Retinal Ganglion Cell Transplantation: Approaches for Overcoming Challenges to Functional Integration
Abstract
1. Introduction
2. RGC Sources for Transplantation
3. Transplanted RGC Survival
3.1. Assessing Donor RGC Survival
- When numbers of surviving donor neurons are low, quantification is ideally performed on retinal flatmounts such that the entire retinal surface area is examined. Histological sections are prone to sampling errors unless graft neurons are evenly spaced. Tiled microscopy of the entire host retinal surface should be imaged at high resolution;
- If donor cells express a fluorescent marker that is cytoplasmic and contained within neurites, colocalization with a secondary nuclear marker is recommended in order to distinguish overlapping donor cells. If human cells are transplanted into nonhuman recipients, antibodies directed against human nuclear antigen may be suitable;
- If donor cells fail to disperse after transplantation, the resulting cell clump is best imaged as a confocal z-stack in order to parse overlapping cells.
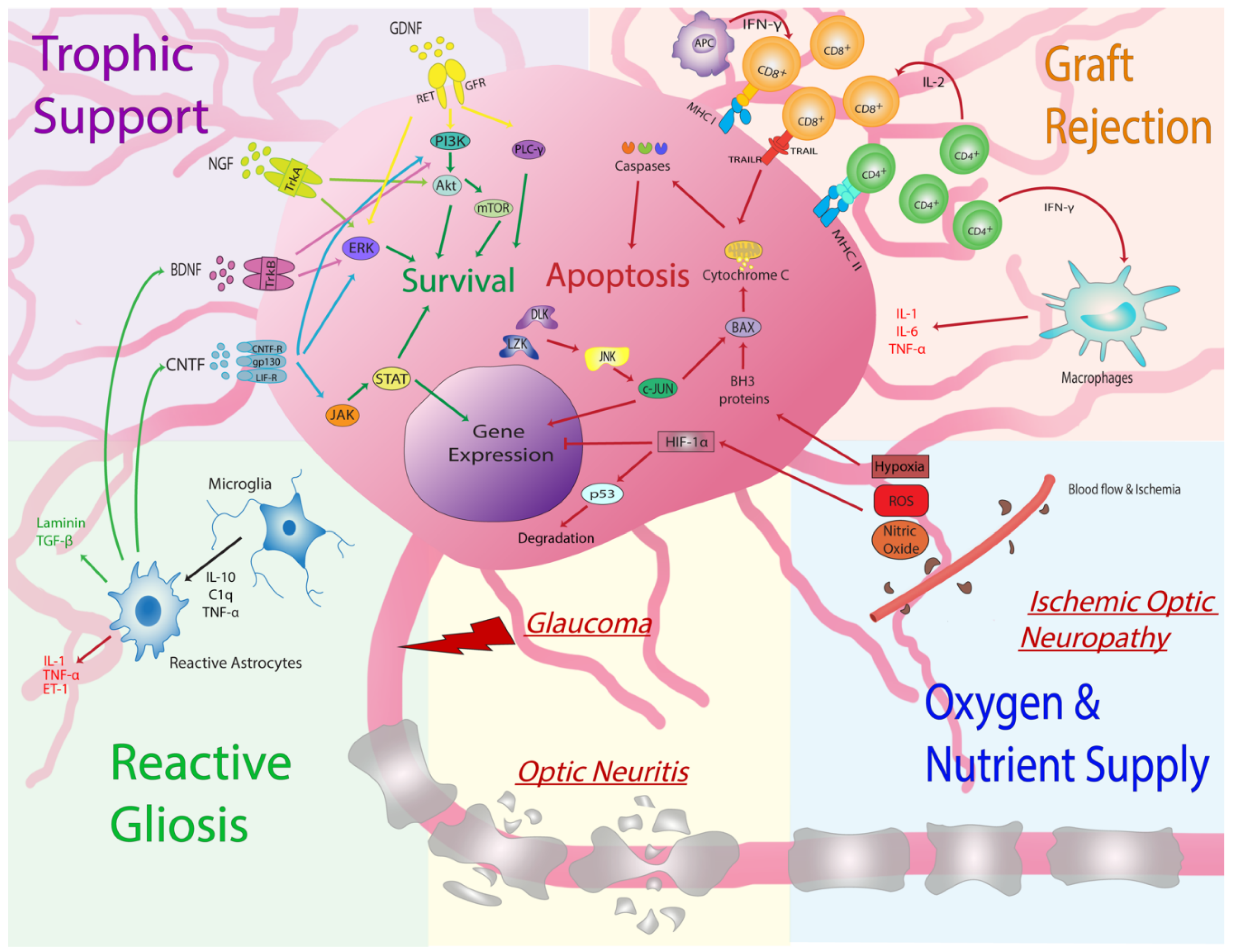
3.2. Increasing Donor RGC Survival: Cell Intrinsic Signaling Pathways
3.3. Increasing Donor RGC Survival: Cell Extrinsic Environmental Factors
4. Transplanted RGC Migration and Somal Integration
4.1. Assessing Donor RGC Laminar Localization
- A combination of analyses using histological sections and en face evaluation of retinal flatmounts will provide a compromise between complete topographic imaging and depth resolution;
- Flatmount microscopy should be performed with high resolution confocal or multiphoton microscopy using a high magnification objective in order to create shallow depth of field;
- High depth resolution is attained by minimizing slice interval distance and pinhole aperture diameter (if single photon microscopy is employed);
- Z-stack reconstructions should be assessed using an orthogonal slice viewer to compare the localization of the donor cell to those of nearby endogenous cells;
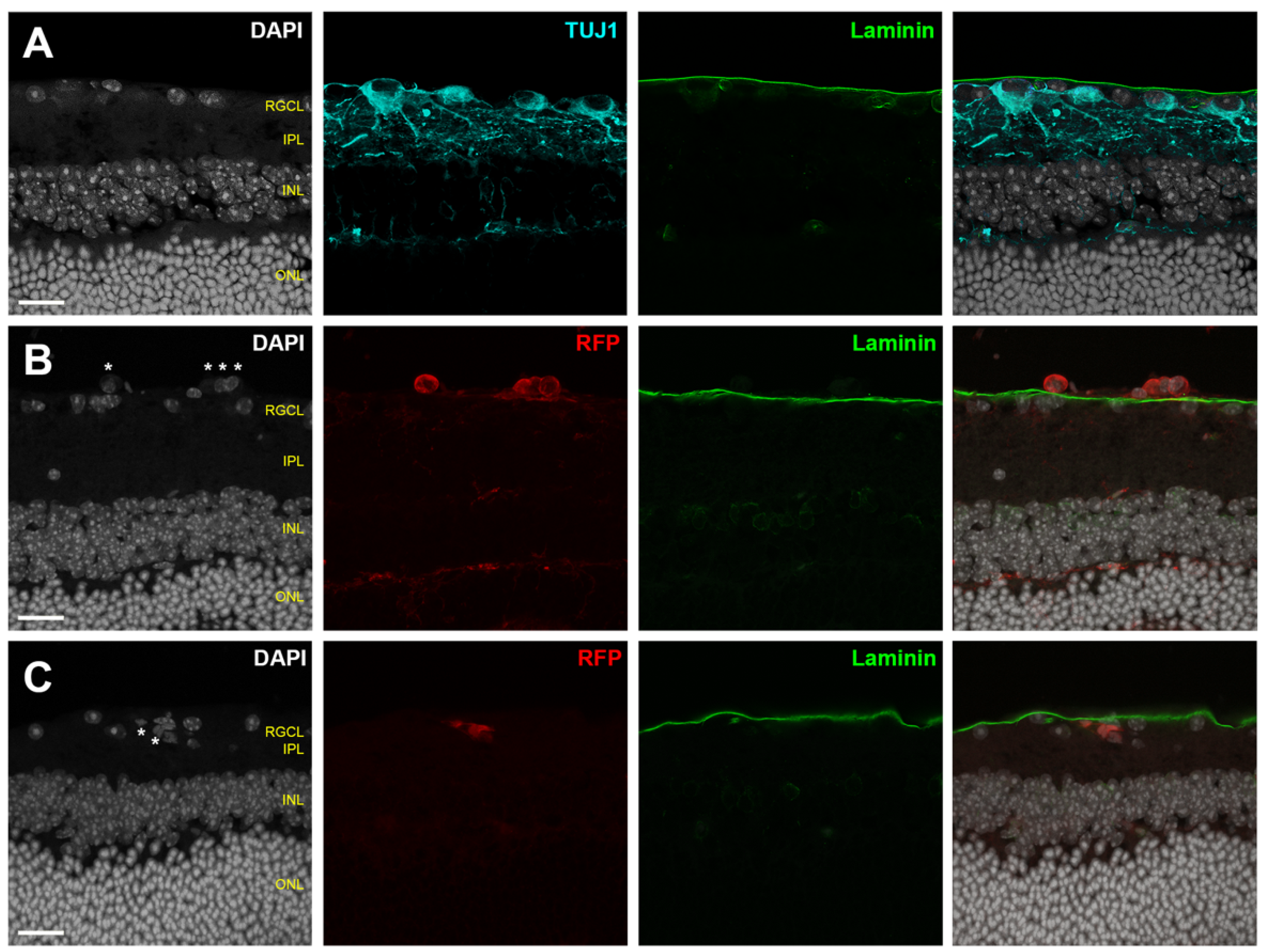
- A secondary marker that outlines the boundary of the retinal parenchyma should be included when possible, such as immunofluorescent laminin delineation of the ILM (Figure 3).
4.2. Assessing Donor RGC Retinotopic Mosaicism
5. Transplanted RGC Dendritogenesis and Afferent Synaptogenesis
5.1. Assessing Donor RGC Neurite Localization
- Retinal flatmounts are superior to histological section for characterizing complex dendritic arbors of RGCs within the IPL;
- Additional secondary tissue samples for histological sectioning can, however, be useful for correlation of sublaminar neurite localization with multiple immunohistochemical markers that define RGC subtype;
- It is of interest to characterize the topographical spacing among integrated RGCs. Reported metrics may include nearest neighbor and density recovery profiles of integrated donor RGCs;
- When possible, metrics describing dendritic architecture should be described for individual cells; if individual arbors are not resolvable from overlapping RGCs, then metrics should be normalized to the number of donor RGCs contained within a region of interest;
- Dendritic architecture and localization within the recipient retina should be assessed using high resolution microscopy; it may be necessary to tile images in order to capture entire dendritic arbors at high resolution;
- Integrated dendritic arbors can be convoluted and should be resolved and traced using 3D rendering software (Figure 5);
- Useful metrics to describe dendritic arbors include total neurite length, number of neurite segments, neurite density, dendritic Sholl analysis [167], and neurite distribution in each retinal layer, and where applicable, within specific IPL sublamina.
5.2. Assessing Donor RGC Functional Connectivity within the Recipient Retinal Neurocircuitry
6. Material Transfer
7. Safety Considerations
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wohl, S.G.; Schmeer, C.W.; Isenmann, S. Neurogenic potential of stem/progenitor-like cells in the adult mammalian eye. Prog. Retin. Eye Res. 2012, 31, 213–242. [Google Scholar] [CrossRef]
- Quigley, H.A. Open-Angle Glaucoma. N. Engl. J. Med. 1993, 328, 1097–1106. [Google Scholar] [CrossRef]
- Maier, P.C.; Funk, J.; Schwarzer, G.; Antes, G.; Falck-Ytter, Y.T. Treatment of ocular hypertension and open angle glaucoma: Meta-analysis of randomised controlled trials. BMJ 2005, 331, 134. [Google Scholar] [CrossRef]
- Sleath, B.; Robin, A.L.; Covert, D.; Byrd, J.E.; Tudor, G.; Svarstad, B. Patient-Reported Behavior and Problems in Using Glaucoma Medications. Ophthalmology 2006, 113, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.F.; Quigley, H.A. Adherence and Persistence with Glaucoma Therapy. Surv. Ophthalmol. 2008, 53, S57–S68. [Google Scholar] [CrossRef]
- Yook, E.; Vinod, K.; Panarelli, J.F. Complications of micro-invasive glaucoma surgery. Curr. Opin. Ophthalmol. 2018, 29, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A. Reduction of Intraocular Pressure and Glaucoma Progression. Arch. Ophthalmol. 2002, 120, 1268. [Google Scholar] [CrossRef]
- Khatib, T.Z.; Martin, K.R. Neuroprotection in Glaucoma: Towards Clinical Trials and Precision Medicine. Curr. Eye Res. 2020, 45, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Welsbie, D.S.; Yang, Z.; Ge, Y.; Mitchell, K.L.; Zhou, X.; Martin, S.E.; Berlinicke, C.A.; Hackler, L.; Fuller, J.; Fu, J.; et al. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc. Natl. Acad. Sci. USA 2013, 110, 4045–4050. [Google Scholar] [CrossRef]
- Laha, B.; Stafford, B.K.; Huberman, A.D. Regenerating optic pathways from the eye to the brain. Science 2017, 356, 1031–1034. [Google Scholar] [CrossRef]
- MacLaren, R.E.; Pearson, R.A.; MacNeil, A.; Douglas, R.H.; Salt, T.E.; Akimoto, M.; Swaroop, A.; Sowden, J.C.; Ali, R.R. Retinal repair by transplantation of photoreceptor precursors. Nature 2006, 444, 203–207. [Google Scholar] [CrossRef]
- Singh, M.S.; Charbel Issa, P.; Butler, R.; Martin, C.; Lipinski, D.M.; Sekaran, S.; Barnard, A.R.; MacLaren, R.E. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc. Natl. Acad. Sci. USA 2013, 110, 1101–1106. [Google Scholar] [CrossRef]
- Zarbin, M.; Sugino, I.; Townes-Anderson, E. Concise Review: Update on Retinal Pigment Epithelium Transplantation for Age-Related Macular Degeneration. Stem Cells Transl. Med. 2019, 8, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Hubschman, J.-P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012, 379, 713–720. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Gu, P. Stem/progenitor cell-based transplantation for retinal degeneration: A review of clinical trials. Cell Death Dis. 2020, 11, 793. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Tuffy, C.; Mertz, J.L.; Quillen, S.; Wechsler, L.; Quigley, H.A.; Zack, D.J.; Johnson, T.V. Role of the Internal Limiting Membrane in Structural Engraftment and Topographic Spacing of Transplanted Human Stem Cell-Derived Retinal Ganglion Cells. Stem Cell Rep. 2021, 16, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Sanes, J.R.; Masland, R.H. The Types of Retinal Ganglion Cells: Current Status and Implications for Neuronal Classification. Annu. Rev. Neurosci. 2015, 38, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.R.; Benowitz, L.I.; Goldberg, J.L.; He, Z. Axon Regeneration in the Mammalian Optic Nerve. Annu. Rev. Vis. Sci. 2020, 6, 195–213. [Google Scholar] [CrossRef]
- Duan, X.; Qiao, M.; Bei, F.; Kim, I.-J.; He, Z.; Sanes, J.R. Subtype-Specific Regeneration of Retinal Ganglion Cells following Axotomy: Effects of Osteopontin and mTOR Signaling. Neuron 2015, 85, 1244–1256. [Google Scholar] [CrossRef]
- Leibinger, M.; Andreadaki, A.; Diekmann, H.; Fischer, D. Neuronal STAT3 activation is essential for CNTF- and inflammatory stimulation-induced CNS axon regeneration. Cell Death Dis. 2013, 4, e805. [Google Scholar] [CrossRef]
- Agostinone, J.; Alarcon-Martinez, L.; Gamlin, C.; Yu, W.-Q.; Wong, R.O.L.; Di Polo, A. Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury. Brain 2018, 141, 1963–1980. [Google Scholar] [CrossRef]
- Zhang, Y.; Williams, P.R.; Jacobi, A.; Wang, C.; Goel, A.; Hirano, A.A.; Brecha, N.C.; Kerschensteiner, D.; He, Z. Elevating Growth Factor Responsiveness and Axon Regeneration by Modulating Presynaptic Inputs. Neuron 2019, 103, 39–51.e5. [Google Scholar] [CrossRef]
- Bray, E.R.; Yungher, B.J.; Levay, K.; Ribeiro, M.; Dvoryanchikov, G.; Ayupe, A.C.; Thakor, K.; Marks, V.; Randolph, M.; Danzi, M.C.; et al. Thrombospondin-1 Mediates Axon Regeneration in Retinal Ganglion Cells. Neuron 2019, 103, 642–657.e7. [Google Scholar] [CrossRef]
- Park, K.K.; Liu, K.; Hu, Y.; Smith, P.D.; Wang, C.; Cai, B.; Xu, B.; Connolly, L.; Kramvis, I.; Sahin, M.; et al. Promoting Axon Regeneration in the Adult CNS by Modulation of the PTEN/mTOR Pathway. Science 2008, 322, 963–966. [Google Scholar] [CrossRef]
- Smith, P.D.; Sun, F.; Park, K.K.; Cai, B.; Wang, C.; Kuwako, K.; Martinez-Carrasco, I.; Connolly, L.; He, Z. SOCS3 Deletion Promotes Optic Nerve Regeneration In Vivo. Neuron 2009, 64, 617–623. [Google Scholar] [CrossRef]
- Sun, F.; Park, K.K.; Belin, S.; Wang, D.; Lu, T.; Chen, G.; Zhang, K.; Yeung, C.; Feng, G.; Yankner, B.A.; et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 2011, 480, 372–375. [Google Scholar] [CrossRef]
- Moore, D.L.; Blackmore, M.G.; Hu, Y.; Kaestner, K.H.; Bixby, J.L.; Lemmon, V.P.; Goldberg, J.L. KLF Family Members Regulate Intrinsic Axon Regeneration Ability. Science 2009, 326, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Lindborg, J.A.; Tran, N.M.; Chenette, D.M.; DeLuca, K.; Foli, Y.; Kannan, R.; Sekine, Y.; Wang, X.; Wollan, M.; Kim, I.-J.; et al. Optic nerve regeneration screen identifies multiple genes restricting adult neural repair. Cell Rep. 2021, 34, 108777. [Google Scholar] [CrossRef]
- Yin, Y.; Henzl, M.T.; Lorber, B.; Nakazawa, T.; Thomas, T.T.; Jiang, F.; Langer, R.; Benowitz, L.I. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat. Neurosci. 2006, 9, 843–852. [Google Scholar] [CrossRef]
- Wang, J.; He, X.; Meng, H.; Li, Y.; Dmitriev, P.; Tian, F.; Page, J.C.; Lu, Q.R.; He, Z. Robust Myelination of Regenerated Axons Induced by Combined Manipulations of GPR17 and Microglia. Neuron 2020, 108, 876–886.e4. [Google Scholar] [CrossRef] [PubMed]
- de Lima, S.; Koriyama, Y.; Kurimoto, T.; Oliveira, J.T.; Yin, Y.; Li, Y.; Gilbert, H.-Y.; Fagiolini, M.; Martinez, A.M.B.; Benowitz, L. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc. Natl. Acad. Sci. USA 2012, 109, 9149–9154. [Google Scholar] [CrossRef]
- Venugopalan, P.; Wang, Y.; Nguyen, T.; Huang, A.; Muller, K.J.; Goldberg, J.L. Transplanted neurons integrate into adult retinas and respond to light. Nat. Commun. 2016, 7, 10472. [Google Scholar] [CrossRef]
- Divya, M.S.; Rasheed, V.A.; Schmidt, T.; Lalitha, S.; Hattar, S.; James, J. Intraocular Injection of ES Cell-Derived Neural Progenitors Improve Visual Function in Retinal Ganglion Cell-Depleted Mouse Models. Front. Cell. Neurosci. 2017, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Rabesandratana, O.; Chaffiol, A.; Mialot, A.; Slembrouck-Brec, A.; Joffrois, C.; Nanteau, C.; Rodrigues, A.; Gagliardi, G.; Reichman, S.; Sahel, J.-A.; et al. Generation of a Transplantable Population of Human iPSC-Derived Retinal Ganglion Cells. Front. Cell Dev. Biol. 2020, 8, 585675. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.-L.; Tang, S.-B. Differentiation of retinal ganglion cells from induced pluripotent stem cells: A review. Int. J. Ophthalmol. 2019, 12, 152–160. [Google Scholar] [CrossRef]
- Sluch, V.M.; Davis, C.O.; Ranganathan, V.; Kerr, J.M.; Krick, K.; Martin, R.; Berlinicke, C.A.; Marsh-Armstrong, N.; Diamond, J.S.; Mao, H.-Q.; et al. Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Sci. Rep. 2015, 5, 16595. [Google Scholar] [CrossRef]
- Tanaka, T.; Yokoi, T.; Tamalu, F.; Watanabe, S.-I.; Nishina, S.; Azuma, N. Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Sci. Rep. 2015, 5, 8344. [Google Scholar] [CrossRef]
- Klimanskaya, I.; Chung, Y.; Becker, S.; Lu, S.-J.; Lanza, R. Human embryonic stem cell lines derived from single blastomeres. Nature 2006, 444, 481–485. [Google Scholar] [CrossRef]
- Lamba, D.A.; Karl, M.O.; Ware, C.B.; Reh, T.A. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 12769–12774. [Google Scholar] [CrossRef]
- Mu, X.; Klein, W.H. A gene regulatory hierarchy for retinal ganglion cell specification and differentiation. Semin. Cell Dev. Biol. 2004, 15, 115–123. [Google Scholar] [CrossRef]
- Meyer-Franke, A.; Kaplan, M.R.; Pfieger, F.W.; Barres, B.A. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron 1995, 15, 805–819. [Google Scholar] [CrossRef]
- Ouchi, Y. Negative regulation of retinal-neurite extension by -catenin signaling pathway. J. Cell Sci. 2005, 118, 4473–4483. [Google Scholar] [CrossRef]
- Louvi, A.; Artavanis-Tsakonas, S. Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 2006, 7, 93–102. [Google Scholar] [CrossRef]
- Nelson, B.R.; Gumuscu, B.; Hartman, B.H.; Reh, T.A. Notch Activity Is Downregulated Just prior to Retinal Ganglion Cell Differentiation. Dev. Neurosci. 2006, 28, 128–141. [Google Scholar] [CrossRef]
- Barnstable, C.J.; Dräger, U.C. Thy-1 antigen: A ganglion cell specific marker in rodent retina. Neuroscience 1984, 11, 847–855. [Google Scholar] [CrossRef]
- Pan, L.; Deng, M.; Xie, X.; Gan, L. ISL1 and BRN3B co-regulate the differentiation of murine retinal ganglion cells. Development 2008, 135, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Surgucheva, I.; Weisman, A.D.; Goldberg, J.L.; Shnyra, A.; Surguchov, A. Gamma-synuclein as a marker of retinal ganglion cells. Mol. Vis. 2008, 14, 1540–1548. [Google Scholar] [PubMed]
- Gill, K.P.; Hung, S.S.C.; Sharov, A.; Lo, C.Y.; Needham, K.; Lidgerwood, G.E.; Jackson, S.; Crombie, D.E.; Nayagam, B.A.; Cook, A.L.; et al. Enriched retinal ganglion cells derived from human embryonic stem cells. Sci. Rep. 2016, 6, 30552. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Ohlemacher, S.K.; Sridhar, A.; Xiao, Y.; Hochstetler, A.E.; Sarfarazi, M.; Cummins, T.R.; Meyer, J.S. Stepwise Differentiation of Retinal Ganglion Cells from Human Pluripotent Stem Cells Enables Analysis of Glaucomatous Neurodegeneration. Stem Cells 2016, 34, 1553–1562. [Google Scholar] [CrossRef]
- Teotia, P.; Chopra, D.A.; Dravid, S.M.; Van Hook, M.J.; Qiu, F.; Morrison, J.; Rizzino, A.; Ahmad, I. Generation of Functional Human Retinal Ganglion Cells with Target Specificity from Pluripotent Stem Cells by Chemically Defined Recapitulation of Developmental Mechanism. Stem Cells 2017, 35, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Chavali, V.R.M.; Haider, N.; Rathi, S.; Vrathasha, V.; Alapati, T.; He, J.; Gill, K.; Nikonov, R.; Duong, T.T.; McDougald, D.S.; et al. Dual SMAD inhibition and Wnt inhibition enable efficient and reproducible differentiations of induced pluripotent stem cells into retinal ganglion cells. Sci. Rep. 2020, 10, 11828. [Google Scholar] [CrossRef]
- Chen, M.; Chen, Q.; Sun, X.; Shen, W.; Liu, B.; Zhong, X.; Leng, Y.; Li, C.; Zhang, W.; Chai, F.; et al. Generation of Retinal Ganglion–like Cells from Reprogrammed Mouse Fibroblasts. Investig. Opthalmology Vis. Sci. 2010, 51, 5970. [Google Scholar] [CrossRef]
- Riazifar, H.; Jia, Y.; Chen, J.; Lynch, G.; Huang, T. Chemically Induced Specification of Retinal Ganglion Cells From Human Embryonic and Induced Pluripotent Stem Cells. Stem Cells Transl. Med. 2014, 3, 424–432. [Google Scholar] [CrossRef]
- Sasai, Y. Next-Generation Regenerative Medicine: Organogenesis from Stem Cells in 3D Culture. Cell Stem Cell 2013, 12, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.J.; El-Hodiri, H.; Zhang, L.; Shah, R.; Mathers, E.H.; Jamrich, M. Regulation of vertebrate eye development by Rx genes. Int. J. Dev. Biol. 2004, 48, 761–770. [Google Scholar] [CrossRef]
- Zhong, X.; Gutierrez, C.; Xue, T.; Hampton, C.; Vergara, M.N.; Cao, L.-H.; Peters, A.; Park, T.S.; Zambidis, E.T.; Meyer, J.S.; et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 2014, 5, 4047. [Google Scholar] [CrossRef]
- Xiao, D.; Deng, Q.; Guo, Y.; Huang, X.; Zou, M.; Zhong, J.; Rao, P.; Xu, Z.; Liu, Y.; Hu, Y.; et al. Generation of self-organized sensory ganglion organoids and retinal ganglion cells from fibroblasts. Sci. Adv. 2020, 6, eaaz5858. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Hoshino, A.; Finkbeiner, C.R.; Chitsazan, A.; Dai, L.; Haugan, A.K.; Eschenbacher, K.M.; Jackson, D.L.; Trapnell, C.; Bermingham-McDonogh, O.; et al. Single-Cell Transcriptomic Comparison of Human Fetal Retina, hPSC-Derived Retinal Organoids, and Long-Term Retinal Cultures. Cell Rep. 2020, 30, 1644–1659.e4. [Google Scholar] [CrossRef] [PubMed]
- Langer, K.B.; Ohlemacher, S.K.; Phillips, M.J.; Fligor, C.M.; Jiang, P.; Gamm, D.M.; Meyer, J.S. Retinal Ganglion Cell Diversity and Subtype Specification from Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 1282–1293. [Google Scholar] [CrossRef]
- Oswald, J.; Kegeles, E.; Minelli, T.; Volchkov, P.; Baranov, P. Transplantation of miPSC/mESC-derived retinal ganglion cells into healthy and glaucomatous retinas. Mol. Ther. Methods Clin. Dev. 2021, 21, 180–198. [Google Scholar] [CrossRef]
- Singh, M.S.; Park, S.S.; Albini, T.A.; Canto-Soler, M.V.; Klassen, H.; MacLaren, R.E.; Takahashi, M.; Nagiel, A.; Schwartz, S.D.; Bharti, K. Retinal stem cell transplantation: Balancing safety and potential. Prog. Retin. Eye Res. 2020, 75, 100779. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.V.; Martin, K.R. Development and Characterization of an Adult Retinal Explant Organotypic Tissue Culture System as an In Vitro Intraocular Stem Cell Transplantation Model. Investig. Opthalmology Vis. Sci. 2008, 49, 3503. [Google Scholar] [CrossRef] [PubMed]
- Danias, J.; Lee, K.C.; Zamora, M.-F.; Chen, B.; Shen, F.; Filippopoulos, T.; Su, Y.; Goldblum, D.; Podos, S.M.; Mittag, T. Quantitative Analysis of Retinal Ganglion Cell (RGC) Loss in Aging DBA/2NNia Glaucomatous Mice: Comparison with RGC Loss in Aging C57/BL6 Mice. Investig. Opthalmology Vis. Sci. 2003, 44, 5151. [Google Scholar] [CrossRef] [PubMed]
- García-Ayuso, D.; Salinas-Navarro, M.; Agudo, M.; Cuenca, N.; Pinilla, I.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Retinal ganglion cell numbers and delayed retinal ganglion cell death in the P23H rat retina. Exp. Eye Res. 2010, 91, 800–810. [Google Scholar] [CrossRef]
- Kerrigan-Baumrind, L.A.; Quigley, H.A.; Pease, M.E.; Kerrigan, D.F.; Mitchell, R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest. Ophthalmol. Vis. Sci. 2000, 41, 741–748. [Google Scholar]
- Medeiros, F.A.; Lisboa, R.; Weinreb, R.N.; Liebmann, J.M.; Girkin, C.; Zangwill, L.M. Retinal ganglion cell count estimates associated with early development of visual field defects in glaucoma. Ophthalmology 2013, 120, 736–744. [Google Scholar] [CrossRef]
- Kerrison, J.B.; Buchanan, K.; Rosenberg, M.L.; Clark, R.; Andreason, K.; Alfaro, D.V.; Grossniklaus, H.E.; Kerrigan-Baumrind, L.A.; Kerrigan, D.F.; Miller, N.R.; et al. Quantification of optic nerve axon loss associated with a relative afferent pupillary defect in the monkey. Arch. Ophthalmol. 2001, 119, 1333–1341. [Google Scholar] [CrossRef][Green Version]
- Chao, J.R.; Lamba, D.A.; Klesert, T.R.; Torre, A.L.; Hoshino, A.; Taylor, R.J.; Jayabalu, A.; Engel, A.L.; Khuu, T.H.; Wang, R.K.; et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Cells into the Subretinal Space of a Non-Human Primate. Transl. Vis. Sci. Technol. 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tenerelli, K.; Wu, S.; Xia, X.; Yokota, S.; Sun, C.; Galvao, J.; Venugopalan, P.; Li, C.; Madaan, A.; et al. Cell transplantation of retinal ganglion cells derived from hESCs. Restor. Neurol. Neurosci. 2020, 38, 131–140. [Google Scholar] [CrossRef]
- Wang, S.-T.; Chen, L.; Zhang, P.; Wang, X.-B.; Sun, Y.; Ma, L.-X.; Liu, Q.; Zhou, G.-M. Transplantation of Retinal Progenitor Cells from Optic Cup-Like Structures Differentiated from Human Embryonic Stem Cells In Vitro and In Vivo Generation of Retinal Ganglion-Like Cells. Stem Cells Dev. 2019, 28, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhong, X.; Yang, S.; Luo, Z.; Li, K.; Liu, Y.; Cai, S.; Gu, H.; Lu, S.; Zhang, H.; et al. HiPSC-derived retinal ganglion cells grow dendritic arbors and functional axons on a tissue-engineered scaffold. Acta Biomater. 2017, 54, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Suen, H.C.; Qian, Y.; Liao, J.; Luk, C.S.; Lee, W.T.; Ng, J.K.W.; Chan, T.T.H.; Hou, H.W.; Li, I.; Li, K.; et al. Transplantation of Retinal Ganglion Cells Derived from Male Germline Stem Cell as a Potential Treatment to Glaucoma. Stem Cells Dev. 2019, 28, 1365–1375. [Google Scholar] [CrossRef]
- Hertz, J.; Qu, B.; Hu, Y.; Patel, R.D.; Valenzuela, D.A.; Goldberg, J.L. Survival and Integration of Developing and Progenitor-Derived Retinal Ganglion Cells following Transplantation. Cell Transplant. 2014, 23, 855–872. [Google Scholar] [CrossRef]
- Wu, S.; Chang, K.-C.; Nahmou, M.; Goldberg, J.L. Induced Pluripotent Stem Cells Promote Retinal Ganglion Cell Survival After Transplant. Investig. Opthalmology Vis. Sci. 2018, 59, 1571. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, T.M.; Astle, D. Photoreceptor number and outer segment disk membrane surface area in the retina of the rat: Stereological data for whole organ and average photoreceptor cell. J. Neurocytol. 1997, 26, 53–61. [Google Scholar] [CrossRef]
- Morquette, B.; Morquette, P.; Agostinone, J.; Feinstein, E.; McKinney, R.A.; Kolta, A.; Di Polo, A. REDD2-mediated inhibition of mTOR promotes dendrite retraction induced by axonal injury. Cell Death Differ. 2015, 22, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Teotia, P.; Van Hook, M.J.; Fischer, D.; Ahmad, I. Human retinal ganglion cell axon regeneration by recapitulating developmental mechanisms: Effects of recruitment of the mTOR pathway. Development 2019, 146, dev178012. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.; Clark, A.; McDowell, C. Subtype-specific response of retinal ganglion cells to optic nerve crush. Cell Death Discov. 2018, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Sarbassov, D.D.; Ali, S.M.; Latek, R.R.; Guntur, K.V.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. GβL, a Positive Regulator of the Rapamycin-Sensitive Pathway Required for the Nutrient-Sensitive Interaction between Raptor and mTOR. Mol. Cell 2003, 11, 895–904. [Google Scholar] [CrossRef]
- Chen, A.; Xiong, L.-J.; Tong, Y.; Mao, M. Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol. Med. Rep. 2013, 8, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and apoptosis: Size matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, Y.; Hayat, U.; Li, S. PTEN inhibition and axon regeneration and neural repair. Neural Regen. Res. 2015, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.A.; Harder, J.M.; John, S.W.; Shrager, P.; Libby, R.T. DLK-dependent signaling is important for somal but not axonal degeneration of retinal ganglion cells following axonal injury. Neurobiol. Dis. 2014, 69, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Nimnual, A.; Zong, W.-X.; Kennedy, N.J.; Flavell, R.A.; Thompson, C.B.; Bar-Sagi, D.; Davis, R.J. The Bax Subfamily of Bcl2-Related Proteins Is Essential for Apoptotic Signal Transduction by c-Jun NH2-Terminal Kinase. Mol. Cell. Biol. 2002, 22, 4929–4942. [Google Scholar] [CrossRef]
- Donahue, R.J.; Maes, M.E.; Grosser, J.A.; Nickells, R.W. BAX-Depleted Retinal Ganglion Cells Survive and Become Quiescent Following Optic Nerve Damage. Mol. Neurobiol. 2020, 57, 1070–1084. [Google Scholar] [CrossRef]
- Watkins, T.A.; Wang, B.; Huntwork-Rodriguez, S.; Yang, J.; Jiang, Z.; Eastham-Anderson, J.; Modrusan, Z.; Kaminker, J.S.; Tessier-Lavigne, M.; Lewcock, J.W. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc. Natl. Acad. Sci. USA 2013, 110, 4039–4044. [Google Scholar] [CrossRef]
- Sluch, V.M.; Chamling, X.; Liu, M.M.; Berlinicke, C.A.; Cheng, J.; Mitchell, K.L.; Welsbie, D.S.; Zack, D.J. Enhanced Stem Cell Differentiation and Immunopurification of Genome Engineered Human Retinal Ganglion Cells. Stem Cells Transl. Med. 2017, 6, 1972–1986. [Google Scholar] [CrossRef]
- Patel, A.K.; Broyer, R.M.; Lee, C.D.; Lu, T.; Louie, M.J.; La Torre, A.; Al-Ali, H.; Vu, M.T.; Mitchell, K.L.; Wahlin, K.J.; et al. Inhibition of GCK-IV kinases dissociates cell death and axon regeneration in CNS neurons. Proc. Natl. Acad. Sci. USA 2020, 117, 33597–33607. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Aquila, S.; Santoro, M.; Caputo, A.; Panno, M.L.; Pezzi, V.; De Amicis, F. The Tumor Suppressor PTEN as Molecular Switch Node Regulating Cell Metabolism and Autophagy: Implications in Immune System and Tumor Microenvironment. Cells 2020, 9, 1725. [Google Scholar] [CrossRef]
- Kimura, A.; Namekata, K.; Guo, X.; Harada, C.; Harada, T. Neuroprotection, Growth Factors and BDNF-TrkB Signalling in Retinal Degeneration. Int. J. Mol. Sci. 2016, 17, 1584. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Colafrancesco, V.; Parisi, V.; Sposato, V.; Rossi, S.; Russo, M.A.; Coassin, M.; Lambiase, A.; Aloe, L. Ocular Application of Nerve Growth Factor Protects Degenerating Retinal Ganglion Cells in a Rat Model of Glaucoma. J. Glaucoma 2011, 20, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Birman, E.; Saragovi, H.U. Neurotrophic rationale in glaucoma: A TrkA agonist, but not NGF or a p75 antagonist, protects retinal ganglion cellsin vivo. Dev. Neurobiol. 2007, 67, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, T.; Tanaka, T.; Matsuzaka, E.; Tamalu, F.; Watanabe, S.-I.; Nishina, S.; Azuma, N. Effects of neuroactive agents on axonal growth and pathfinding of retinal ganglion cells generated from human stem cells. Sci. Rep. 2017, 7, 16757. [Google Scholar] [CrossRef]
- Slack, S.E.; Grist, J.; Mac, Q.; McMahon, S.B.; Pezet, S. TrkB expression and phospho-ERK activation by brain-derived neurotrophic factor in rat spinothalamic tract neurons. J. Comp. Neurol. 2005, 489, 59–68. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, P.S.; Friedman, B.; Radziejewski, C.; Alexander, C.; Boland, P.; Schick, C.M.; Lindsay, R.M.; Wiegand, S.J. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron 1992, 8, 983–993. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood–brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Shiozawa, A.L.; Igarashi, T.; Kobayashi, M.; Nakamoto, K.; Kameya, S.; Fujishita, S.; Takahashi, H.; Okada, T. Tyrosine triple mutated AAV2-BDNF gene therapy in an inner retinal injury model induced by intravitreal injection of N-methyl-D-aspartate (NMDA). Mol. Vis. 2020, 26, 409–422. [Google Scholar] [PubMed]
- Osborne, A.; Khatib, T.Z.; Songra, L.; Barber, A.C.; Hall, K.; Kong, G.Y.X.; Widdowson, P.S.; Martin, K.R. Neuroprotection of retinal ganglion cells by a novel gene therapy construct that achieves sustained enhancement of brain-derived neurotrophic factor/tropomyosin-related kinase receptor-B signaling. Cell Death Dis. 2018, 9, 1007. [Google Scholar] [CrossRef]
- Khatib, T.Z.; Osborne, A.; Yang, S.; Ali, Z.; Jia, W.; Manyakin, I.; Hall, K.; Watt, R.; Widdowson, P.S.; Martin, K.R. Receptor-ligand supplementation via a self-cleaving 2A peptide–based gene therapy promotes CNS axonal transport with functional recovery. Sci. Adv. 2021, 7, eabd2590. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.M.; Grozdanic, S.D.; Blits, B.; Kuehn, M.H.; Zamzow, D.; Buss, J.E.; Kardon, R.H.; Sakaguchi, D.S. Transplantation of BDNF-Secreting Mesenchymal Stem Cells Provides Neuroprotection in Chronically Hypertensive Rat Eyes. Investig. Opthalmology Vis. Sci. 2011, 52, 4506. [Google Scholar] [CrossRef]
- Harada, T.; Harada, C.; Kohsaka, S.; Wada, E.; Yoshida, K.; Ohno, S.; Mamada, H.; Tanaka, K.; Parada, L.F.; Wada, K. Microglia–Müller Glia Cell Interactions Control Neurotrophic Factor Production during Light-Induced Retinal Degeneration. J. Neurosci. 2002, 22, 9228–9236. [Google Scholar] [CrossRef]
- Harada, C.; Harada, T.; Quah, H.-M.; Maekawa, F.; Yoshida, K.; Ohno, S.; Wada, K.; Parada, L..; Tanaka, K. Potential role of glial cell line-derived neurotrophic factor receptors in Müller glial cells during light-induced retinal degeneration. Neuroscience 2003, 122, 229–235. [Google Scholar] [CrossRef]
- Hauck, S.M.; Kinkl, N.; Deeg, C.A.; Swiatek-de Lange, M.; Schöffmann, S.; Ueffing, M. GDNF family ligands trigger indirect neuroprotective signaling in retinal glial cells. Mol. Cell. Biol. 2006, 26, 2746–2757. [Google Scholar] [CrossRef] [PubMed]
- Stahl, N.; Yancopoulos, G.D. The tripartite CNTF receptor complex: Activation and signaling involves components shared with other cytokines. J. Neurobiol. 1994, 25, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.-P.; Liang, J.-J.; Chen, J.; Harvey, A.R.; Ng, T.K.; Zhang, M.; Pang, C.P.; Cui, Q.; Fan, Y.-M. AAV-mediated transfer of RhoA shRNA and CNTF promotes retinal ganglion cell survival and axon regeneration. Neuroscience 2017, 343, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Pease, M.E.; Zack, D.J.; Berlinicke, C.; Bloom, K.; Cone, F.; Wang, Y.; Klein, R.L.; Hauswirth, W.W.; Quigley, H.A. Effect of CNTF on Retinal Ganglion Cell Survival in Experimental Glaucoma. Investig. Opthalmology Vis. Sci. 2009, 50, 2194. [Google Scholar] [CrossRef]
- Mathews, M.K.; Guo, Y.; Langenberg, P.; Bernstein, S.L. Ciliary neurotrophic factor (CNTF)-mediated ganglion cell survival in a rodent model of non-arteritic anterior ischaemic optic neuropathy (NAION). Br. J. Ophthalmol. 2015, 99, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Song, Y.; Kjellstrom, S.; Tanikawa, A.; Liu, Y.; Li, Y.; Zhao, L.; Bush, R.A.; Laties, A.M.; Sieving, P.A. Regulation of Rod Phototransduction Machinery by Ciliary Neurotrophic Factor. J. Neurosci. 2006, 26, 13523–13530. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yin, Y.; Benowitz, L. Chemokine CCL5 promotes robust optic nerve regeneration and mediates many of the effects of CNTF gene therapy. Proc. Natl. Acad. Sci. USA 2021, 118, e2017282118. [Google Scholar] [CrossRef] [PubMed]
- Flachsbarth, K.; Kruszewski, K.; Jung, G.; Jankowiak, W.; Riecken, K.; Wagenfeld, L.; Richard, G.; Fehse, B.; Bartsch, U. Neural Stem Cell–Based Intraocular Administration of Ciliary Neurotrophic Factor Attenuates the Loss of Axotomized Ganglion Cells in Adult Mice. Investig. Opthalmology Vis. Sci. 2014, 55, 7029. [Google Scholar] [CrossRef]
- Sieving, P.A.; Caruso, R.C.; Tao, W.; Coleman, H.R.; Thompson, D.J.S.; Fullmer, K.R.; Bush, R.A. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: Phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc. Natl. Acad. Sci. USA 2006, 103, 3896–3901. [Google Scholar] [CrossRef]
- Millán-Rivero, J.E.; Nadal-Nicolás, F.M.; García-Bernal, D.; Sobrado-Calvo, P.; Blanquer, M.; Moraleda, J.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Human Wharton’s jelly mesenchymal stem cells protect axotomized rat retinal ganglion cells via secretion of anti-inflammatory and neurotrophic factors. Sci. Rep. 2018, 8, 16299. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-L.; Li, N.; Wei, X.; Tang, L.; Wang, T.-H.; Chen, X.-M. Neuroprotective effects of BDNF and GDNF in intravitreally transplanted mesenchymal stem cells after optic nerve crush in mice. Int. J. Ophthalmol. 2017, 10, 35–42. [Google Scholar] [CrossRef]
- Johnson, T.V.; Martin, K.R. Cell transplantation approaches to retinal ganglion cell neuroprotection in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 78–82. [Google Scholar] [CrossRef]
- Boia, R.; Ruzafa, N.; Aires, I.D.; Pereiro, X.; Ambrósio, A.F.; Vecino, E.; Santiago, A.R. Neuroprotective strategies for retinal ganglion cell degeneration: Current status and challenges ahead. Int. J. Mol. Sci. 2020, 21, 2262. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Z.-N.; Rong, Z.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Xian, B.; Huang, B. The immune response of stem cells in subretinal transplantation. Stem Cell Res. Ther. 2015, 6, 161. [Google Scholar] [CrossRef]
- Boyd, A.; Higashi, Y.; Wood, K. Transplanting stem cells: Potential targets for immune attack. Modulating the immune response against embryonic stem cell transplantation. Adv. Drug Deliv. Rev. 2005, 57, 1944–1969. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Q.; Temple, S. Stem cell therapies for retinal diseases: Recapitulating development to replace degenerated cells. Development 2017, 144, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Bessoles, S.; Grandclément, C.; Alari-Pahissa, E.; Gehrig, J.; Jeevan-Raj, B.; Held, W. Adaptations of Natural Killer Cells to Self-MHC Class I. Front. Immunol. 2014, 5, 349. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Shirouzu, T.; Nakata, K.; Yoshimura, N.; Ushigome, H. The Role of Major Histocompatibility Complex in Organ Transplantation- Donor Specific Anti-Major Histocompatibility Complex Antibodies Analysis Goes to the Next Stage. Int. J. Mol. Sci. 2019, 20, 4544. [Google Scholar] [CrossRef]
- Taylor, A.L.; Negus, S.L.; Negus, M.; Bolton, E.M.; Bradley, J.A.; Pettigrew, G.J. Pathways of Helper CD4 T Cell Allorecognition in Generating Alloantibody and CD8 T Cell Alloimmunity. Transplantation 2007, 83, 931–937. [Google Scholar] [CrossRef]
- Diehl, R.; Ferrara, F.; Müller, C.; Dreyer, A.Y.; McLeod, D.D.; Fricke, S.; Boltze, J. Immunosuppression for in vivo research: State-of-the-art protocols and experimental approaches. Cell. Mol. Immunol. 2017, 14, 146–179. [Google Scholar] [CrossRef]
- Lilienfeld, B.G.; Crew, M.D.; Forte, P.; Baumann, B.C.; Seebach, J.D. Transgenic expression of HLA-E single chain trimer protects porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Xenotransplantation 2007, 14, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, B.; Ono, M.; Kagita, A.; Fujii, K.; Sasakawa, N.; Ueda, T.; Gee, P.; Nishikawa, M.; Nomura, M.; et al. Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell 2019, 24, 566–578.e7. [Google Scholar] [CrossRef]
- Swijnenburg, R.-J.; Schrepfer, S.; Govaert, J.A.; Cao, F.; Ransohoff, K.; Sheikh, A.Y.; Haddad, M.; Connolly, A.J.; Davis, M.M.; Robbins, R.C.; et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc. Natl. Acad. Sci. USA 2008, 105, 12991–12996. [Google Scholar] [CrossRef]
- Saint-Geniez, M.; D’amore, P.A. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int. J. Dev. Biol. 2004, 48, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef]
- Mélik Parsadaniantz, S.; Réaux-le Goazigo, A.; Sapienza, A.; Habas, C.; Baudouin, C. Glaucoma: A Degenerative Optic Neuropathy Related to Neuroinflammation? Cells 2020, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- García-López, M.; Arenas, J.; Gallardo, M.E. Hereditary Optic Neuropathies: Induced Pluripotent Stem Cell-Based 2D/3D Approaches. Genes 2021, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Ceyzériat, K.; Abjean, L.; Carrillo-de Sauvage, M.-A.; Ben Haim, L.; Escartin, C. The complex STATes of astrocyte reactivity: How are they controlled by the JAK–STAT3 pathway? Neuroscience 2016, 330, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Gibson, S.A.; Buckley, J.A.; Qin, H.; Benveniste, E.N. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin. Immunol. 2018, 189, 4–13. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Reactive Astrocytes in Neural Repair and Protection. Neuroscientist 2005, 11, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Sterling, J.K.; Adetunji, M.O.; Guttha, S.; Bargoud, A.R.; Uyhazi, K.E.; Ross, A.G.; Dunaief, J.L.; Cui, Q.N. GLP-1 Receptor Agonist NLY01 Reduces Retinal Inflammation and Neuron Death Secondary to Ocular Hypertension. Cell Rep. 2020, 33, 108271. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef]
- Luo, H.; Zhuang, J.; Hu, P.; Ye, W.; Chen, S.; Pang, Y.; Li, N.; Deng, C.; Zhang, X. Resveratrol Delays Retinal Ganglion Cell Loss and Attenuates Gliosis-Related Inflammation From Ischemia-Reperfusion Injury. Investig. Opthalmology Vis. Sci. 2018, 59, 3879. [Google Scholar] [CrossRef] [PubMed]
- Pirhan, D.; Yüksel, N.; Emre, E.; Cengiz, A.; Kürşat Yıldız, D. Riluzole- and Resveratrol-Induced Delay of Retinal Ganglion Cell Death in an Experimental Model of Glaucoma. Curr. Eye Res. 2016, 41, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Surviving ischemia: Adaptive responses mediated by hypoxia-inducible factor 1. J. Clin. Invest. 2000, 106, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Nakanishitoda, M. Nitric oxide: Ocular blood flow, glaucoma, and diabetic retinopathy. Prog. Retin. Eye Res. 2007, 26, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Zubrow, A.B.; Delivoria-Papadopoulos, M.; Ashraf, Q.M.; Ballesteros, J.R.; Fritz, K.I.; Mishra, O.P. Nitric oxide-mediated expression of Bax protein and DNA fragmentation during hypoxia in neuronal nuclei from newborn piglets. Brain Res. 2002, 954, 60–67. [Google Scholar] [CrossRef]
- Bull, N.D.; Limb, G.A.; Martin, K.R. Human Müller Stem Cell (MIO-M1) Transplantation in a Rat Model of Glaucoma: Survival, Differentiation, and Integration. Investig. Opthalmology Vis. Sci. 2008, 49, 3449. [Google Scholar] [CrossRef]
- Singhal, S.; Lawrence, J.M.; Bhatia, B.; Ellis, J.S.; Kwan, A.S.; MacNeil, A.; Luthert, P.J.; Fawcett, J.W.; Perez, M.-T.; Khaw, P.T.; et al. Chondroitin Sulfate Proteoglycans and Microglia Prevent Migration and Integration of Grafted Müller Stem Cells into Degenerating Retina. Stem Cells 2008, 26, 1074–1082. [Google Scholar] [CrossRef]
- Johnson, T.V.; Bull, N.D.; Hunt, D.P.; Marina, N.; Tomarev, S.I.; Martin, K.R. Neuroprotective Effects of Intravitreal Mesenchymal Stem Cell Transplantation in Experimental Glaucoma. Investig. Opthalmology Vis. Sci. 2010, 51, 2051. [Google Scholar] [CrossRef]
- Singhal, S.; Bhatia, B.; Jayaram, H.; Becker, S.; Jones, M.F.; Cottrill, P.B.; Khaw, P.T.; Salt, T.E.; Limb, G.A. Human Müller Glia with Stem Cell Characteristics Differentiate into Retinal Ganglion Cell (RGC) Precursors In Vitro and Partially Restore RGC Function In Vivo Following Transplantation. Stem Cells Transl. Med. 2012, 1, 188–199. [Google Scholar] [CrossRef]
- Becker, S.; Eastlake, K.; Jayaram, H.; Jones, M.F.; Brown, R.A.; McLellan, G.J.; Charteris, D.G.; Khaw, P.T.; Limb, G.A. Allogeneic Transplantation of Müller-Derived Retinal Ganglion Cells Improves Retinal Function in a Feline Model of Ganglion Cell Depletion. Stem Cells Transl. Med. 2016, 5, 192–205. [Google Scholar] [CrossRef]
- Reinhard, J.; Wiemann, S.; Hildebrandt, S.; Faissner, A. Extracellular Matrix Remodeling in the Retina and Optic Nerve of a Novel Glaucoma Mouse Model. Biology 2021, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, M.; Chintala, S.K. Kainic Acid–Mediated Upregulation of Matrix Metalloproteinase-9 Promotes Retinal Degeneration. Investig. Opthalmology Vis. Sci. 2004, 45, 2374. [Google Scholar] [CrossRef] [PubMed]
- Amini, R.; Rocha-Martins, M.; Norden, C. Neuronal Migration and Lamination in the Vertebrate Retina. Front. Neurosci. 2018, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Icha, J.; Kunath, C.; Rocha-Martins, M.; Norden, C. Independent modes of ganglion cell translocation ensure correct lamination of the zebrafish retina. J. Cell Biol. 2016, 215, 259–275. [Google Scholar] [CrossRef]
- Keeley, P.W.; Eglen, S.J.; Reese, B.E. From random to regular: Variation in the patterning of retinal mosaics. J. Comp. Neurol. 2020, 528, 2135–2160. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.L.; Espinosa, J.S.; Xu, Y.; Davidson, N.; Kovacs, G.T.A.; Barres, B.A. Retinal Ganglion Cells Do Not Extend Axons by Default. Neuron 2002, 33, 689–702. [Google Scholar] [CrossRef]
- Randlett, O.; Poggi, L.; Zolessi, F.R.; Harris, W.A. The Oriented Emergence of Axons from Retinal Ganglion Cells Is Directed by Laminin Contact In Vivo. Neuron 2011, 70, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Riccomagno, M.M.; Sun, L.O.; Brady, C.M.; Alexandropoulos, K.; Seo, S.; Kurokawa, M.; Kolodkin, A.L. Cas Adaptor Proteins Organize the Retinal Ganglion Cell Layer Downstream of Integrin Signaling. Neuron 2014, 81, 779–786. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Johnson, T.V. The internal limiting membrane: Roles in retinal development and implications for emerging ocular therapies. Exp. Eye Res. 2021, 206, 108545. [Google Scholar] [CrossRef] [PubMed]
- Tian, N. Developmental mechanisms that regulate retinal ganglion cell dendritic morphology. Dev. Neurobiol. 2011, 71, 1297–1309. [Google Scholar] [CrossRef]
- Duan, X.; Krishnaswamy, A.; De la Huerta, I.; Sanes, J.R. Type II Cadherins Guide Assembly of a Direction-Selective Retinal Circuit. Cell 2014, 158, 793–807. [Google Scholar] [CrossRef]
- Matsuoka, R.L.; Chivatakarn, O.; Badea, T.C.; Samuels, I.S.; Cahill, H.; Katayama, K.; Kumar, S.R.; Suto, F.; Chédotal, A.; Peachey, N.S.; et al. Class 5 Transmembrane Semaphorins Control Selective Mammalian Retinal Lamination and Function. Neuron 2011, 71, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Reggiani, J.D.S.; Laboulaye, M.A.; Pandey, S.; Chen, B.; Rubenstein, J.L.R.; Krishnaswamy, A.; Sanes, J.R. Tbr1 instructs laminar patterning of retinal ganglion cell dendrites. Nat. Neurosci. 2018, 21, 659–670. [Google Scholar] [CrossRef]
- Peng, Y.-R.; Tran, N.M.; Krishnaswamy, A.; Kostadinov, D.; Martersteck, E.M.; Sanes, J.R. Satb1 Regulates Contactin 5 to Pattern Dendrites of a Mammalian Retinal Ganglion Cell. Neuron 2017, 95, 869–883.e6. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, M.; Sanes, J.R. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature 2008, 451, 465–469. [Google Scholar] [CrossRef]
- Sholl, D.A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953, 87, 387–406. [Google Scholar] [PubMed]
- Kosik, K.; Finch, E. MAP2 and tau segregate into dendritic and axonal domains after the elaboration of morphologically distinct neurites: An immunocytochemical study of cultured rat cerebrum. J. Neurosci. 1987, 7, 3142–3153. [Google Scholar] [CrossRef]
- Dehmelt, L.; Halpain, S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005, 6, 204. [Google Scholar] [CrossRef] [PubMed]
- Wässle, H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 2004, 5, 747–757. [Google Scholar] [CrossRef]
- Kuypers, H.G.J.M.; Ugolini, G. Viruses as transneuronal tracers. Trends Neurosci. 1990, 13, 71–75. [Google Scholar] [CrossRef]
- Zemanick, M.C.; Strick, P.L.; Dix, R.D. Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proc. Natl. Acad. Sci. USA 1991, 88, 8048–8051. [Google Scholar] [CrossRef]
- Zingg, B.; Chou, X.; Zhang, Z.; Mesik, L.; Liang, F.; Tao, H.W.; Zhang, L.I. AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron 2017, 93, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Card, J.P. Practical Considerations for the Use of Pseudorabies Virus in Transneuronal Studies of Neural Circuitry. Neurosci. Biobehav. Rev. 1998, 22, 685–694. [Google Scholar] [CrossRef]
- Strack, A.; Loewy, A. Pseudorabies virus: A highly specific transneuronal cell body marker in the sympathetic nervous system. J. Neurosci. 1990, 10, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.F.; Björklund, A.; Parmar, M. Transsynaptic tracing and its emerging use to assess graft-reconstructed neural circuits. Stem Cells 2020, 38, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, T.; Dong, Y.; Kondoh, K.; Lu, Z. Trans-synaptic Neural Circuit-Tracing with Neurotropic Viruses. Neurosci. Bull. 2019, 35, 909–920. [Google Scholar] [CrossRef]
- Adler, A.F.; Cardoso, T.; Nolbrant, S.; Mattsson, B.; Hoban, D.B.; Jarl, U.; Wahlestedt, J.N.; Grealish, S.; Björklund, A.; Parmar, M. hESC-Derived Dopaminergic Transplants Integrate into Basal Ganglia Circuitry in a Preclinical Model of Parkinson’s Disease. Cell Rep. 2019, 28, 3462–3473.e5. [Google Scholar] [CrossRef]
- Siegel, M.S.; Isacoff, E.Y. A Genetically Encoded Optical Probe of Membrane Voltage. Neuron 1997, 19, 735–741. [Google Scholar] [CrossRef]
- Nakai, J.; Ohkura, M.; Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat. Biotechnol. 2001, 19, 137–141. [Google Scholar] [CrossRef]
- Heidelberger, R.; Thoreson, W.B.; Witkovsky, P. Synaptic transmission at retinal ribbon synapses. Prog. Retin. Eye Res. 2005, 24, 682–720. [Google Scholar] [CrossRef]
- Brandstätter, J.H.; Fletcher, E.L.; Garner, C.C.; Gundelfinger, E.D.; Wässle, H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. Eur. J. Neurosci. 1999, 11, 3683–3693. [Google Scholar] [CrossRef] [PubMed]
- Dick, O.; Hack, I.; Altrock, W.D.; Garner, C.C.; Gundelfinger, E.D.; Brandstätter, J.H. Localization of the presynaptic cytomatrix protein Piccolo at ribbon and conventional synapses in the rat retina: Comparison with Bassoon. J. Comp. Neurol. 2001, 439, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Brandstätter, J.H.; Löhrke, S.; Morgans, C.W.; Wässle, H. Distributions of two homologous synaptic vesicle proteins, synaptoporin and synaptophysin, in the mammalian retina. J. Comp. Neurol. 1996, 370, 1–10. [Google Scholar] [CrossRef]
- Sterling, P.; Matthews, G. Structure and function of ribbon synapses. Trends Neurosci. 2005, 28, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Elegheert, J.; Song, I.; Sasakura, H.; Senkov, O.; Matsuda, K.; Kakegawa, W.; Clayton, A.J.; Chang, V.T.; Ferrer-Ferrer, M.; et al. A synthetic synaptic organizer protein restores glutamatergic neuronal circuits. Science 2020, 369, eabb4853. [Google Scholar] [CrossRef]
- Singh, M.S.; Balmer, J.; Barnard, A.R.; Aslam, S.A.; Moralli, D.; Green, C.M.; Barnea-Cramer, A.; Duncan, I.; MacLaren, R.E. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat. Commun. 2016, 7, 1–5. [Google Scholar] [CrossRef]
- Pearson, R.A.; Gonzalez-Cordero, A.; West, E.L.; Ribeiro, J.R.; Aghaizu, N.; Goh, D.; Sampson, R.D.; Georgiadis, A.; Waldron, P.V.; Duran, Y.; et al. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat. Commun. 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Santos-Ferreira, T.; Llonch, S.; Borsch, O.; Postel, K.; Haas, J.; Ader, M. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Decembrini, S.; Martin, C.; Sennlaub, F.; Chemtob, S.; Biel, M.; Samardzija, M.; Moulin, A.; Behar-Cohen, F.; Arsenijevic, Y. Cone Genesis Tracing by the Chrnb4-EGFP Mouse Line: Evidences of Cellular Material Fusion after Cone Precursor Transplantation. Mol. Ther. 2017, 25, 634–653. [Google Scholar] [CrossRef]
- Narsinh, K.H.; Sun, N.; Sanchez-Freire, V.; Lee, A.S.; Almeida, P.; Hu, S.; Jan, T.; Wilson, K.D.; Leong, D.; Rosenberg, J.; et al. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J. Clin. Investig. 2011, 121, 1217–1221. [Google Scholar] [CrossRef]
- Moon, S.-H.; Kim, J.-S.; Park, S.-J.; Lim, J.-J.; Lee, H.-J.; Lee, S.M.; Chung, H.-M. Effect of chromosome instability on the maintenance and differentiation of human embryonic stem cells in vitro and in vivo. Stem Cell Res. 2011, 6, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Conesa, C.; Doss, M.X.; Antzelevitch, C.; Sachinidis, A.; Sancho, J.; Carrodeguas, J.A. Identification of Specific Pluripotent Stem Cell Death—Inducing Small Molecules by Chemical Screening. Stem Cell Rev. Rep. 2012, 8, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Satarian, L.; Nourinia, R.; Safi, S.; Kanavi, M.; Jarughi, N.; Daftarian, N.; Arab, L.; Aghdami, N.; Ahmadieh, H.; Baharvand, H. Intravitreal injection of bone marrow mesenchymal stem cells in patients with advanced retinitis pigmentosa; a safety study. J. Ophthalmic Vis. Res. 2017, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Tuekprakhon, A.; Sangkitporn, S.; Trinavarat, A.; Pawestri, A.R.; Vamvanij, V.; Ruangchainikom, M.; Luksanapruksa, P.; Pongpaksupasin, P.; Khorchai, A.; Dambua, A.; et al. Intravitreal autologous mesenchymal stem cell transplantation: A non-randomized phase I clinical trial in patients with retinitis pigmentosa. Stem Cell Res. Ther. 2021, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Kuriyan, A.E.; Albini, T.A.; Townsend, J.H.; Rodriguez, M.; Pandya, H.K.; Leonard, R.E.; Parrott, M.B.; Rosenfeld, P.J.; Flynn, H.W.; Goldberg, J.L. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N. Engl. J. Med. 2017, 376, 1047–1053. [Google Scholar] [CrossRef]
- Wen, Y.-T.; Ho, Y.-C.; Lee, Y.-C.; Ding, D.-C.; Liu, P.-K.; Tsai, R.-K. The Benefits and Hazards of Intravitreal Mesenchymal Stem Cell (MSC) Based-Therapies in the Experimental Ischemic Optic Neuropathy. Int. J. Mol. Sci. 2021, 22, 2117. [Google Scholar] [CrossRef]
- Tedeschi, A.; Bradke, F. Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr. Opin. Neurobiol. 2017, 42, 118–127. [Google Scholar] [CrossRef]
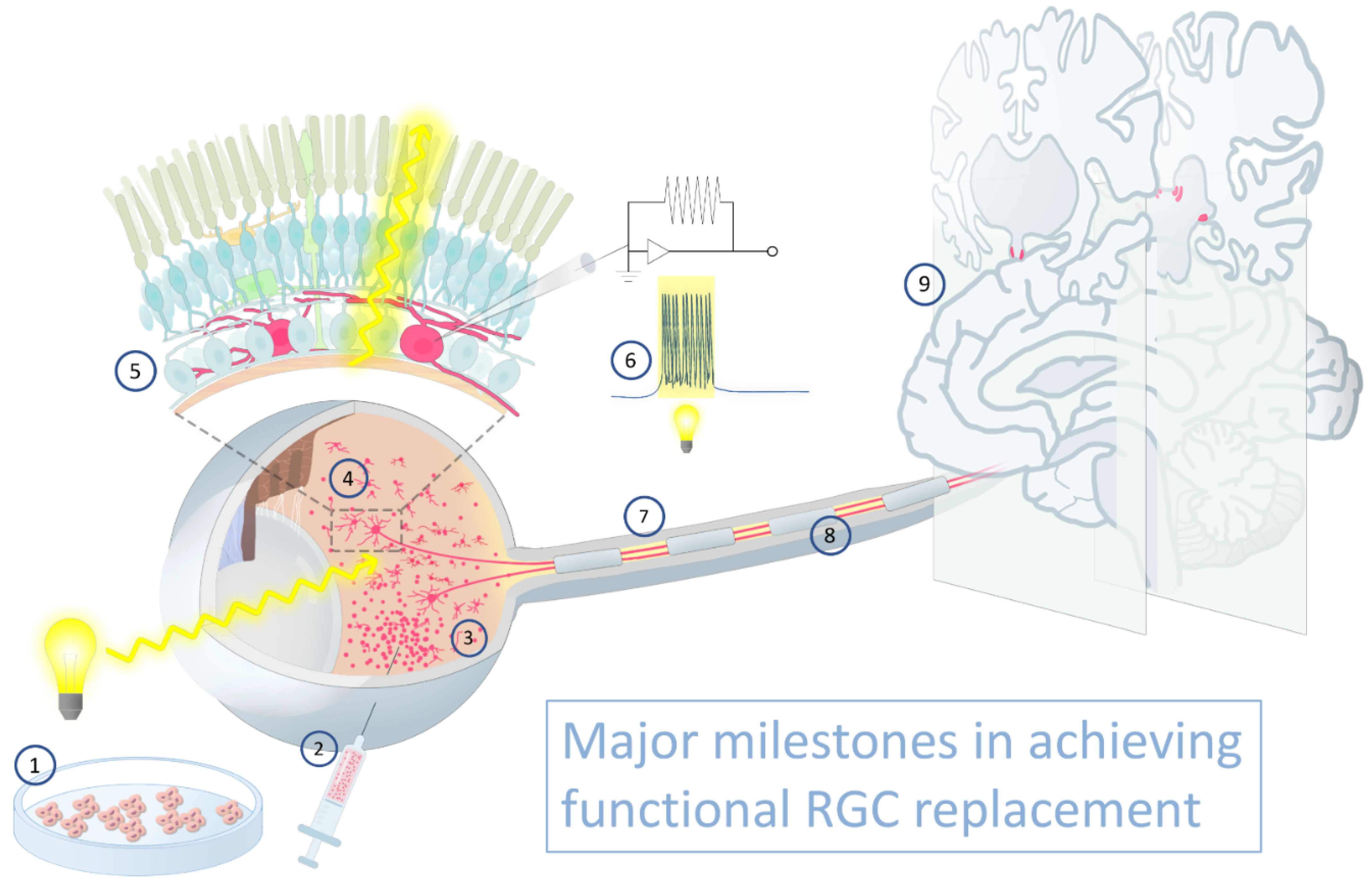


| Donor Cell Source | Advantages | Disadvantages |
|---|---|---|
| Primary murine RGCs | Compatible allogeneic transplantation in murine recipeints Bona fide RGCs based on normal development | Limited scalability Limited/no clinical potential |
| Stem cell-derived murine RGCs | Autologous or allogenic transplantation in murine hosts Multiple published differentiation protocols Scaleable and renewable | Potential teratogenicity Limited/no clinical potential |
| Human ESC-derived RGCs | Scalable and renewable Multiple published differentiation protocols Translational potential | Ethical concerns Potential teratogenicity Line-to-line heterogenity Finite number of parental lines; limitations in establishing new lines |
| Human iPSC-derived RGCs | Scalable and renewable Multiple published differentiation protocols Potential for autologous transplantation Unlimited ability to establish new and specialized cell lines Translational potential | Line-to-line heterogenity Potential teratogenicity |
| Donor Cell Source | Host Species | Disease Model | Injection Route | Injection Vehicle | Immuno-Suppressive Regiment | # of Donor Cells in Transplant | Experiment Duration | Presence of Donor Cells in Host Eyes | RGC Survival Rate | Host Retina Localization | Neurite Formation | Functional Improvement | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hESC-RPCs | NHP | Healthy eyes | Subretinal | Media (DMEM/F12, N2, B27, NEAA, pen/strep, DAPT) | None | 1 × 106/eye | 1–3 months | Yes | Not reported | RGCL, INL | Yes (Projections toward ONH) | Not reported | Chao et al. (2017) [70] |
| hESC-RGCs | Rat | Healthy eyes | Intravitreal | Media (Sato medium containing DAPT) | None | 5 × 104/eye | 1 week | Yes (5/5 eyes w/ detectable donor cells) | 19–25 cells/mm2 | RGCL (HuNu+ RGCs near Tuj1+ retinal layer) | Not reported | Not reported | Zhang et al. (2020) [71] |
| hESC-RPCs | Mouse | NMDA excito-toxicity | Intravitreal | Media (DMEM/F12, N2, KOSR, L-glutamine, non-essential amino acids, nicotinamide) | None | 2 × 104/eye | 4–5 weeks | Yes | Not reported | RGCL (HuNu+ donor cells near Brn3a+ host cells) | Not reported | Not reported | Wang et al. (2019) [72] |
| hiPSC-RGCs | Rabbit, Monkey | Healthy eyes | Intravitreal | PLGA scaffold | None | 1 × 105/scaffold | 1 week–3 months | Yes | Not reported | Not reported | Yes (RGCs on scaffolds form dendrites; express Neuro-filament) | Not reported (Donor RGCs express voltage-gated Na+ channels) | Li et al. (2017) [73] |
| hiPSC-RGCs | Mouse | ONC | Intravitreal | MACS buffer | Cyclosporine (210 mg/L) in drinking water | 2 × 105/eye | 1–4 weeks | Yes (10 of 17 eyes) | Not reported | RGCL (9/17 eyes showed donor cells in close proximity to host RGCL) | No | Not reported | Rabesandratana et al. (2020) [34] |
| hSSC-RGCs | Mouse | NMDAexcito-toxicity | Intravitreal | FACS buffer | None | 1 × 104/eye | 10 days | Yes (unspecified fraction of eyes demonstrated survival) | Not reported | RGCL (donor cells found nearby endogenous RGCs) | No | Not reported | Suen et al. (2019) [74] |
| rRGCs | Rat | Healthy eyes | Intravitreal | PBS | None | 5 × 104/eye | 1–7 days | Yes | ~3% on day 1 ~1% on day 7 | NFL (donor cells along the host Tuj1+ NFL) RGCL (proportion of donor cells intermingled w/ host RGCL) | No (In vivo: not reported; ex vivo ~75% neurite outgrowth in developing RGCs, ~20% in adult donor RGCs) | Not reported | Hertz et al. (2014) [75] |
| mRGCs | Rat | Healthy eyes | Intravitreal | Media (Neurobasal, insulin, pyruvate, L-glutamine, T3, NAC, GS21, BDNF, CNTF, forskolin) | None | 4-6 × 104/eye | 1–4 weeks | Yes (15 of 152 eyes) | <1–7% | RGCL (15/152 eyes) | Yes (>90% of surviving cells, some of complex morphology) | Yes (PSD95+ donor neurites; light-evoked postsynaptic current) | Venugopalan et al. (2016) [32] |
| mRGCs | Rat | Healthy eyes | Intravitreal | Cotransplantation w/ hiPSCs in media (StemMACS iPS-Brew XF, Miltenyi Biotec) | None | 4 × 104/eye | 1 week | Yes (20% of experiments w/ retinal engraftment) | <1% increasing to approx. 3.5% with iPSC co-transplant | Not reported | Yes (Increased by hIPSC co-transplant) | Not reported | Wu et al. (2018) [76] |
| mESC-RPCs | Mouse | NMDA- excito-toxicity | Intravitreal | PBS with 10 ng/mL FGF2 | None | 1 × 106/eye | 2 months | Yes | Not reported | RGCL (flat mount and sectioned retina w/ GFP+ donor cells near host RGCL) | Yes (GFP+ cells w/ neurite morphology resembling endogenous RGC) | Yes (optokinetic tracking and light avoidance, c-Fos expression) | Divya et al. (2017) [33] |
| DBA/2J mice | Intravitreal | None | 1 × 106/eye | 2 months | Not reported | Not reported | Not reported | Not reported | No improved visual acuity | ||||
| miPSC/mESC-RGCs | Mouse | Healthy eyes | (1) Intravitreal; (2) Subretinal | Media (DMEM, Glutamax, non-essential amino acids, pyruvate, lipid concentrate, antibiotics, b-mercaptoethanol, NS21, NAC) | None | 2 × 104/eye (adult recipient); 1 × 104/eye (early postnatal recipient) | 2 weeks–12 months | Yes (At 2 weeks: 8/10 adults; 9/9 pups. At 12 months: 2/4 mice) | 0.5–5% | RGCL (Thy1-GFP+ donor cells adjacent to host RGCL) ONH (some donor cells migrated into the nerve head) | Yes (diverse morphology, ranging from no neurite to laminated processes) | Not reported, but evidence of synaptic connection with host by WGA tracing | Oswald et al. (2021) [62] |
| Microbead-induced high IOP; | Intravitreal | None | 2 × 104/eye | 2 weeks | Yes (4/6 mice) | 0.1–1% | Not reported | Yes (~50% of cells formed neurites) | Not reported | ||||
| NMDAexcito-toxicity | Intravitreal | None | 2 × 104/eye | 2 weeks | Yes (4/6 mice) | 0.1–1% | Not reported | Yes (~25% of cells formed neurites) | Not reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.Y.; Aguzzi, E.A.; Johnson, T.V. Retinal Ganglion Cell Transplantation: Approaches for Overcoming Challenges to Functional Integration. Cells 2021, 10, 1426. https://doi.org/10.3390/cells10061426
Zhang KY, Aguzzi EA, Johnson TV. Retinal Ganglion Cell Transplantation: Approaches for Overcoming Challenges to Functional Integration. Cells. 2021; 10(6):1426. https://doi.org/10.3390/cells10061426
Chicago/Turabian StyleZhang, Kevin Y., Erika A. Aguzzi, and Thomas V. Johnson. 2021. "Retinal Ganglion Cell Transplantation: Approaches for Overcoming Challenges to Functional Integration" Cells 10, no. 6: 1426. https://doi.org/10.3390/cells10061426
APA StyleZhang, K. Y., Aguzzi, E. A., & Johnson, T. V. (2021). Retinal Ganglion Cell Transplantation: Approaches for Overcoming Challenges to Functional Integration. Cells, 10(6), 1426. https://doi.org/10.3390/cells10061426






