Airway-On-A-Chip: Designs and Applications for Lung Repair and Disease
Abstract
1. Introduction
2. Modeling Lung Biology On-Chip
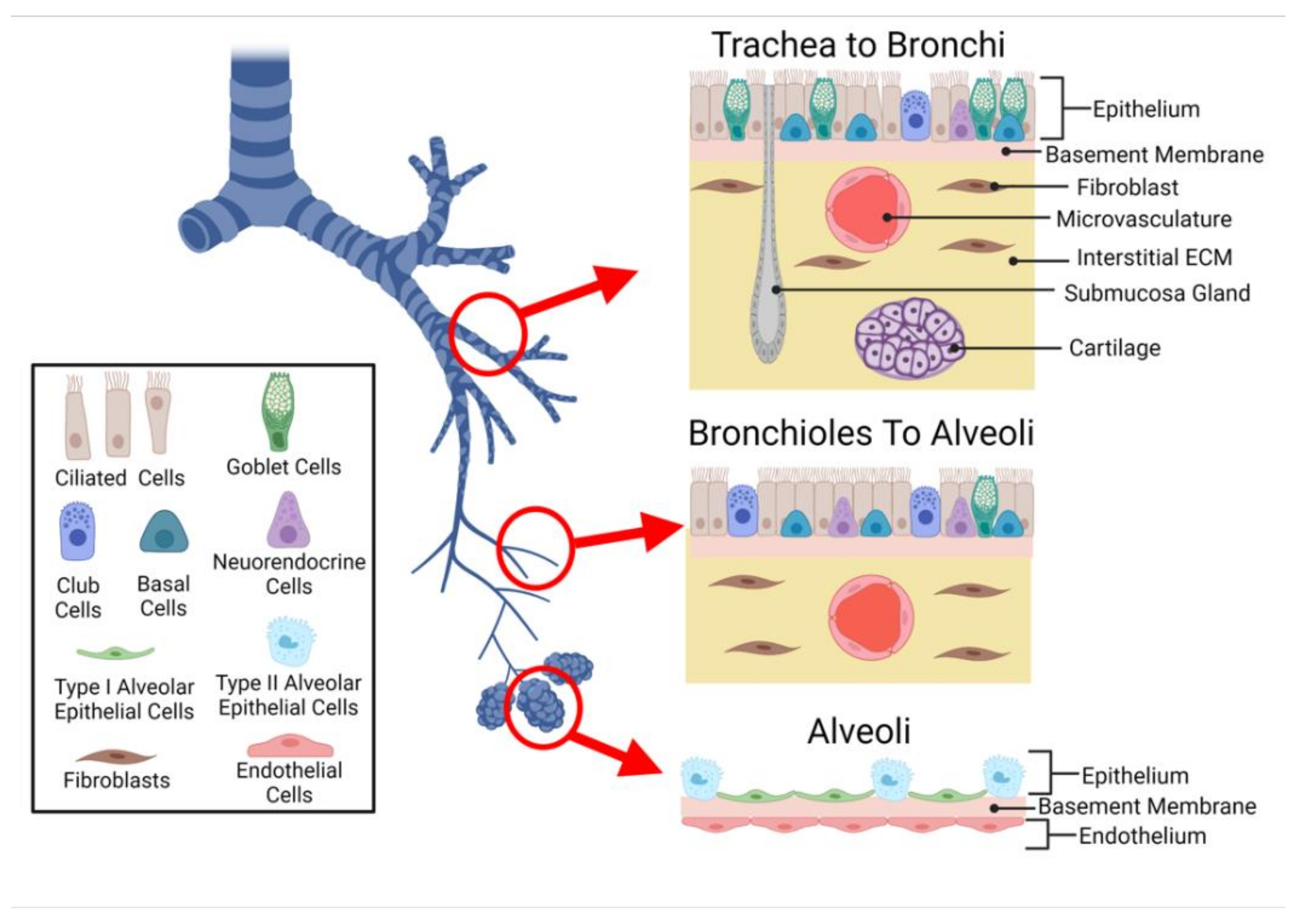
2.1. Cellular Composition
2.1.1. Epithelium
2.1.2. Submucosa
2.1.3. Lung Microvasculature
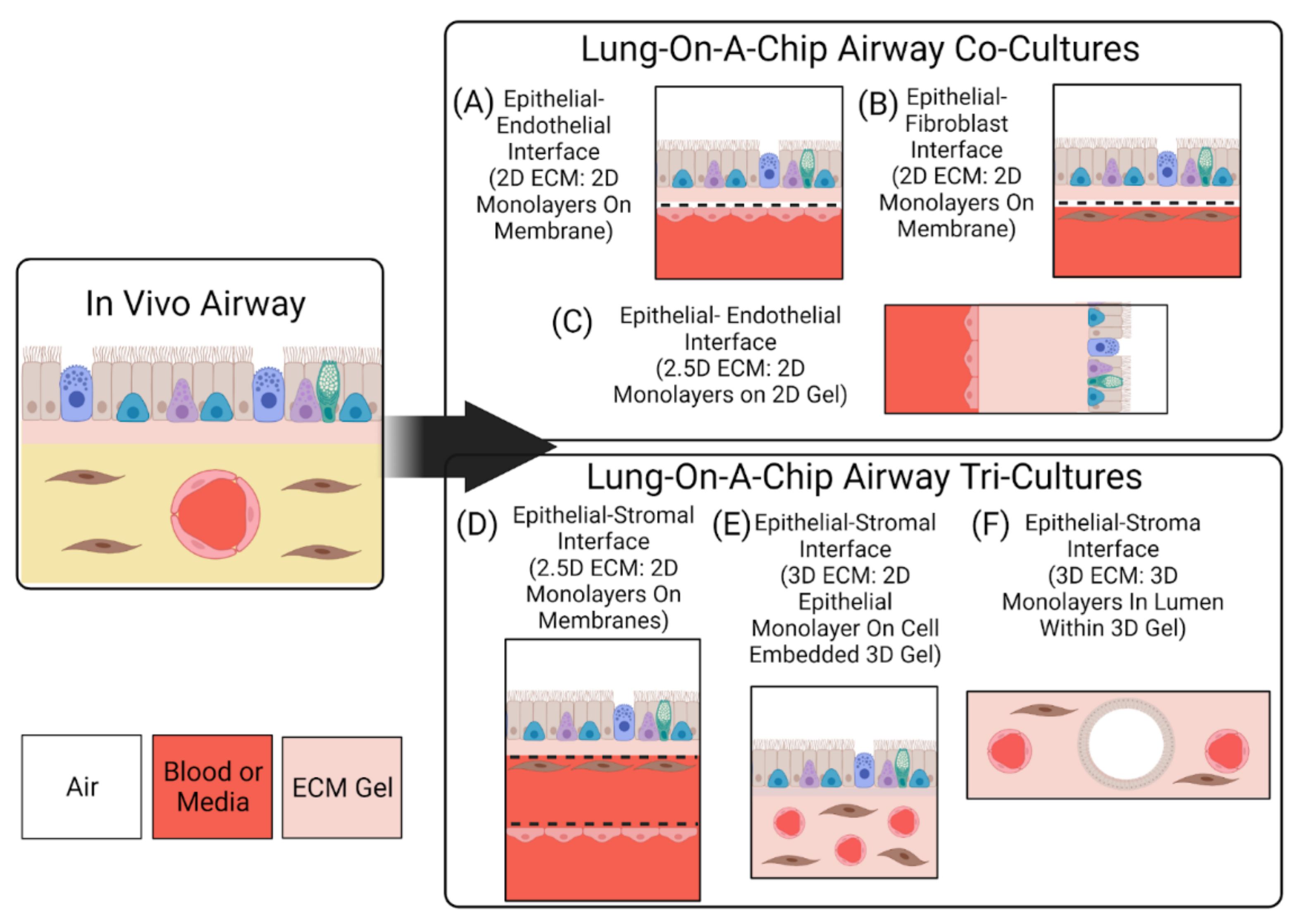
2.2. Interfaces
2.3. Interactions
3. Designing ECM Substitutes On-Chip
3.1. Lung ECM
3.1.1. ECM Structure
3.1.2. ECM Composition, Biomechanics, and Stiffness
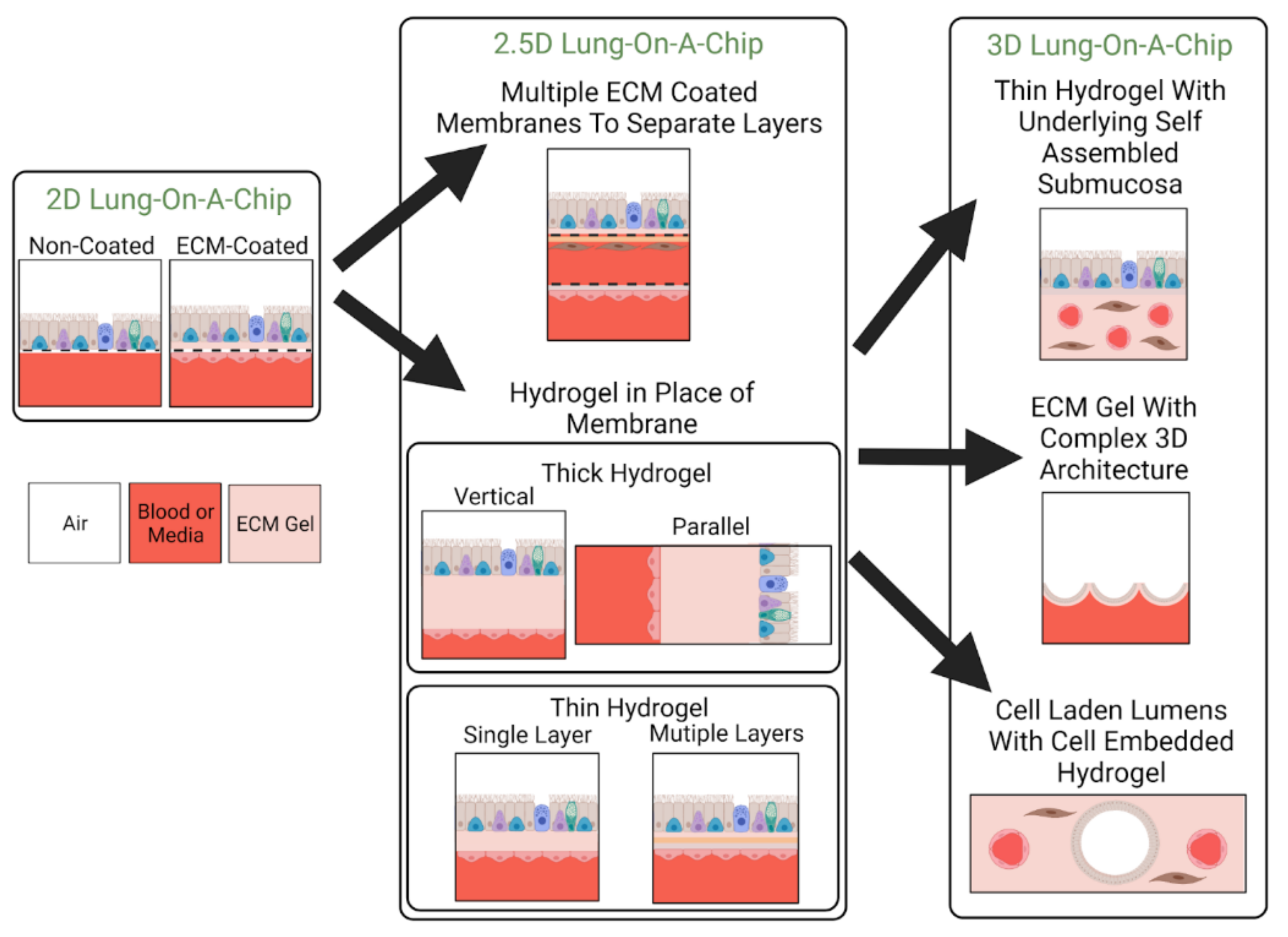
3.2. Classification by ECM Dimensionality
3.2.1. 2D Models: Non-ECM Coated
3.2.2. 2D Models: ECM Coated
3.2.3. 2.5D Models
3.2.4. 3D Models
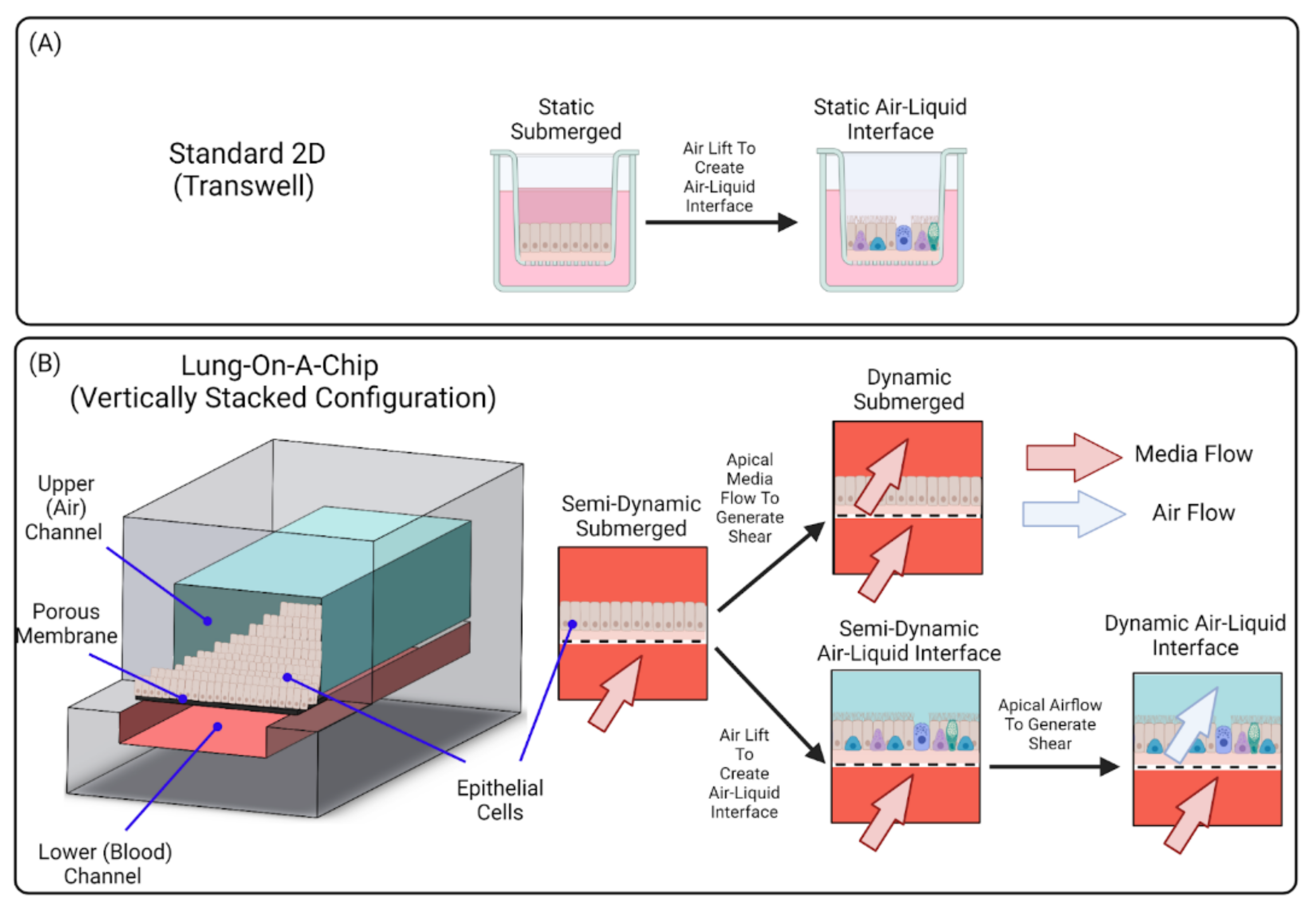
4. Leveraging Airway On-Chip Technology for Inhalation Assays
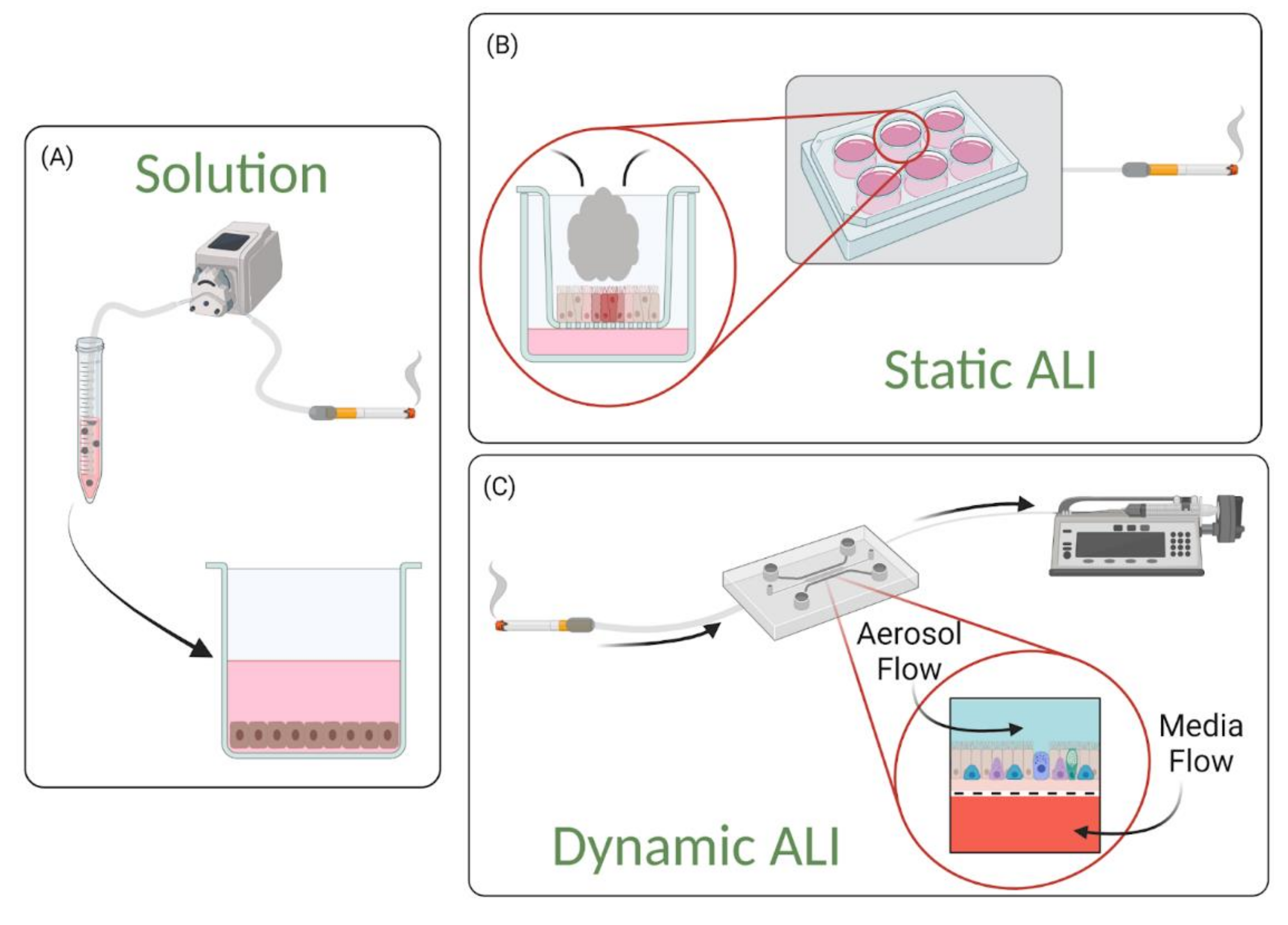
4.1. Solution-Based Exposures
4.2. Static Air–Liquid Interface (ALI) Exposures
4.3. Dynamic Air–Liquid Interface (ALI) Exposures
5. Readouts from Lung-On-A-Chip Models
5.1. In Situ Characterization and Real Time Analysis
5.1.1. Microscopy and Imaging Capabilities
5.1.2. Effluent Collection and Supernatant Based Assays
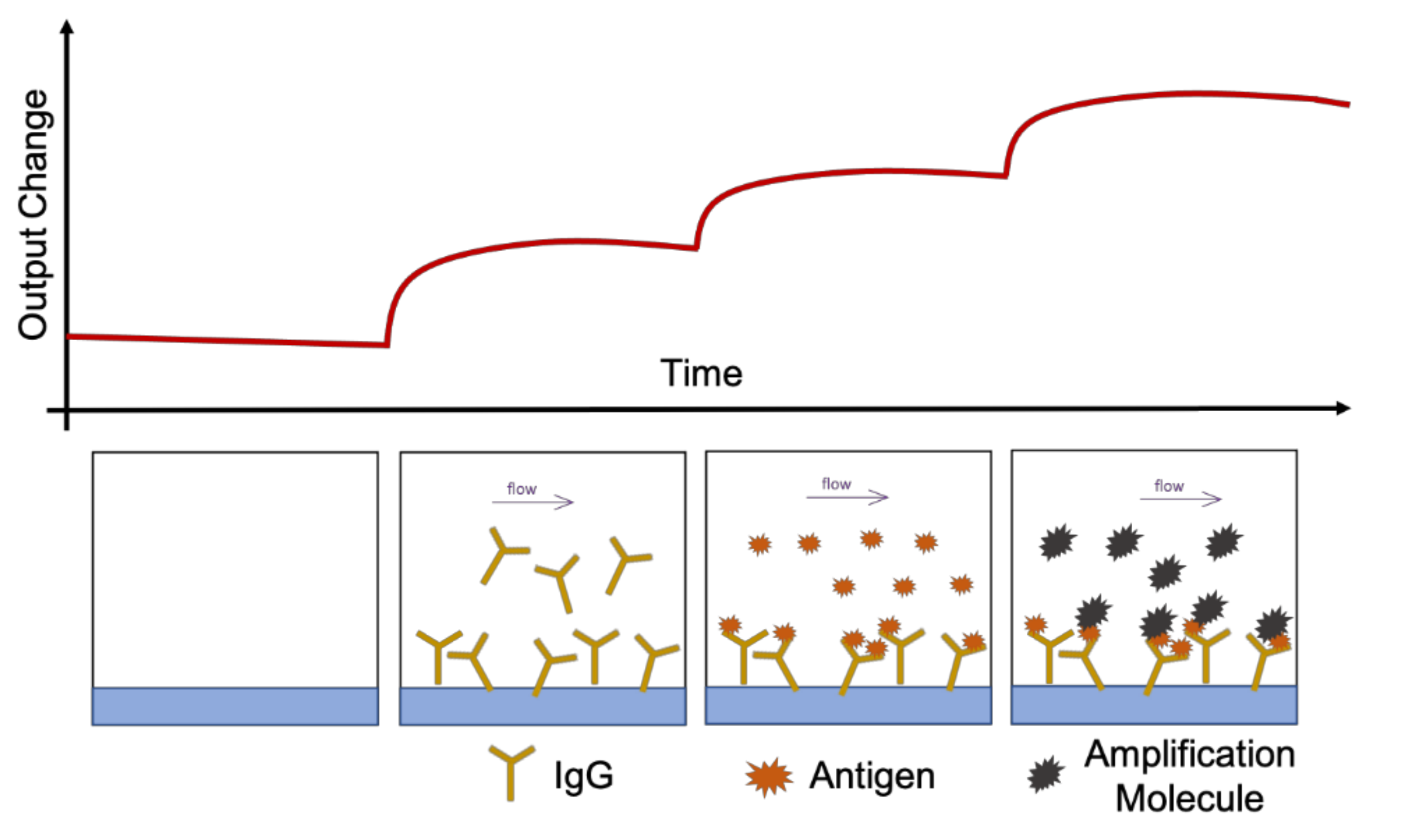
5.1.3. Integrated On-Chip Sensors
5.1.4. Inline Sensors
5.1.5. Cellular Cross Talk
5.2. Endpoint Analysis
6. Discussion
Hurdles to Translation and Remaining Challenges
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rothbauer, M.; Zirath, H.; Ertl, P. Recent Advances in Microfluidic Technologies for Cell-to-Cell Interaction Studies. Lab Chip 2018, 18, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Djukanovic, R.; Tashkin, D.P.; Millar, A.B.; du Bois, R.M.; Orr, P.A. The Role of Small Airways in Lung Disease. Respir. Med. 2002, 96, 67–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martin, C.; Frija, J.; Burgel, P.-R. Dysfunctional Lung Anatomy and Small Airways Degeneration in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2013, 8, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Gohy, S.; Hupin, C.; Ladjemi, M.Z.; Hox, V.; Pilette, C. Key Role of the Epithelium in Chronic Upper Airways Diseases. Clin. Exp. Allergy 2020, 50, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Nicod, L.P. Pulmonary Defence Mechanisms. Respiration 1999, 66, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Artzy-Schnirman, A.; Hobi, N.; Schneider-Daum, N.; Guenat, O.T.; Lehr, C.-M.; Sznitman, J. Advanced in Vitro Lung-on-Chip Platforms for Inhalation Assays: From Prospect to Pipeline. Eur. J. Pharm. Biopharm. 2019, 144, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Hernández, J.E.; Villalba-Rodríguez, A.M.; Romero-Castillo, K.D.; Aguilar-Aguila-Isaías, M.A.; García-Reyes, I.E.; Hernández-Antonio, A.; Ahmed, I.; Sharma, A.; Parra-Saldívar, R.; Iqbal, H.M.N. Organs-on-a-Chip Module: A Review from the Development and Applications Perspective. Micromachines 2018, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-Chip: Recent Breakthroughs and Future Prospects. Biomed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef]
- Figeys, D.; Pinto, D. Lab-on-a-Chip: A Revolution in Biological and Medical Sciences. Anal. Chem. 2000, 72, 330A–335A. [Google Scholar] [CrossRef]
- Haeberle, S.; Zengerle, R. Microfluidic Platforms for Lab-on-a-Chip Applications. Lab Chip 2007, 7, 1094–1110. [Google Scholar] [CrossRef]
- Nossa, R.; Costa, J.; Cacopardo, L.; Ahluwalia, A. Breathing in Vitro: Designs and Applications of Engineered Lung Models. J. Tissue Eng. 2021, 12, 20417314211008696. [Google Scholar] [CrossRef]
- Miller, A.J.; Spence, J.R. In Vitro Models to Study Human Lung Development, Disease and Homeostasis. Physiology 2017, 32, 246–260. [Google Scholar] [CrossRef]
- Nichols, J.E.; Niles, J.A.; Vega, S.P.; Argueta, L.B.; Eastaway, A.; Cortiella, J. Modeling the Lung: Design and Development of Tissue Engineered Macro- and Micro-Physiologic Lung Models for Research Use. Exp. Biol. Med. 2014, 239, 1135–1169. [Google Scholar] [CrossRef]
- Franks, T.J.; Colby, T.V.; Travis, W.D.; Tuder, R.M.; Reynolds, H.Y.; Brody, A.R.; Cardoso, W.V.; Crystal, R.G.; Drake, C.J.; Engelhardt, J.; et al. Resident Cellular Components of the Human Lung: Current Knowledge and Goals for Research on Cell Phenotyping and Function. Proc. Am. Thorac. Soc. 2008, 5, 763–766. [Google Scholar] [CrossRef]
- Nawroth, J.C.; Barrile, R.; Conegliano, D.; van Riet, S.; Hiemstra, P.S.; Villenave, R. Stem Cell-Based Lung-on-Chips: The Best of Both Worlds? Adv. Drug Deliv. Rev. 2019, 140, 12–32. [Google Scholar] [CrossRef]
- Felder, M.; Stucki, A.O.; Stucki, J.D.; Geiser, T.; Guenat, O.T. The Potential of Microfluidic Lung Epithelial Wounding: Towards in Vivo-like Alveolar Microinjuries. Integr. Biol. 2014, 6, 1132–1140. [Google Scholar] [CrossRef]
- Felder, M.; Trueeb, B.; Stucki, A.O.; Borcard, S.; Stucki, J.D.; Schnyder, B.; Geiser, T.; Guenat, O.T. Impaired Wound Healing of Alveolar Lung Epithelial Cells in a Breathing Lung-On-A-Chip. Front. Bioeng. Biotechnol. 2019, 7, 3. [Google Scholar] [CrossRef]
- Nalayanda, D.D.; Puleo, C.; Fulton, W.B.; Sharpe, L.M.; Wang, T.-H.; Abdullah, F. An Open-Access Microfluidic Model for Lung-Specific Functional Studies at an Air-Liquid Interface. Biomed. Microdevices 2009, 11, 1081–1089. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, C.; Jiang, L.; Qin, J. A 3D Human Lung-on-a-Chip Model for Nanotoxicity Testing. Toxicol. Res. 2018, 7, 1048–1060. [Google Scholar] [CrossRef]
- Hou, W.; Hu, S.; Yong, K.; Zhang, J.; Ma, H. Cigarette Smoke-Induced Malignant Transformation via STAT3 Signalling in Pulmonary Epithelial Cells in a Lung-on-a-Chip Model. Bio-Des. Manuf. 2020, 3, 383–395. [Google Scholar] [CrossRef]
- Humayun, M.; Chow, C.-W.; Young, E.W.K. Microfluidic Lung Airway-on-a-Chip with Arrayable Suspended Gels for Studying Epithelial and Smooth Muscle Cell Interactions. Lab Chip 2018, 18, 1298–1309. [Google Scholar] [CrossRef]
- Benam, K.H.; Villenave, R.; Lucchesi, C.; Varone, A.; Hubeau, C.; Lee, H.-H.; Alves, S.E.; Salmon, M.; Ferrante, T.C.; Weaver, J.C.; et al. Small Airway-on-a-Chip Enables Analysis of Human Lung Inflammation and Drug Responses in Vitro. Nat. Methods 2016, 13, 151–157. [Google Scholar] [CrossRef]
- Sellgren, K.L.; Butala, E.J.; Gilmour, B.P.; Randell, S.H.; Grego, S. A Biomimetic Multicellular Model of the Airways Using Primary Human Cells. Lab Chip 2014, 14, 3349–3358. [Google Scholar] [CrossRef]
- Huang, D.; Liu, T.; Liao, J.; Maharjan, S.; Xie, X.; Pérez, M.; Anaya, I.; Wang, S.; Tirado Mayer, A.; Kang, Z.; et al. Reversed-Engineered Human Alveolar Lung-on-a-Chip Model. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Barkal, L.J.; Procknow, C.L.; Álvarez-García, Y.R.; Niu, M.; Jiménez-Torres, J.A.; Brockman-Schneider, R.A.; Gern, J.E.; Denlinger, L.C.; Theberge, A.B.; Keller, N.P.; et al. Microbial Volatile Communication in Human Organotypic Lung Models. Nat. Commun. 2017, 8, 1770. [Google Scholar] [CrossRef]
- Park, J.Y.; Ryu, H.; Lee, B.; Ha, D.-H.; Ahn, M.; Kim, S.; Kim, J.Y.; Jeon, N.L.; Cho, D.-W. Development of a Functional Airway-on-a-Chip by 3D Cell Printing. Biofabrication 2018, 11, 015002. [Google Scholar] [CrossRef]
- Li, E.; Xu, Z.; Liu, F.; Wang, H.; Wen, J.; Shao, S.; Zhang, L.; Wang, L.; Liu, C.; Lu, J.; et al. Continual Exposure to Cigarette Smoke Extracts Induces Tumor-like Transformation of Human Nontumor Bronchial Epithelial Cells in a Microfluidic Chip. J. Thorac. Oncol. 2014, 9, 1091–1100. [Google Scholar] [CrossRef]
- Gerritsen, J. Host Defence Mechanisms of the Respiratory System. Paediatr. Respir. Rev. 2000, 1, 128–134. [Google Scholar]
- Amatngalim, G.D.; Hiemstra, P.S. Airway Epithelial Cell Function and Respiratory Host Defense in Chronic Obstructive Pulmonary Disease. Chin. Med. J. 2018, 131, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Crystal, R.G.; Randell, S.H.; Engelhardt, J.F.; Voynow, J.; Sunday, M.E. Airway Epithelial Cells: Current Concepts and Challenges. Proc. Am. Thorac. Soc. 2008, 5, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Ayers, M.M.; Jeffery, P.K. Proliferation and Differentiation in Mammalian Airway Epithelium. Eur. Respir. J. 1988, 1, 58–80. [Google Scholar] [PubMed]
- Knight, D.A.; Holgate, S.T. The Airway Epithelium: Structural and Functional Properties in Health and Disease. Respirology 2003, 8, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.D.; Wypych, T.P. Cellular and Functional Heterogeneity of the Airway Epithelium. Mucosal Immunol. 2021. [Google Scholar] [CrossRef]
- Fahy, J.V.; Dickey, B.F. Airway Mucus Function and Dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef]
- Kuek, L.E.; Lee, R.J. First Contact: The Role of Respiratory Cilia in Host-Pathogen Interactions in the Airways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L603–L619. [Google Scholar] [CrossRef]
- Yang, J.; Hernandez, B.J.; Martinez Alanis, D.; Narvaez del Pilar, O.; Vila-Ellis, L.; Akiyama, H.; Evans, S.E.; Ostrin, E.J.; Chen, J. The Development and Plasticity of Alveolar Type 1 Cells. Development 2016, 143, 54–65. [Google Scholar] [CrossRef]
- Mason, R.J. Biology of Alveolar Type II Cells. Respirology 2006, 11, S12–S15. [Google Scholar] [CrossRef]
- Douville, N.J.; Zamankhan, P.; Tung, Y.-C.; Li, R.; Vaughan, B.L.; Tai, C.-F.; White, J.; Christensen, P.J.; Grotberg, J.B.; Takayama, S. Combination of Fluid and Solid Mechanical Stresses Contribute to Cell Death and Detachment in a Microfluidic Alveolar Model. Lab Chip 2011, 11, 609–619. [Google Scholar] [CrossRef]
- White, E.S. Lung Extracellular Matrix and Fibroblast Function. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S1), S30–S33. [Google Scholar] [CrossRef]
- D’Urso, M.; Kurniawan, N.A. Mechanical and Physical Regulation of Fibroblast-Myofibroblast Transition: From Cellular Mechanoresponse to Tissue Pathology. Front. Bioeng. Biotechnol. 2020, 8, 609653. [Google Scholar] [CrossRef]
- Sakai, N.; Tager, A.M. Fibrosis of Two: Epithelial Cell-Fibroblast Interactions in Pulmonary Fibrosis. Biochim. Biophys. Acta 2013, 1832, 911–921. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Buchanan, C.F.; Verbridge, S.S.; Vlachos, P.P.; Rylander, M.N. Flow Shear Stress Regulates Endothelial Barrier Function and Expression of Angiogenic Factors in a 3D Microfluidic Tumor Vascular Model. Cell Adh. Migr. 2014, 8, 517–524. [Google Scholar] [CrossRef]
- Pezzulo, A.A.; Starner, T.D.; Scheetz, T.E.; Traver, G.L.; Tilley, A.E.; Harvey, B.-G.; Crystal, R.G.; McCray, P.B.; Zabner, J. The Air-Liquid Interface and Use of Primary Cell Cultures Are Important to Recapitulate the Transcriptional Profile of in Vivo Airway Epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 300, L25–L31. [Google Scholar] [CrossRef]
- Jain, A.; Barrile, R.; van der Meer, A.D.; Mammoto, A.; Mammoto, T.; De Ceunynck, K.; Aisiku, O.; Otieno, M.A.; Louden, C.S.; Hamilton, G.A.; et al. Primary Human Lung Alveolus-on-a-Chip Model of Intravascular Thrombosis for Assessment of Therapeutics. Clin. Pharmacol. Ther. 2018, 103, 332–340. [Google Scholar] [CrossRef]
- Nawroth, J.C.; Lucchesi, C.; Cheng, D.; Shukla, A.; Ngyuen, J.; Shroff, T.; Varone, A.; Karalis, K.; Lee, H.-H.; Alves, S.; et al. A Microengineered Airway Lung Chip Models Key Features of Viral-Induced Exacerbation of Asthma. Am. J. Respir. Cell Mol. Biol. 2020, 63, 591–600. [Google Scholar] [CrossRef]
- Punde, T.H.; Wu, W.-H.; Lien, P.-C.; Chang, Y.-L.; Kuo, P.-H.; Chang, M.D.-T.; Lee, K.-Y.; Huang, C.-D.; Kuo, H.-P.; Chan, Y.-F.; et al. A Biologically Inspired Lung-on-a-Chip Device for the Study of Protein-Induced Lung Inflammation. Integr. Biol. 2015, 7, 162–169. [Google Scholar] [CrossRef]
- Stucki, A.O.; Stucki, J.D.; Hall, S.R.R.; Felder, M.; Mermoud, Y.; Schmid, R.A.; Geiser, T.; Guenat, O.T. A Lung-on-a-Chip Array with an Integrated Bio-Inspired Respiration Mechanism. Lab Chip 2015, 15, 1302–1310. [Google Scholar] [CrossRef]
- Zamprogno, P.; Wüthrich, S.; Achenbach, S.; Thoma, G.; Stucki, J.D.; Hobi, N.; Schneider-Daum, N.; Lehr, C.-M.; Huwer, H.; Geiser, T.; et al. Second-Generation Lung-on-a-Chip with an Array of Stretchable Alveoli Made with a Biological Membrane. Commun. Biol. 2021, 4, 168. [Google Scholar] [CrossRef]
- Virumbrales-Muñoz, M.; Ayuso, J.M.; Gong, M.M.; Humayun, M.; Livingston, M.K.; Lugo-Cintrón, K.M.; McMinn, P.; Álvarez-García, Y.R.; Beebe, D.J. Microfluidic Lumen-Based Systems for Advancing Tubular Organ Modeling. Chem. Soc. Rev. 2020, 49, 6402–6442. [Google Scholar] [CrossRef]
- Bischel, L.L.; Sung, K.E.; Jiménez-Torres, J.A.; Mader, B.; Keely, P.J.; Beebe, D.J. The Importance of Being a Lumen. FASEB J. 2014, 28, 4583–4590. [Google Scholar] [CrossRef]
- Paek, J.; Park, S.E.; Lu, Q.; Park, K.-T.; Cho, M.; Oh, J.M.; Kwon, K.W.; Yi, Y.-S.; Song, J.W.; Edelstein, H.I.; et al. Microphysiological Engineering of Self-Assembled and Perfusable Microvascular Beds for the Production of Vascularized Three-Dimensional Human Microtissues. ACS Nano 2019, 13, 7627–7643. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.D. A Human Breathing Lung-on-a-Chip. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S1), S42–S44. [Google Scholar] [CrossRef]
- Stucki, J.D.; Hobi, N.; Galimov, A.; Stucki, A.O.; Schneider-Daum, N.; Lehr, C.-M.; Huwer, H.; Frick, M.; Funke-Chambour, M.; Geiser, T.; et al. Medium Throughput Breathing Human Primary Cell Alveolus-on-Chip Model. Sci. Rep. 2018, 8, 14359. [Google Scholar] [CrossRef]
- Lenz, A.-G.; Karg, E.; Brendel, E.; Hinze-Heyn, H.; Maier, K.L.; Eickelberg, O.; Stoeger, T.; Schmid, O. Inflammatory and Oxidative Stress Responses of an Alveolar Epithelial Cell Line to Airborne Zinc Oxide Nanoparticles at the Air-Liquid Interface: A Comparison with Conventional, Submerged Cell-Culture Conditions. Biomed Res. Int. 2013, 2013, 652632. [Google Scholar] [CrossRef]
- Sidhaye, V.K.; Schweitzer, K.S.; Caterina, M.J.; Shimoda, L.; King, L.S. Shear Stress Regulates Aquaporin-5 and Airway Epithelial Barrier Function. Proc. Natl. Acad. Sci. USA 2008, 105, 3345–3350. [Google Scholar] [CrossRef]
- Benam, K.H. Disrupting Experimental Strategies for Inhalation Toxicology: The Emergence of Microengineered Breathing-Smoking Human Lung-on-a-Chip. Appl. In Vitro Toxicol. 2018, 4, 107–114. [Google Scholar] [CrossRef]
- Benam, K.H.; Novak, R.; Nawroth, J.; Hirano-Kobayashi, M.; Ferrante, T.C.; Choe, Y.; Prantil-Baun, R.; Weaver, J.C.; Bahinski, A.; Parker, K.K.; et al. Matched-Comparative Modeling of Normal and Diseased Human Airway Responses Using a Microengineered Breathing Lung Chip. Cell Syst. 2016, 3, 456–466.e4. [Google Scholar] [CrossRef]
- Boitano, S.; Safdar, Z.; Welsh, D.G.; Bhattacharya, J.; Koval, M. Cell-Cell Interactions in Regulating Lung Function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L455–L459. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, M.; Chen, W.; Jiang, L.; Chen, C.; Qin, J. Assessment of Air Pollutant PM2.5 Pulmonary Exposure Using a 3D Lung-on-Chip Model. ACS Biomater. Sci. Eng. 2020, 6, 3081–3090. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.C.; Borok, Z. TGF-Beta-Induced EMT: Mechanisms and Implications for Fibrotic Lung Disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L525–L534. [Google Scholar] [CrossRef]
- Hackett, T.-L. Epithelial-Mesenchymal Transition in the Pathophysiology of Airway Remodelling in Asthma. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 53–59. [Google Scholar] [CrossRef]
- Sohal, S.S. Epithelial and Endothelial Cell Plasticity in Chronic Obstructive Pulmonary Disease (COPD). Respir. Investig. 2017, 55, 104–113. [Google Scholar] [CrossRef]
- Gohy, S.T.; Hupin, C.; Fregimilicka, C.; Detry, B.R.; Bouzin, C.; Gaide Chevronay, H.; Lecocq, M.; Weynand, B.; Ladjemi, M.Z.; Pierreux, C.E.; et al. Imprinting of the COPD Airway Epithelium for Dedifferentiation and Mesenchymal Transition. Eur. Respir. J. 2015, 45, 1258–1272. [Google Scholar] [CrossRef]
- Mertz, D.R.; Ahmed, T.; Takayama, S. Engineering Cell Heterogeneity into Organs-on-a-Chip. Lab Chip 2018, 18, 2378–2395. [Google Scholar] [CrossRef]
- Gkatzis, K.; Taghizadeh, S.; Huh, D.; Stainier, D.Y.R.; Bellusci, S. Use of Three-Dimensional Organoids and Lung-on-a-Chip Methods to Study Lung Development, Regeneration and Disease. Eur. Respir. J. 2018, 52. [Google Scholar] [CrossRef]
- Mondrinos, M.J.; Yi, Y.-S.; Wu, N.-K.; Ding, X.; Huh, D. Native Extracellular Matrix-Derived Semipermeable, Optically Transparent, and Inexpensive Membrane Inserts for Microfluidic Cell Culture. Lab Chip 2017, 17, 3146–3158. [Google Scholar] [CrossRef]
- Burgess, J.K.; Mauad, T.; Tjin, G.; Karlsson, J.C.; Westergren-Thorsson, G. The Extracellular Matrix-the under-Recognized Element in Lung Disease? J. Pathol. 2016, 240, 397–409. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Bailey, K.E.; Floren, M.L.; D’Ovidio, T.J.; Lammers, S.R.; Stenmark, K.R.; Magin, C.M. Tissue-Informed Engineering Strategies for Modeling Human Pulmonary Diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L303–L320. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular Matrix: A Dynamic Microenvironment for Stem Cell Niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.-M.; Fabre, A.; Débarre, D.; Marchal-Somme, J.; Crestani, B.; Martin, J.-L.; Beaurepaire, E.; Schanne-Klein, M.-C. Three-Dimensional Investigation and Scoring of Extracellular Matrix Remodeling during Lung Fibrosis Using Multiphoton Microscopy. Microsc. Res. Tech. 2007, 70, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Black, P.N.; Ching, P.S.T.; Beaumont, B.; Ranasinghe, S.; Taylor, G.; Merrilees, M.J. Changes in Elastic Fibres in the Small Airways and Alveoli in COPD. Eur. Respir. J. 2008, 31, 998–1004. [Google Scholar] [CrossRef]
- Sundarakrishnan, A.; Chen, Y.; Black, L.D.; Aldridge, B.B.; Kaplan, D.L. Engineered Cell and Tissue Models of Pulmonary Fibrosis. Adv. Drug Deliv. Rev. 2018, 129, 78–94. [Google Scholar] [CrossRef]
- Pelosi, P.; Rocco, P.R.M.; Negrini, D.; Passi, A. The Extracellular Matrix of the Lung and Its Role in Edema Formation. An. Acad. Bras. Cienc. 2007, 79, 285–297. [Google Scholar] [CrossRef]
- Burgstaller, G.; Oehrle, B.; Gerckens, M.; White, E.S.; Schiller, H.B.; Eickelberg, O. The Instructive Extracellular Matrix of the Lung: Basic Composition and Alterations in Chronic Lung Disease. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- Morrissey, M.A.; Sherwood, D.R. An Active Role for Basement Membrane Assembly and Modification in Tissue Sculpting. J. Cell Sci. 2015, 128, 1661–1668. [Google Scholar] [CrossRef]
- Mak, K.M.; Mei, R. Basement Membrane Type IV Collagen and Laminin: An Overview of Their Biology and Value as Fibrosis Biomarkers of Liver Disease. Anat. Rec. 2017, 300, 1371–1390. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Suki, B.; Ito, S.; Stamenovic, D.; Lutchen, K.R.; Ingenito, E.P. Biomechanics of the Lung Parenchyma: Critical Roles of Collagen and Mechanical Forces. J. Appl. Physiol. 2005, 98, 1892–1899. [Google Scholar] [CrossRef]
- Yue, B. Biology of the Extracellular Matrix: An Overview. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and Extracellular Matrix Homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- Cavalcante, F.S.A.; Ito, S.; Brewer, K.; Sakai, H.; Alencar, A.M.; Almeida, M.P.; Andrade, J.S.; Majumdar, A.; Ingenito, E.P.; Suki, B. Mechanical Interactions between Collagen and Proteoglycans: Implications for the Stability of Lung Tissue. J. Appl. Physiol. 2005, 98, 672–679. [Google Scholar] [CrossRef]
- Faffe, D.S.; Zin, W.A. Lung Parenchymal Mechanics in Health and Disease. Physiol. Rev. 2009, 89, 759–775. [Google Scholar] [CrossRef]
- Dunsmore, S.E.; Rannels, D.E. Extracellular Matrix Biology in the Lung. Am. J. Physiol. 1996, 270, L3–L27. [Google Scholar] [CrossRef]
- Booth, A.J.; Hadley, R.; Cornett, A.M.; Dreffs, A.A.; Matthes, S.A.; Tsui, J.L.; Weiss, K.; Horowitz, J.C.; Fiore, V.F.; Barker, T.H.; et al. Acellular Normal and Fibrotic Human Lung Matrices as a Culture System for in Vitro Investigation. Am. J. Respir. Crit. Care Med. 2012, 186, 866–876. [Google Scholar] [CrossRef]
- Schiller, H.B.; Fässler, R. Mechanosensitivity and Compositional Dynamics of Cell-Matrix Adhesions. EMBO Rep. 2013, 14, 509–519. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Pierschbacher, M.D. New Perspectives in Cell Adhesion: RGD and Integrins. Science 1987, 238, 491–497. [Google Scholar] [CrossRef]
- Engler, A.J.; Humbert, P.O.; Wehrle-Haller, B.; Weaver, V.M. Multiscale Modeling of Form and Function. Science 2009, 324, 208–212. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Herrera, J.; Henke, C.A.; Bitterman, P.B. Extracellular Matrix as a Driver of Progressive Fibrosis. J. Clin. Investig. 2018, 128, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, C.; Failla, M.; Crimi, N.; Raghu, G. Idiopathic Pulmonary Fibrosis: A Disease with Similarities and Links to Cancer Biology. Eur. Respir. J. 2010, 35, 496–504. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Gu, X.; Leong, K.W. Biophysical Regulation of Cell Behavior-Cross Talk between Substrate Stiffness and Nanotopography. Engineering 2017, 3, 36–54. [Google Scholar] [CrossRef]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A Tense Situation: Forcing Tumour Progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Hassell, B.A.; Goyal, G.; Lee, E.; Sontheimer-Phelps, A.; Levy, O.; Chen, C.S.; Ingber, D.E. Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy in Vitro. Cell Rep. 2018, 23, 3698. [Google Scholar] [CrossRef]
- Konar, D.; Devarasetty, M.; Yildiz, D.V.; Atala, A.; Murphy, S.V. Lung-On-A-Chip Technologies for Disease Modeling and Drug Development. Biomed. Eng. Comput. Biol. 2016, 7, 17–27. [Google Scholar] [CrossRef]
- Huh, D.; Fujioka, H.; Tung, Y.-C.; Futai, N.; Paine, R.; Grotberg, J.B.; Takayama, S. Acoustically Detectable Cellular-Level Lung Injury Induced by Fluid Mechanical Stresses in Microfluidic Airway Systems. Proc. Natl. Acad. Sci. USA 2007, 104, 18886–18891. [Google Scholar] [CrossRef] [PubMed]
- Kaya, D.; Keçeci, K. Review—Track-Etched Nanoporous Polymer Membranes as Sensors: A Review. J. Electrochem. Soc. 2020, 167, 037543. [Google Scholar] [CrossRef]
- Pasman, T.; Grijpma, D.; Stamatialis, D.; Poot, A. Flat and Microstructured Polymeric Membranes in Organs-on-Chips. J. R. Soc. Interface 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.H.; Vincent, L.G.; Fuhrmann, A.; Choi, Y.S.; Hribar, K.C.; Taylor-Weiner, H.; Chen, S.; Engler, A.J. Interplay of Matrix Stiffness and Protein Tethering in Stem Cell Differentiation. Nat. Mater. 2014, 13, 979–987. [Google Scholar] [CrossRef]
- Seghir, R.; Arscott, S. Extended PDMS Stiffness Range for Flexible Systems. Sens. Actuators A Phys. 2015, 230, 33–39. [Google Scholar] [CrossRef]
- van Meer, B.J.; de Vries, H.; Firth, K.S.A.; van Weerd, J.; Tertoolen, L.G.J.; Karperien, H.B.J.; Jonkheijm, P.; Denning, C.; IJzerman, A.P.; Mummery, C.L. Small Molecule Absorption by PDMS in the Context of Drug Response Bioassays. Biochem. Biophys. Res. Commun. 2017, 482, 323–328. [Google Scholar] [CrossRef]
- Tavana, H.; Zamankhan, P.; Christensen, P.J.; Grotberg, J.B.; Takayama, S. Epithelium Damage and Protection during Reopening of Occluded Airways in a Physiologic Microfluidic Pulmonary Airway Model. Biomed. Microdevices 2011, 13, 731–742. [Google Scholar] [CrossRef]
- Huh, D.; Leslie, D.C.; Matthews, B.D.; Fraser, J.P.; Jurek, S.; Hamilton, G.A.; Thorneloe, K.S.; McAlexander, M.A.; Ingber, D.E. A Human Disease Model of Drug Toxicity-Induced Pulmonary Edema in a Lung-on-a-Chip Microdevice. Sci. Transl. Med. 2012, 4, 159ra147. [Google Scholar] [CrossRef]
- Trieu, D.; Waddell, T.K.; McGuigan, A.P. A Microfluidic Device to Apply Shear Stresses to Polarizing Ciliated Airway Epithelium Using Air Flow. Biomicrofluidics 2014, 8, 064104. [Google Scholar] [CrossRef]
- Bellis, S.L. Advantages of RGD Peptides for Directing Cell Association with Biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Wu, X.; Peters-Hall, J.R.; Bose, S.; Peña, M.T.; Rose, M.C. Human Bronchial Epithelial Cells Differentiate to 3D Glandular Acini on Basement Membrane Matrix. Am. J. Respir. Cell Mol. Biol. 2011, 44, 914–921. [Google Scholar] [CrossRef]
- LaValley, D.J.; Reinhart-King, C.A. Matrix Stiffening in the Formation of Blood Vessels. Adv. Regen. Biol. 2014, 1, 25247. [Google Scholar] [CrossRef]
- Tran, V.T.; Mredha, M.T.I.; Jeon, I. High-Water-Content Hydrogels Exhibiting Superior Stiffness, Strength, and Toughness. Extrem. Mech. Lett. 2020, 37, 100691. [Google Scholar] [CrossRef]
- Doherty, E.L.; Aw, W.Y.; Hickey, A.J.; Polacheck, W.J. Microfluidic and Organ-on-a-Chip Approaches to Investigate Cellular and Microenvironmental Contributions to Cardiovascular Function and Pathology. Front. Bioeng. Biotechnol. 2021, 9, 624435. [Google Scholar] [CrossRef]
- Lee, S.H.; Shim, K.Y.; Kim, B.; Sung, J.H. Hydrogel-Based Three-Dimensional Cell Culture for Organ-on-a-Chip Applications. Biotechnol. Prog. 2017, 33, 580–589. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design Properties of Hydrogel Tissue-Engineering Scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural Polymers for the Microencapsulation of Cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- GBD 2015 Tobacco Collaborators Smoking Prevalence and Attributable Disease Burden in 195 Countries and Territories, 1990-2015: A Systematic Analysis from the Global Burden of Disease Study 2015. Lancet 2017, 389, 1885–1906. [CrossRef]
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, Regional, and National Comparative Risk Assessment of 79 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks, 1990-2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Dai, H.; Leventhal, A.M. Prevalence of E-Cigarette Use Among Adults in the United States, 2014–2018. JAMA 2019, 322, 1824–1827. [Google Scholar] [CrossRef]
- Lin, C.; Baiocchi, M.; Halpern-Felsher, B. Longitudinal Trends in E-Cigarette Devices Used by Californian Youth, 2014–2018. Addict. Behav. 2020, 108, 106459. [Google Scholar] [CrossRef]
- Fadus, M.C.; Smith, T.T.; Squeglia, L.M. The Rise of E-Cigarettes, Pod Mod Devices, and JUUL among Youth: Factors Influencing Use, Health Implications, and Downstream Effects. Drug Alcohol Depend. 2019, 201, 85–93. [Google Scholar] [CrossRef]
- Callahan-Lyon, P. Electronic Cigarettes: Human Health Effects. Tob. Control 2014, 23 (Suppl. S2), ii36–ii40. [Google Scholar] [CrossRef]
- Hajek, P.; Etter, J.-F.; Benowitz, N.; Eissenberg, T.; McRobbie, H. Electronic Cigarettes: Review of Use, Content, Safety, Effects on Smokers and Potential for Harm and Benefit. Addiction 2014, 109, 1801–1810. [Google Scholar] [CrossRef]
- McKee, M.; Capewell, S. Evidence about Electronic Cigarettes: A Foundation Built on Rock or Sand? BMJ 2015, 351, h4863. [Google Scholar] [CrossRef]
- Pyror, W.A. Biological Effects of Cigarette Smoke, Wood Smoke, and the Smoke from Plastics: The Use of Electron Spin Resonance. Free Radic. Biol. Med. 1992, 13, 659–676. [Google Scholar] [CrossRef]
- Van der Vaart, H.; Postma, D.S.; Timens, W.; ten Hacken, N.H.T. Acute Effects of Cigarette Smoke on Inflammation and Oxidative Stress: A Review. Thorax 2004, 59, 713–721. [Google Scholar] [CrossRef]
- Rennard, S.I. Cigarette Smoke in Research. Am. J. Respir. Cell Mol. Biol. 2004, 31, 479–480. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-H.; Chen, P.; Chen, Y.; He, S.-D.; Ye, J.-R.; Zhang, H.-L.; Cao, J. Comparison between Cigarette Smoke-Induced Emphysema and Cigarette Smoke Extract-Induced Emphysema. Tob. Induc. Dis. 2015, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, P.S.; Bals, R. Basic Science of Electronic Cigarettes: Assessment in Cell Culture and in Vivo Models. Respir. Res. 2016, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Even-Tzur, N.; Kloog, Y.; Wolf, M.; Elad, D. Mucus Secretion and Cytoskeletal Modifications in Cultured Nasal Epithelial Cells Exposed to Wall Shear Stresses. Biophys. J. 2008, 95, 2998–3008. [Google Scholar] [CrossRef] [PubMed]
- Guirao, B.; Meunier, A.; Mortaud, S.; Aguilar, A.; Corsi, J.-M.; Strehl, L.; Hirota, Y.; Desoeuvre, A.; Boutin, C.; Han, Y.-G.; et al. Coupling between Hydrodynamic Forces and Planar Cell Polarity Orients Mammalian Motile Cilia. Nat. Cell Biol. 2010, 12, 341–350. [Google Scholar] [CrossRef]
- Hoshino, Y.; Mio, T.; Nagai, S.; Miki, H.; Ito, I.; Izumi, T. Cytotoxic Effects of Cigarette Smoke Extract on an Alveolar Type II Cell-Derived Cell Line. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L509–L516. [Google Scholar] [CrossRef]
- Higham, A.; Bostock, D.; Booth, G.; Dungwa, J.V.; Singh, D. The Effect of Electronic Cigarette and Tobacco Smoke Exposure on COPD Bronchial Epithelial Cell Inflammatory Responses. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 989–1000. [Google Scholar] [CrossRef]
- Zeglinski, M.R.; Turner, C.T.; Zeng, R.; Schwartz, C.; Santacruz, S.; Pawluk, M.A.; Zhao, H.; Chan, A.W.H.; Carlsten, C.; Granville, D.J. Soluble Wood Smoke Extract Promotes Barrier Dysfunction in Alveolar Epithelial Cells through a MAPK Signaling Pathway. Sci. Rep. 2019, 9, 10027. [Google Scholar] [CrossRef]
- Johnson, M.D.; Schilz, J.; Djordjevic, M.V.; Rice, J.R.; Shields, P.G. Evaluation of in Vitro Assays for Assessing the Toxicity of Cigarette Smoke and Smokeless Tobacco. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3263–3304. [Google Scholar] [CrossRef]
- Carnevali, S.; Petruzzelli, S.; Longoni, B.; Vanacore, R.; Barale, R.; Cipollini, M.; Scatena, F.; Paggiaro, P.; Celi, A.; Giuntini, C. Cigarette Smoke Extract Induces Oxidative Stress and Apoptosis in Human Lung Fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L955–L963. [Google Scholar] [CrossRef]
- Heijink, I.H.; Brandenburg, S.M.; Postma, D.S.; van Oosterhout, A.J.M. Cigarette Smoke Impairs Airway Epithelial Barrier Function and Cell-Cell Contact Recovery. Eur. Respir. J. 2012, 39, 419–428. [Google Scholar] [CrossRef]
- Oudijk, E.J.D.; Lammers, J.W.J.; Koenderman, L. Systemic Inflammation in Chronic Obstructive Pulmonary Disease. Eur. Respir. J. Suppl. 2003, 46, 5s–13s. [Google Scholar] [CrossRef]
- Laniado-Laborín, R. Smoking and Chronic Obstructive Pulmonary Disease (COPD). Parallel Epidemics of the 21 Century. Int. J. Environ. Res. Public Health 2009, 6, 209–224. [Google Scholar] [CrossRef]
- Pauwels, R.A.; Rabe, K.F. Burden and Clinical Features of Chronic Obstructive Pulmonary Disease (COPD). Lancet 2004, 364, 613–620. [Google Scholar] [CrossRef]
- Keatings, V.M.; Collins, P.D.; Scott, D.M.; Barnes, P.J. Differences in Interleukin-8 and Tumor Necrosis Factor-Alpha in Induced Sputum from Patients with Chronic Obstructive Pulmonary Disease or Asthma. Am. J. Respir. Crit. Care Med. 1996, 153, 530–534. [Google Scholar] [CrossRef]
- Mio, T.; Romberger, D.J.; Thompson, A.B.; Robbins, R.A.; Heires, A.; Rennard, S.I. Cigarette Smoke Induces Interleukin-8 Release from Human Bronchial Epithelial Cells. Am. J. Respir. Crit. Care Med. 1997, 155, 1770–1776. [Google Scholar] [CrossRef]
- Richter, A.; O’Donnell, R.A.; Powell, R.M.; Sanders, M.W.; Holgate, S.T.; Djukanović, R.; Davies, D.E. Autocrine Ligands for the Epidermal Growth Factor Receptor Mediate Interleukin-8 Release from Bronchial Epithelial Cells in Response to Cigarette Smoke. Am. J. Respir. Cell Mol. Biol. 2002, 27, 85–90. [Google Scholar] [CrossRef]
- Witherden, I.R.; Vanden Bon, E.J.; Goldstraw, P.; Ratcliffe, C.; Pastorino, U.; Tetley, T.D. Primary Human Alveolar Type II Epithelial Cell Chemokine Release: Effects of Cigarette Smoke and Neutrophil Elastase. Am. J. Respir. Cell Mol. Biol. 2004, 30, 500–509. [Google Scholar] [CrossRef]
- Kode, A.; Yang, S.-R.; Rahman, I. Differential Effects of Cigarette Smoke on Oxidative Stress and Proinflammatory Cytokine Release in Primary Human Airway Epithelial Cells and in a Variety of Transformed Alveolar Epithelial Cells. Respir. Res. 2006, 7, 132. [Google Scholar] [CrossRef]
- Thompson, A.B.; Daughton, D.; Robbins, R.A.; Ghafouri, M.A.; Oehlerking, M.; Rennard, S.I. Intraluminal Airway Inflammation in Chronic Bronchitis. Characterization and Correlation with Clinical Parameters. Am. Rev. Respir. Dis. 1989, 140, 1527–1537. [Google Scholar] [CrossRef]
- Osei, E.T.; Noordhoek, J.A.; Hackett, T.L.; Spanjer, A.I.R.; Postma, D.S.; Timens, W.; Brandsma, C.-A.; Heijink, I.H. Interleukin-1α Drives the Dysfunctional Cross-Talk of the Airway Epithelium and Lung Fibroblasts in COPD. Eur. Respir. J. 2016, 48, 359–369. [Google Scholar] [CrossRef]
- Herrington, J.S.; Myers, C. Electronic Cigarette Solutions and Resultant Aerosol Profiles. J. Chromatogr. A 2015, 1418, 192–199. [Google Scholar] [CrossRef]
- Behar, R.Z.; Luo, W.; McWhirter, K.J.; Pankow, J.F.; Talbot, P. Analytical and Toxicological Evaluation of Flavor Chemicals in Electronic Cigarette Refill Fluids. Sci. Rep. 2018, 8, 8288. [Google Scholar] [CrossRef]
- Wang, G.; Liu, W.; Song, W. Toxicity Assessment of Electronic Cigarettes. Inhal. Toxicol. 2019, 31, 259–273. [Google Scholar] [CrossRef]
- Marques, P.; Piqueras, L.; Sanz, M.-J. An Updated Overview of E-Cigarette Impact on Human Health. Respir. Res. 2021, 22, 151. [Google Scholar] [CrossRef]
- Gotts, J.E.; Jordt, S.-E.; McConnell, R.; Tarran, R. What Are the Respiratory Effects of E-Cigarettes? BMJ 2019, 366, l5275. [Google Scholar] [CrossRef]
- Leslie, L.J.; Vasanthi Bathrinarayanan, P.; Jackson, P.; Mabiala Ma Muanda, J.A.; Pallett, R.; Stillman, C.J.P.; Marshall, L.J. A Comparative Study of Electronic Cigarette Vapor Extracts on Airway-Related Cell Lines in Vitro. Inhal. Toxicol. 2017, 29, 126–136. [Google Scholar] [CrossRef]
- Higham, A.; Rattray, N.J.W.; Dewhurst, J.A.; Trivedi, D.K.; Fowler, S.J.; Goodacre, R.; Singh, D. Electronic Cigarette Exposure Triggers Neutrophil Inflammatory Responses. Respir. Res. 2016, 17, 56. [Google Scholar] [CrossRef]
- Cervellati, F.; Muresan, X.M.; Sticozzi, C.; Gambari, R.; Montagner, G.; Forman, H.J.; Torricelli, C.; Maioli, E.; Valacchi, G. Comparative Effects between Electronic and Cigarette Smoke in Human Keratinocytes and Epithelial Lung Cells. Toxicol In Vitro 2014, 28, 999–1005. [Google Scholar] [CrossRef]
- Putzhammer, R.; Doppler, C.; Jakschitz, T.; Heinz, K.; Förste, J.; Danzl, K.; Messner, B.; Bernhard, D. Vapours of US and EU Market Leader Electronic Cigarette Brands and Liquids Are Cytotoxic for Human Vascular Endothelial Cells. PLoS ONE 2016, 11, e0157337. [Google Scholar] [CrossRef]
- Bengalli, R.; Ferri, E.; Labra, M.; Mantecca, P. Lung Toxicity of Condensed Aerosol from E-CIG Liquids: Influence of the Flavor and the In Vitro Model Used. Int. J. Environ. Res. Public Health 2017, 14, 1254. [Google Scholar] [CrossRef]
- Romagna, G.; Allifranchini, E.; Bocchietto, E.; Todeschi, S.; Esposito, M.; Farsalinos, K.E. Cytotoxicity Evaluation of Electronic Cigarette Vapor Extract on Cultured Mammalian Fibroblasts (ClearStream-LIFE): Comparison with Tobacco Cigarette Smoke Extract. Inhal. Toxicol. 2013, 25, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Carr, T.; Oke, O.; Jaunky, T.; Breheny, D.; Lowe, F.; Gaça, M. E-Cigarette Aerosols Induce Lower Oxidative Stress in Vitro When Compared to Tobacco Smoke. Toxicol. Mech. Methods 2016, 26, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Jaunky, T.; Hewitt, K.; Breheny, D.; Lowe, F.; Fearon, I.M.; Gaca, M. A Comparative Assessment of E-Cigarette Aerosols and Cigarette Smoke on in Vitro Endothelial Cell Migration. Toxicol. Lett. 2017, 277, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Karp, P.H.; Moninger, T.O.; Weber, S.P.; Nesselhauf, T.S.; Launspach, J.L.; Zabner, J.; Welsh, M.J. An in Vitro Model of Differentiated Human Airway Epithelia. Methods for Establishing Primary Cultures. Methods Mol. Biol. 2002, 188, 115–137. [Google Scholar] [CrossRef]
- Bluhmki, T.; Bitzer, S.; Gindele, J.A.; Schruf, E.; Kiechle, T.; Webster, M.; Schymeinsky, J.; Ries, R.; Gantner, F.; Bischoff, D.; et al. Development of a Miniaturized 96-Transwell Air-Liquid Interface Human Small Airway Epithelial Model. Sci. Rep. 2020, 10, 13022. [Google Scholar] [CrossRef]
- Haghi, M.; Ong, H.X.; Traini, D.; Young, P. Across the Pulmonary Epithelial Barrier: Integration of Physicochemical Properties and Human Cell Models to Study Pulmonary Drug Formulations. Pharmacol. Ther. 2014, 144, 235–252. [Google Scholar] [CrossRef]
- Dong, W.; Matsuno, Y.; Kameyama, A. A Procedure for Alcian Blue Staining of Mucins on Polyvinylidene Difluoride Membranes. Anal. Chem. 2012, 84, 8461–8466. [Google Scholar] [CrossRef]
- Ong, H.X.; Jackson, C.L.; Cole, J.L.; Lackie, P.M.; Traini, D.; Young, P.M.; Lucas, J.; Conway, J. Primary Air-Liquid Interface Culture of Nasal Epithelium for Nasal Drug Delivery. Mol. Pharm. 2016, 13, 2242–2252. [Google Scholar] [CrossRef]
- Grau-Bartual, S.; Al-Jumaily, A.M.; Young, P.M.; Traini, D.; Ghadiri, M. Effect of Continuous Positive Airway Pressure Treatment on Permeability, Inflammation and Mucus Production of Human Epithelial Cells. ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef]
- Abdullah, L.H.; Coakley, R.; Webster, M.J.; Zhu, Y.; Tarran, R.; Radicioni, G.; Kesimer, M.; Boucher, R.C.; Davis, C.W.; Ribeiro, C.M.P. Mucin Production and Hydration Responses to Mucopurulent Materials in Normal versus Cystic Fibrosis Airway Epithelia. Am. J. Respir. Crit. Care Med. 2018, 197, 481–491. [Google Scholar] [CrossRef]
- Abdullah, L.H.; Wolber, C.; Kesimer, M.; Sheehan, J.K.; Davis, C.W. Studying Mucin Secretion from Human Bronchial Epithelial Cell Primary Cultures. Methods Mol. Biol. 2012, 842, 259–277. [Google Scholar] [CrossRef]
- Michi, A.N.; Proud, D. A Toolbox for Studying Respiratory Viral Infections Using Air-Liquid Interface Cultures of Human Airway Epithelial Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021. [Google Scholar] [CrossRef]
- Kuehn, D.; Majeed, S.; Guedj, E.; Dulize, R.; Baumer, K.; Iskandar, A.; Boue, S.; Martin, F.; Kostadinova, R.; Mathis, C.; et al. Impact Assessment of Repeated Exposure of Organotypic 3D Bronchial and Nasal Tissue Culture Models to Whole Cigarette Smoke. J. Vis. Exp. 2015. [Google Scholar] [CrossRef]
- Vasanthi Bathrinarayanan, P.; Brown, J.E.P.; Marshall, L.J.; Leslie, L.J. An Investigation into E-Cigarette Cytotoxicity in-Vitro Using a Novel 3D Differentiated Co-Culture Model of Human Airways. Toxicol. In Vitro 2018, 52, 255–264. [Google Scholar] [CrossRef]
- Ghosh, B.; Reyes-Caballero, H.; Akgün-Ölmez, S.G.; Nishida, K.; Chandrala, L.; Smirnova, L.; Biswal, S.; Sidhaye, V.K. Effect of Sub-Chronic Exposure to Cigarette Smoke, Electronic Cigarette and Waterpipe on Human Lung Epithelial Barrier Function. BMC Pulm. Med. 2020, 20, 216. [Google Scholar] [CrossRef]
- Aufderheide, M.; Scheffler, S.; Ito, S.; Ishikawa, S.; Emura, M. Ciliatoxicity in Human Primary Bronchiolar Epithelial Cells after Repeated Exposure at the Air-Liquid Interface with Native Mainstream Smoke of K3R4F Cigarettes with and without Charcoal Filter. Exp. Toxicol. Pathol. 2015, 67, 407–411. [Google Scholar] [CrossRef]
- Li, X. In Vitro Toxicity Testing of Cigarette Smoke Based on the Air-Liquid Interface Exposure: A Review. Toxicol. In Vitro 2016, 36, 105–113. [Google Scholar] [CrossRef]
- Iskandar, A.R.; Xiang, Y.; Frentzel, S.; Talikka, M.; Leroy, P.; Kuehn, D.; Guedj, E.; Martin, F.; Mathis, C.; Ivanov, N.V.; et al. Impact Assessment of Cigarette Smoke Exposure on Organotypic Bronchial Epithelial Tissue Cultures: A Comparison of Mono-Culture and Coculture Model Containing Fibroblasts. Toxicol. Sci. 2015, 147, 207–221. [Google Scholar] [CrossRef]
- Phillips, J.; Kluss, B.; Richter, A.; Massey, E. Exposure of Bronchial Epithelial Cells to Whole Cigarette Smoke: Assessment of Cellular Responses. Altern. Lab. Anim. 2005, 33, 239–248. [Google Scholar] [CrossRef]
- Beisswenger, C.; Platz, J.; Seifart, C.; Vogelmeier, C.; Bals, R. Exposure of Differentiated Airway Epithelial Cells to Volatile Smoke in Vitro. Respiration 2004, 71, 402–409. [Google Scholar] [CrossRef]
- Aufderheide, M.; Mohr, U. CULTEX—An Alternative Technique for Cultivation and Exposure of Cells of the Respiratory Tract to Airborne Pollutants at the Air/Liquid Interface. Exp. Toxicol. Pathol. 2000, 52, 265–270. [Google Scholar] [CrossRef]
- Aufderheide, M.; Halter, B.; Möhle, N.; Hochrainer, D. The CULTEX RFS: A Comprehensive Technical Approach for the in Vitro Exposure of Airway Epithelial Cells to the Particulate Matter at the Air-Liquid Interface. Biomed Res. Int. 2013, 2013, 734137. [Google Scholar] [CrossRef]
- Lucci, F.; Castro, N.D.; Rostami, A.A.; Oldham, M.J.; Hoeng, J.; Pithawalla, Y.B.; Kuczaj, A.K. Characterization and Modeling of Aerosol Deposition in Vitrocell® Exposure Systems-Exposure Well Chamber Deposition Efficiency. J. Aerosol Sci. 2018, 123, 141–160. [Google Scholar] [CrossRef]
- Majeed, S.; Frentzel, S.; Wagner, S.; Kuehn, D.; Leroy, P.; Guy, P.A.; Knorr, A.; Hoeng, J.; Peitsch, M.C. Characterization of the Vitrocell® 24/48 in Vitro Aerosol Exposure System Using Mainstream Cigarette Smoke. Chem. Cent. J. 2014, 8, 62. [Google Scholar] [CrossRef][Green Version]
- St-Laurent, J.; Proulx, L.-I.; Boulet, L.-P.; Bissonnette, E. Comparison of Two in Vitro Models of Cigarette Smoke Exposure. Inhal. Toxicol. 2009, 21, 1148–1153. [Google Scholar] [CrossRef]
- Iskandar, A.R.; Gonzalez-Suarez, I.; Majeed, S.; Marescotti, D.; Sewer, A.; Xiang, Y.; Leroy, P.; Guedj, E.; Mathis, C.; Schaller, J.-P.; et al. A Framework for in Vitro Systems Toxicology Assessment of E-Liquids. Toxicol. Mech. Methods 2016, 26, 389–413. [Google Scholar] [CrossRef]
- Scheffler, S.; Dieken, H.; Krischenowski, O.; Förster, C.; Branscheid, D.; Aufderheide, M. Evaluation of E-Cigarette Liquid Vapor and Mainstream Cigarette Smoke after Direct Exposure of Primary Human Bronchial Epithelial Cells. Int. J. Environ. Res. Public Health 2015, 12, 3915–3925. [Google Scholar] [CrossRef]
- Scheffler, S.; Dieken, H.; Krischenowski, O.; Aufderheide, M. Cytotoxic Evaluation of E-Liquid Aerosol Using Different Lung-Derived Cell Models. Int. J. Environ. Res. Public Health 2015, 12, 12466–12474. [Google Scholar] [CrossRef]
- Lerner, C.A.; Sundar, I.K.; Yao, H.; Gerloff, J.; Ossip, D.J.; McIntosh, S.; Robinson, R.; Rahman, I. Vapors Produced by Electronic Cigarettes and E-Juices with Flavorings Induce Toxicity, Oxidative Stress, and Inflammatory Response in Lung Epithelial Cells and in Mouse Lung. PLoS ONE 2015, 10, e0116732. [Google Scholar] [CrossRef]
- Anthérieu, S.; Garat, A.; Beauval, N.; Soyez, M.; Allorge, D.; Garçon, G.; Lo-Guidice, J.-M. Comparison of Cellular and Transcriptomic Effects between Electronic Cigarette Vapor and Cigarette Smoke in Human Bronchial Epithelial Cells. Toxicol. In Vitro 2017, 45, 417–425. [Google Scholar] [CrossRef]
- Hwang, J.H.; Lyes, M.; Sladewski, K.; Enany, S.; McEachern, E.; Mathew, D.P.; Das, S.; Moshensky, A.; Bapat, S.; Pride, D.T.; et al. Electronic Cigarette Inhalation Alters Innate Immunity and Airway Cytokines While Increasing the Virulence of Colonizing Bacteria. J. Mol. Med. 2016, 94, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Herr, C.; Tsitouras, K.; Niederstraßer, J.; Backes, C.; Beisswenger, C.; Dong, L.; Guillot, L.; Keller, A.; Bals, R. Cigarette Smoke and Electronic Cigarettes Differentially Activate Bronchial Epithelial Cells. Respir. Res. 2020, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, K.; Nordström, A.; Thimraj, T.A.; Rahman, M.; Ramström, M.; Sompa, S.I.; Lin, E.Z.; O’Brien, F.; Koelmel, J.; Ernstgård, L.; et al. Addressing the Challenges of E-Cigarette Safety Profiling by Assessment of Pulmonary Toxicological Response in Bronchial and Alveolar Mucosa Models. Sci. Rep. 2020, 10, 20460. [Google Scholar] [CrossRef] [PubMed]
- Amin Arefi, S.M.; Tony Yang, C.W.; Sin, D.D.; Feng, J.J. Simulation of Nanoparticle Transport and Adsorption in a Microfluidic Lung-on-a-Chip Device. Biomicrofluidics 2020, 14, 044117. [Google Scholar] [CrossRef]
- Conway, J. Lung Imaging-Two Dimensional Gamma Scintigraphy, SPECT, CT and PET. Adv. Drug Deliv. Rev. 2012, 64, 357–368. [Google Scholar] [CrossRef]
- Benam, K.H.; Novak, R.; Ferrante, T.C.; Choe, Y.; Ingber, D.E. Biomimetic Smoking Robot for in Vitro Inhalation Exposure Compatible with Microfluidic Organ Chips. Nat. Protoc. 2020, 15, 183–206. [Google Scholar] [CrossRef]
- Kurth, F.; Györvary, E.; Heub, S.; Ledroit, D.; Paoletti, S.; Renggli, K.; Revol, V.; Verhulsel, M.; Weder, G.; Loizeau, F. Organs-on-a-chip engineering. In Organ-on-a-Chip; Elsevier: Amsterdam, The Netherlands, 2020; pp. 47–130. ISBN 9780128172025. [Google Scholar]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Cole, R. Live-Cell Imaging. Cell Adh. Migr. 2014, 8, 452–459. [Google Scholar] [CrossRef]
- St Croix, C.M.; Shand, S.H.; Watkins, S.C. Confocal Microscopy: Comparisons, Applications, and Problems. BioTechniques 2005, 39, S2–S5. [Google Scholar] [CrossRef]
- Hamm, A.; Krott, N.; Breibach, I.; Blindt, R.; Bosserhoff, A.K. Efficient Transfection Method for Primary Cells. Tissue Eng. 2002, 8, 235–245. [Google Scholar] [CrossRef]
- Kintaka, R.; Makanae, K.; Moriya, H. Cellular Growth Defects Triggered by an Overload of Protein Localization Processes. Sci. Rep. 2016, 6, 31774. [Google Scholar] [CrossRef]
- Link, C.D.; Fonte, V.; Hiester, B.; Yerg, J.; Ferguson, J.; Csontos, S.; Silverman, M.A.; Stein, G.H. Conversion of Green Fluorescent Protein into a Toxic, Aggregation-Prone Protein by C-Terminal Addition of a Short Peptide. J. Biol. Chem. 2006, 281, 1808–1816. [Google Scholar] [CrossRef]
- Xu, H.-X.; Tan, Y.; Wang, D.; Wang, X.-L.; An, W.-L.; Xu, P.-P.; Xu, S.; Wang, Y.-Z. Autofluorescence of Hydrogels without a Fluorophore. Soft Matter 2019, 15, 3588–3594. [Google Scholar] [CrossRef]
- Nava, R.G.; Li, W.; Gelman, A.E.; Krupnick, A.S.; Miller, M.J.; Kreisel, D. Two-Photon Microscopy in Pulmonary Research. Semin. Immunopathol. 2010, 32, 297–304. [Google Scholar] [CrossRef][Green Version]
- Benninger, R.K.P.; Piston, D.W. Two-Photon Excitation Microscopy for the Study of Living Cells and Tissues. Curr. Protoc. Cell Biol. 2013. [Google Scholar] [CrossRef]
- Mostaço-Guidolin, L.; Rosin, N.L.; Hackett, T.-L. Imaging Collagen in Scar Tissue: Developments in Second Harmonic Generation Microscopy for Biomedical Applications. Int. J. Mol. Sci. 2017, 18, 1772. [Google Scholar] [CrossRef]
- Xydias, D.; Ziakas, G.; Psilodimitrakopoulos, S.; Lemonis, A.; Bagli, E.; Fotsis, T.; Gravanis, A.; Tzeranis, D.S.; Stratakis, E. Three-Dimensional Characterization of Collagen Remodeling in Cell-Seeded Collagen Scaffolds via Polarization Second Harmonic Generation. Biomed. Opt. Express 2021, 12, 1136–1153. [Google Scholar] [CrossRef]
- Pavlova, I.; Hume, K.R.; Yazinski, S.A.; Peters, R.M.; Weiss, R.S.; Webb, W.W. Multiphoton Microscopy as a Diagnostic Imaging Modality for Lung Cancer. Proc. SPIE 2010, 7569, 756918. [Google Scholar] [CrossRef]
- Mostaço-Guidolin, L.B.; Osei, E.T.; Ullah, J.; Hajimohammadi, S.; Fouadi, M.; Li, X.; Li, V.; Shaheen, F.; Yang, C.X.; Chu, F.; et al. Defective Fibrillar Collagen Organization by Fibroblasts Contributes to Airway Remodeling in Asthma. Am. J. Respir. Crit. Care Med. 2019, 200, 431–443. [Google Scholar] [CrossRef]
- Cohen, S.; Valm, A.M.; Lippincott-Schwartz, J. Multispectral Live-Cell Imaging. Curr. Protoc. Cell Biol. 2018, 79, e46. [Google Scholar] [CrossRef]
- Garini, Y.; Young, I.T.; McNamara, G. Spectral Imaging: Principles and Applications. Cytometry A 2006, 69, 735–747. [Google Scholar] [CrossRef]
- Li, Q.; He, X.; Wang, Y.; Liu, H.; Xu, D.; Guo, F. Review of Spectral Imaging Technology in Biomedical Engineering: Achievements and Challenges. J. Biomed. Opt. 2013, 18, 100901. [Google Scholar] [CrossRef]
- Lu, Y.; Kamel-El Sayed, S.A.; Wang, K.; Tiede-Lewis, L.M.; Grillo, M.A.; Veno, P.A.; Dusevich, V.; Phillips, C.L.; Bonewald, L.F.; Dallas, S.L. Live Imaging of Type I Collagen Assembly Dynamics in Osteoblasts Stably Expressing GFP and MCherry-Tagged Collagen Constructs. J. Bone Miner. Res. 2018, 33, 1166–1182. [Google Scholar] [CrossRef]
- Akther, F.; Little, P.; Li, Z.; Nguyen, N.-T.; Ta, H.T. Hydrogels as Artificial Matrices for Cell Seeding in Microfluidic Devices. RSC Adv. 2020, 10, 43682–43703. [Google Scholar] [CrossRef]
- Thacker, V.V.; Dhar, N.; Sharma, K.; Barrile, R.; Karalis, K.; McKinney, J.D. A Lung-on-Chip Model of Early Mycobacterium Tuberculosis Infection Reveals an Essential Role for Alveolar Epithelial Cells in Controlling Bacterial Growth. eLife 2020, 9. [Google Scholar] [CrossRef]
- Peel, S.; Corrigan, A.M.; Ehrhardt, B.; Jang, K.-J.; Caetano-Pinto, P.; Boeckeler, M.; Rubins, J.E.; Kodella, K.; Petropolis, D.B.; Ronxhi, J.; et al. Introducing an Automated High Content Confocal Imaging Approach for Organs-on-Chips. Lab Chip 2019, 19, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Junaid, A.; Mashaghi, A.; Hankemeier, T.; Vulto, P. An End-User Perspective on Organ-on-a-Chip: Assays and Usability Aspects. Curr. Opin. Biomed. Eng. 2017, 1, 15–22. [Google Scholar] [CrossRef]
- Olson, N.; Hristova, M.; Heintz, N.H.; Lounsbury, K.M.; van der Vliet, A. Activation of Hypoxia-Inducible Factor-1 Protects Airway Epithelium against Oxidant-Induced Barrier Dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L993–L1002. [Google Scholar] [CrossRef]
- Frost, T.S.; Jiang, L.; Lynch, R.M.; Zohar, Y. Permeability of Epithelial/Endothelial Barriers in Transwells and Microfluidic Bilayer Devices. Micromachines 2019, 10, 533. [Google Scholar] [CrossRef]
- Rao, M.S.; Van Vleet, T.R.; Ciurlionis, R.; Buck, W.R.; Mittelstadt, S.W.; Blomme, E.A.G.; Liguori, M.J. Comparison of RNA-Seq and Microarray Gene Expression Platforms for the Toxicogenomic Evaluation of Liver from Short-Term Rat Toxicity Studies. Front. Genet. 2018, 9, 636. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A Revolutionary Tool for Transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.A.U.; Kim, Y.S.; Ali, M.; Lee, B.G.; Cho, Y.-J.; Choi, K.H. A Lung Cancer-on-Chip Platform with Integrated Biosensors for Physiological Monitoring and Toxicity Assessment. Biochem. Eng. J. 2020, 155, 107469. [Google Scholar] [CrossRef]
- Henry, O.Y.F.; Villenave, R.; Cronce, M.J.; Leineweber, W.D.; Benz, M.A.; Ingber, D.E. Organs-on-Chips with Integrated Electrodes for Trans-Epithelial Electrical Resistance (TEER) Measurements of Human Epithelial Barrier Function. Lab Chip 2017, 17, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Maoz, B.M.; Herland, A.; Henry, O.Y.F.; Leineweber, W.D.; Yadid, M.; Doyle, J.; Mannix, R.; Kujala, V.J.; FitzGerald, E.A.; Parker, K.K.; et al. Organs-on-Chips with Combined Multi-Electrode Array and Transepithelial Electrical Resistance Measurement Capabilities. Lab Chip 2017, 17, 2294–2302. [Google Scholar] [CrossRef]
- Van der Helm, M.W.; Odijk, M.; Frimat, J.-P.; van der Meer, A.D.; Eijkel, J.C.T.; van den Berg, A.; Segerink, L.I. Fabrication and Validation of an Organ-on-Chip System with Integrated Electrodes to Directly Quantify Transendothelial Electrical Resistance. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Svensjö, E.; Bouskela, E. Endothelial barrier: Factors that regulate its permeability. In Endothelium and Cardiovascular Diseases; Elsevier: Amsterdam, The Netherlands, 2018; pp. 37–48. ISBN 9780128123485. [Google Scholar]
- Mousavi Shaegh, S.A.; De Ferrari, F.; Zhang, Y.S.; Nabavinia, M.; Binth Mohammad, N.; Ryan, J.; Pourmand, A.; Laukaitis, E.; Banan Sadeghian, R.; Nadhman, A.; et al. A Microfluidic Optical Platform for Real-Time Monitoring of PH and Oxygen in Microfluidic Bioreactors and Organ-on-Chip Devices. Biomicrofluidics 2016, 10, 044111. [Google Scholar] [CrossRef]
- Weltin, A.; Hammer, S.; Noor, F.; Kaminski, Y.; Kieninger, J.; Urban, G.A. Accessing 3D Microtissue Metabolism: Lactate and Oxygen Monitoring in Hepatocyte Spheroids. Biosens. Bioelectron. 2017, 87, 941–948. [Google Scholar] [CrossRef]
- Clarke, G.A.; Hartse, B.X.; Niaraki Asli, A.E.; Taghavimehr, M.; Hashemi, N.; Abbasi Shirsavar, M.; Montazami, R.; Alimoradi, N.; Nasirian, V.; Ouedraogo, L.J.; et al. Advancement of Sensor Integrated Organ-on-Chip Devices. Sensors 2021, 21, 1367. [Google Scholar] [CrossRef]
- Grist, S.M.; Chrostowski, L.; Cheung, K.C. Optical Oxygen Sensors for Applications in Microfluidic Cell Culture. Sensors 2010, 10, 9286–9316. [Google Scholar] [CrossRef]
- Luan, E.; Shoman, H.; Ratner, D.M.; Cheung, K.C.; Chrostowski, L. Silicon Photonic Biosensors Using Label-Free Detection. Sensors 2018, 18, 3519. [Google Scholar] [CrossRef]
- Chrostowski, L.; Leanne, D.; Matthew, M.; Connor, M.; Luan, E.; Al-Qadasi, M.; Avineet, R.; Mojaver, H.R.; Lyall, E.; Gervais, A.; et al. A silicon photonic evanescent-field sensor architecture using a fixed-wavelength laser. In Proceedings of the Optical Interconnects XXI, (Online Only Conference), 5 March 2021; Schröder, H., Chen, R.T., Eds.; SPIE (USA): Bellingham, WA, USA; p. 29. [Google Scholar]
- Standiford, T.J.; Kunkel, S.L.; Basha, M.A.; Chensue, S.W.; Lynch, J.P.; Toews, G.B.; Westwick, J.; Strieter, R.M. Interleukin-8 Gene Expression by a Pulmonary Epithelial Cell Line. A Model for Cytokine Networks in the Lung. J. Clin. Investig. 1990, 86, 1945–1953. [Google Scholar] [CrossRef]
- Löfdahl, M.; Kaarteenaho, R.; Lappi-Blanco, E.; Tornling, G.; Sköld, M.C. Tenascin-C and Alpha-Smooth Muscle Actin Positive Cells Are Increased in the Large Airways in Patients with COPD. Respir. Res. 2011, 12, 48. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-Chips: Into the next Decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef]
- Cirit, M.; Stokes, C.L. Maximizing the Impact of Microphysiological Systems with in Vitro-in Vivo Translation. Lab Chip 2018, 18, 1831–1837. [Google Scholar] [CrossRef]
- Pound, P. Are Animal Models Needed to Discover, Develop and Test Pharmaceutical Drugs for Humans in the 21st Century? Animals 2020, 10, 2455. [Google Scholar] [CrossRef]
- Movia, D.; Prina-Mello, A. Preclinical Development of Orally Inhaled Drugs (OIDs)-Are Animal Models Predictive or Shall We Move Towards In Vitro Non-Animal Models? Animals 2020, 10, 1259. [Google Scholar] [CrossRef]
- Barnes, P.J.; Bonini, S.; Seeger, W.; Belvisi, M.G.; Ward, B.; Holmes, A. Barriers to New Drug Development in Respiratory Disease. Eur. Respir. J. 2015, 45, 1197–1207. [Google Scholar] [CrossRef]
- van den Berg, A.; Mummery, C.L.; Passier, R.; van der Meer, A.D. Personalised Organs-on-Chips: Functional Testing for Precision Medicine. Lab Chip 2019, 19, 198–205. [Google Scholar] [CrossRef]
- Franzen, N.; van Harten, W.H.; Retèl, V.P.; Loskill, P.; van den Eijnden-van Raaij, J.; IJzerman, M. Impact of Organ-on-a-Chip Technology on Pharmaceutical R&D Costs. Drug Discov. Today 2019, 24, 1720–1724. [Google Scholar] [CrossRef]
- Ewart, L.; Roth, A. Opportunities and Challenges with Microphysiological Systems: A Pharma End-User Perspective. Nat. Rev. Drug Discov. 2020. [Google Scholar] [CrossRef]
- Mastrangeli, M.; Millet, S.; Orchid Partners, T.; Van den Eijnden-van Raaij, J. Organ-on-Chip in Development: Towards a Roadmap for Organs-on-Chip. ALTEX 2019, 36, 650–668. [Google Scholar] [CrossRef]
- Klapperich, C.M. Microfluidic Diagnostics: Time for Industry Standards. Expert Rev. Med. Devices 2009, 6, 211–213. [Google Scholar] [CrossRef]
- Becker, H. Mind the Gap! Lab Chip 2010, 10, 271–273. [Google Scholar] [CrossRef]
- Probst, C.; Schneider, S.; Loskill, P. High-Throughput Organ-on-a-Chip Systems: Current Status and Remaining Challenges. Curr. Opin. Biomed. Eng. 2018, 6, 33–41. [Google Scholar] [CrossRef]
- Reyes, D.R.; van Heeren, H.; Guha, S.; Herbertson, L.; Tzannis, A.P.; Ducrée, J.; Bissig, H.; Becker, H. Accelerating Innovation and Commercialization through Standardization of Microfluidic-Based Medical Devices. Lab Chip 2021, 21, 9–21. [Google Scholar] [CrossRef]
- Ramadan, Q.; Zourob, M. Organ-on-a-Chip Engineering: Toward Bridging the Gap between Lab and Industry. Biomicrofluidics 2020, 14, 041501. [Google Scholar] [CrossRef] [PubMed]
- Sakolish, C.; Weber, E.J.; Kelly, E.J.; Himmelfarb, J.; Mouneimne, R.; Grimm, F.A.; House, J.S.; Wade, T.; Han, A.; Chiu, W.A.; et al. Technology Transfer of the Microphysiological Systems: A Case Study of the Human Proximal Tubule Tissue Chip. Sci. Rep. 2018, 8, 14882. [Google Scholar] [CrossRef]
- Patel, B.; Gauvin, R.; Absar, S.; Gupta, V.; Gupta, N.; Nahar, K.; Khademhosseini, A.; Ahsan, F. Computational and Bioengineered Lungs as Alternatives to Whole Animal, Isolated Organ, and Cell-Based Lung Models. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L733–L747. [Google Scholar] [CrossRef][Green Version]
- Rifaioglu, A.S.; Atas, H.; Martin, M.J.; Cetin-Atalay, R.; Atalay, V.; Doğan, T. Recent Applications of Deep Learning and Machine Intelligence on in Silico Drug Discovery: Methods, Tools and Databases. Brief. Bioinform. 2019, 20, 1878–1912. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Furlong, L.I.; Sanz, F. In Silico Models in Drug Development: Where We Are. Curr. Opin. Pharmacol. 2018, 42, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of Machine Learning in Drug Discovery and Development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, T.B.; Keller, D.A.; Sander, M.; Carney, E.W.; Doerrer, N.G.; Eaton, D.L.; Fitzpatrick, S.C.; Hastings, K.L.; Mendrick, D.L.; Tice, R.R.; et al. FutureTox II: In Vitro Data and in Silico Models for Predictive Toxicology. Toxicol. Sci. 2015, 143, 256–267. [Google Scholar] [CrossRef] [PubMed]

| Area of Interest | Culture Type | Cells | Cell Origin | Tissue Interface | Air–Liquid Interface | Ref |
|---|---|---|---|---|---|---|
| Alveolus | Monoculture | Lung alveolar (type II) epithelial-like cells (A549) | Cell Line | Epithelium | No | [17] |
| Alveolus | Monoculture | Lung alveolar (type II) epithelial-like cells (A549) | Cell Line | Epithelium | No | [18] |
| Alveolus | Monoculture | Lung alveolar (type II) epithelial-like cells (A549) | Cell Line | Epithelium | Yes | [19] |
| Alveolus | Co-Culture | Human alveolar epithelial cells (hAECs) Human pulmonary microvascular endothelial cells | Primary | Alveolar– Capillary | Yes | [26] |
| Alveolus | Co-Culture | Bronchial epithelial cells (16 HBE14 o-)/Primary human pulmonary alveolar epithelial cells (pHPAECs) Human umbilical vein endothelial cells (pHUVEC) | Cell Lines/Primary | Alveolar– Capillary | Yes | [50] |
| Alveolus | Co-Culture | Lung alveolar (type II) epithelial-like cells (A549)/Murine alveolar epithelial cells (AECs) | Cell Lines/Primary | Alveolar– Capillary | Yes | [40] |
| Alveolus | Co-Culture | Human pulmonary alveolar epithelial cells (HPAEpiCs) Human umbilical vein endothelial cells (HUVEC) | Cell Lines | Alveolar– Capillary | No | [20] |
| Alveolus | Co-Culture | Human alveolar epithelial cells Human lung microvascular endothelial cells | Primary | Alveolar–Capillary | Yes | [47] |
| Alveoli | Monoculture | Human alveolar epithelial cells (hAECs) | Primary | Epithelium | Yes | [25] |
| Alveoli | Co-Culture | Human bronchial epithelial cells (BEAS-2 B) Human vascular endothelial cells (HUVECs) | Cell Lines | Alveolar–Capillary | Yes | [21] |
| Alveoli | Co-Culture | Human alveolar epithelial cells (hAECs) Human lung endothelial cells | Primary | Alveolar–Capillary | Yes | [51] |
| Airway | Co-Culture | Human airway epithelial cells (hAECs) Human pulmonary microvascular endothelial cells | Primary | Epithelial– Endothelial | Yes | [23] |
| Airway | Co-Culture | Human airway epithelial cells (Calu-3) Human bronchial smooth muscle cells (hBSMC) | Cell Lines | Epithelial–Mesenchymal | Yes | [22] |
| Airway | Tri-Culture | Human tracheo-bronchial epithelial cells Human lung fibroblasts Human lung microvascular endothelial cells | Primary | Epithelial–Stromal/Vascular | Yes | [24] |
| Airway | Tri-Culture | Human tracheal epithelial cells Human Lung fibroblasts Human dermal microvascular endothelial cells | Primary | Epithelial–Stromal/Vascular | Yes | [28] |
| Airway | Tri-Culture | Human bronchial epithelial cells Normal pulmonary fibroblasts Human lung microvascular endothelial cells | Primary | Epithelial–Stromal/Vascular | Yes | [27] |
| Dimension | ECM Substitute Type | ECM Substitution Material | Lung ECM Replicated | Area of Focus | Reference |
|---|---|---|---|---|---|
| 2 | Non-Coated Membrane | Polyester | Basement Membrane | Airway | [101] |
| 2 | Non-Coated Membrane | PET | Basement Membrane | Alveolus | [19] |
| 2 | ECM Coated Membrane | PDMS | Basement Membrane | Alveolus | [40] |
| 2 | ECM Coated Membrane | PDMS | Basement Membrane | Alveolus | [108] |
| 2 | ECM Coated Membrane | PDMS | Basement Membrane | Alveolus | [24] |
| 2 | ECM Coated Membrane | Polyester | Basement Membrane | Airway | [50] |
| 2–2.5 | ECM Coated Membrane Combined With Additional Channel | PTFE and PET | Interstitial Layer | Airway | [23] |
| 2.5 | ECM Thin Film | Collagen and Elastin | Basement Membrane | Alveoli | [27,69] |
| 2.5 | ECM Thin Films | Collagen I and Matrigel | Basement Membrane | Airway | [27,69] |
| 2.5–3 | ECM Hydrogel | Collagen and Matrigel | Interstitial Matrix | Airway | [22] |
| 3 | ECM Hydrogel | Collagen and Fibrinogen | Interstitial Matrix | Airway | [28] |
| 3 | ECM Hydrogel | Decellularized ECM | Interstitial Matrix | Airway | [51] |
| 3 | ECM Hydrogel | GelMA | Interstitial Matrix | Alveoli | [25] |
| Reference | CSE Concentration | Solvent | Notes |
|---|---|---|---|
| Hoshino et al. [135] | 0.5 filtered cigarettes/mL | DMEM |
|
| Carnevali et al. [139] | 0.04 filterless cigarettes/mL | DMEM |
|
| Heijink et al. [140] | 0.08 filterless reference cigarettes/mL | EMEM |
|
| Richter et al. [146] | 0.04 filterless reference cigarettes/mL | RPMI 1640 |
|
| Kode et al. [148] | 0.1 reference cigarettes/mL | DMEM, RPMI 1640 |
|
| Witherden et al. [147] | 1 filterless cigarette/mL | LPHM |
|
| Reference | Cells | eCVE Preparation | Results |
|---|---|---|---|
| Romegna et al. [161] | BALB/3T3 fibroblasts (mouse) | 200 mg e-liquid extracted into 20 mL culture medium | One of 21 extracts was cytotoxic (51% viability) at highest (undiluted concentration); all others not cytotoxic |
| Cervellati et al. [158] | A549 | Whole smoke delivered to incubator with lids of culture plate removed | Flavored e-liquids and e-liquids with nicotine led to decreased in viability (LDH) |
| Higham et al. [157] | Neutrophils isolated from peripheral donor blood | 50–300 mL aerosol bubbled through RPMI 1640 culture medium (volume unspecified). Normalized to OD at 320 nm in culture medium. 0.22 micron filtered | Increase in MMP-9 and IL-8 release with exposure to eCVE |
| Putzhammer et al. [159] | HUVEC | 88.5 mg liquid (equivalent to 700 mL aerosol) extracted into 8 mL culture medium. 0.2 micron filtered. Prepared freshly prior to experiments | Some e-liquid aerosols decreased viability, one of 11 tested increased oxidative stress. Results were liquid dependent. The same electronic cigarettes were used with different liquids; authors isolated effects to liquids. |
| Taylor et al. [162] | NCI-H292 | 550 mL aerosol bubbled through 20 mL DMEM/F12. Nicotine content characterized by GC-MS, tar by OD at 320 nm | No cytotoxic effects or oxidative stress induced by eCVE |
| Leslie et al. [156] | BEAS-2 B, IB3-1, C38 (human bronchial epithelium cell lines), Wi-38 fibroblasts, J774 THP-1 macrophages | 490 mL aerosol extracted into 10 mL DMEM/F12, EMEM, or RPMI 1640. Used within 1 h | Some extracts reduced viability below 70% (considered cytotoxic), varied depending on flavor and cell line |
| Taylor et al. [163] | HUVEC | 550 mL aerosol extracted into 20 mL Vasculife culture medium. Nicotine concentration qualified via GC-MS | eCVE did not inhibit endothelial cell migration |
| Bengalli et al. [160] | A549, NCI H441 | 11 L aerosol extracted into 25 mL OPTIMEM culture medium, 0.2 micron filtered and frozen at −20 until use | Viability and barrier integrity decreased with exposure to certain flavors, one of which led to an increase in IL-8 and MCP-1 release. Unflavored liquids produced insignificant effects. Nicotine was not found to be a factor. |
| Higham et al. [136] | Calu-3, primary bronchial epithelial cells (from healthy and COPD patients). Cultured at ALI but exposed to liquid solution. | 50–300 mL aerosol bubbled through DMEM/F12 culture medium (volume unspecified). Normalized to OD at 320 nm in culture medium. 0.22 micron filtered | eCVE had cytotoxic effects and increased IL-6 and IL-8 while decreasing TEER, indicating a decrease in barrier integrity |
| Reference | Exposure System | Cells | Findings |
|---|---|---|---|
| Scheffler et al. 2015 a [187] | CULTEX® | Primary BECs | Higher oxidative stress and decreased viability found |
| Scheffler et al. 2015 b [188] | CULTEX® | PBECs, CL-1548, A549 | Decrease in viability, with sensitivity dependent on cell line |
| Lerner et al. [189] | Custom configuration | NCI-H292, BEAS-2 B | Increased IL-6 and IL-8 secretion |
| Iskandar et al. [186] | VITROCELL® | Primary BECs | Viability and ciliary beating unchanged, IL-8 increased |
| Antherieu et al. [190] | VITROCELL® | BEAS-2 B | Viability and oxidative stress levels unchanged, modest increase in IL-6 |
| Hwang et al. [191] | Custom configuration | A549 | Necrotic cell death induced, host defense decreased |
| Herr et al. [192] | Custom configuration | Primary BECs, Calu-3, HCI-H292 | IL-8 secretion increased; no significant change in barrier integrity |
| Bathrinarayana et al. [174] | Custom configuration | CALU-3 (HBEC cell line) with MRC-5 (human pulmonary fibroblast cell line) in co-culture | ECA decreased viability at exposure times greater than three hours, increased IL-6 and IL-8 secretion, and increased oxidative stress (via hydrogen-peroxide assay) |
| Ghosh et al. [175] | Custom configuration | Primary human BECs | Upon exposure to ECA barrier integrity (as assessed by TEER and permeability to FITC-dextran) decreased, ciliary beat frequency was reduced, E-cadherin expression insignificantly affected |
| Ganguli et al. [193] | Custom configuration | Primary human BECs, NCI-H441 (human alveolar EC line) | Aerosolization wattage positively correlated with concentration of ECA particulates and presence of nicotine in e-liquid; transcription of genes related to oxidative-stress, inflammatory mediator expression (MMP-1, interleukins 1 B, 6, 8, 10) and depended on e-liquid flavor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennet, T.J.; Randhawa, A.; Hua, J.; Cheung, K.C. Airway-On-A-Chip: Designs and Applications for Lung Repair and Disease. Cells 2021, 10, 1602. https://doi.org/10.3390/cells10071602
Bennet TJ, Randhawa A, Hua J, Cheung KC. Airway-On-A-Chip: Designs and Applications for Lung Repair and Disease. Cells. 2021; 10(7):1602. https://doi.org/10.3390/cells10071602
Chicago/Turabian StyleBennet, Tanya J., Avineet Randhawa, Jessica Hua, and Karen C. Cheung. 2021. "Airway-On-A-Chip: Designs and Applications for Lung Repair and Disease" Cells 10, no. 7: 1602. https://doi.org/10.3390/cells10071602
APA StyleBennet, T. J., Randhawa, A., Hua, J., & Cheung, K. C. (2021). Airway-On-A-Chip: Designs and Applications for Lung Repair and Disease. Cells, 10(7), 1602. https://doi.org/10.3390/cells10071602






