A Robust Protocol for Decellularized Human Lung Bioink Generation Amenable to 2D and 3D Lung Cell Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Decellularization
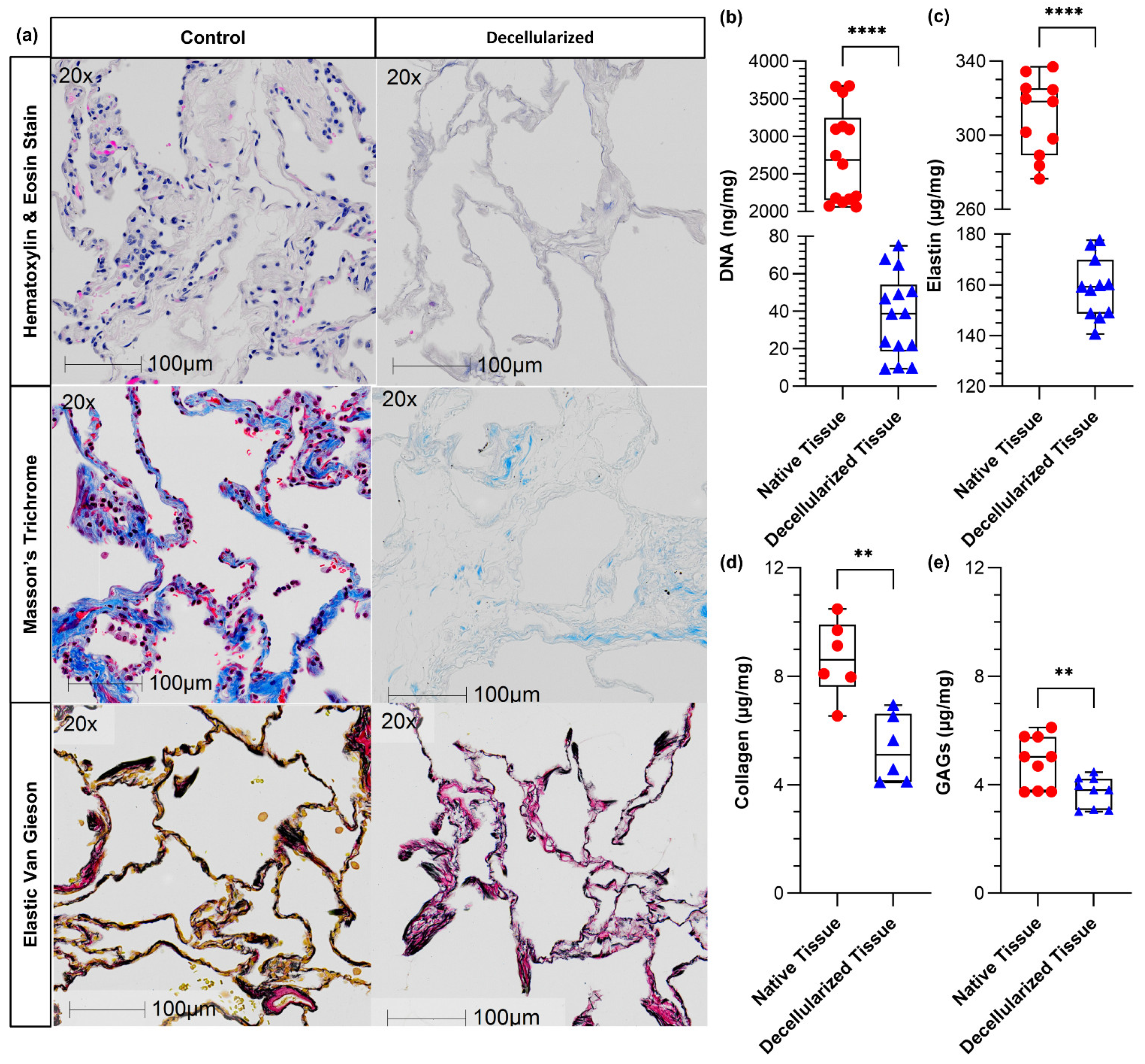
2.2. Tissue Histology
2.3. Biochemical Assays
2.3.1. DNA
2.3.2. Collagen
2.3.3. GAGs
2.3.4. Elastin
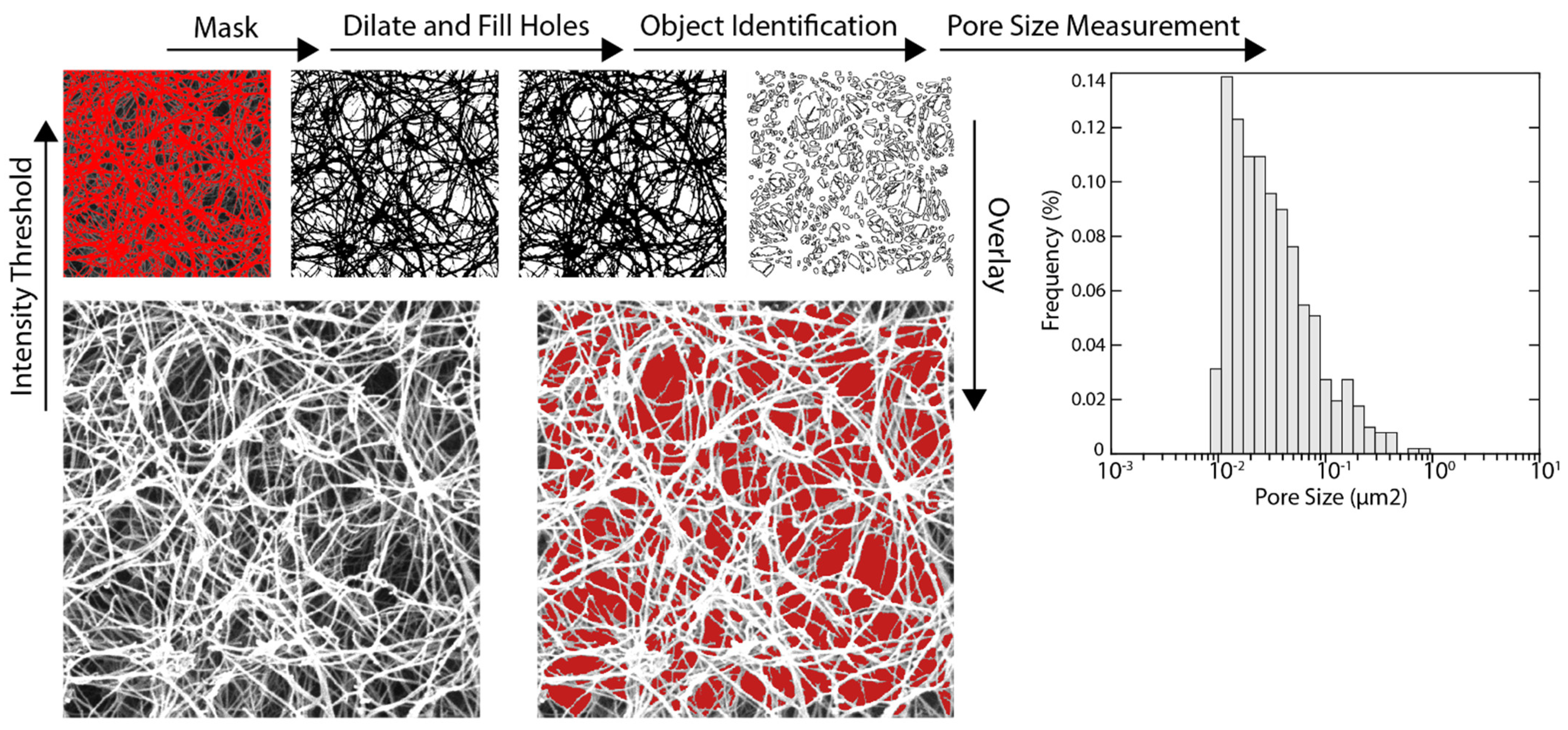
2.4. Evaluation of Hydrogel Fiber Orientation Using Scanning Electron Microscopy
2.5. Human Lung dECM Based Hydrogels
2.6. Surface Modification with dECM Precursor
2.7. Turbidimetric Gelation Kinetics
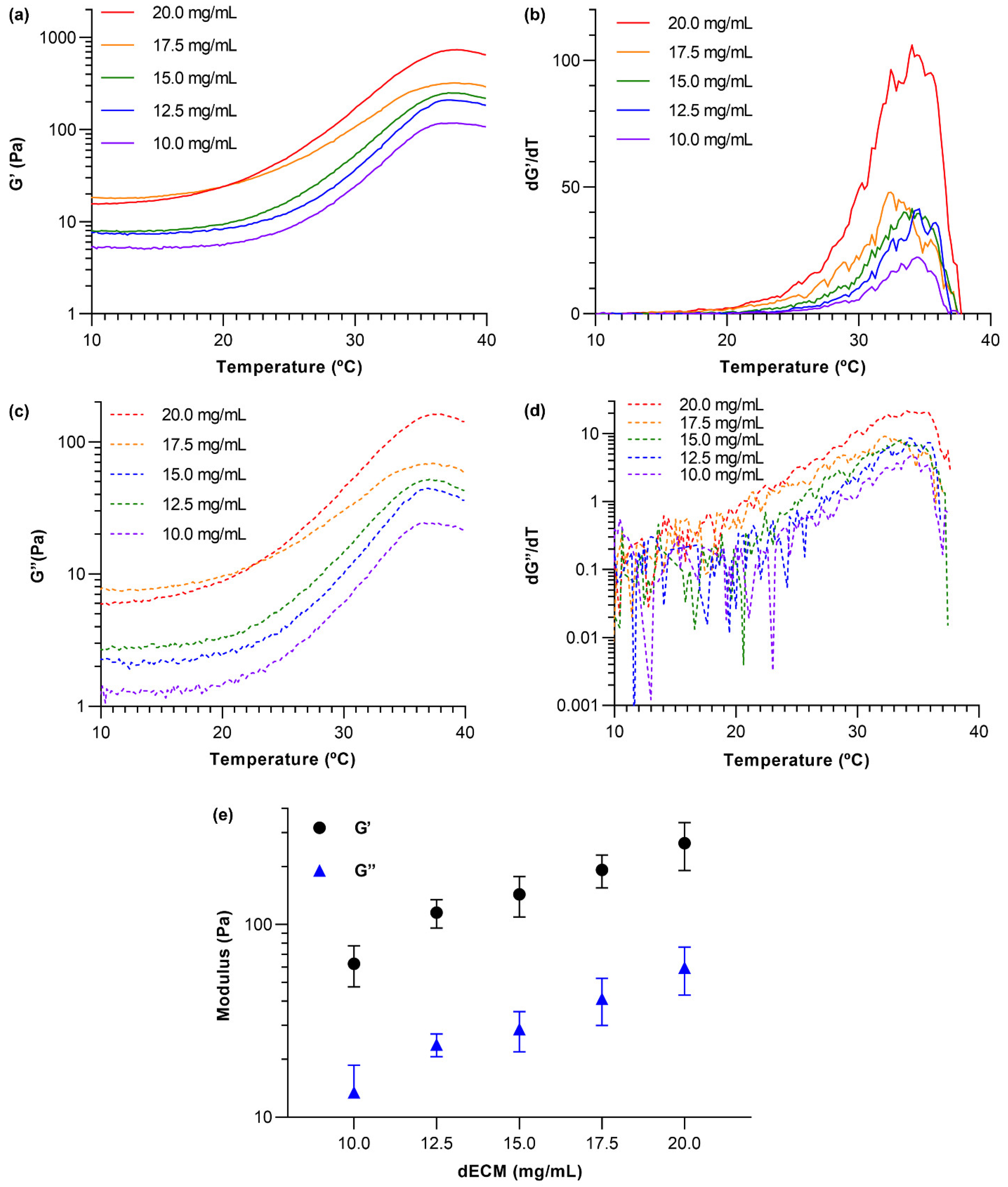
2.8. dECM Hydrogel Rheology Measurements
2.9. Cell Culture and Staining
2.10. Contraction of dECM Hydrogels
2.11. Transepithelial Electrical Resistance (TEER) Measurements
2.12. Dextran Permeability Assay
2.13. Statistical Analysis
3. Results
3.1. Histologic and Biochemical Matrix Analysis
3.2. Turbidimetric Gelation Kinetics
3.3. dECM Hydrogel Rheology
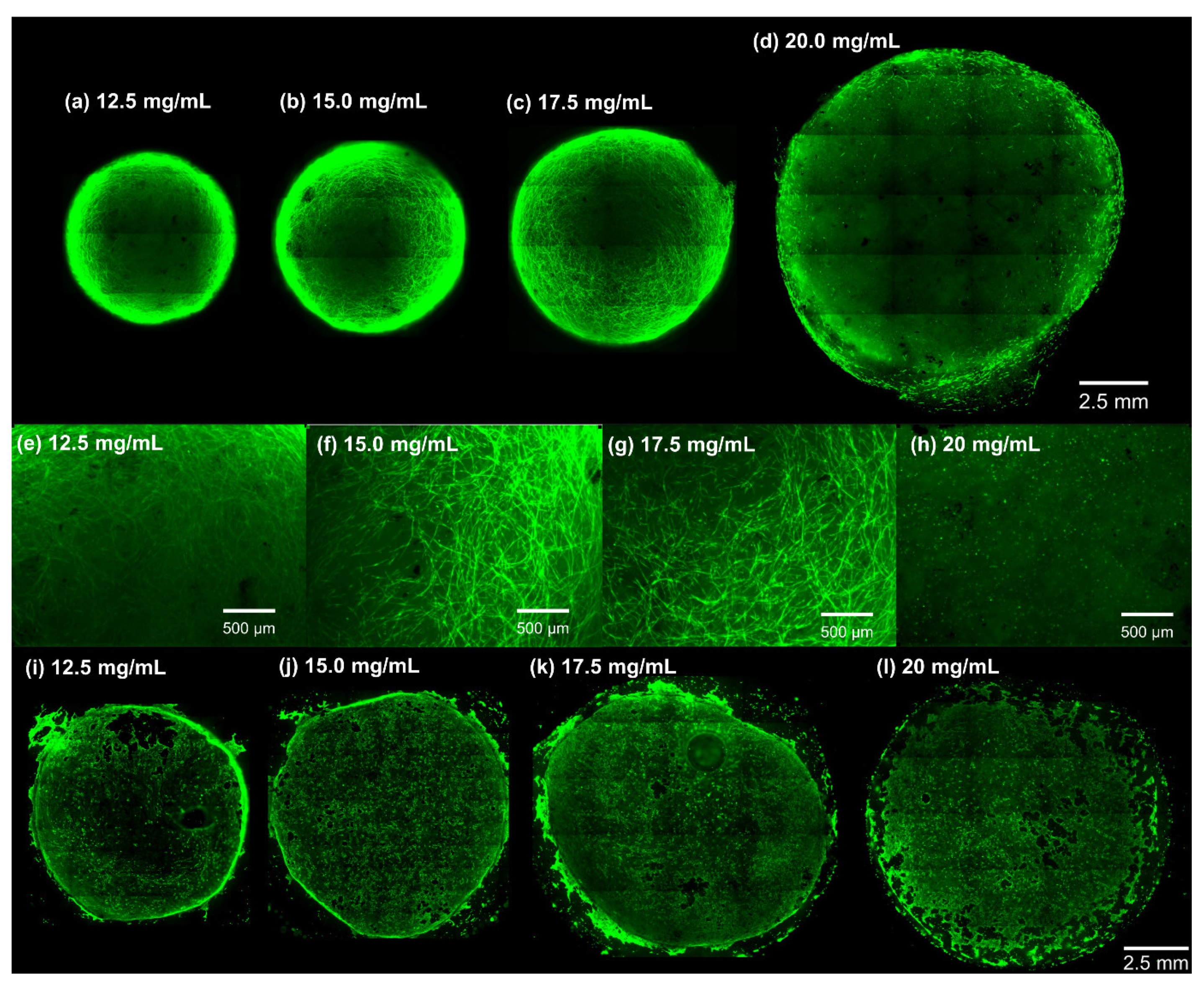
3.4. Macroscopic and Microscopic Appearance of dECM Hydrogels
3.5. In Vitro Cell Culture and Viability Assessment
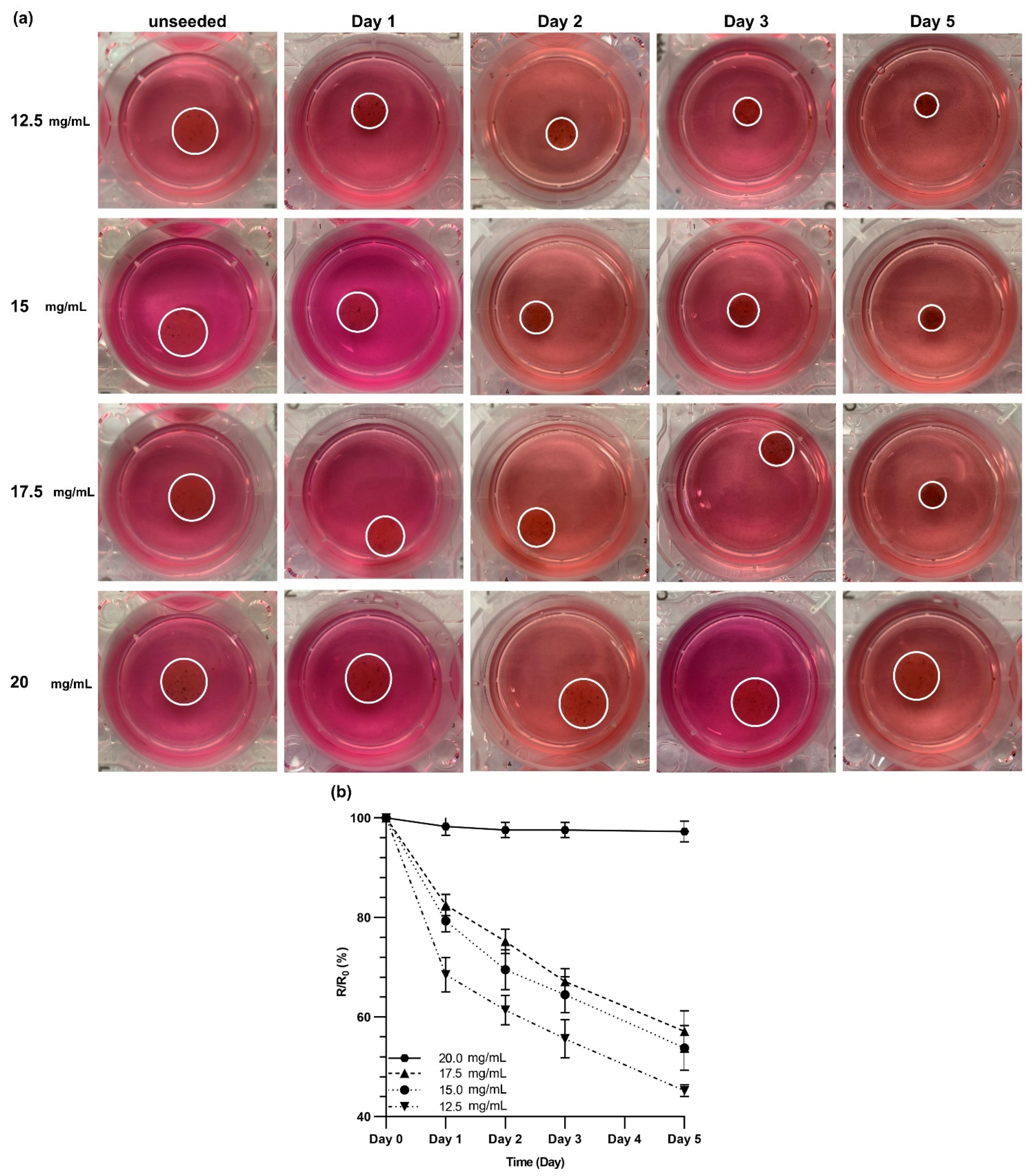
3.6. Contraction of dECM Hydrogels
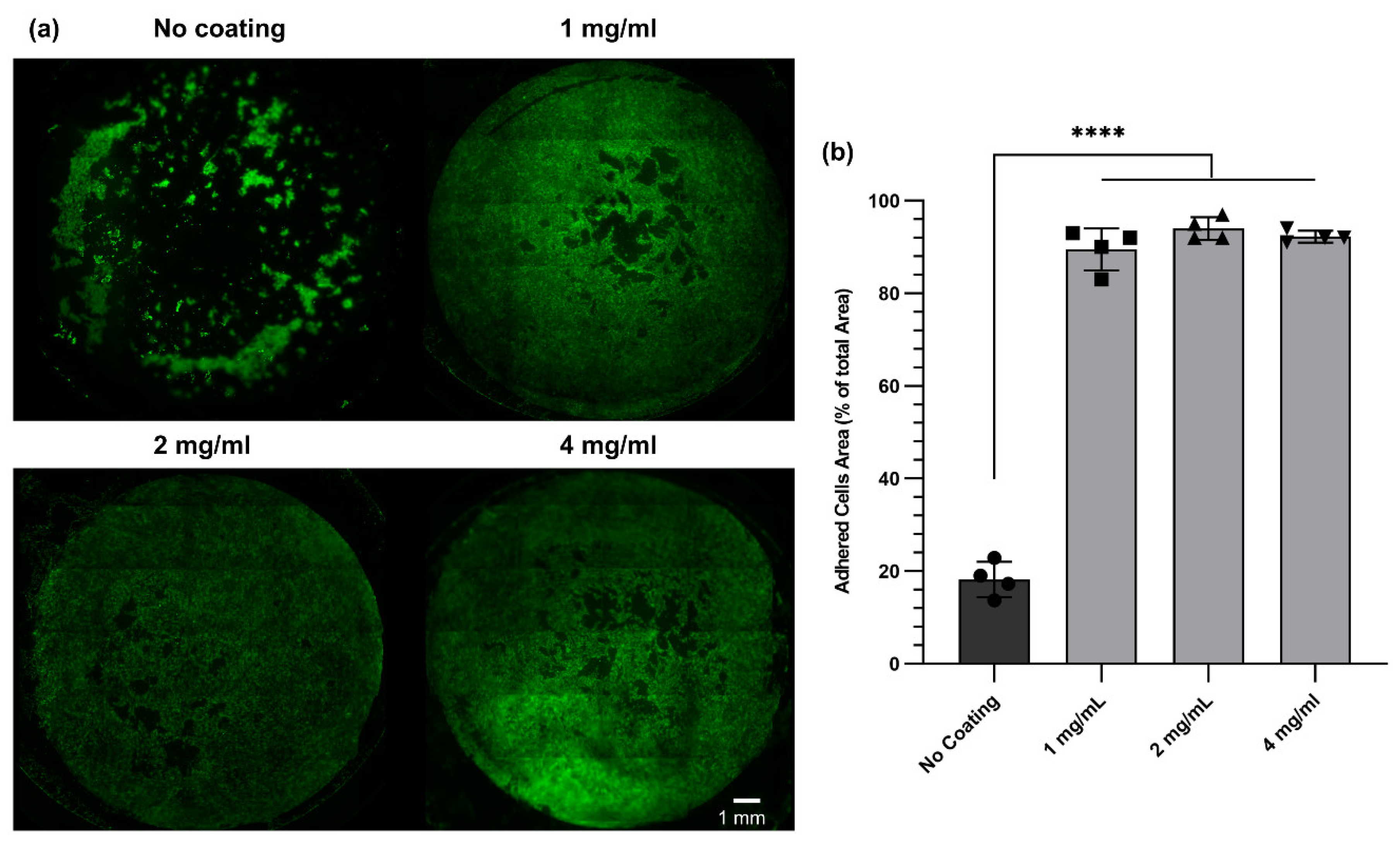
3.7. The Impact of dECM Coating on Cell Adhesion and Proliferation
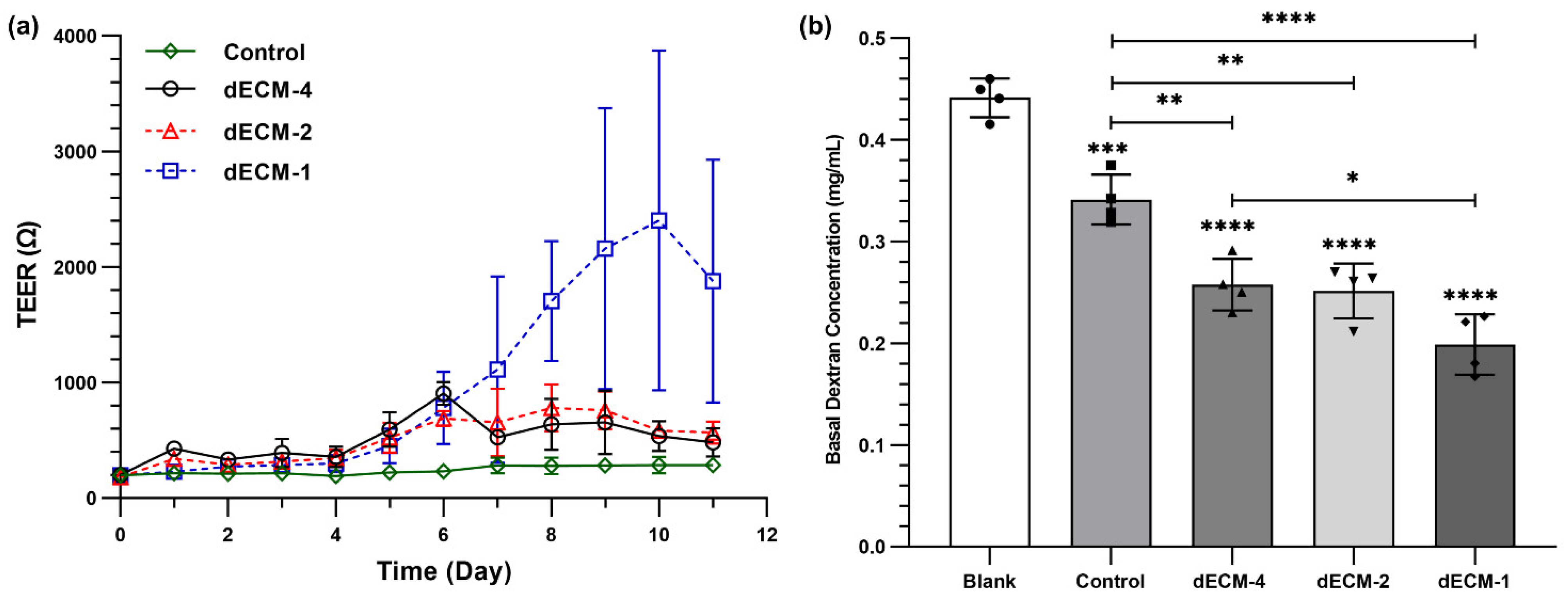
3.8. The Barrier Function of Primary Human Bronchial Epithelial Cells
4. Discussion
5. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hogg, J.C.; Paré, P.D.; Hackett, T.L. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol. Rev. 2017, 97, 529–552. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.L.; Freeman, C.M.; Hogg, J.C. The immunopathogenesis of chronic obstructive pulmonary disease: Insights from recent research. Proc. Am. Thorac. Soc. 2007, 4, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Khakban, A.; Sin, D.D.; FitzGerald, J.M.; McManus, B.M.; Ng, R.; Hollander, Z.; Sadatsafavi, M. The projected epidemic of chronic obstructive pulmonary disease hospitalizations over the next 15 years a population-based perspective. Am. J. Respir. Crit. Care Med. 2017, 195, 287–291. [Google Scholar] [CrossRef]
- Doryab, A.; Tas, S.; Taskin, M.B.; Yang, L.; Hilgendorff, A.; Groll, J.; Wagner, D.E.; Schmid, O. Evolution of Bioengineered Lung Models: Recent Advances and Challenges in Tissue Mimicry for Studying the Role of Mechanical Forces in Cell Biology. Adv. Funct. Mater. 2019, 29, 1–20. [Google Scholar] [CrossRef]
- Sedláková, V.; Kloučková, M.; Garlíková, Z.; Vašíčková, K.; Jaroš, J.; Kandra, M.; Kotasová, H.; Hampl, A. Options for modeling the respiratory system: Inserts, scaffolds and microfluidic chips. Drug Discov. Today 2019, 24, 971–982. [Google Scholar] [CrossRef]
- Barros, A.S.; Costa, A.; Sarmento, B. Building three-dimensional lung models for studying pharmacokinetics of inhaled drugs. Adv. Drug Deliv. Rev. 2021, 170, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Doryab, A.; Amoabediny, G.; Salehi-Najafabadi, A. Advances in pulmonary therapy and drug development: Lung tissue engineering to lung-on-a-chip. Biotechnol. Adv. 2016, 34, 588–596. [Google Scholar] [CrossRef]
- Bove, P.F.; Dang, H.; Cheluvaraju, C.; Jones, L.C.; Liu, X.; O’Neal, W.K.; Randell, S.H.; Schlegel, R.; Boucher, R.C. Breaking the In Vitro Alveolar Type II Cell Proliferation Barrier while Retaining Ion Transport Properties. Am. J. Respir. Cell Mol. Biol. 2014, 50, 767–776. [Google Scholar] [CrossRef]
- Poon, J.C.H.; Liao, Z.; Suzuki, T.; Carleton, M.M.; Soleas, J.P.; Aitchison, J.S.; Karoubi, G.; McGuigan, A.P.; Waddell, T.K. Design of biomimetic substrates for long-term maintenance of alveolar epithelial cells. Biomater. Sci. 2018, 6, 292–303. [Google Scholar] [CrossRef]
- Roan, E.; Waters, C.M. What do we know about mechanical strain in lung alveoli? Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, 625–635. [Google Scholar] [CrossRef]
- Sokocevic, D.; Bonenfant, N.R.; Wagner, D.E.; Borg, Z.D.; Lathrop, M.J.; Lam, Y.W.; Deng, B.; DeSarno, M.J.; Ashikaga, T.; Loi, R.; et al. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials 2013, 34, 3256–3269. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Benam, K.H.; Villenave, R.; Lucchesi, C.; Varone, A.; Hubeau, C.; Lee, H.H.; Alves, S.E.; Salmon, M.; Ferrante, T.C.; Weaver, J.C.; et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat. Methods 2016, 13, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Benam, K.H.; Novak, R.; Nawroth, J.; Hirano-Kobayashi, M.; Ferrante, T.C.; Choe, Y.; Prantil-Baun, R.; Weaver, J.C.; Bahinski, A.; Parker, K.K.; et al. Matched-Comparative Modeling of Normal and Diseased Human Airway Responses Using a Microengineered Breathing Lung Chip. Cell Syst. 2016, 3, 456–466.e4. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Hogg, J.C.; Timens, W. The pathology of chronic obstructive pulmonary disease. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 435–459. [Google Scholar] [CrossRef]
- Burgess, J.K.; Mauad, T.; Tjin, G.; Karlsson, J.C.; Westergren-Thorsson, G. The extracellular matrix—The under-recognized element in lung disease? J. Pathol. 2016, 240, 397–409. [Google Scholar] [CrossRef]
- Yanagihara, T.; Chong, S.G.; Vierhout, M.; Hirota, J.A.; Ask, K.; Kolb, M. Current models of pulmonary fibrosis for future drug discovery efforts. Expert Opin. Drug Discov. 2020, 15, 931–941. [Google Scholar] [CrossRef]
- Zuo, H.; Han, B.; Poppinga, W.J.; Ringnalda, L.; Kistemaker, L.E.M.; Halayko, A.J.; Gosens, R.; Nikolaev, V.O.; Schmidt, M. Cigarette smoke up-regulates PDE3 and PDE4 to decrease cAMP in airway cells. Br. J. Pharmacol. 2018, 175, 2988–3006. [Google Scholar] [CrossRef]
- Ingber, D.E. Is it Time for Reviewer 3 to Request Human Organ Chip Experiments Instead of Animal Validation Studies? Adv. Sci. 2020, 7, 1–15. [Google Scholar] [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Front. Bioeng. Biotechnol. 2017, 5, 1–22. [Google Scholar] [CrossRef]
- Park, J.; Wetzel, I.; Dréau, D.; Cho, H. 3D Miniaturization of Human Organs for Drug Discovery. Adv. Healthc. Mater. 2018, 7, 1–26. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Tun, H.W.; Wang, T.; Naing, M.W. Organ-Derived Decellularized Extracellular Matrix: A Game Changer for Bioink Manufacturing? Trends Biotechnol. 2018, 36, 787–805. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.M.; Alsafadi, H.N.; Tas, S.; Bölükbas, D.A.; Prithiviraj, S.; Da Silva, I.A.N.; Mittendorfer, M.; Ota, C.; Stegmayr, J.; Daoud, F.; et al. Extracellular-Matrix-Reinforced Bioinks for 3D Bioprinting Human Tissue. Adv. Mater. 2021, 33. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G.; Al-Sadi, O.; Ertel, W.; Lohan, A. Decellularized Tendon Extracellular Matrix—A Valuable Approach for Tendon Reconstruction? Cells 2012, 1, 1010–1028. [Google Scholar] [CrossRef]
- Mazza, G.; Telese, A.; Al-Akkad, W.; Frenguelli, L.; Levi, A.; Marrali, M.; Longato, L.; Thanapirom, K.; Vilia, M.G.; Lombardi, B.; et al. Cirrhotic Human Liver Extracellular Matrix 3D Scaffolds Promote Smad-Dependent TGF-β1 Epithelial Mesenchymal Transition. Cells 2019, 9, 83. [Google Scholar] [CrossRef]
- Alevra Sarika, N.; Payen, V.L.; Fléron, M.; Ravau, J.; Brusa, D.; Najimi, M.; De Pauw, E.; Eppe, G.; Mazzucchelli, G.; Sokal, E.M.; et al. El Human Liver-Derived Extracellular Matrix for the Culture of Distinct Human Primary Liver Cells. Cells 2020, 9, 1357. [Google Scholar] [CrossRef]
- Gilpin, S.E.; Guyette, J.P.; Gonzalez, G.; Ren, X.; Asara, J.M.; Mathisen, D.J.; Vacanti, J.P.; Ott, H.C. Perfusion decellularization of human and porcine lungs: Bringing the matrix to clinical scale. J. Heart Lung Transplant. 2014, 33, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Moser, P.T.; Gilpin, S.E.; Okamoto, T.; Wu, T.; Tapias, L.F.; Mercier, F.E.; Xiong, L.; Ghawi, R.; Scadden, D.T.; et al. Engineering pulmonary vasculature in decellularized rat and human lungs. Nat. Biotechnol. 2015, 33, 1097–1102. [Google Scholar] [CrossRef]
- Godin, L.M.; Sandri, B.J.; Wagner, D.E.; Meyer, C.M.; Price, A.P.; Akinnola, I.; Weiss, D.J.; Panoskaltsis-Mortari, A.P.M. Decreased laminin expression by human lung epithelial cells and fibroblasts cultured in acellular lung scaffolds from aged mice. PLoS ONE 2016, 11, e0150966. [Google Scholar] [CrossRef] [PubMed]
- Young, B.M.; Shankar, K.; Tho, C.K.; Pellegrino, A.R.; Heise, R.L. Laminin-driven Epac/Rap1 regulation of epithelial barriers on decellularized matrix. Acta Biomater. 2019, 100, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, J.L.; Gard, A.L.; Gerhold, K.A.; Wilcox, E.C.; Liu, A.; Schwan, J.; Le, A.V.; Baevova, P.; Dimitrievska, S.; Zhao, L.; et al. Comparative biology of decellularized lung matrix: Implications of species mismatch in regenerative medicine. Biomaterials 2016, 102, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, H.; Gao, G.; Jang, J.; Cho, D.W. Decellularized extracellular matrix: A step towards the next generation source for bioink manufacturing. Biofabrication 2017, 9, 034104. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pérez, J.; Ahearne, M. The impact of decellularization methods on extracellular matrix derived hydrogels. Sci. Rep. 2019, 9, 14933. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.L.; Sidney, L.E.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Corneal Decellularization: A Method of Recycling Unsuitable Donor Tissue for Clinical Translation? Curr. Eye Res. 2016, 41, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.T.; Daly, K.A.; Brennan-Pierce, E.P.; Johnson, S.A.; Carruthers, C.A.; D’Amore, A.; Nagarkar, S.P.; Velankar, S.S.; Badylak, S.F. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 2012, 33, 7028–7038. [Google Scholar] [CrossRef]
- Pouliot, R.A.; Young, B.M.; Link, P.A.; Park, H.E.; Kahn, A.R.; Shankar, K.; Schneck, M.B.; Weiss, D.J.; Heise, R.L. Porcine Lung-Derived Extracellular Matrix Hydrogel Properties Are Dependent on Pepsin Digestion Time. Tissue Eng.-Part C Methods 2020, 26, 332–346. [Google Scholar] [CrossRef]
- Chandiramohan, A.; Dabaghi, M.; Aguiar, J.A.; Tiessen, N.; Stewart, M.; Cao, Q.T.; Nguyen, J.P.; Makhdami, N.; Cox, G.; Doxey, A.C.; et al. Development and validation of an open-source, disposable, 3D-printed in vitro environmental exposure system for Transwell culture inserts. ERJ Open Res. 2021, 7, 00705–02020. [Google Scholar] [CrossRef]
- Vllasaliu, D.; Falcone, F.H.; Stolnik, S.; Garnett, M. Basement membrane influences intestinal epithelial cell growth and presents a barrier to the movement of macromolecules. Exp. Cell Res. 2014, 323, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Koval, M.; Ward, C.; Findley, M.K.; Roser-Page, S.; Helms, M.N.; Roman, J. Extracellular matrix influences alveolar epithelial claudin expression and barrier function. Am. J. Respir. Cell Mol. Biol. 2010, 42, 172–180. [Google Scholar] [CrossRef]
- Gilpin, S.E.; Li, Q.; Evangelista-Leite, D.; Ren, X.; Reinhardt, D.P.; Frey, B.L.; Ott, H.C. Fibrillin-2 and Tenascin-C bridge the age gap in lung epithelial regeneration. Biomaterials 2017, 140, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535. [Google Scholar] [CrossRef] [PubMed]
- Rosales, A.M.; Anseth, K.S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater. 2016, 1, 15012. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Liu, H.; Wu, M.; Jia, Y.; Niu, L.; Huang, G.; Xu, F. Control of fibroblast shape in sequentially formed 3D hybrid hydrogels regulates cellular responses to microenvironmental cues. NPG Asia Mater. 2020, 12, 45. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A Common Pathway to Organ Injury and Failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Gaggar, A.; Duncan, S.R. Fibrosis: Ultimate and proximate causes. J. Clin. Investig. 2014, 124, 4673–4677. [Google Scholar] [CrossRef]
- Zeisberg, M.; Kalluri, R. Cellular Mechanisms of Tissue Fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am. J. Physiol. Cell Physiol. 2013, 304, 216–225. [Google Scholar] [CrossRef]
- Jeon, H.; Simon, C.G.; Kim, G. A mini-review: Cell response to microscale, nanoscale, and hierarchical patterning of surface structure. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1580–1594. [Google Scholar] [CrossRef] [PubMed]
- Higuita-Castro, N.; Nelson, M.T.; Shukla, V.; Agudelo-Garcia, P.A.; Zhang, W.; Duarte-Sanmiguel, S.M.; Englert, J.A.; Lannutti, J.J.; Hansford, D.J.; Ghadiali, S.N. Using a Novel Microfabricated Model of the Alveolar-Capillary Barrier to Investigate the Effect of Matrix Structure on Atelectrauma. Sci. Rep. 2017, 7, 11623. [Google Scholar] [CrossRef]
- Baptista, D.; Teixeira, L.M.; Birgani, Z.T.; van Riet, S.; Pasman, T.; Poot, A.; Stamatialis, D.; Rottier, R.J.; Hiemstra, P.S.; Habibović, P.; et al. 3D alveolar in vitro model based on epithelialized biomimetically curved culture membranes. Biomaterials 2021, 266, 120436. [Google Scholar] [CrossRef]
- Shin, Y.J.; Shafranek, R.T.; Tsui, J.H.; Walcott, J.; Nelson, A.; Kim, D.H. 3D bioprinting of mechanically tuned bioinks derived from cardiac decellularized extracellular matrix. Acta Biomater. 2021, 119, 75–88. [Google Scholar] [CrossRef]
- Ning, L.; Mehta, R.; Cao, C.; Theus, A.; Tomov, M.; Zhu, N.; Weeks, E.R.; Bauser-Heaton, H.; Serpooshan, V. Embedded 3D Bioprinting of Gelatin Methacryloyl-Based Constructs with Highly Tunable Structural Fidelity. ACS Appl. Mater. Interfaces 2020, 12, 44563–44577. [Google Scholar] [CrossRef]
- Liu, W.; Heinrich, M.A.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A.; et al. Extrusion Bioprinting of Shear-Thinning Gelatin Methacryloyl Bioinks. Adv. Healthc. Mater. 2017, 6, 1601451. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015, 7, 035006. [Google Scholar] [CrossRef]
- Moncal, K.K.; Ozbolat, V.; Datta, P.; Heo, D.N.; Ozbolat, I.T. Thermally-controlled extrusion-based bioprinting of collagen. J. Mater. Sci. Mater. Med. 2019, 30, 55. [Google Scholar] [CrossRef]

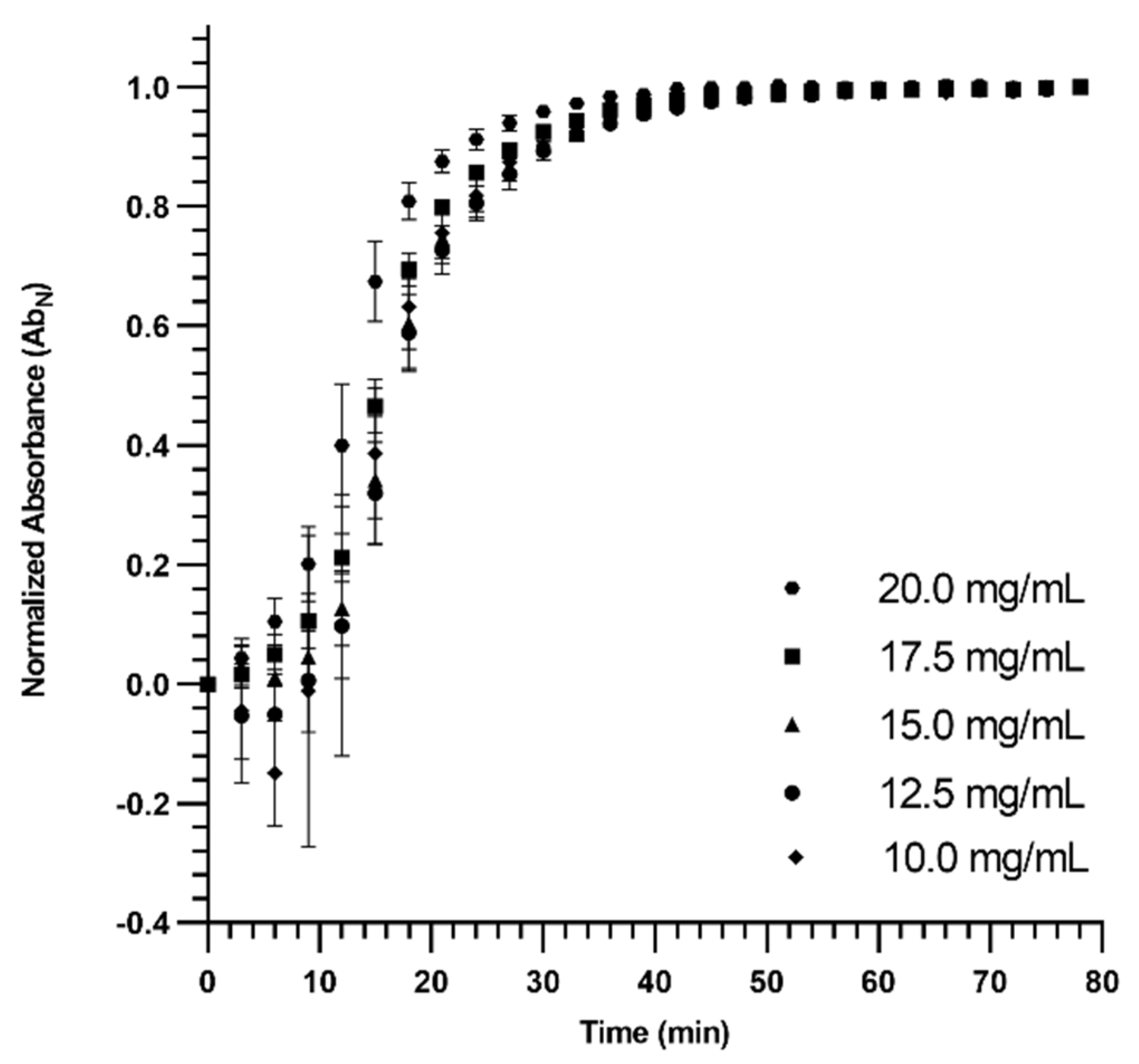

| Parameter | Slope | t1/2 | t95% | tlag | ||||
|---|---|---|---|---|---|---|---|---|
| Concentration | Average | ±SD | Average | ±SD | Average | ±SD | Average | ±SD |
| 10.0 mg/mL | 0.034 | 0.007 | 20.79 | 5.21 | 36.26 | 1.07 | 9.57 | 2.78 |
| 12.5 mg/mL | 0.034 | 0.002 | 19.83 | 1.42 | 38.22 | 2.01 | 9.98 | 3.56 |
| 15.0 mg/mL | 0.030 | 0.005 | 20.39 | 3.03 | 36.89 | 2.43 | 9.15 | 1.69 |
| 17.5 mg/mL | 0.035 | 0.003 | 17.14 | 0.62 | 33.53 | 2.20 | 6.10 | 2.24 |
| 20.0 mg/mL | 0.041 | 0.004 | 14.16 | 1.54 | 29.23 | 1.17 | 3.52 | 1.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabaghi, M.; Saraei, N.; Carpio, M.B.; Nanduri, V.; Ungureanu, J.; Babi, M.; Chandiramohan, A.; Noble, A.; Revill, S.D.; Zhang, B.; et al. A Robust Protocol for Decellularized Human Lung Bioink Generation Amenable to 2D and 3D Lung Cell Culture. Cells 2021, 10, 1538. https://doi.org/10.3390/cells10061538
Dabaghi M, Saraei N, Carpio MB, Nanduri V, Ungureanu J, Babi M, Chandiramohan A, Noble A, Revill SD, Zhang B, et al. A Robust Protocol for Decellularized Human Lung Bioink Generation Amenable to 2D and 3D Lung Cell Culture. Cells. 2021; 10(6):1538. https://doi.org/10.3390/cells10061538
Chicago/Turabian StyleDabaghi, Mohammadhossein, Neda Saraei, Mabel Barreiro Carpio, Vibudha Nanduri, Julia Ungureanu, Mouhanad Babi, Abiram Chandiramohan, Alexander Noble, Spencer D. Revill, Boyang Zhang, and et al. 2021. "A Robust Protocol for Decellularized Human Lung Bioink Generation Amenable to 2D and 3D Lung Cell Culture" Cells 10, no. 6: 1538. https://doi.org/10.3390/cells10061538
APA StyleDabaghi, M., Saraei, N., Carpio, M. B., Nanduri, V., Ungureanu, J., Babi, M., Chandiramohan, A., Noble, A., Revill, S. D., Zhang, B., Ask, K., Kolb, M., Shargall, Y., Moran-Mirabal, J., & Hirota, J. A. (2021). A Robust Protocol for Decellularized Human Lung Bioink Generation Amenable to 2D and 3D Lung Cell Culture. Cells, 10(6), 1538. https://doi.org/10.3390/cells10061538






