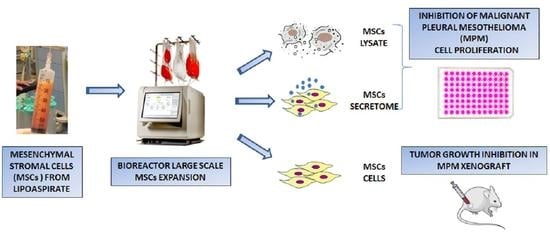
Inhibition of Human Malignant Pleural Mesothelioma Growth by Mesenchymal Stromal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Cell Lines
2.2. Mesenchymal Stromal Cells (MSCs) Expansion and Characterization
2.3. MSCs for In Vitro Studies
2.4. Large Scale Expansion of MSCs
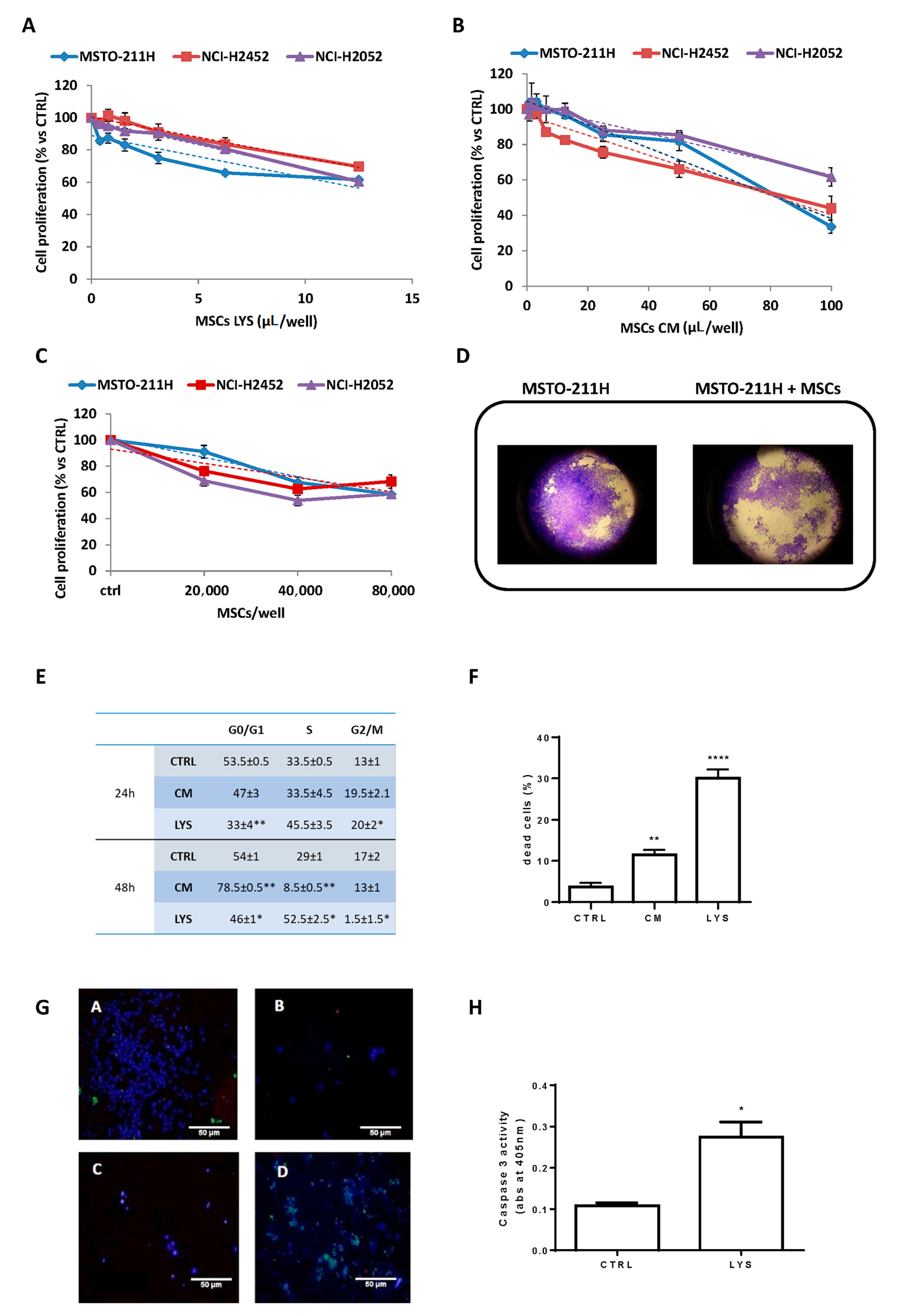
2.5. Analysis of Cell Proliferation, Cell Death and Cell Cycle
2.6. Transwell Assay
2.7. Cytokines Measure in MSCs Secretome
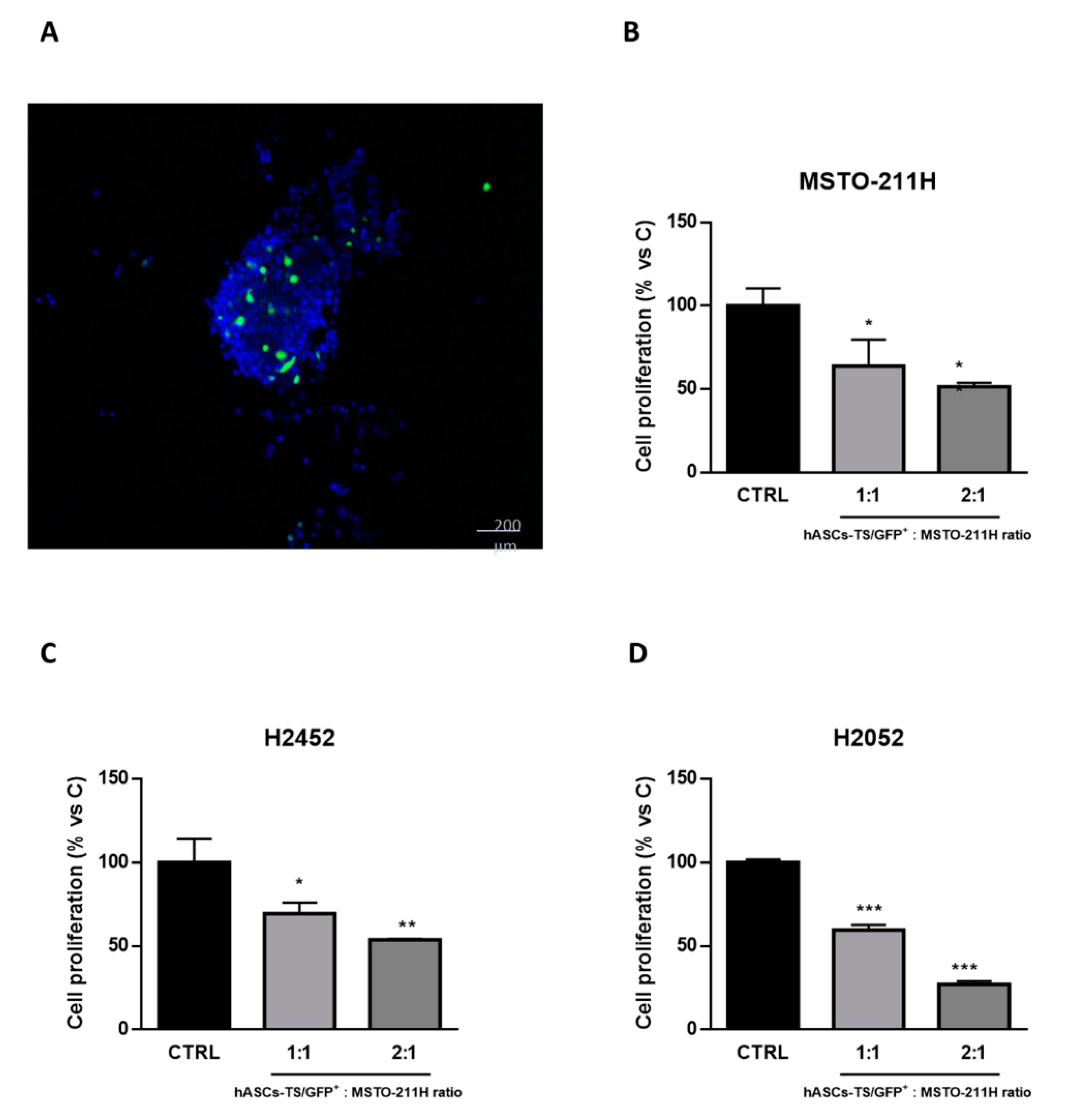
2.8. Direct Tumor MSCs Cells Interaction
2.9. In Vivo Experiments
2.10. Histological and Morphometric Analysis of Tumor Xenografts
2.11. Statistical Analysis
3. Results
3.1. Effects of MSCs Lysate and Secretome on Proliferation of Mesothelioma Cell Lines
3.2. Direct Effect of MSCs on Proliferation of Mesothelioma Cells
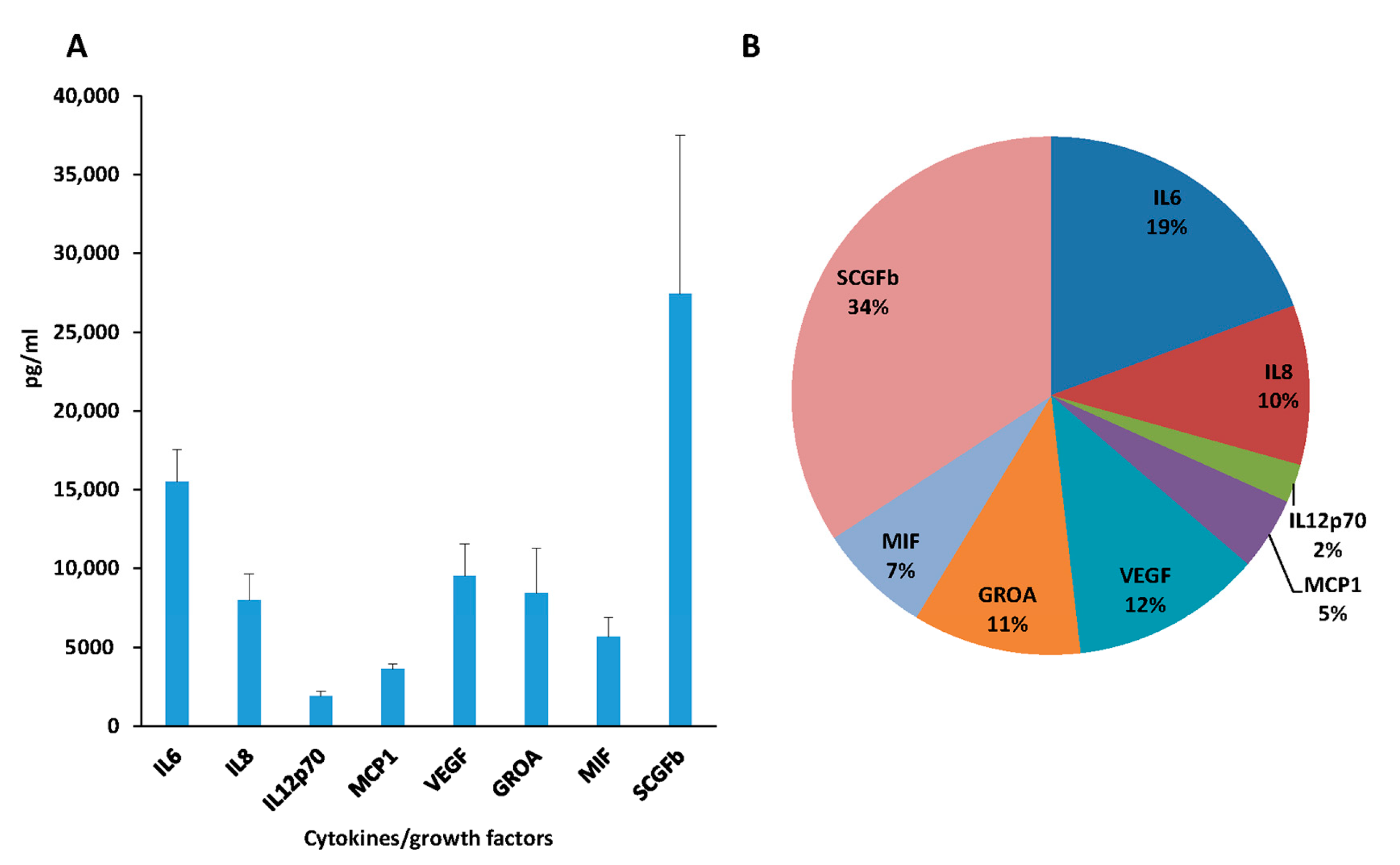
3.3. Cytokines Analysis of Secretome
3.4. Large-Scale Expansion
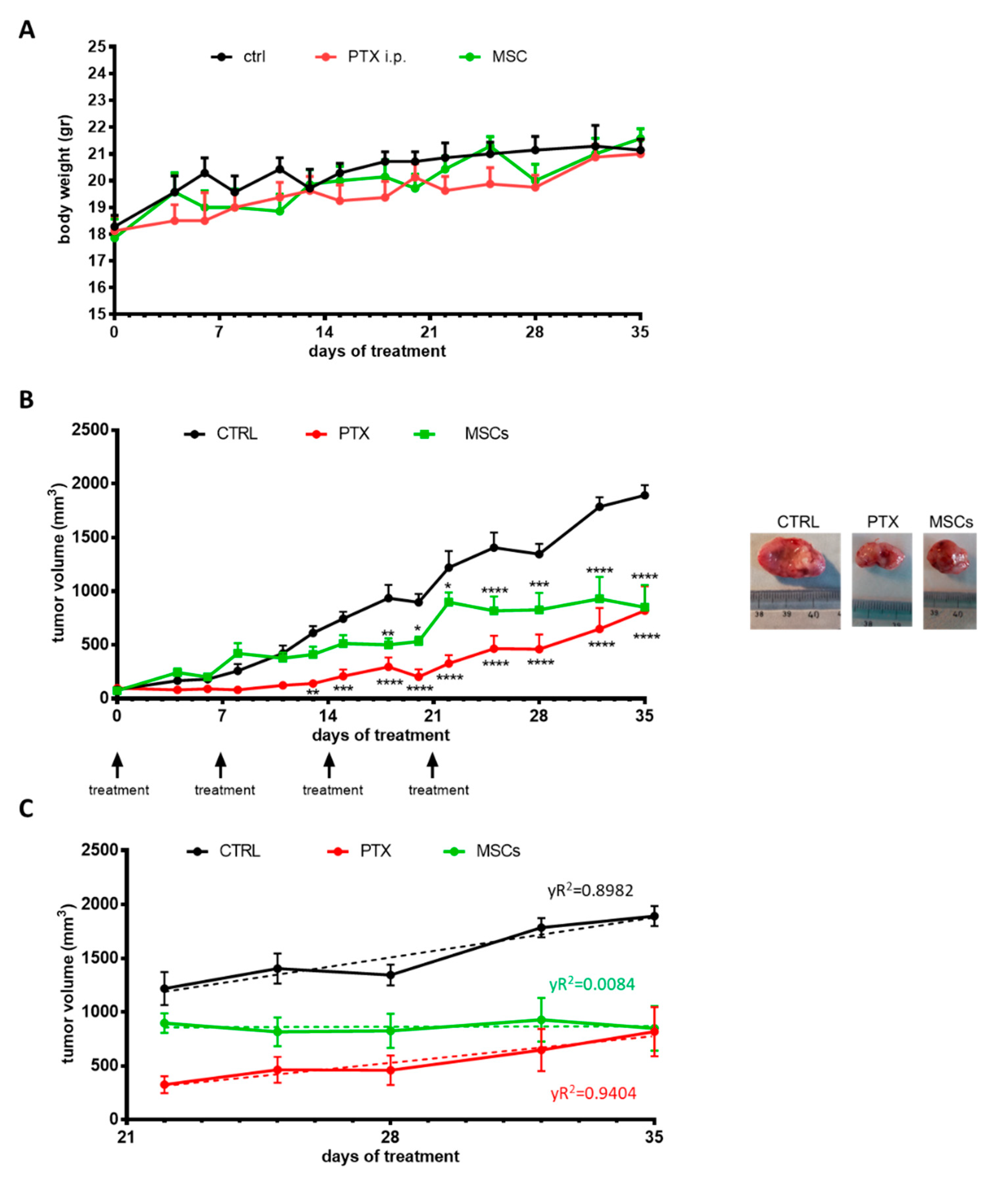
3.5. In Vivo Efficacy of MSCs
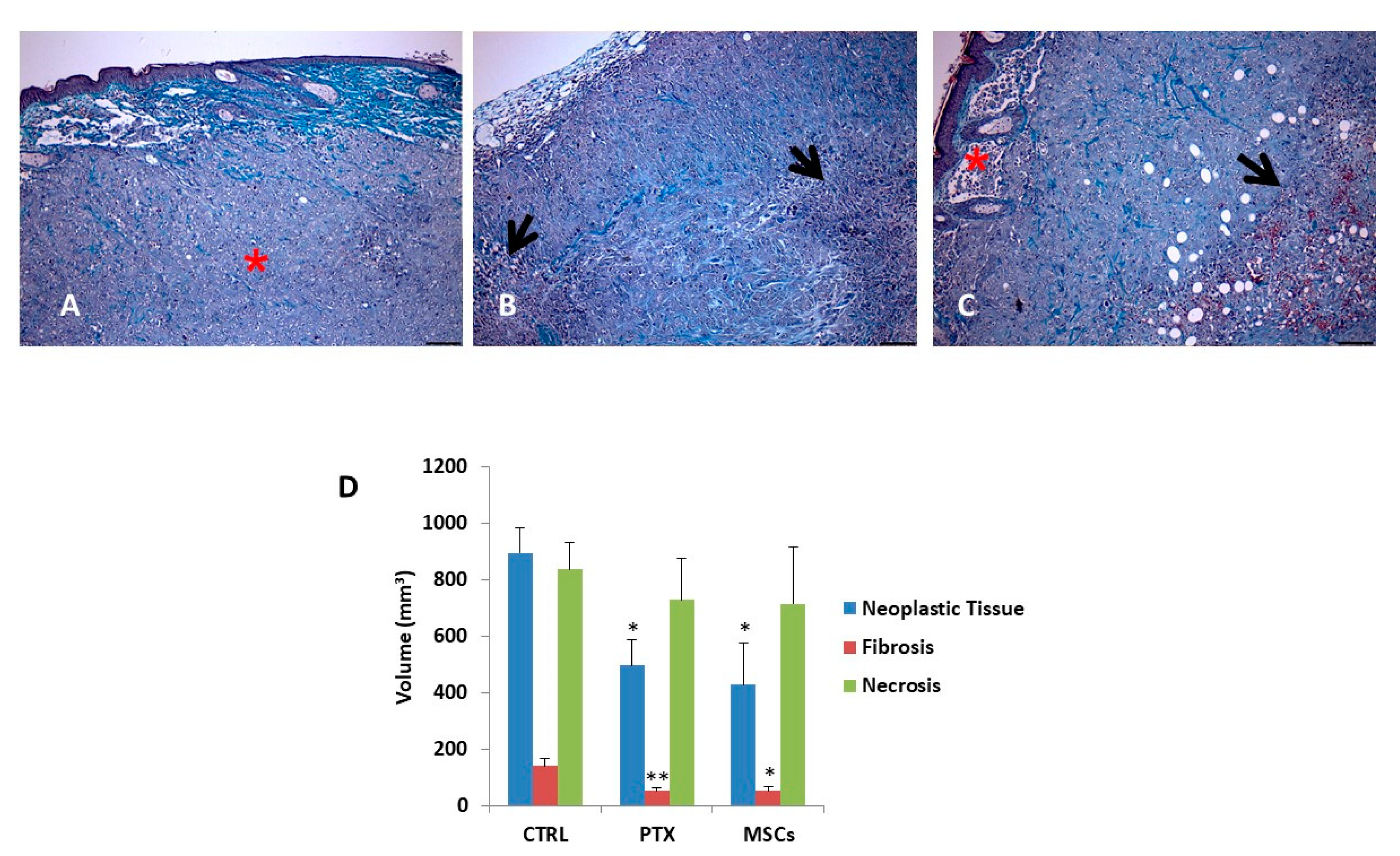
3.6. Histology and Morphometric Analysis of Xenograft
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carbone, M.; Ly, B.H.; Dodson, R.F.; Pagano, I.; Morris, P.T.; Dogan, U.A.; Gazdar, A.F.; Pass, H.I.; Yang, H. Malignant mesothelioma: Facts, myths, and hypotheses. J. Cell Physiol. 2012, 227, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Peto, J.; Decarli, A.; La Vecchia, C.; Levi, F.; Negri, E. The European mesothelioma epidemic. Br. J. Cancer 1999, 79, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Fennell, D.A.; Gaudino, G.; O’Byrne, K.J.; Mutti, L.; van Meerbeeck, J. Advances in the systemic therapy of malignant pleural mesothelioma. Nat. Clin. Pract. Oncol. 2008, 5, 136–147. [Google Scholar] [CrossRef]
- Krug, L.M.; Pass, H.I.; Rusch, V.W.; Kindler, H.L.; Sugarbaker, D.J.; Rosenzweig, K.E.; Flores, R.; Friedberg, J.S.; Pisters, K.; Monberg, M.; et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J. Clin. Oncol. 2009, 27, 3007–3013. [Google Scholar] [CrossRef]
- Mutti, L.; Peikert, T.; Robinson, B.W.S.; Scherpereel, A.; Tsao, A.S.; de Perrot, M.; Woodard, G.A.; Jablons, D.M.; Wiens, J.; Hirsch, F.R.; et al. Scientific Advances and New Frontiers in Mesothelioma Therapeutics. J. Thorac. Oncol. 2018, 13, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, Y.; Yang, J.; Zhang, X.; Zhang, H.; Zhang, T.; Zhao, S.; Zheng, P.; Huo, J.; Wu, H. Gastric cancer-derived mesenchymal stem cells prompt gastric cancer progression through secretion of interleukin-8. J. Exp. Clin. Cancer Res. 2015, 34, 52. [Google Scholar] [CrossRef]
- Li, G.C.; Zhang, H.W.; Zhao, Q.C.; Sun, L.I.; Yang, J.J.; Hong, L.; Feng, F.; Cai, L. Mesenchymal stem cells promote tumor angiogenesis via the action of transforming growth factor β1. Oncol. Lett. 2016, 11, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Houthuijzen, J.M.; Daenen, L.G.; Roodhart, J.M.; Voest, E.E. The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. Br. J. Cancer 2012, 106, 1901–1906. [Google Scholar] [CrossRef]
- Ramasamy, R.; Lam, E.W.; Soeiro, I.; Tisato, V.; Bonnet, D.; Dazzi, F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: Impact on in vivo tumor growth. Leukemia 2007, 21, 304–310. [Google Scholar] [CrossRef]
- Chanda, D.; Isayeva, T.; Kumar, S.; Hensel, J.A.; Sawant, A.; Ramaswamy, G.; Siegal, G.P.; Beatty, M.S.; Ponnazhagan, S. Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in prostate cancer bone metastasis. Clin. Cancer Res. 2009, 15, 7175–7185. [Google Scholar] [CrossRef]
- Ohlsson, L.B.; Varas, L.; Kjellman, C.; Edvardsen, K.; Lindvall, M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp. Mol. Pathol. 2003, 75, 248–255. [Google Scholar] [CrossRef]
- Ayuzawa, R.; Doi, C.; Rachakatla, R.S.; Pyle, M.M.; Maurya, D.K.; Troyer, D.; Tamura, M. Naïve human umbilical cord matrix derived stem cells significantly attenuate growth of human breast cancer cells in vitro and in vivo. Cancer Lett. 2009, 280, 31–37. [Google Scholar] [CrossRef]
- Yuan, Z.; Kolluri, K.K.; Gowers, K.H.; Janes, S.M. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J. Extracell. Vesicles 2017, 6, 1265291. [Google Scholar] [CrossRef]
- Li, L.; Tian, H.; Chen, Z.; Yue, W.; Li, S.; Li, W. Inhibition of lung cancer cell proliferation mediated by human mesenchymal stem cells. Acta Biochim. Biophys. Sin. (Shanghai) 2011, 43, 143–148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramdasi, S.; Sarang, S.; Viswanathan, C. Potential of Mesenchymal Stem Cell based application in Cancer. Int. J. Hematol. Oncol. Stem Cell Res. 2015, 9, 95–103. [Google Scholar]
- Galland, S.; Stamenkovic, I. Mesenchymal stromal cells in cancer: A review of their immunomodulatory functions and dual effects on tumor progression. J. Pathol. 2020, 250, 555–572. [Google Scholar] [CrossRef]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Nwabo Kamdje, A.H.; Kamga, P.T.; Simo, R.T.; Vecchio, L.; Seke Etet, P.F.; Muller, J.M.; Bassi, G.; Lukong, E.; Goel, R.K.; Amvene, J.M.; et al. Mesenchymal stromal cells’ role in tumor microenvironment: Involvement of signaling pathways. Cancer Biol. Med. 2017, 14, 129–141. [Google Scholar] [CrossRef]
- Cortes-Dericks, L.; Froment, L.; Kocher, G.; Schmid, R.A. Human lung-derived mesenchymal stem cell-conditioned medium exerts in vitro antitumor effects in malignant pleural mesothelioma cell lines. Stem Cell Res. Ther. 2016, 7, 25. [Google Scholar] [CrossRef]
- Lathrop, M.J.; Sage, E.K.; Macura, S.L.; Brooks, E.M.; Cruz, F.; Bonenfant, N.R.; Sokocevic, D.; MacPherson, M.B.; Beuschel, S.L.; Dunaway, C.W.; et al. Antitumor effects of TRAIL-expressing mesenchymal stromal cells in a mouse xenograft model of human mesothelioma. Cancer Gene Ther. 2015, 22, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Sage, E.K.; Kolluri, K.K.; McNulty, K.; Lourenco Sda, S.; Kalber, T.L.; Ordidge, K.L.; Davies, D.; Gary Lee, Y.C.; Giangreco, A.; Janes, S.M. Systemic but not topical TRAIL-expressing mesenchymal stem cells reduce tumour growth in malignant mesothelioma. Thorax 2014, 69, 638–647. [Google Scholar] [CrossRef]
- Lisini, D.; Nava, S.; Pogliani, S.; Avanzini, M.A.; Lenta, E.; Bedini, G.; Mantelli, M.; Pecciarini, L.; Croce, S.; Boncoraglio, G.; et al. Adipose tissue-derived mesenchymal stromal cells for clinical application: An efficient isolation approach. Curr. Res. Transl. Med. 2019, 67, 20–27. [Google Scholar] [CrossRef]
- Lisini, D.; Nava, S.; Frigerio, S.; Pogliani, S.; Maronati, G.; Marcianti, A.; Coccè, V.; Bondiolotti, G.; Cavicchini, L.; Paino, F.; et al. Automated Large-Scale Production of Paclitaxel Loaded Mesenchymal Stromal Cells for Cell Therapy Applications. Pharmaceutics 2020, 12, 411. [Google Scholar] [CrossRef] [PubMed]
- Petrella, F.; Coccè, V.; Masia, C.; Milani, M.; Salè, E.O.; Alessandri, G.; Parati, E.; Sisto, F.; Pentimalli, F.; Brini, A.T.; et al. Paclitaxel-releasing mesenchymal stromal cells inhibit in vitro proliferation of human mesothelioma cells. Biomed. Pharmacother. 2017, 87, 755–758. [Google Scholar] [CrossRef]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fumarola, C.; La Monica, S.; Alfieri, R.R.; Borra, E.; Guidotti, G.G. Cell size reduction induced by inhibition of the mTOR/S6K-signaling pathway protects Jurkat cells from apoptosis. Cell Death Differ. 2005, 12, 1344–1357. [Google Scholar] [CrossRef]
- Balducci, L.; Blasi, A.; Saldarelli, M.; Soleti, A.; Pessina, A.; Bonomi, A.; Coccè, V.; Dossena, M.; Tosetti, V.; Ceserani, V.; et al. Immortalization of human adipose-derived stromal cells: Production of cell lines with high growth rate, mesenchymal marker expression and capability to secrete high levels of angiogenic factors. Stem Cell Res. Ther. 2014, 5, 63. [Google Scholar] [CrossRef]
- Coccè, V.; Farronato, D.; Brini, A.T.; Masia, C.; Giannì, A.B.; Piovani, G.; Sisto, F.; Alessandri, G.; Angiero, F.; Pessina, A. Drug Loaded Gingival Mesenchymal Stromal Cells (GinPa-MSCs) Inhibit In Vitro Proliferation of Oral Squamous Cell Carcinoma. Sci. Rep. 2017, 7, 9376. [Google Scholar] [CrossRef]
- Coccè, V.; Balducci, L.; Falchetti, M.L.; Pascucci, L.; Ciusani, E.; Brini, A.T.; Sisto, F.; Piovani, G.; Alessandri, G.; Parati, E.; et al. Fluorescent Immortalized Human Adipose Derived Stromal Cells (hASCs-TS/GFP+) for Studying Cell Drug Delivery Mediated by Microvesicles. Anticancer Agents Med. Chem. 2017, 17, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Berenzi, A.; Steimberg, N.; Boniotti, J.; Mazzoleni, G. MRT letter: 3D culture of isolated cells: A fast and efficient method for optimizing their histochemical and immunocytochemical analyses. Microsc. Res. Tech. 2015, 78, 249–254. [Google Scholar] [CrossRef]
- La Monica, S.; Cretella, D.; Bonelli, M.; Fumarola, C.; Cavazzoni, A.; Digiacomo, G.; Flammini, L.; Barocelli, E.; Minari, R.; Naldi, N.; et al. Trastuzumab emtansine delays and overcomes resistance to the third-generation EGFR-TKI osimertinib in NSCLC EGFR mutated cell lines. J. Exp. Clin. Cancer Res. 2017, 36, 174. [Google Scholar] [CrossRef]
- La Monica, S.; Minari, R.; Cretella, D.; Flammini, L.; Fumarola, C.; Bonelli, M.; Cavazzoni, A.; Digiacomo, G.; Galetti, M.; Madeddu, D.; et al. Third generation EGFR inhibitor osimertinib combined with pemetrexed or cisplatin exerts long-lasting anti-tumor effect in EGFR-mutated pre-clinical models of NSCLC. J. Exp. Clin. Cancer Res. 2019, 38, 222. [Google Scholar] [CrossRef]
- Christodoulou, I.; Goulielmaki, M.; Devetzi, M.; Panagiotidis, M.; Koliakos, G.; Zoumpourlis, V. Mesenchymal stem cells in preclinical cancer cytotherapy: A systematic review. Stem Cell Res. Ther. 2018, 9, 336. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, K.S.; Bae, S.; Son, H.K.; Myung, P.K.; Hong, H.J.; Kim, H. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int. J. Stem Cells 2009, 2, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hoogduijn, M.J.; Baan, C.C.; Korevaar, S.S.; de Kuiper, R.; Yan, L.; Wang, L.; van Besouw, N.M. Adipose Tissue-Derived Mesenchymal Stem Cells Have a Heterogenic Cytokine Secretion Profile. Stem Cells Int. 2017, 2017, 4960831. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahim, S.N.; Ho, G.Y.; Coward, J.I. The role of interleukin-6 in malignant mesothelioma. Transl. Lung Cancer Res. 2015, 4, 55–66. [Google Scholar]
- Assoni, A.; Coatti, G.; Valadares, M.C.; Beccari, M.; Gomes, J.; Pelatti, M.; Mitne-Neto, M.; Carvalho, V.M.; Zatz, M. Different Donors Mesenchymal Stromal Cells Secretomes Reveal Heterogeneous Profile of Relevance for Therapeutic Use. Stem Cells Dev. 2017, 26, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Shrihari, T.G. Dual role of inflammatory mediators in cancer. Ecancermedicalscience 2017, 11, 721. [Google Scholar] [CrossRef]
- Lee, M.W.; Ryu, S.; Kim, D.S.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Mesenchymal stem cells in suppression or progression of hematologic malignancy: Current status and challenges. Leukemia 2019, 33, 597–611. [Google Scholar] [CrossRef]
- Pokrovskaya, L.A.; Zubareva, E.V.; Nadezhdin, S.V.; Lysenko, A.S.; Litovkina, T.L. Biological activity of mesenchymal stem cells secretome as a basis for cell-free therapeutic approach. Res. Results Pharmacol. 2020, 6, 57–68. [Google Scholar] [CrossRef]
- Mirabdollahi, M.; Haghjooyjavanmard, S.; Sadeghi-Aliabadi, H. An anticancer effect of umbilical cord-derived mesenchymal stem cell secretome on the breast cancer cell line. Cell Tissue Bank 2019, 20, 423–434. [Google Scholar] [CrossRef]
- Leuning, D.G.; Beijer, N.R.M.; du Fossé, N.A.; Vermeulen, S.; Lievers, E.; van Kooten, C.; Rabelink, T.J.; Boer, J. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci. Rep. 2018, 8, 7716. [Google Scholar] [CrossRef] [PubMed]
- Otsu, K.; Das, S.; Houser, S.D.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood 2009, 113, 4197–4205. [Google Scholar] [CrossRef]
- Pessina, A.; Bonomi, A.; Coccè, V.; Invernici, G.; Navone, S.; Cavicchini, L.; Sisto, F.; Ferrari, M.; Viganò, L.; Locatelli, A.; et al. Mesenchymal stromal cells primed with paclitaxel provide a new approach for cancer therapy. PLoS ONE 2011, 6, e28321. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, A.; Sordi, V.; Dugnani, E.; Ceserani, V.; Dossena, M.; Coccè, V.; Cavicchini, L.; Ciusani, E.; Bondiolotti, G.; Piovani, G.; et al. Gemcitabine-releasing mesenchymal stromal cells inhibit in vitro proliferation of human pancreatic carcinoma cells. Cytotherapy 2015, 17, 1687–1695. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coccè, V.; La Monica, S.; Bonelli, M.; Alessandri, G.; Alfieri, R.; Lagrasta, C.A.; Madeddu, D.; Frati, C.; Flammini, L.; Lisini, D.; et al. Inhibition of Human Malignant Pleural Mesothelioma Growth by Mesenchymal Stromal Cells. Cells 2021, 10, 1427. https://doi.org/10.3390/cells10061427
Coccè V, La Monica S, Bonelli M, Alessandri G, Alfieri R, Lagrasta CA, Madeddu D, Frati C, Flammini L, Lisini D, et al. Inhibition of Human Malignant Pleural Mesothelioma Growth by Mesenchymal Stromal Cells. Cells. 2021; 10(6):1427. https://doi.org/10.3390/cells10061427
Chicago/Turabian StyleCoccè, Valentina, Silvia La Monica, Mara Bonelli, Giulio Alessandri, Roberta Alfieri, Costanza Annamaria Lagrasta, Denise Madeddu, Caterina Frati, Lisa Flammini, Daniela Lisini, and et al. 2021. "Inhibition of Human Malignant Pleural Mesothelioma Growth by Mesenchymal Stromal Cells" Cells 10, no. 6: 1427. https://doi.org/10.3390/cells10061427
APA StyleCoccè, V., La Monica, S., Bonelli, M., Alessandri, G., Alfieri, R., Lagrasta, C. A., Madeddu, D., Frati, C., Flammini, L., Lisini, D., Marcianti, A., Parati, E., Paino, F., Giannì, A., Farronato, G., Falco, A., Spaggiari, L., Petrella, F., & Pessina, A. (2021). Inhibition of Human Malignant Pleural Mesothelioma Growth by Mesenchymal Stromal Cells. Cells, 10(6), 1427. https://doi.org/10.3390/cells10061427