; Löwer, A.; Langer, A.; Merdes, G.; Paro, R.; et al. Homo- and heterodimerization of APP family members promotes intercellular adhesion. EMBO J. 2005, 24, 3624–3634. [Google Scholar] [CrossRef]
- Zheng, H.; Jiang, M.; Trumbauer, M.E.; Sirinathsinghji, D.J.; Hopkins, R.; Smith, D.W.; Heavens, R.P.; Dawson, G.R.; Boyce, S.; Conner, M.W.; et al. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell 1995, 81, 525–531. [Google Scholar] [CrossRef]
- Müller, U.; Cristina, N.; Li, Z.W.; Wolfer, D.P.; Lipp, H.P.; Rülicke, T.; Brandner, S.; Aguzzi, A.; Weissmann, C. Behavioral and anatomical deficits in mice homozygous for a modified beta-amyloid precursor protein gene. Cell 1994, 79, 755–765. [Google Scholar] [CrossRef]
- Tremml, P.; Lipp, H.P.; Müller, U.; Ricceri, L.; Wolfer, D.P. Neurobehavioral development, adult openfield exploration and swimming navigation learning in mice with a modified beta-amyloid precursor protein gene. Behav. Brain Res. 1998, 95, 65–76. [Google Scholar] [CrossRef]
- Müller, U.; Cristina, N.; Li, Z.W.; Wolfer, D.P.; Lipp, H.P.; Rülicke, T.; Brandner, S.; Aguzzi, A.; Weissman, C. Mice homozygous for a modified beta-amyloid precursor protein (beta APP) gene show impaired behavior and high incidence of agenesis of the corpus callosum. Ann. N. Y. Acad. Sci. 1996, 777, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Osterhout, J.A.; Stafford, B.K.; Nguyen, P.L.; Yoshihara, Y.; Huberman, A.D. Contactin-4 mediates axon-target specificity and functional development of the accessory optic system. Neuron 2015, 86, 985–999. [Google Scholar] [CrossRef]
- Marik, S.A.; Olsen, O.; Tessier-Lavigne, M.; Gilbert, C.D. Physiological role for amyloid precursor protein in adult experience-dependent plasticity. Proc. Natl. Acad. Sci. USA. 2016, 113, 7912–7917. [Google Scholar] [CrossRef]
- Southam, K.A.; Stennard, F.; Pavez, C.; Small, D.H. Knockout of Amyloid β Protein Precursor (APP) Expression Alters Synaptogenesis, Neurite Branching and Axonal Morphology of Hippocampal Neurons. Neurochem. Res. 2019, 44, 1346–1355. [Google Scholar] [CrossRef]
- Magara, F.; Müller, U.; Li, Z.W.; Lipp, H.P.; Weissmann, C.; Stagljar, M.; Wolfer, D.P. Genetic background changes the pattern of forebrain commissure defects in transgenic mice underexpressing the beta-amyloid-precursor protein. Proc. Natl. Acad. Sci. USA 1999, 96, 4656–4661. [Google Scholar] [CrossRef]
- Dawson, G.R.; Seabrook, G.R.; Zheng, H.; Smith, D.W.; Graham, S.; O’Dowd, G.; Bowery, B.J.; Boyce, S.; Trumbauer, M.E.; Chen, H.Y.; et al. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience 1999, 90, 1–13. [Google Scholar] [CrossRef]
- Perez, R.G.; Zheng, H.; van der Ploeg, L.H.T.; Koo, E.H. The β-Amyloid Precursor Protein of Alzheimer’s Disease Enhances Neuron Viability and Modulates Neuronal Polarity. J. Neurosci. 1997, 17, 9407–9414. [Google Scholar] [CrossRef]
- Seabrook, G.R.; Smith, D.W.; Bowery, B.J.; Easter, A.; Reynolds, T.; Fitzjohn, S.M.; Morton, R.A.; Zheng, H.; Dawson, G.R.; Sirinathsinghji, D.J.; et al. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology 1999, 38, 349–359. [Google Scholar] [CrossRef]
- Tyan, S.-H.; Shih, A.Y.-J.; Walsh, J.J.; Maruyama, H.; Sarsoza, F.; Ku, L.; Eggert, S.; Hof, P.R.; Koo, E.H.; Dickstein, D.L. Amyloid precursor protein (APP) regulates synaptic structure and function. Mol. Cell. Neurosci. 2012, 51, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Z.; Sun, L.; Yang, L.; Li, H.; Cole, A.L.; Rodriguez-Rivera, J.; Lu, H.-C.; Zheng, H. The amyloid precursor protein controls adult hippocampal neurogenesis through GABAergic interneurons. J. Neurosci. 2014, 34, 13314–13325. [Google Scholar] [CrossRef]
- Bittner, T.; Fuhrmann, M.; Burgold, S.; Jung, C.K.E.; Volbracht, C.; Steiner, H.; Mitteregger, G.; Kretzschmar, H.A.; Haass, C.; Herms, J. Gamma-secretase inhibition reduces spine density in vivo via an amyloid precursor protein-dependent pathway. J. Neurosci. 2009, 29, 10405–10409. [Google Scholar] [CrossRef]
- Weyer, S.W.; Zagrebelsky, M.; Herrmann, U.; Hick, M.; Ganss, L.; Gobbert, J.; Gruber, M.; Altmann, C.; Korte, M.; Deller, T.; et al. Comparative analysis of single and combined APP/APLP knockouts reveals reduced spine density in APP-KO mice that is prevented by APPsα expression. Acta Neuropathol. Commun. 2014, 2, 36. [Google Scholar] [CrossRef]
- Lee, K.J.; Moussa, C.E.H.; Lee, Y.; Sung, Y.; Howell, B.W.; Turner, R.S.; Pak, D.T.S.; Hoe, H.S. Beta amyloid-independent role of amyloid precursor protein in generation and maintenance of dendritic spines. Neuroscience 2010, 169, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Galanis, C.; Fellenz, M.; Becker, D.; Bold, C.; Lichtenthaler, S.F.; Müller, U.C.; Deller, T.; Vlachos, A. Amyloid-beta mediates homeostatic synaptic plasticity. J. Neurosci. 2021. [Google Scholar] [CrossRef]
- Zou, C.; Crux, S.; Marinesco, S.; Montagna, E.; Sgobio, C.; Shi, Y.; Shi, S.; Zhu, K.; Dorostkar, M.M.; Müller, U.C.; et al. Amyloid precursor protein maintains constitutive and adaptive plasticity of dendritic spines in adult brain by regulating D-serine homeostasis. EMBO J. 2016, 35, 2213–2222. [Google Scholar] [CrossRef]
- Steubler, V.; Erdinger, S.; Back, M.K.; Ludewig, S.; Fässler, D.; Richter, M.; Han, K.; Slomianka, L.; Amrein, I.; von Engelhardt, J.; et al. Loss of all three APP family members during development impairs synaptic function and plasticity, disrupts learning, and causes an autism-like phenotype. EMBO J. 2021, e107471. [Google Scholar] [CrossRef]
- Menzies, A.S.; Aszodi, A.; Williams, S.E.; Pfeifer, A.; Wehman, A.M.; Goh, K.L.; Mason, C.A.; Fassler, R.; Gertler, F.B. Mena and vasodilator-stimulated phosphoprotein are required for multiple actin-dependent processes that shape the vertebrate nervous system. J. Neurosci. 2004, 24, 8029–8038. [Google Scholar] [CrossRef]
- Wang, P. Defective Neuromuscular Synapses in Mice Lacking Amyloid Precursor Protein (APP) and APP-Like Protein 2. J. Neurosci. 2005, 25, 1219–1225. [Google Scholar] [CrossRef]
- Phinney, A.L.; Calhoun, M.E.; Wolfer, D.P.; Lipp, H.P.; Zheng, H.; Jucker, M. No hippocampal neuron or synaptic bouton loss in learning-impaired aged beta-amyloid precursor protein-null mice. Neuroscience 1999, 90, 1207–1216. [Google Scholar] [CrossRef]
- Ring, S.; Weyer, S.W.; Kilian, S.B.; Waldron, E.; Pietrzik, C.U.; Filippov, M.A.; Herms, J.; Buchholz, C.; Eckman, C.B.; Korte, M.; et al. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J. Neurosci. 2007, 27, 7817–7826. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, J.P.; Müller, U.; Leist, M.; Li, Z.W.; Nicotera, P.; Aguzzi, A. Hypersensitivity to seizures in beta-amyloid precursor protein deficient mice. Cell Death Differ. 1998, 5, 858–866. [Google Scholar] [CrossRef]
- Jedlicka, P.; Owen, M.; Vnencak, M.; Tschäpe, J.-A.; Hick, M.; Müller, U.C.; Deller, T. Functional consequences of the lack of amyloid precursor protein in the mouse dentate gyrus in vivo. Exp. Brain Res. 2012, 217, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, B.; Long, C.; Wu, G.; Zheng, H. Increased asynchronous release and aberrant calcium channel activation in amyloid precursor protein deficient neuromuscular synapses. Neuroscience 2007, 149, 768–778. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, J.; Ho, A.; Watanabe, H.; Bolshakov, V.Y.; Shen, J. APP Family Regulates Neuronal Excitability and Synaptic Plasticity but Not Neuronal Survival. Neuron 2020, 108, 676–690. [Google Scholar] [CrossRef]
- Furman, C.; Sieminski, A.L.; Kwiatkowski, A.V.; Rubinson, D.A.; Vasile, E.; Bronson, R.T.; Fässler, R.; Gertler, F.B. Ena/VASP is required for endothelial barrier function in vivo. J. Cell Biol. 2007, 179, 761–775. [Google Scholar] [CrossRef]
- Guénette, S.Y.; Chen, J.; Ferland, A.; Haass, C.; Capell, A.; Tanzi, R.E. hFE65L Influences Amyloid Precursor Protein Maturation and Secretion. J. Neurochem. 2001, 73, 985–993. [Google Scholar] [CrossRef]
- Ferreira, A.; Caceres, A.; Kosik, K.S. Intraneuronal compartments of the amyloid precursor protein. J. Neurosci. 1993, 13, 3112–3123. [Google Scholar] [CrossRef]
- Yamazaki, T.; Koo, E.H.; Selkoe, D.J. Cell Surface Amyloid β-Protein Precursor Colocalizes with β1 Integrins at Substrate Contact Sites in Neural Cells. J. Neurosci. 1997, 17, 1004–1010. [Google Scholar] [CrossRef]
- Ikin, A.F.; Annaert, W.G.; Takei, K.; de Camilli, P.; Jahn, R.; Greengard, P.; Buxbaum, J.D. Alzheimer amyloid protein precursor is localized in nerve terminal preparations to Rab5-containing vesicular organelles distinct from those implicated in the synaptic vesicle pathway. J. Biol. Chem. 1996, 271, 31783–31786. [Google Scholar] [CrossRef]
- Yamazaki, T.; Selkoe, D.J.; Koo, E.H. Trafficking of cell surface beta-amyloid precursor protein: Retrograde and transcytotic transport in cultured neurons. J. Cell Biol. 1995, 129, 431–442. [Google Scholar] [CrossRef]
- Koo, E.H.; Sisodia, S.S.; Cork, L.C.; Unterbeck, A.; Bayney, R.M.; Price, D.L. Differential expression of amyloid precursor protein mRNAs in cases of Alzheimer’s disease and in aged nonhuman primates. Neuron 1990, 4, 97–104. [Google Scholar] [CrossRef]
- Schilling, S.; Mehr, A.; Ludewig, S.; Stephan, J.; Zimmermann, M.; August, A.; Strecker, P.; Korte, M.; Koo, E.H.; Müller, U.C.; et al. APLP1 Is a Synaptic Cell Adhesion Molecule, Supporting Maintenance of Dendritic Spines and Basal Synaptic Transmission. J. Neurosci. 2017, 37, 5345–5365. [Google Scholar] [CrossRef]
- Laßek, M.; Weingarten, J.; Einsfelder, U.; Brendel, P.; Müller, U.; Volknandt, W. Amyloid precursor proteins are constituents of the presynaptic active zone. J. Neurochem. 2013, 127, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hoe, H.-S.; Fu, Z.; Makarova, A.; Lee, J.-Y.; Lu, C.; Feng, L.; Pajoohesh-Ganji, A.; Matsuoka, Y.; Hyman, B.T.; Ehlers, M.D.; et al. The effects of amyloid precursor protein on postsynaptic composition and activity. J. Biol. Chem. 2009, 284, 8495–8506. [Google Scholar] [CrossRef]
- Wilhelm, B.G.; Mandad, S.; Truckenbrodt, S.; Kröhnert, K.; Schäfer, C.; Rammner, B.; Koo, S.J.; Claßen, G.A.; Krauss, M.; Haucke, V.; et al. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 2014, 344, 1023–1028. [Google Scholar] [CrossRef]
- Cousins, S.L.; Hoey, S.E.A.; Anne Stephenson, F.; Perkinton, M.S. Amyloid precursor protein 695 associates with assembled NR2A- and NR2B-containing NMDA receptors to result in the enhancement of their cell surface delivery. J. Neurochem. 2009, 111, 1501–1513. [Google Scholar] [CrossRef]
- Cousins, S.L.; Innocent, N.; Stephenson, F.A. Neto1 associates with the NMDA receptor/amyloid precursor protein complex. J. Neurochem. 2013, 126, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Cousins, S.L.; Dai, W.; Stephenson, F.A. APLP1 and APLP2, members of the APP family of proteins, behave similarly to APP in that they associate with NMDA receptors and enhance NMDA receptor surface expression. J. Neurochem. 2015, 133, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Hoe, H.-S.; Lee, H.-K.; Pak, D.T.S. The upside of APP at synapses. CNS Neurosci. Ther. 2012, 18, 47–56. [Google Scholar] [CrossRef]
- Sheng, Z.; Prorok, M.; Brown, B.E.; Castellino, F.J. N-methyl-D-aspartate receptor inhibition by an apolipoprotein E-derived peptide relies on low-density lipoprotein receptor-associated protein. Neuropharmacology 2008, 55, 204–214. [Google Scholar] [CrossRef]
- Bacskai, B.J.; Xia, M.Q.; Strickland, D.K.; Rebeck, G.W.; Hyman, B.T. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 11551–11556. [Google Scholar] [CrossRef]
- Pietrzik, C.U.; Yoon, I.-S.; Jaeger, S.; Busse, T.; Weggen, S.; Koo, E.H. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J. Neurosci. 2004, 24, 4259–4265. [Google Scholar] [CrossRef]
- Liu, Q.; Zerbinatti, C.V.; Zhang, J.; Hoe, H.-S.; Wang, B.; Cole, S.L.; Herz, J.; Muglia, L.; Bu, G. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron 2007, 56, 66–78. [Google Scholar] [CrossRef]
- Jaeger, S.; Pietrzik, C.U. Functional role of lipoprotein receptors in Alzheimer’s disease. Curr. Alzheimer Res. 2008, 5, 15–25. [Google Scholar] [CrossRef]
- Sabo, S.L.; Lanier, L.M.; Ikin, A.F.; Khorkova, O.; Sahasrabudhe, S.; Greengard, P.; Buxbaum, J.D. Regulation of beta-amyloid secretion by FE65, an amyloid protein precursor-binding protein. J. Biol. Chem. 1999, 274, 7952–7957. [Google Scholar] [CrossRef]
- Chang, Y.; Tesco, G.; Jeong, W.J.; Lindsley, L.; Eckman, E.A.; Eckman, C.B.; Tanzi, R.E.; Guénette, S.Y. Generation of the beta-amyloid peptide and the amyloid precursor protein C-terminal fragment gamma are potentiated by FE65L1. J. Biol. Chem. 2003, 278, 51100–51107. [Google Scholar] [CrossRef]
- Hu, Q.; Kukull, W.A.; Bressler, S.L.; Gray, M.D.; Cam, J.A.; Larson, E.B.; Martin, G.M.; Deeb, S.S. The human FE65 gene: Genomic structure and an intronic biallelic polymorphism associated with sporadic dementia of the Alzheimer type. Hum. Genet. 1998, 103, 295–303. [Google Scholar] [CrossRef]
- Lambert, J.-C.; Mann, D.; Goumidi, L.; Harris, J.; Pasquier, F.; Frigard, B.; Cottel, D.; Lendon, C.; Iwatsubo, T.; Amouyel, P.; et al. A FE65 polymorphism associated with risk of developing sporadic late-onset Alzheimer’s disease but not with Aβ loading in brains. Neurosci. Lett. 2000, 293, 29–32. [Google Scholar] [CrossRef]
- Tanahashi, H.; Asada, T.; Tabira, T. c954C>T polymorphism in the Fe65L2 gene is associated with early-onset Alzheimer’s disease. Ann. Neurol. 2002, 52, 691–693. [Google Scholar] [CrossRef]
- Jung, C.K.E.; Herms, J. Role of APP for dendritic spine formation and stability. Exp. Brain Res. 2012, 217, 463–470. [Google Scholar] [CrossRef]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; DeKosky, S.T.; Mufson, E.J. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 2007, 68, 1501–1508. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Meyer-Luehmann, M.; Osetek, J.D.; Jones, P.B.; Stern, E.A.; Bacskai, B.J.; Hyman, B.T. Impaired spine stability underlies plaque-related spine loss in an Alzheimer’s disease mouse model. Am. J. Pathol. 2007, 171, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, S.; Vandermeulen, L.; Pizzamiglio, L.; Aksan, B.; Yan, J.; Konietzny, A.; Bonomi, E.; Borroni, B.; Padovani, A.; Rust, M.B.; et al. Cyclase-associated protein 2 dimerization regulates cofilin in synaptic plasticity and Alzheimer’s disease. Brain Commun. 2020, 2, fcaa086. [Google Scholar] [CrossRef]
- Pelucchi, S.; Stringhi, R.; Marcello, E. Dendritic Spines in Alzheimer’s Disease: How the Actin Cytoskeleton Contributes to Synaptic Failure. Int. J. Mol. Sci. 2020, 21, 908. [Google Scholar] [CrossRef]
- Rush, T.; Martinez-Hernandez, J.; Dollmeyer, M.; Frandemiche, M.L.; Borel, E.; Boisseau, S.; Jacquier-Sarlin, M.; Buisson, A. Synaptotoxicity in Alzheimer’s Disease Involved a Dysregulation of Actin Cytoskeleton Dynamics through Cofilin 1 Phosphorylation. J. Neurosci. 2018, 38, 10349–10361. [Google Scholar] [CrossRef] [PubMed]
- Lauterborn, J.C.; Cox, C.D.; Chan, S.W.; Vanderklish, P.W.; Lynch, G.; Gall, C.M. Synaptic actin stabilization protein loss in Down syndrome and Alzheimer disease. Brain Pathol. 2020, 30, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Borovac, J.; Bosch, M.; Okamoto, K. Regulation of actin dynamics during structural plasticity of dendritic spines: Signaling messengers and actin-binding proteins. Mol. Cell. Neurosci. 2018, 91, 122–130. [Google Scholar] [CrossRef]
- Shirao, T.; Hanamura, K.; Koganezawa, N.; Ishizuka, Y.; Yamazaki, H.; Sekino, Y. The role of drebrin in neurons. J. Neurochem. 2017, 141, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Ben Zablah, Y.; Merovitch, N.; Jia, Z. The Role of ADF/Cofilin in Synaptic Physiology and Alzheimer’s Disease. Front. Cell Dev. Biol. 2020, 8, 594998. [Google Scholar] [CrossRef]
- Gordon-Weeks, P.R. The role of the drebrin/EB3/Cdk5 pathway in dendritic spine plasticity, implications for Alzheimer’s disease. Brain Res. Bull. 2016, 126, 293–299. [Google Scholar] [CrossRef]
- Patnaik, A.; Zagrebelsky, M.; Korte, M.; Holz, A. Signaling via the p75 neurotrophin receptor facilitates amyloid-β-induced dendritic spine pathology. Sci. Rep. 2020, 10, 13322. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, M.; Sun, T.; Chen, Y.; Lan, Z.; Lian, B.; Zhao, C.; Liu, Z.; Zhang, J.; Liu, Y. Age-related changes in hippocampal AD pathology, actin remodeling proteins and spatial memory behavior of male APP/PS1 mice. Behav. Brain Res. 2019, 376, 112182. [Google Scholar] [CrossRef]
- Yang, G.; Gong, Y.-D.; Gong, K.; Jiang, W.-L.; Kwon, E.; Wang, P.; Zheng, H.; Zhang, X.-F.; Gan, W.-B.; Zhao, N.-M. Reduced synaptic vesicle density and active zone size in mice lacking amyloid precursor protein (APP) and APP-like protein 2. Neurosci. Lett. 2005, 384, 66–71. [Google Scholar] [CrossRef]
- Weber, A.J.; Herskowitz, J.H. Perspectives on ROCK2 as a Therapeutic Target for Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 636017. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).