How SARS-CoV-2 and Other Viruses Build an Invasion Route to Hijack the Host Nucleocytoplasmic Trafficking System
Abstract
1. Introduction
2. Fundamentals of Nucleocytoplasmic Trafficking
3. Mechanisms of the Host Nuclear Transport Machinery Hijacking by Viruses
3.1. Viruses That Replicate in the Cytoplasm
3.1.1. β-Coronaviruses (SARS-CoV-2, SARS-CoV-1, and MERS-CoV)
3.1.2. Ebola Virus (Zaire Ebolavirus)
3.1.3. Dengue Virus (DENV) and Zika Virus (ZIKV)
3.1.4. Chikungunya Virus (CHIKV)
3.2. Viruses That Replicate in the Nucleus
3.2.1. Human Immunodeficiency Virus (HIV)
3.2.2. Influenza A Virus (IAV)
3.2.3. Human Papilloma Virus (HPV)
3.2.4. Hepatitis B Virus (HBV)
3.2.5. Herpes Simplex Virus Type-1, Human Cytomegalovirus, and Epstein–Barr Virus
4. Potential Antiviral Drugs That Target the Host Nuclear Transport Machinery
4.1. Host-Specific Nuclear Import Inhibitors
4.2. Host-Specific Nuclear Export Inhibitors
4.3. Viral-Specific Nuclear Transport Inhibitors
4.4. Clinical Translation of Nuclear Transport Inhibitors against Viral Infections
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Korobeinikov, A. Immune response and within-host viral evolution: Immune response can accelerate evolution. J. Theor. Biol. 2018, 456, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Görlich, D.; Kutay, U. Transport between the cell nucleus and the cytoplasm. Ann. Rev. Cell Develop. Biol. 1999, 15, 607–660. [Google Scholar] [CrossRef]
- Makhnevych, T.; Lusk, C.P.; Anderson, A.M.; Aitchison, J.D.; Wozniak, R.W. Cell cycle regulated transport controlled by alterations in the nuclear pore complex. Cell 2003, 115, 813–823. [Google Scholar] [CrossRef]
- Nakano, H.; Funasaka, T.; Hashizume, C.; Wong, R.W. Nucleoporin translocated promoter region (Tpr) associates with dynein complex, preventing chromosome lagging formation during mitosis. J. Biol. Chem. 2010, 285, 10841–10849. [Google Scholar] [CrossRef]
- Perera, R.M.; Stoykova, S.; Nicolay, B.N.; Ross, K.N.; Fitamant, J.; Boukhali, M.; Lengrand, J.; Deshpande, V.; Selig, M.K.; Ferrone, C.R.; et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature 2015, 524, 361–365. [Google Scholar] [CrossRef]
- Dewi, F.R.P.; Jiapaer, S.; Kobayashi, A.; Hazawa, M.; Ikliptikawati, D.K.; Hartono; Sabit, H.; Nakada, M.; Wong, R.W. Nucleoporin TPR (translocated promoter region, nuclear basket protein) upregulation alters MTOR-HSF1 trails and suppresses autophagy induction in ependymoma. Autophagy 2021, 17, 1001–1012. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Y.; Yildirim, E. The nuclear pore complex in cell type-specific chromatin structure and gene regulation. Trends Genet. TIG 2019, 35, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zebell, S.G.; Liang, Z.; Wang, S.; Kang, B.H.; Dong, X. Nuclear pore permeabilization is a convergent signaling event in effector-triggered immunity. Cell 2016, 166, 1526–1538. [Google Scholar] [CrossRef]
- Lezon, T.R.; Sali, A.; Bahar, I. Global motions of the nuclear pore complex: Insights from elastic network models. PLoS Comput. Biol. 2009, 5, e1000496. [Google Scholar] [CrossRef] [PubMed]
- Bui, K.H.; von Appen, A.; DiGuilio, A.L.; Ori, A.; Sparks, L.; Mackmull, M.T.; Bock, T.; Hagen, W.; Andrés-Pons, A.; Glavy, J.S.; et al. Integrated structural analysis of the human nuclear pore complex scaffold. Cell 2013, 155, 1233–1243. [Google Scholar] [CrossRef]
- Eibauer, M.; Pellanda, M.; Turgay, Y.; Dubrovsky, A.; Wild, A.; Medalia, O. Structure and gating of the nuclear pore complex. Nat. Commun. 2015, 6, 7532. [Google Scholar] [CrossRef]
- Hülsmann, B.B.; Labokha, A.A.; Görlich, D. The permeability of reconstituted nuclear pores provides direct evidence for the selective phase model. Cell 2012, 150, 738–751. [Google Scholar] [CrossRef]
- Lim, R.Y.; Huang, N.P.; Köser, J.; Deng, J.; Lau, K.H.; Schwarz-Herion, K.; Fahrenkrog, B.; Aebi, U. Flexible phenylalanine-glycine nucleoporins as entropic barriers to nucleocytoplasmic transport. Proc. Nat. Acad. Sci. USA 2006, 103, 9512–9517. [Google Scholar] [CrossRef]
- Frey, S.; Görlich, D. A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell 2007, 130, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Peters, R. Translocation through the nuclear pore complex: Selectivity and speed by reduction-of-dimensionality. Traffic 2005, 6, 421–427. [Google Scholar] [CrossRef]
- Patel, S.S.; Belmont, B.J.; Sante, J.M.; Rexach, M.F. Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell 2007, 129, 83–96. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Hazawa, M.; Kobayashi, A.; Guillaud, L.; Watanabe-Nakayama, T.; Nakayama, M.; Wang, H.; Kodera, N.; Oshima, M.; Ando, T.; et al. Spatiotemporally tracking of nano-biofilaments inside the nuclear pore complex core. Biomaterials 2020, 256, 120198. [Google Scholar] [CrossRef]
- Dworetzky, S.I.; Lanford, R.E.; Feldherr, C.M. The effects of variations in the number and sequence of targeting signals on nuclear uptake. J. Cell Biol. 1988, 107, 1279–1287. [Google Scholar] [CrossRef]
- Panté, N.; Kann, M. Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Mol. Biol. Cell 2002, 13, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Pumroy, R.A.; Cingolani, G. Diversification of importin-α isoforms in cellular trafficking and disease states. Biochem. J. 2015, 466, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Mosammaparast, N.; Pemberton, L.F. Karyopherins: From nuclear-transport mediators to nuclear-function regulators. Trends Cell Biol. 2004, 14, 547–556. [Google Scholar] [CrossRef]
- Fontes, M.R.; Teh, T.; Kobe, B. Structural basis of recognition of monopartite and bipartite nuclear localization sequences by mammalian importin-alpha. J. Mol. Biol. 2000, 297, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Herold, A.; Truant, R.; Wiegand, H.; Cullen, B.R. Determination of the functional domain organization of the importin alpha nuclear import factor. J. Cell Biol. 1998, 143, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Conti, E.; Uy, M.; Leighton, L.; Blobel, G.; Kuriyan, J. Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin alpha. Cell 1998, 94, 193–204. [Google Scholar] [CrossRef]
- Kalderon, D.; Richardson, W.D.; Markham, A.F.; Smith, A.E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature 1984, 311, 33–38. [Google Scholar] [CrossRef]
- Robbins, J.; Dilworth, S.M.; Laskey, R.A.; Dingwall, C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: Identification of a class of bipartite nuclear targeting sequence. Cell 1991, 64, 615–623. [Google Scholar] [CrossRef]
- Cingolani, G.; Petosa, C.; Weis, K.; Müller, C.W. Structure of importin-beta bound to the IBB domain of importin-alpha. Nature 1999, 399, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, R.; Littlewood, T.; Stewart, M. Structural basis for the interaction between FxFG nucleoporin repeats and importin-beta in nuclear trafficking. Cell 2000, 102, 99–108. [Google Scholar] [CrossRef]
- Conti, E.; Izaurralde, E. Nucleocytoplasmic transport enters the atomic age. Curr. Opin. Cell Biol. 2001, 13, 310–319. [Google Scholar] [CrossRef]
- Lee, S.J.; Matsuura, Y.; Liu, S.M.; Stewart, M. Structural basis for nuclear import complex dissociation by RanGTP. Nature 2005, 435, 693–696. [Google Scholar] [CrossRef]
- Görlich, D.; Panté, N.; Kutay, U.; Aebi, U.; Bischoff, F.R. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 1996, 15, 5584–5594. [Google Scholar] [CrossRef]
- Chi, N.C.; Adam, E.J.; Visser, G.D.; Adam, S.A. RanBP1 stabilizes the interaction of Ran with p97 nuclear protein import. J. Cell Biol. 1996, 135, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Rexach, M.; Blobel, G. Protein import into nuclei: Association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell 1995, 83, 683–692. [Google Scholar] [CrossRef]
- Kutay, U.; Bischoff, F.R.; Kostka, S.; Kraft, R.; Görlich, D. Export of importin alpha from the nucleus is mediated by a specific nuclear transport factor. Cell 1997, 90, 1061–1071. [Google Scholar] [CrossRef]
- Bischoff, F.R.; Klebe, C.; Kretschmer, J.; Wittinghofer, A.; Ponstingl, H. RanGAP1 induces GTPase activity of nuclear Ras-related Ran. Proc. Nat. Acad. Sci. USA 1994, 91, 2587–2591. [Google Scholar] [CrossRef]
- Bischoff, F.R.; Krebber, H.; Kempf, T.; Hermes, I.; Ponstingl, H. Human RanGTPase-activating protein RanGAP1 is a homologue of yeast Rna1p involved in mRNA processing and transport. Proc. Nat. Acad. Sci. USA 1995, 92, 1749–1753. [Google Scholar] [CrossRef]
- Bischoff, F.R.; Krebber, H.; Smirnova, E.; Dong, W.; Ponstingl, H. Co-activation of RanGTPase and inhibition of GTP dissociation by Ran-GTP binding protein RanBP1. EMBO J. 1995, 14, 705–715. [Google Scholar] [CrossRef]
- Coutavas, E.; Ren, M.; Oppenheim, J.D.; D’Eustachio, P.; Rush, M.G. Characterization of proteins that interact with the cell-cycle regulatory protein Ran/TC4. Nature 1993, 366, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, R.; Ribbeck, K.; Akin, D.; Kent, H.M.; Feldherr, C.M.; Görlich, D.; Stewart, M. Interaction between NTF2 and xFxFG-containing nucleoporins is required to mediate nuclear import of RanGDP. J. Mol. Biol. 1999, 293, 579–593. [Google Scholar] [CrossRef]
- Bischoff, F.R.; Ponstingl, H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature 1991, 354, 80–82. [Google Scholar] [CrossRef]
- Fischer, U.; Huber, J.; Boelens, W.C.; Mattaj, I.W.; Lührmann, R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell 1995, 82, 475–483. [Google Scholar] [CrossRef]
- Kırlı, K.; Karaca, S.; Dehne, H.J.; Samwer, M.; Pan, K.T.; Lenz, C.; Urlaub, H.; Görlich, D. A deep proteomics perspective on CRM1-mediated nuclear export and nucleocytoplasmic partitioning. eLife 2015, 4. [Google Scholar] [CrossRef]
- Lindsay, M.E.; Holaska, J.M.; Welch, K.; Paschal, B.M.; Macara, I.G. Ran-binding protein 3 is a cofactor for Crm1-mediated nuclear protein export. J. Cell Biol. 2001, 153, 1391–1402. [Google Scholar] [CrossRef]
- Hutten, S.; Kehlenbach, R.H. Nup214 is required for CRM1-dependent nuclear protein export in vivo. Mol. Cell. Biol. 2006, 26, 6772–6785. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Min, S.; Zhang, Y.; Zhang, Y.; Zhou, Q.; Shen, X.; Jia, D.; Han, J.; Sun, Q. Distinct RanBP1 nuclear export and cargo dissociation mechanisms between fungi and animals. eLife 2019, 8. [Google Scholar] [CrossRef]
- Engelsma, D.; Bernad, R.; Calafat, J.; Fornerod, M. Supraphysiological nuclear export signals bind CRM1 independently of RanGTP and arrest at Nup358. EMBO J. 2004, 23, 3643–3652. [Google Scholar] [CrossRef] [PubMed]
- Kehlenbach, R.H.; Dickmanns, A.; Kehlenbach, A.; Guan, T.; Gerace, L. A role for RanBP1 in the release of CRM1 from the nuclear pore complex in a terminal step of nuclear export. J. Cell Biol. 1999, 145, 645–657. [Google Scholar] [CrossRef]
- Herold, A.; Suyama, M.; Rodrigues, J.P.; Braun, I.C.; Kutay, U.; Carmo-Fonseca, M.; Bork, P.; Izaurralde, E. TAP (NXF1) belongs to a multigene family of putative RNA export factors with a conserved modular architecture. Mol. Cell. Biol. 2000, 20, 8996–9008. [Google Scholar] [CrossRef] [PubMed]
- Stage-Zimmermann, T.; Schmidt, U.; Silver, P.A. Factors affecting nuclear export of the 60S ribosomal subunit in vivo. Mol. Biol. Cell 2000, 11, 3777–3789. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Segref, A.; Bachi, A.; Wilm, M.; Mattaj, I.W. PHAX, a mediator of U snRNA nuclear export whose activity is regulated by phosphorylation. Cell 2000, 101, 187–198. [Google Scholar] [CrossRef]
- Stade, K.; Ford, C.S.; Guthrie, C.; Weis, K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 1997, 90, 1041–1050. [Google Scholar] [CrossRef]
- Kutay, U.; Lipowsky, G.; Izaurralde, E.; Bischoff, F.R.; Schwarzmaier, P.; Hartmann, E.; Görlich, D. Identification of a tRNA-specific nuclear export receptor. Mol. Cell 1998, 1, 359–369. [Google Scholar] [CrossRef]
- Arts, G.J.; Fornerod, M.; Mattaj, I.W. Identification of a nuclear export receptor for tRNA. Curr. Biol. CB 1998, 8, 305–314. [Google Scholar] [CrossRef]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Hautbergue, G.M.; Hung, M.L.; Golovanov, A.P.; Lian, L.Y.; Wilson, S.A. Mutually exclusive interactions drive handover of mRNA from export adaptors to TAP. Proc. Nat. Acad. Sci. USA 2008, 105, 5154–5159. [Google Scholar] [CrossRef]
- Braun, I.C.; Herold, A.; Rode, M.; Izaurralde, E. Nuclear export of mRNA by TAP/NXF1 requires two nucleoporin-binding sites but not p15. Mol. Cell. Biol. 2002, 22, 5405–5418. [Google Scholar] [CrossRef] [PubMed]
- Farny, N.G.; Hurt, J.A.; Silver, P.A. Definition of global and transcript-specific mRNA export pathways in metazoans. Genes Dev. 2008, 22, 66–78. [Google Scholar] [CrossRef]
- Guzik, B.W.; Levesque, L.; Prasad, S.; Bor, Y.C.; Black, B.E.; Paschal, B.M.; Rekosh, D.; Hammarskjöld, M.L. NXT1 (p15) is a crucial cellular cofactor in TAP-dependent export of intron-containing RNA in mammalian cells. Mol. Cell. Biol. 2001, 21, 2545–2554. [Google Scholar] [CrossRef]
- Grüter, P.; Tabernero, C.; von Kobbe, C.; Schmitt, C.; Saavedra, C.; Bachi, A.; Wilm, M.; Felber, B.K.; Izaurralde, E. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell 1998, 1, 649–659. [Google Scholar] [CrossRef]
- Blevins, M.B.; Smith, A.M.; Phillips, E.M.; Powers, M.A. Complex formation among the RNA export proteins Nup98, Rae1/Gle2, and TAP. J. Biol. Chem. 2003, 278, 20979–20988. [Google Scholar] [CrossRef]
- Fribourg, S.; Braun, I.C.; Izaurralde, E.; Conti, E. Structural basis for the recognition of a nucleoporin FG repeat by the NTF2-like domain of the TAP/p15 mRNA nuclear export factor. Mol. Cell 2001, 8, 645–656. [Google Scholar] [CrossRef]
- Braun, I.C.; Rohrbach, E.; Schmitt, C.; Izaurralde, E. TAP binds to the constitutive transport element (CTE) through a novel RNA-binding motif that is sufficient to promote CTE-dependent RNA export from the nucleus. EMBO J. 1999, 18, 1953–1965. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ren, Y. Mechanisms of nuclear mRNA export: A structural perspective. Traffic 2019, 20, 829–840. [Google Scholar] [CrossRef]
- Brennan, C.M.; Gallouzi, I.E.; Steitz, J.A. Protein ligands to HuR modulate its interaction with target mRNAs in vivo. J. Cell Biol. 2000, 151, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bogerd, H.P.; Wang, P.J.; Page, D.C.; Cullen, B.R. Two closely related human nuclear export factors utilize entirely distinct export pathways. Mol. Cell 2001, 8, 397–406. [Google Scholar] [CrossRef]
- Volpon, L.; Culjkovic-Kraljacic, B.; Sohn, H.S.; Blanchet-Cohen, A.; Osborne, M.J.; Borden, K.L.B. A biochemical framework for eIF4E-dependent mRNA export and nuclear recycling of the export machinery. RNA 2017, 23, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Trotta, C.R.; Lund, E.; Kahan, L.; Johnson, A.W.; Dahlberg, J.E. Coordinated nuclear export of 60S ribosomal subunits and NMD3 in vertebrates. EMBO J. 2003, 22, 2841–2851. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.R.; Navas-Martin, S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. MMBR 2005, 69, 635–664. [Google Scholar] [CrossRef]
- Bleibtreu, A.; Bertine, M.; Bertin, C.; Houhou-Fidouh, N.; Visseaux, B. Focus on Middle East respiratory syndrome coronavirus (MERS-CoV). Med. Mal. Infect. 2020, 50, 243–251. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Eng. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Snijder, E.J.; Limpens, R.; de Wilde, A.H.; de Jong, A.W.M.; Zevenhoven-Dobbe, J.C.; Maier, H.J.; Faas, F.; Koster, A.J.; Bárcena, M. A unifying structural and functional model of the coronavirus replication organelle: Tracking down RNA synthesis. PLoS Biol. 2020, 18, e3000715. [Google Scholar] [CrossRef]
- Wolff, G.; Limpens, R.; Zevenhoven-Dobbe, J.C.; Laugks, U.; Zheng, S.; de Jong, A.W.M.; Koning, R.I.; Agard, D.A.; Grünewald, K.; Koster, A.J.; et al. A molecular pore spans the double membrane of the coronavirus replication organelle. Science 2020, 369, 1395–1398. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Miorin, L.; Kehrer, T.; Sanchez-Aparicio, M.T.; Zhang, K.; Cohen, P.; Patel, R.S.; Cupic, A.; Makio, T.; Mei, M.; Moreno, E.; et al. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc. Nat. Acad. Sci. USA 2020, 117, 28344–28354. [Google Scholar] [CrossRef]
- Kato, K.; Ikliptikawati, D.K.; Kobayashi, A.; Kondo, H.; Lim, K.; Hazawa, M.; Wong, R.W. Overexpression of SARS-CoV-2 protein ORF6 dislocates RAE1 and NUP98 from the nuclear pore complex. Biochem. Biophys. Res. Commun. 2021, 536, 59–66. [Google Scholar] [CrossRef]
- Addetia, A.; Lieberman, N.A.P.; Phung, Q.; Hsiang, T.Y.; Xie, H.; Roychoudhury, P.; Shrestha, L.; Loprieno, M.A.; Huang, M.L.; Gale, M., Jr.; et al. SARS-CoV-2 ORF6 Disrupts Bidirectional Nucleocytoplasmic Transport through Interactions with Rae1 and Nup98. mBio 2021, 12. [Google Scholar] [CrossRef]
- Xia, H.; Cao, Z.; Xie, X.; Zhang, X.; Chen, J.Y.; Wang, H.; Menachery, V.D.; Rajsbaum, R.; Shi, P.Y. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020, 33, 108234. [Google Scholar] [CrossRef]
- Frieman, M.; Yount, B.; Heise, M.; Kopecky-Bromberg, S.A.; Palese, P.; Baric, R.S. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J. Virol. 2007, 81, 9812–9824. [Google Scholar] [CrossRef]
- Kopecky-Bromberg, S.A.; Martínez-Sobrido, L.; Frieman, M.; Baric, R.A.; Palese, P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007, 81, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Izaurralde, E.; Jarmolowski, A.; Beisel, C.; Mattaj, I.W.; Dreyfuss, G.; Fischer, U. A role for the M9 transport signal of hnRNP A1 in mRNA nuclear export. J. Cell Biol. 1997, 137, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Durie, D.; Li, H.; Liu, B.Q.; Skehel, J.M.; Mauri, F.; Cuorvo, L.V.; Barbareschi, M.; Guo, L.; Holcik, M.; et al. hnRNPA1 couples nuclear export and translation of specific mRNAs downstream of FGF-2/S6K2 signalling. Nucl. Acids Res. 2014, 42, 12483–12497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Miorin, L.; Makio, T.; Dehghan, I.; Gao, S.; Xie, Y.; Zhong, H.; Esparza, M.; Kehrer, T.; Kumar, A.; et al. Nsp1 protein of SARS-CoV-2 disrupts the mRNA export machinery to inhibit host gene expression. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef]
- Gomez, G.N.; Abrar, F.; Dodhia, M.P.; Gonzalez, F.G.; Nag, A. SARS coronavirus protein nsp1 disrupts localization of Nup93 from the nuclear pore complex. Biochem. Cell Biol. Biochim. Biol. Cell. 2019, 97, 758–766. [Google Scholar] [CrossRef]
- Sharma, K.; Åkerström, S.; Sharma, A.K.; Chow, V.T.; Teow, S.; Abrenica, B.; Booth, S.A.; Booth, T.F.; Mirazimi, A.; Lal, S.K. SARS-CoV 9b protein diffuses into nucleus, undergoes active Crm1 mediated nucleocytoplasmic export and triggers apoptosis when retained in the nucleus. PLoS ONE 2011, 6, e19436. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, F.; Zhu, N.; Wang, W.; Deng, Y.; Zhao, Z.; Tan, W. Middle East respiratory syndrome coronavirus ORF4b protein inhibits type I interferon production through both cytoplasmic and nuclear targets. Sci. Rep. 2015, 5, 17554. [Google Scholar] [CrossRef] [PubMed]
- Canton, J.; Fehr, A.R.; Fernandez-Delgado, R.; Gutierrez-Alvarez, F.J.; Sanchez-Aparicio, M.T.; García-Sastre, A.; Perlman, S.; Enjuanes, L.; Sola, I. MERS-CoV 4b protein interferes with the NF-κB-dependent innate immune response during infection. PLoS Pathog. 2018, 14, e1006838. [Google Scholar] [CrossRef]
- Schmidt, M.L.; Hoenen, T. Characterization of the catalytic center of the Ebola virus L polymerase. PLoS Negl. Trop. Dis. 2017, 11, e0005996. [Google Scholar] [CrossRef]
- Bodmer, B.S.; Greßler, J.; Schmidt, M.L.; Holzerland, J.; Brandt, J.; Braun, S.; Groseth, A.; Hoenen, T. Differences in Viral RNA Synthesis but Not Budding or Entry Contribute to the In Vitro Attenuation of Reston Virus Compared to Ebola Virus. Microorganisms 2020, 8, 1215. [Google Scholar] [CrossRef]
- Reid, S.P.; Valmas, C.; Martinez, O.; Sanchez, F.M.; Basler, C.F. Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin alpha proteins with activated STAT1. J. Virol. 2007, 81, 13469–13477. [Google Scholar] [CrossRef]
- Reid, S.P.; Leung, L.W.; Hartman, A.L.; Martinez, O.; Shaw, M.L.; Carbonnelle, C.; Volchkov, V.E.; Nichol, S.T.; Basler, C.F. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J. Virol. 2006, 80, 5156–5167. [Google Scholar] [CrossRef]
- Xu, W.; Edwards, M.R.; Borek, D.M.; Feagins, A.R.; Mittal, A.; Alinger, J.B.; Berry, K.N.; Yen, B.; Hamilton, J.; Brett, T.J.; et al. Ebola virus VP24 targets a unique NLS binding site on karyopherin alpha 5 to selectively compete with nuclear import of phosphorylated STAT1. Cell Host Microbe 2014, 16, 187–200. [Google Scholar] [CrossRef]
- He, F.; Melén, K.; Maljanen, S.; Lundberg, R.; Jiang, M.; Österlund, P.; Kakkola, L.; Julkunen, I. Ebolavirus protein VP24 interferes with innate immune responses by inhibiting interferon-λ1 gene expression. Virology 2017, 509, 23–34. [Google Scholar] [CrossRef]
- Zhang, A.P.; Bornholdt, Z.A.; Liu, T.; Abelson, D.M.; Lee, D.E.; Li, S.; Woods, V.L., Jr.; Saphire, E.O. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog. 2012, 8, e1002550. [Google Scholar] [CrossRef]
- Marg, A.; Shan, Y.; Meyer, T.; Meissner, T.; Brandenburg, M.; Vinkemeier, U. Nucleocytoplasmic shuttling by nucleoporins Nup153 and Nup214 and CRM1-dependent nuclear export control the subcellular distribution of latent Stat1. J. Cell Biol. 2004, 165, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee-Kishore, M.; Wright, K.L.; Ting, J.P.; Stark, G.R. How Stat1 mediates constitutive gene expression: A complex of unphosphorylated Stat1 and IRF1 supports transcription of the LMP2 gene. EMBO J. 2000, 19, 4111–4122. [Google Scholar] [CrossRef]
- Shabman, R.S.; Gulcicek, E.E.; Stone, K.L.; Basler, C.F. The Ebola virus VP24 protein prevents hnRNP C1/C2 binding to karyopherin α1 and partially alters its nuclear import. J. Infect. Dis. 2011, 204, S904–S910. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Paek, K.Y.; Choi, K.; Kim, T.D.; Hahm, B.; Kim, K.T.; Jang, S.K. Heterogeneous nuclear ribonucleoprotein C modulates translation of c-myc mRNA in a cell cycle phase-dependent manner. Mol. Cell. Biol. 2003, 23, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, I.; Elsby, R.; Fernandez, M.; Faria, P.A.; Nussenzveig, D.R.; Lossos, I.S.; Fontoura, B.M.; Martin, W.D.; Barber, G.N. NFAR-1 and -2 modulate translation and are required for efficient host defense. Proc. Nat. Acad. Sci. USA 2008, 105, 4173–4178. [Google Scholar] [CrossRef]
- Brunner, J.E.; Nguyen, J.H.; Roehl, H.H.; Ho, T.V.; Swiderek, K.M.; Semler, B.L. Functional interaction of heterogeneous nuclear ribonucleoprotein C with poliovirus RNA synthesis initiation complexes. J. Virol. 2005, 79, 3254–3266. [Google Scholar] [CrossRef]
- Gontarek, R.R.; Gutshall, L.L.; Herold, K.M.; Tsai, J.; Sathe, G.M.; Mao, J.; Prescott, C.; Del Vecchio, A.M. hnRNP C and polypyrimidine tract-binding protein specifically interact with the pyrimidine-rich region within the 3′NTR of the HCV RNA genome. Nucl. Acids Res. 1999, 27, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Sokolowski, M.; Schwartz, S. Heterogeneous nuclear ribonucleoprotein C binds exclusively to the functionally important UUUUU-motifs in the human papillomavirus type-1 AU-rich inhibitory element. Virus Res. 2001, 73, 163–175. [Google Scholar] [CrossRef]
- Miyake, T.; Farley, C.M.; Neubauer, B.E.; Beddow, T.P.; Hoenen, T.; Engel, D.A. ebola virus inclusion body formation and RNA synthesis are controlled by a novel domain of nucleoprotein interacting with VP35. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Gabriel, G.; Feldmann, F.; Reimer, R.; Thiele, S.; Fischer, M.; Hartmann, E.; Bader, M.; Ebihara, H.; Hoenen, T.; Feldmann, H. Importin-α7 Is Involved in the Formation of Ebola Virus Inclusion Bodies but Is Not Essential for Pathogenicity in Mice. J. Infect. Dis. 2015, 212, S316–S321. [Google Scholar] [CrossRef][Green Version]
- Wendt, L.; Brandt, J.; Bodmer, B.S.; Reiche, S.; Schmidt, M.L.; Traeger, S.; Hoenen, T. The ebola virus nucleoprotein recruits the nuclear RNA Export Factor NXF1 into inclusion bodies to facilitate viral protein expression. Cells 2020, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, J.; Dias Junior, A.G.; Rigby, R.E.; Donald, C.L.; Mayer, A.; Sezgin, E.; Song, C.; Jin, B.; Hublitz, P.; Eggeling, C.; et al. Infection with a Brazilian isolate of Zika virus generates RIG-I stimulatory RNA and the viral NS5 protein blocks type I IFN induction and signaling. Eur. J. Immunol. 2018, 48, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, L.K.; Hoenen, A.; Morgan, G.; Mackenzie, J.M. The endoplasmic reticulum provides the membrane platform for biogenesis of the flavivirus replication complex. J. Virol. 2010, 84, 10438–10447. [Google Scholar] [CrossRef] [PubMed]
- Welsch, S.; Miller, S.; Romero-Brey, I.; Merz, A.; Bleck, C.K.; Walther, P.; Fuller, S.D.; Antony, C.; Krijnse-Locker, J.; Bartenschlager, R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 2009, 5, 365–375. [Google Scholar] [CrossRef]
- Yu, L.; Takeda, K.; Markoff, L. Protein-protein interactions among West Nile non-structural proteins and transmembrane complex formation in mammalian cells. Virology 2013, 446, 365–377. [Google Scholar] [CrossRef]
- Ashour, J.; Laurent-Rolle, M.; Shi, P.Y.; García-Sastre, A. NS5 of dengue virus mediates STAT2 binding and degradation. J. Virol. 2009, 83, 5408–5418. [Google Scholar] [CrossRef]
- Brooks, A.J.; Johansson, M.; John, A.V.; Xu, Y.; Jans, D.A.; Vasudevan, S.G. The interdomain region of dengue NS5 protein that binds to the viral helicase NS3 contains independently functional importin beta 1 and importin alpha/beta-recognized nuclear localization signals. J. Biol. Chem. 2002, 277, 36399–36407. [Google Scholar] [CrossRef]
- Pryor, M.J.; Rawlinson, S.M.; Butcher, R.E.; Barton, C.L.; Waterhouse, T.A.; Vasudevan, S.G.; Bardin, P.G.; Wright, P.J.; Jans, D.A.; Davidson, A.D. Nuclear localization of dengue virus nonstructural protein 5 through its importin alpha/beta-recognized nuclear localization sequences is integral to viral infection. Traffic 2007, 8, 795–807. [Google Scholar] [CrossRef]
- Potisopon, S.; Priet, S.; Collet, A.; Decroly, E.; Canard, B.; Selisko, B. The methyltransferase domain of dengue virus protein NS5 ensures efficient RNA synthesis initiation and elongation by the polymerase domain. Nucl. Acids Res. 2014, 42, 11642–11656. [Google Scholar] [CrossRef]
- Hannemann, H.; Sung, P.Y.; Chiu, H.C.; Yousuf, A.; Bird, J.; Lim, S.P.; Davidson, A.D. Serotype-specific differences in dengue virus non-structural protein 5 nuclear localization. J. Biol. Chem. 2013, 288, 22621–22635. [Google Scholar] [CrossRef]
- Tay, M.Y.; Smith, K.; Ng, I.H.; Chan, K.W.; Zhao, Y.; Ooi, E.E.; Lescar, J.; Luo, D.; Jans, D.A.; Forwood, J.K.; et al. The C-terminal 18 Amino Acid Region of Dengue Virus NS5 Regulates its Subcellular Localization and Contains a Conserved Arginine Residue Essential for Infectious Virus Production. PLoS Pathog. 2016, 12, e1005886. [Google Scholar] [CrossRef]
- Rawlinson, S.M.; Pryor, M.J.; Wright, P.J.; Jans, D.A. CRM1-mediated nuclear export of dengue virus RNA polymerase NS5 modulates interleukin-8 induction and virus production. J. Biol. Chem. 2009, 284, 15589–15597. [Google Scholar] [CrossRef]
- De Jesús-González, L.A.; Cervantes-Salazar, M.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Farfán-Morales, C.N.; Palacios-Rápalo, S.N.; Pérez-Olais, J.H.; Cordero-Rivera, C.D.; Hurtado-Monzón, A.M.; Ruíz-Jiménez, F.; et al. The nuclear pore complex: A Target for NS3 protease of dengue and zika viruses. Viruses 2020, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Rápalo, S.N.; De Jesús-González, L.A.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Farfan-Morales, C.N.; Gutiérrez-Escolano, A.L.; Del Ángel, R.M. Nuclear localization of non-structural protein 3 (NS3) during dengue virus infection. Arch. Virol. 2021, 166, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Gulland, A. Zika virus is a global public health emergency, declares WHO. BMJ 2016, 352, i657. [Google Scholar] [CrossRef] [PubMed]
- Kleber de Oliveira, W.; Cortez-Escalante, J.; De Oliveira, W.T.; do Carmo, G.M.; Henriques, C.M.; Coelho, G.E.; Araújo de França, G.V. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed zika virus transmission during the first trimester of pregnancy-brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 242–247. [Google Scholar] [CrossRef]
- Yang, L.; Wang, R.; Yang, S.; Ma, Z.; Lin, S.; Nan, Y.; Li, Q.; Tang, Q.; Zhang, Y.J. Karyopherin alpha 6 is required for replication of porcine reproductive and respiratory syndrome virus and zika virus. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Luo, G. Zika virus NS5 nuclear accumulation is protective of protein degradation and is required for viral RNA replication. Virology 2020, 541, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Ng, I.H.W.; Chan, K.W.; Tan, M.J.A.; Gwee, C.P.; Smith, K.M.; Jeffress, S.J.; Saw, W.G.; Swarbrick, C.M.D.; Watanabe, S.; Jans, D.A.; et al. Zika Virus NS5 forms supramolecular nuclear bodies that sequester importin-α and modulate the host immune and pro-inflammatory response in neuronal cells. ACS Infect. Dis. 2019, 5, 932–948. [Google Scholar] [CrossRef]
- Ye, J.; Chen, Z.; Li, Y.; Zhao, Z.; He, W.; Zohaib, A.; Song, Y.; Deng, C.; Zhang, B.; Chen, H.; et al. Japanese encephalitis virus NS5 Inhibits Type I Interferon (IFN) production by blocking the nuclear translocation of ifn regulatory factor 3 and NF-κB. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Lin, S.; Yang, S.; He, J.; Guest, J.D.; Ma, Z.; Yang, L.; Pierce, B.G.; Tang, Q.; Zhang, Y.J. Zika virus NS5 protein antagonizes type I interferon production via blocking TBK1 activation. Virology 2019, 527, 180–187. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, L.; Chang, P.; Yang, S.; Lin, S.; Tang, Q.; Wang, X.; Zhang, Y.J. Zika virus NS2A protein induces the degradation of KPNA2 (karyopherin subunit alpha 2) via chaperone-mediated autophagy. Autophagy 2020, 16, 2238–2251. [Google Scholar] [CrossRef]
- Remenyi, R.; Gao, Y.; Hughes, R.E.; Curd, A.; Zothner, C.; Peckham, M.; Merits, A.; Harris, M. Persistent replication of a chikungunya virus replicon in human cells is associated with presence of stable cytoplasmic granules containing nonstructural protein 3. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Hawman, D.W.; Stoermer, K.A.; Montgomery, S.A.; Pal, P.; Oko, L.; Diamond, M.S.; Morrison, T.E. Chronic joint disease caused by persistent Chikungunya virus infection is controlled by the adaptive immune response. J. Virol. 2013, 87, 13878–13888. [Google Scholar] [CrossRef]
- Thomas, S.; Rai, J.; John, L.; Schaefer, S.; Pützer, B.M.; Herchenröder, O. Chikungunya virus capsid protein contains nuclear import and export signals. Virol. J. 2013, 10, 269. [Google Scholar] [CrossRef]
- Jacobs, S.C.; Taylor, A.; Herrero, L.J.; Mahalingam, S.; Fazakerley, J.K. Mutation of a conserved nuclear export sequence in chikungunya virus capsid protein disrupts host cell nuclear import. Viruses 2017, 9, 306. [Google Scholar] [CrossRef]
- Webb, L.G.; Veloz, J.; Pintado-Silva, J.; Zhu, T.; Rangel, M.V.; Mutetwa, T.; Zhang, L.; Bernal-Rubio, D.; Figueroa, D.; Carrau, L.; et al. Chikungunya virus antagonizes cGAS-STING mediated type-I interferon responses by degrading cGAS. PLoS Pathog. 2020, 16, e1008999. [Google Scholar] [CrossRef]
- Göertz, G.P.; McNally, K.L.; Robertson, S.J.; Best, S.M.; Pijlman, G.P.; Fros, J.J. The methyltransferase-like domain of chikungunya virus nsP2 inhibits the interferon response by promoting the nuclear export of STAT1. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Fros, J.J.; Liu, W.J.; Prow, N.A.; Geertsema, C.; Ligtenberg, M.; Vanlandingham, D.L.; Schnettler, E.; Vlak, J.M.; Suhrbier, A.; Khromykh, A.A.; et al. Chikungunya virus nonstructural protein 2 inhibits type I/II interferon-stimulated JAK-STAT signaling. J. Virol. 2010, 84, 10877–10887. [Google Scholar] [CrossRef] [PubMed]
- Fros, J.J.; van der Maten, E.; Vlak, J.M.; Pijlman, G.P. The C-terminal domain of chikungunya virus nsP2 independently governs viral RNA replication, cytopathicity, and inhibition of interferon signaling. J. Virol. 2013, 87, 10394–10400. [Google Scholar] [CrossRef]
- Wong, K.Z.; Chu, J.J.H. The interplay of viral and host factors in chikungunya virus infection: Targets for antiviral strategies. Viruses 2018, 10, 294. [Google Scholar] [CrossRef]
- Fros, J.J.; Major, L.D.; Scholte, F.E.M.; Gardner, J.; van Hemert, M.J.; Suhrbier, A.; Pijlman, G.P. Chikungunya virus non-structural protein 2-mediated host shut-off disables the unfolded protein response. J. Gen. Virol. 2015, 96, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Nat. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef]
- Barré-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vézinet-Brun, F.; Rouzioux, C.; et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Engelman, A.N.; Singh, P.K. Cellular and molecular mechanisms of HIV-1 integration targeting. Cell. Mol. Life Sci. 2018, 75, 2491–2507. [Google Scholar] [CrossRef]
- Wong, R.W.; Mamede, J.I.; Hope, T.J. Impact of nucleoporin-mediated chromatin localization and nuclear architecture on HIV integration site selection. J. Virol. 2015, 89, 9702–9705. [Google Scholar] [CrossRef][Green Version]
- Popov, S.; Rexach, M.; Ratner, L.; Blobel, G.; Bukrinsky, M. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J. Biol. Chem. 1998, 273, 13347–13352. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, M.; Huang, Q.; Gates, A.T.; Zhang, X.D.; Castle, J.C.; Stec, E.; Ferrer, M.; Strulovici, B.; Hazuda, D.J.; et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe 2008, 4, 495–504. [Google Scholar] [CrossRef]
- König, R.; Zhou, Y.; Elleder, D.; Diamond, T.L.; Bonamy, G.M.; Irelan, J.T.; Chiang, C.Y.; Tu, B.P.; De Jesus, P.D.; Lilley, C.E.; et al. Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell 2008, 135, 49–60. [Google Scholar] [CrossRef]
- Brass, A.L.; Dykxhoorn, D.M.; Benita, Y.; Yan, N.; Engelman, A.; Xavier, R.J.; Lieberman, J.; Elledge, S.J. Identification of host proteins required for HIV infection through a functional genomic screen. Science 2008, 319, 921–926. [Google Scholar] [CrossRef]
- Imbeault, M.; Ouellet, M.; Tremblay, M.J. Microarray study reveals that HIV-1 induces rapid type-I interferon-dependent p53 mRNA up-regulation in human primary CD4+ T cells. Retrovirology 2009, 6, 5. [Google Scholar] [CrossRef]
- Tsurutani, N.; Kubo, M.; Maeda, Y.; Ohashi, T.; Yamamoto, N.; Kannagi, M.; Masuda, T. Identification of critical amino acid residues in human immunodeficiency virus type 1 IN required for efficient proviral DNA formation at steps prior to integration in dividing and nondividing cells. J. Virol. 2000, 74, 4795–4806. [Google Scholar] [CrossRef] [PubMed]
- Gallay, P.; Stitt, V.; Mundy, C.; Oettinger, M.; Trono, D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J. Virol. 1996, 70, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Fouchier, R.A.; Meyer, B.E.; Simon, J.H.; Fischer, U.; Malim, M.H. HIV-1 infection of non-dividing cells: Evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 1997, 16, 4531–4539. [Google Scholar] [CrossRef]
- Ganser-Pornillos, B.K.; Cheng, A.; Yeager, M. Structure of full-length HIV-1 CA: A model for the mature capsid lattice. Cell 2007, 131, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Arhel, N.J.; Souquere-Besse, S.; Munier, S.; Souque, P.; Guadagnini, S.; Rutherford, S.; Prévost, M.C.; Allen, T.D.; Charneau, P. HIV-1 DNA Flap formation promotes uncoating of the pre-integration complex at the nuclear pore. EMBO J. 2007, 26, 3025–3037. [Google Scholar] [CrossRef]
- Li, C.; Burdick, R.C.; Nagashima, K.; Hu, W.S.; Pathak, V.K. HIV-1 cores retain their integrity until minutes before uncoating in the nucleus. Proc. Nat. Acad. Sci. USA 2021, 118, e2019467118. [Google Scholar] [CrossRef]
- Francis, A.C.; Marin, M.; Prellberg, M.J.; Palermino-Rowland, K.; Melikyan, G.B. HIV-1 Uncoating and Nuclear Import Precede the Completion of Reverse Transcription in Cell Lines and in Primary Macrophages. Viruses 2020, 12, 1234. [Google Scholar] [CrossRef]
- Dharan, A.; Bachmann, N.; Talley, S.; Zwikelmaier, V.; Campbell, E.M. Nuclear pore blockade reveals that HIV-1 completes reverse transcription and uncoating in the nucleus. Nat. Microbiol. 2020, 5, 1088–1095. [Google Scholar] [CrossRef]
- Zila, V.; Margiotta, E.; Turoňová, B.; Müller, T.G.; Zimmerli, C.E.; Mattei, S.; Allegretti, M.; Börner, K.; Rada, J.; Müller, B.; et al. Cone-shaped HIV-1 capsids are transported through intact nuclear pores. Cell 2021, 184, 1032–1046.e18. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Sumner, R.P.; Rasaiyaah, J.; Tan, C.P.; Rodriguez-Plata, M.T.; Van Tulleken, C.; Fink, D.; Zuliani-Alvarez, L.; Thorne, L.; Stirling, D.; et al. HIV-1 Vpr antagonizes innate immune activation by targeting karyopherin-mediated NF-κB/IRF3 nuclear transport. eLife 2020, 9, e60821. [Google Scholar] [CrossRef]
- Karni, O.; Friedler, A.; Zakai, N.; Gilon, C.; Loyter, A. A peptide derived from the N-terminal region of HIV-1 Vpr promotes nuclear import in permeabilized cells: Elucidation of the NLS region of the Vpr. FEBS Lett. 1998, 429, 421–425. [Google Scholar] [CrossRef]
- Nitahara-Kasahara, Y.; Kamata, M.; Yamamoto, T.; Zhang, X.; Miyamoto, Y.; Muneta, K.; Iijima, S.; Yoneda, Y.; Tsunetsugu-Yokota, Y.; Aida, Y. Novel nuclear import of Vpr promoted by importin alpha is crucial for human immunodeficiency virus type 1 replication in macrophages. J. Virol. 2007, 81, 5284–5293. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, H.; Sanjoh, A.; Murakami, T.; Murakami, H.; Matsuda, G.; Hagiwara, K.; Yokoyama, M.; Sato, H.; Miyamoto, Y.; Dohmae, N.; et al. Molecular Mechanism of HIV-1 Vpr for Binding to Importin-α. J. Mol. Biol. 2016, 428, 2744–2757. [Google Scholar] [CrossRef]
- Pollard, V.W.; Malim, M.H. The HIV-1 Rev protein. Ann. Rev. Microbiol. 1998, 52, 491–532. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.R.; Percipalle, P. Interactions between HIV Rev and nuclear import and export factors: The Rev nuclear localisation signal mediates specific binding to human importin-beta. J. Mol. Biol. 1997, 274, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Emerman, M.; Malim, M.H. HIV-1 regulatory/accessory genes: Keys to unraveling viral and host cell biology. Science 1998, 280, 1880–1884. [Google Scholar] [CrossRef]
- Bogerd, H.P.; Echarri, A.; Ross, T.M.; Cullen, B.R. Inhibition of human immunodeficiency virus Rev and human T-cell leukemia virus Rex function, but not Mason-Pfizer monkey virus constitutive transport element activity, by a mutant human nucleoporin targeted to Crm1. J. Virol. 1998, 72, 8627–8635. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, W.; Reichart, B.; Ewald, A.; Müller, E.; Schmitt, I.; Stauber, R.H.; Lottspeich, F.; Jockusch, B.M.; Scheer, U.; Hauber, J.; et al. Cofactor requirements for nuclear export of Rev response element (RRE)- and constitutive transport element (CTE)-containing retroviral RNAs. An unexpected role for actin. J. Cell Biol. 2001, 152, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Ullman, K.S.; Shah, S.; Powers, M.A.; Forbes, D.J. The nucleoporin nup153 plays a critical role in multiple types of nuclear export. Mol. Biol. Cell 1999, 10, 649–664. [Google Scholar] [CrossRef]
- Zolotukhin, A.S.; Felber, B.K. Nucleoporins nup98 and nup214 participate in nuclear export of human immunodeficiency virus type 1 Rev. J. Virol. 1999, 73, 120–127. [Google Scholar] [CrossRef]
- Monette, A.; Panté, N.; Mouland, A.J. HIV-1 remodels the nuclear pore complex. J. Cell Biol. 2011, 193, 619–631. [Google Scholar] [CrossRef]
- Taniguchi, I.; Mabuchi, N.; Ohno, M. HIV-1 Rev protein specifies the viral RNA export pathway by suppressing TAP/NXF1 recruitment. Nucl. Acids Res. 2014, 42, 6645–6658. [Google Scholar] [CrossRef] [PubMed]
- McCauley, S.M.; Kim, K.; Nowosielska, A.; Dauphin, A.; Yurkovetskiy, L.; Diehl, W.E.; Luban, J. Intron-containing RNA from the HIV-1 provirus activates type I interferon and inflammatory cytokines. Nat. Commun. 2018, 9, 5305. [Google Scholar] [CrossRef]
- Te Velthuis, A.J.W.; Grimes, J.M.; Fodor, E. Structural insights into RNA polymerases of negative-sense RNA viruses. Nat. Rev. Microbiol. 2021, 19, 303–318. [Google Scholar] [CrossRef]
- O’Neill, R.E.; Jaskunas, R.; Blobel, G.; Palese, P.; Moroianu, J. Nuclear import of influenza virus RNA can be mediated by viral nucleoprotein and transport factors required for protein import. J. Biol. Chem. 1995, 270, 22701–22704. [Google Scholar] [CrossRef]
- Wang, P.; Palese, P.; O’Neill, R.E. The NPI-1/NPI-3 (karyopherin alpha) binding site on the influenza a virus nucleoprotein NP is a nonconventional nuclear localization signal. J. Virol. 1997, 71, 1850–1856. [Google Scholar] [CrossRef]
- Weber, F.; Kochs, G.; Gruber, S.; Haller, O. A classical bipartite nuclear localization signal on Thogoto and influenza A virus nucleoproteins. Virology 1998, 250, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Cros, J.F.; García-Sastre, A.; Palese, P. An unconventional NLS is critical for the nuclear import of the influenza A virus nucleoprotein and ribonucleoprotein. Traffic 2005, 6, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Donchet, A.; Vassal-Stermann, E.; Gérard, F.C.A.; Ruigrok, R.W.H.; Crépin, T. Differential behaviours and preferential bindings of influenza nucleoproteins on importins-α. Viruses 2020, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Tarendeau, F.; Boudet, J.; Guilligay, D.; Mas, P.J.; Bougault, C.M.; Boulo, S.; Baudin, F.; Ruigrok, R.W.; Daigle, N.; Ellenberg, J.; et al. Structure and nuclear import function of the C-terminal domain of influenza virus polymerase PB2 subunit. Nat. Struct. Mol. Biol. 2007, 14, 229–233. [Google Scholar] [CrossRef]
- Gabriel, G.; Herwig, A.; Klenk, H.D. Interaction of polymerase subunit PB2 and NP with importin alpha1 is a determinant of host range of influenza A virus. PLoS Pathog. 2008, 4, e11. [Google Scholar] [CrossRef] [PubMed]
- Hudjetz, B.; Gabriel, G. Human-like PB2 627K influenza virus polymerase activity is regulated by importin-α1 and -α7. PLoS Pathog. 2012, 8, e1002488. [Google Scholar] [CrossRef]
- Pumroy, R.A.; Ke, S.; Hart, D.J.; Zachariae, U.; Cingolani, G. Molecular determinants for nuclear import of influenza A PB2 by importin α isoforms 3 and 7. Structure 2015, 23, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Thiele, S.; Stanelle-Bertram, S.; Beck, S.; Kouassi, N.M.; Zickler, M.; Müller, M.; Tuku, B.; Resa-Infante, P.; van Riel, D.; Alawi, M.; et al. Cellular Importin-α3 Expression Dynamics in the Lung Regulate Antiviral Response Pathways against Influenza A Virus Infection. Cell Rep. 2020, 31, 107549. [Google Scholar] [CrossRef]
- Swale, C.; Monod, A.; Tengo, L.; Labaronne, A.; Garzoni, F.; Bourhis, J.M.; Cusack, S.; Schoehn, G.; Berger, I.; Ruigrok, R.W.; et al. Structural characterization of recombinant IAV polymerase reveals a stable complex between viral PA-PB1 heterodimer and host RanBP5. Sci. Rep. 2016, 6, 24727. [Google Scholar] [CrossRef]
- Swale, C.; Da Costa, B.; Sedano, L.; Garzoni, F.; McCarthy, A.A.; Berger, I.; Bieniossek, C.; Ruigrok, R.W.H.; Delmas, B.; Crépin, T. X-ray structure of the human karyopherin RanBP5, an essential factor for influenza polymerase nuclear trafficking. J. Mol. Biol. 2020, 432, 3353–3359. [Google Scholar] [CrossRef]
- Brunotte, L.; Flies, J.; Bolte, H.; Reuther, P.; Vreede, F.; Schwemmle, M. The nuclear export protein of H5N1 influenza A viruses recruits Matrix 1 (M1) protein to the viral ribonucleoprotein to mediate nuclear export. J. Biol. Chem. 2014, 289, 20067–20077. [Google Scholar] [CrossRef]
- Gao, S.; Wang, S.; Cao, S.; Sun, L.; Li, J.; Bi, Y.; Gao, G.F.; Liu, W. Characteristics of nucleocytoplasmic transport of H1N1 influenza A virus nuclear export protein. J. Virol. 2014, 88, 7455–7463. [Google Scholar] [CrossRef]
- Satterly, N.; Tsai, P.L.; van Deursen, J.; Nussenzveig, D.R.; Wang, Y.; Faria, P.A.; Levay, A.; Levy, D.E.; Fontoura, B.M. Influenza virus targets the mRNA export machinery and the nuclear pore complex. Proc. Nat. Acad. Sci. USA 2007, 104, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xie, Y.; Muñoz-Moreno, R.; Wang, J.; Zhang, L.; Esparza, M.; García-Sastre, A.; Fontoura, B.M.A.; Ren, Y. Structural basis for influenza virus NS1 protein block of mRNA nuclear export. Nat. Microb. 2019, 4, 1671–1679. [Google Scholar] [CrossRef]
- Nemeroff, M.E.; Barabino, S.M.; Li, Y.; Keller, W.; Krug, R.M. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′end formation of cellular pre-mRNAs. Mol. Cell 1998, 1, 991–1000. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Krug, R.M. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3′-end processing machinery. EMBO J. 1999, 18, 2273–2283. [Google Scholar] [CrossRef]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef]
- Van Doorslaer, K.; Chen, Z.; Bernard, H.U.; Chan, P.K.S.; DeSalle, R.; Dillner, J.; Forslund, O.; Haga, T.; McBride, A.A.; Villa, L.L.; et al. ICTV Virus Taxonomy Profile: Papillomaviridae. J. Gen. Virol. 2018, 99, 989–990. [Google Scholar] [CrossRef]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Eng. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef]
- Aydin, I.; Weber, S.; Snijder, B.; Samperio Ventayol, P.; Kühbacher, A.; Becker, M.; Day, P.M.; Schiller, J.T.; Kann, M.; Pelkmans, L.; et al. Large scale RNAi reveals the requirement of nuclear envelope breakdown for nuclear import of human papillomaviruses. PLoS Pathog. 2014, 10, e1004162. [Google Scholar] [CrossRef]
- Pyeon, D.; Pearce, S.M.; Lank, S.M.; Ahlquist, P.; Lambert, P.F. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog. 2009, 5, e1000318. [Google Scholar] [CrossRef]
- Tao, M.; Kruhlak, M.; Xia, S.; Androphy, E.; Zheng, Z.M. Signals that dictate nuclear localization of human papillomavirus type 16 oncoprotein E6 in living cells. J. Virol. 2003, 77, 13232–13247. [Google Scholar] [CrossRef]
- Le Roux, L.G.; Moroianu, J. Nuclear entry of high-risk human papillomavirus type 16 E6 oncoprotein occurs via several pathways. J. Virol. 2003, 77, 2330–2337. [Google Scholar] [CrossRef]
- Masson, M.; Hindelang, C.; Sibler, A.P.; Schwalbach, G.; Travé, G.; Weiss, E. Preferential nuclear localization of the human papillomavirus type 16 E6 oncoprotein in cervical carcinoma cells. J. Gen. Virol. 2003, 84, 2099–2104. [Google Scholar] [CrossRef]
- Freedman, D.A.; Levine, A.J. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol. Cell. Biol. 1998, 18, 7288–7293. [Google Scholar] [CrossRef]
- Stewart, D.; Ghosh, A.; Matlashewski, G. Involvement of nuclear export in human papillomavirus type 18 E6-mediated ubiquitination and degradation of p53. J. Virol. 2005, 79, 8773–8783. [Google Scholar] [CrossRef][Green Version]
- McLaughlin-Drubin, M.E.; Münger, K. The human papillomavirus E7 oncoprotein. Virology 2009, 384, 335–344. [Google Scholar] [CrossRef]
- Angeline, M.; Merle, E.; Moroianu, J. The E7 oncoprotein of high-risk human papillomavirus type 16 enters the nucleus via a nonclassical Ran-dependent pathway. Virology 2003, 317, 13–23. [Google Scholar] [CrossRef]
- Knapp, A.A.; McManus, P.M.; Bockstall, K.; Moroianu, J. Identification of the nuclear localization and export signals of high risk HPV16 E7 oncoprotein. Virology 2009, 383, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, J.; Onder, Z.; Moroianu, J. Nuclear import of high risk HPV16 E7 oncoprotein is mediated by its zinc-binding domain via hydrophobic interactions with Nup62. Virology 2013, 446, 334–345. [Google Scholar] [CrossRef]
- Onder, Z.; Moroianu, J. Nuclear import of cutaneous beta genus HPV8 E7 oncoprotein is mediated by hydrophobic interactions between its zinc-binding domain and FG nucleoporins. Virology 2014, 449, 150–162. [Google Scholar] [CrossRef][Green Version]
- McKee, C.H.; Onder, Z.; Ashok, A.; Cardoso, R.; Moroianu, J. Characterization of the transport signals that mediate the nucleocytoplasmic traffic of low risk HPV11 E7. Virology 2013, 443, 113–122. [Google Scholar] [CrossRef]
- Deng, W.; Lin, B.Y.; Jin, G.; Wheeler, C.G.; Ma, T.; Harper, J.W.; Broker, T.R.; Chow, L.T. Cyclin/CDK regulates the nucleocytoplasmic localization of the human papillomavirus E1 DNA helicase. J. Virol. 2004, 78, 13954–13965. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Wang, Q.; Griffin, H.M.; Murakami, I.; Jackson, D.; Mahmood, R.; Doorbar, J. HPV16 and 18 genome amplification show different E4-dependence, with 16E4 enhancing E1 nuclear accumulation and replicative efficiency via its cell cycle arrest and kinase activation functions. PLoS Pathog. 2017, 13, e1006282. [Google Scholar] [CrossRef]
- Yu, J.H.; Lin, B.Y.; Deng, W.; Broker, T.R.; Chow, L.T. Mitogen-activated protein kinases activate the nuclear localization sequence of human papillomavirus type 11 E1 DNA helicase to promote efficient nuclear import. J. Virol. 2007, 81, 5066–5078. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.L.; Rosas-Acosta, G.; Wu, Y.C.; Wilson, V.G. Nuclear import of bovine papillomavirus type 1 E1 protein is mediated by multiple alpha importins and is negatively regulated by phosphorylation near a nuclear localization signal. J. Virol. 2007, 81, 2899–2908. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fradet-Turcotte, A.; Moody, C.; Laimins, L.A.; Archambault, J. Nuclear export of human papillomavirus type 31 E1 is regulated by Cdk2 phosphorylation and required for viral genome maintenance. J. Virol. 2010, 84, 11747–11760. [Google Scholar] [CrossRef] [PubMed]
- Blachon, S.; Bellanger, S.; Demeret, C.; Thierry, F. Nucleo-cytoplasmic shuttling of high risk human Papillomavirus E2 proteins induces apoptosis. J. Biol. Chem. 2005, 280, 36088–36098. [Google Scholar] [CrossRef] [PubMed]
- Klucevsek, K.; Wertz, M.; Lucchi, J.; Leszczynski, A.; Moroianu, J. Characterization of the nuclear localization signal of high risk HPV16 E2 protein. Virology 2007, 360, 191–198. [Google Scholar] [CrossRef]
- Nelson, L.M.; Rose, R.C.; Moroianu, J. Nuclear import strategies of high risk HPV16 L1 major capsid protein. J. Biol. Chem. 2002, 277, 23958–23964. [Google Scholar] [CrossRef] [PubMed]
- Darshan, M.S.; Lucchi, J.; Harding, E.; Moroianu, J. The l2 minor capsid protein of human papillomavirus type 16 interacts with a network of nuclear import receptors. J. Virol. 2004, 78, 12179–12188. [Google Scholar] [CrossRef] [PubMed]
- Papaevangelou, G.; Roumeliotou-Karayannis, A.; Tassopoulos, N.; Kolaitis, N.; Contoyannis, P.; Krugman, S. Post-exposure hepatitis B vaccination of sexual partners of acute viral hepatitis patients. J. Infect. 1983, 7, 63–67. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Singh, A.; Dubey, S.K.; Hetta, H.F.; John, J.; Singh, M.P. Molecular mechanistic insight of hepatitis B virus mediated hepatocellular carcinoma. Microb. Pathog. 2019, 128, 184–194. [Google Scholar] [CrossRef]
- Nassal, M. HBV cccDNA: Viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015, 64, 1972–1984. [Google Scholar] [CrossRef]
- Kann, M.; Sodeik, B.; Vlachou, A.; Gerlich, W.H.; Helenius, A. Phosphorylation-dependent binding of hepatitis B virus core particles to the nuclear pore complex. J. Cell Biol. 1999, 145, 45–55. [Google Scholar] [CrossRef]
- Rabe, B.; Vlachou, A.; Panté, N.; Helenius, A.; Kann, M. Nuclear import of hepatitis B virus capsids and release of the viral genome. Proc. Nat. Acad. Sci. USA 2003, 100, 9849–9854. [Google Scholar] [CrossRef]
- Guo, H.; Mao, R.; Block, T.M.; Guo, J.T. Production and function of the cytoplasmic deproteinized relaxed circular DNA of hepadnaviruses. J. Virol. 2010, 84, 387–396. [Google Scholar] [CrossRef]
- Schmitz, A.; Schwarz, A.; Foss, M.; Zhou, L.; Rabe, B.; Hoellenriegel, J.; Stoeber, M.; Panté, N.; Kann, M. Nucleoporin 153 arrests the nuclear import of hepatitis B virus capsids in the nuclear basket. PLoS Pathog. 2010, 6, e1000741. [Google Scholar] [CrossRef]
- Li, H.C.; Huang, E.Y.; Su, P.Y.; Wu, S.Y.; Yang, C.C.; Lin, Y.S.; Chang, W.C.; Shih, C. Nuclear export and import of human hepatitis B virus capsid protein and particles. PLoS Pathog. 2010, 6, e1001162. [Google Scholar] [CrossRef]
- Nair, S.; Zlotnick, A. HBV Core protein is in flux between cytoplasmic, nuclear, and nucleolar compartments. mBio 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J.C.; Pierson, E.E.; Keifer, D.Z.; Delaleau, M.; Gallucci, L.; Cazenave, C.; Kann, M.; Jarrold, M.F.; Zlotnick, A. Importin β can bind hepatitis b virus core protein and empty core-like particles and induce structural changes. PLoS Pathog. 2016, 12, e1005802. [Google Scholar] [CrossRef]
- Mitra, B.; Wang, J.; Kim, E.S.; Mao, R.; Dong, M.; Liu, Y.; Zhang, J.; Guo, H. Hepatitis B Virus Precore Protein p22 Inhibits Alpha Interferon Signaling by Blocking STAT Nuclear Translocation. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Dai, X.; Zhou, Z.H. Structure of the herpes simplex virus 1 capsid with associated tegument protein complexes. Science 2018, 360, eaao7298. [Google Scholar] [CrossRef]
- Pasdeloup, D.; Blondel, D.; Isidro, A.L.; Rixon, F.J. Herpesvirus capsid association with the nuclear pore complex and viral DNA release involve the nucleoporin CAN/Nup214 and the capsid protein pUL25. J. Virol. 2009, 83, 6610–6623. [Google Scholar] [CrossRef]
- Copeland, A.M.; Newcomb, W.W.; Brown, J.C. Herpes simplex virus replication: Roles of viral proteins and nucleoporins in capsid-nucleus attachment. J. Virol. 2009, 83, 1660–1668. [Google Scholar] [CrossRef]
- Abaitua, F.; Hollinshead, M.; Bolstad, M.; Crump, C.M.; O’Hare, P. A Nuclear localization signal in herpesvirus protein VP1-2 is essential for infection via capsid routing to the nuclear pore. J. Virol. 2012, 86, 8998–9014. [Google Scholar] [CrossRef] [PubMed]
- Ojala, P.M.; Sodeik, B.; Ebersold, M.W.; Kutay, U.; Helenius, A. Herpes simplex virus type 1 entry into host cells: Reconstitution of capsid binding and uncoating at the nuclear pore complex in vitro. Mol. Cell. Biol. 2000, 20, 4922–4931. [Google Scholar] [CrossRef] [PubMed]
- Döhner, K.; Ramos-Nascimento, A.; Bialy, D.; Anderson, F.; Hickford-Martinez, A.; Rother, F.; Koithan, T.; Rudolph, K.; Buch, A.; Prank, U.; et al. Importin α1 is required for nuclear import of herpes simplex virus proteins and capsid assembly in fibroblasts and neurons. PLoS Pathog. 2018, 14, e1006823. [Google Scholar] [CrossRef] [PubMed]
- Marsden, H.S.; Murphy, M.; McVey, G.L.; MacEachran, K.A.; Owsianka, A.M.; Stow, N.D. Role of the carboxy terminus of herpes simplex virus type 1 DNA polymerase in its interaction with UL42. J. Gen. Virol. 1994, 75, 3127–3135. [Google Scholar] [CrossRef]
- Loregian, A.; Piaia, E.; Cancellotti, E.; Papini, E.; Marsden, H.S.; Palù, G. The catalytic subunit of herpes simplex virus type 1 DNA polymerase contains a nuclear localization signal in the UL42-binding region. Virology 2000, 273, 139–148. [Google Scholar] [CrossRef]
- Alvisi, G.; Musiani, D.; Jans, D.A.; Ripalti, A. An importin alpha/beta-recognized bipartite nuclear localization signal mediates targeting of the human herpes simplex virus type 1 DNA polymerase catalytic subunit pUL30 to the nucleus. Biochemistry 2007, 46, 9155–9163. [Google Scholar] [CrossRef] [PubMed]
- Alvisi, G.; Avanzi, S.; Musiani, D.; Camozzi, D.; Leoni, V.; Ly-Huynh, J.D.; Ripalti, A. Nuclear import of HSV-1 DNA polymerase processivity factor UL42 is mediated by a C-terminally located bipartite nuclear localization signal. Biochemistry 2008, 47, 13764–13777. [Google Scholar] [CrossRef]
- Yusuf, B.; Gopurappilly, R.; Dadheech, N.; Gupta, S.; Bhonde, R.; Pal, R. Embryonic fibroblasts represent a connecting link between mesenchymal and embryonic stem cells. Dev. Growth Differ. 2013, 55, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.W.; Malik, P.; Clements, J.B. The herpes simplex virus ICP27 protein: A multifunctional post-transcriptional regulator of gene expression. Biochem. Soc. Trans. 2005, 33, 499–501. [Google Scholar] [CrossRef]
- Sandri-Goldin, R.M. The many roles of the regulatory protein ICP27 during herpes simplex virus infection. Front. Biosci. J. Virtual Librar. 2008, 13, 5241–5256. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.H.; Li, L.; Silva, L.; Sandri-Goldin, R.M. ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export. J. Virol. 2005, 79, 3949–3961. [Google Scholar] [CrossRef]
- Escudero-Paunetto, L.; Li, L.; Hernandez, F.P.; Sandri-Goldin, R.M. SR proteins SRp20 and 9G8 contribute to efficient export of herpes simplex virus 1 mRNAs. Virology 2010, 401, 155–164. [Google Scholar] [CrossRef]
- Koffa, M.D.; Clements, J.B.; Izaurralde, E.; Wadd, S.; Wilson, S.A.; Mattaj, I.W.; Kuersten, S. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 2001, 20, 5769–5778. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Tabarraei, A.; Kehlenbach, R.H.; Korfali, N.; Iwasawa, R.; Graham, S.V.; Schirmer, E.C. Herpes simplex virus ICP27 protein directly interacts with the nuclear pore complex through Nup62, inhibiting host nucleocytoplasmic transport pathways. J. Biol. Chem. 2012, 287, 12277–12292. [Google Scholar] [CrossRef]
- Boruchowicz, H.; Hawkins, J.; Cruz-Palomar, K.; Lippé, R. The XPO6 Exportin Mediates Herpes Simplex Virus 1 gM Nuclear Release Late in Infection. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Walter, G.; Richert, Q.; Ponnampalam, A.; Sharma, A. Acute superior mesenteric vein thrombosis in the setting of cytomegalovirus mononucleosis: A case report and review of the literature. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Bowen, L.N.; Smith, B.; Reich, D.; Quezado, M.; Nath, A. HIV-associated opportunistic CNS infections: Pathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2016, 12, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.B.; James, S.H.; Prichard, M.N. Human cytomegalovirus UL97 kinase alters the accumulation of CDK1. J. Gen. Virol. 2012, 93, 1743–1755. [Google Scholar] [CrossRef]
- Webel, R.; Solbak, S.; Held, C.; Milbradt, J.; Groß, A.; Eichler, J.; Wittenberg, T.; Jardin, C.; Sticht, H.; Fossen, T.; et al. Nuclear import of isoforms of the cytomegalovirus kinase pUL97 is mediated by differential activity of NLS1 and NLS2 both acting through classical importin-α binding. J. Gen. Virol. 2012, 93, 1756–1768. [Google Scholar] [CrossRef]
- Perng, Y.C.; Campbell, J.A.; Lenschow, D.J.; Yu, D. Human cytomegalovirus pUL79 is an elongation factor of RNA polymerase II for viral gene transcription. PLoS Pathog. 2014, 10, e1004350. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Cai, M.; Xing, J.; Wang, S.; Zheng, C. A PY-nuclear localization signal is required for nuclear accumulation of HCMV UL79 protein. Med. Microbiol. Immunol. 2012, 201, 381–387. [Google Scholar] [CrossRef]
- Gao, Y.; Kagele, D.; Smallenberg, K.; Pari, G.S. Nucleocytoplasmic shuttling of human cytomegalovirus UL84 is essential for virus growth. J. Virol. 2010, 84, 8484–8494. [Google Scholar] [CrossRef]
- Lischka, P.; Sorg, G.; Kann, M.; Winkler, M.; Stamminger, T. A nonconventional nuclear localization signal within the UL84 protein of human cytomegalovirus mediates nuclear import via the importin alpha/beta pathway. J. Virol. 2003, 77, 3734–3748. [Google Scholar] [CrossRef][Green Version]
- Lischka, P.; Rauh, C.; Mueller, R.; Stamminger, T. Human cytomegalovirus UL84 protein contains two nuclear export signals and shuttles between the nucleus and the cytoplasm. J. Virol. 2006, 80, 10274–10280. [Google Scholar] [CrossRef][Green Version]
- Toth, Z.; Lischka, P.; Stamminger, T. RNA-binding of the human cytomegalovirus transactivator protein UL69, mediated by arginine-rich motifs, is not required for nuclear export of unspliced RNA. Nucl. Acids Res. 2006, 34, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Zielke, B.; Thomas, M.; Giede-Jeppe, A.; Müller, R.; Stamminger, T. Characterization of the betaherpesviral pUL69 protein family reveals binding of the cellular mRNA export factor UAP56 as a prerequisite for stimulation of nuclear mRNA export and for efficient viral replication. J. Virol. 2011, 85, 1804–1819. [Google Scholar] [CrossRef] [PubMed]
- Stamminger, T. Interactions of human cytomegalovirus proteins with the nuclear transport machinery. Curr. Top. Microbiol. Immunol. 2008, 325, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Lischka, P.; Rosorius, O.; Trommer, E.; Stamminger, T. A novel transferable nuclear export signal mediates CRM1-independent nucleocytoplasmic shuttling of the human cytomegalovirus transactivator protein pUL69. EMBO J. 2001, 20, 7271–7283. [Google Scholar] [CrossRef][Green Version]
- Rechter, S.; Scott, G.M.; Eickhoff, J.; Zielke, K.; Auerochs, S.; Müller, R.; Stamminger, T.; Rawlinson, W.D.; Marschall, M. Cyclin-dependent Kinases Phosphorylate the Cytomegalovirus RNA Export Protein pUL69 and Modulate Its Nuclear Localization and Activity. J. Biol. Chem. 2009, 284, 8605–8613. [Google Scholar] [CrossRef] [PubMed]
- Münz, C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat. Rev. Microbiol. 2019, 17, 691–700. [Google Scholar] [CrossRef]
- Ito, S.; Ikeda, M.; Kato, N.; Matsumoto, A.; Ishikawa, Y.; Kumakubo, S.; Yanagi, K. Epstein-barr virus nuclear antigen-1 binds to nuclear transporter karyopherin alpha1/NPI-1 in addition to karyopherin alpha2/Rch1. Virology 2000, 266, 110–119. [Google Scholar] [CrossRef][Green Version]
- Chang, C.W.; Lee, C.P.; Su, M.T.; Tsai, C.H.; Chen, M.R. BGLF4 kinase modulates the structure and transport preference of the nuclear pore complex to facilitate nuclear import of Epstein-Barr virus lytic proteins. J. Virol. 2015, 89, 1703–1718. [Google Scholar] [CrossRef]
- Lee, C.P.; Huang, Y.H.; Lin, S.F.; Chang, Y.; Chang, Y.H.; Takada, K.; Chen, M.R. Epstein-Barr virus BGLF4 kinase induces disassembly of the nuclear lamina to facilitate virion production. J. Virol. 2008, 82, 11913–11926. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kamil, J.P.; Coughlin, M.; Reim, N.I.; Coen, D.M. Human cytomegalovirus UL50 and UL53 recruit viral protein kinase UL97, not protein kinase C, for disruption of nuclear lamina and nuclear egress in infected cells. J. Virol. 2014, 88, 249–262. [Google Scholar] [CrossRef]
- Yang, Y.C.; Sugden, B. Epstein-Barr Virus Limits the Accumulation of IPO7, an Essential Gene Product. Front. Microbiol. 2021, 12, 643327. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Gradoville, L.; Miller, G. Cellular immediate-early gene expression occurs kinetically upstream of Epstein-Barr virus bzlf1 and brlf1 following cross-linking of the B cell antigen receptor in the Akata Burkitt lymphoma cell line. J. Virol. 2010, 84, 12405–12418. [Google Scholar] [CrossRef]
- Boyle, S.M.; Ruvolo, V.; Gupta, A.K.; Swaminathan, S. Association with the cellular export receptor CRM 1 mediates function and intracellular localization of Epstein-Barr virus SM protein, a regulator of gene expression. J. Virol. 1999, 73, 6872–6881. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.; Ott, M.; Raschbichler, V.; Nagel, C.H.; Binz, A.; Sodeik, B.; Bauerfeind, R.; Bailer, S.M. The herpes simplex virus protein pUL31 escorts nucleocapsids to sites of nuclear egress, a process coordinated by its N-terminal domain. PLoS Pathog. 2015, 11, e1004957. [Google Scholar] [CrossRef]
- Gonnella, R.; Farina, A.; Santarelli, R.; Raffa, S.; Feederle, R.; Bei, R.; Granato, M.; Modesti, A.; Frati, L.; Delecluse, H.J.; et al. Characterization and intracellular localization of the Epstein-Barr virus protein BFLF2: Interactions with BFRF1 and with the nuclear lamina. J. Virol. 2005, 79, 3713–3727. [Google Scholar] [CrossRef] [PubMed]
- Schmeiser, C.; Borst, E.; Sticht, H.; Marschall, M.; Milbradt, J. The cytomegalovirus egress proteins pUL50 and pUL53 are translocated to the nuclear envelope through two distinct modes of nuclear import. J. Gen. Virol. 2013, 94, 2056–2069. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Zou, X.; Xu, Z.; Wang, Y.; Wang, P.; Ou, X.; Li, Y.; Chen, D.; Peng, T.; et al. Characterization of the nucleocytoplasmic transport mechanisms of epstein-barr virus BFLF2. Cell. Physiol. Biochem. 2018, 51, 1500–1517. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, S.; Mo, C.; Zeng, Z.; Li, X.; Chen, C.; Yang, Y.; Wang, J.; Huang, J.; Chen, D.; et al. Identification of molecular determinants for the nuclear import of pseudorabies virus UL31. Arch. Biochem. Biophys. 2015, 587, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Chen, D.; Zeng, Z.; Yang, H.; Jiang, S.; Li, X.; Mai, J.; Peng, T.; Li, M. Characterization of the nuclear import signal of herpes simplex virus 1 UL31. Arch. Virol. 2016, 161, 2379–2385. [Google Scholar] [CrossRef]
- Paßvogel, L.; Klupp, B.G.; Granzow, H.; Fuchs, W.; Mettenleiter, T.C. Functional characterization of nuclear trafficking signals in pseudorabies virus pUL31. J. Virol. 2015, 89, 2002–2012. [Google Scholar] [CrossRef]
- Funk, C.; Raschbichler, V.; Lieber, D.; Wetschky, J.; Arnold, E.K.; Leimser, J.; Biggel, M.; Friedel, C.C.; Ruzsics, Z.; Bailer, S.M. Comprehensive analysis of nuclear export of herpes simplex virus type 1 tegument proteins and their Epstein-Barr virus orthologs. Traffic 2019, 20, 152–167. [Google Scholar] [CrossRef]
- Gabriel, G.; Klingel, K.; Otte, A.; Thiele, S.; Hudjetz, B.; Arman-Kalcek, G.; Sauter, M.; Shmidt, T.; Rother, F.; Baumgarte, S.; et al. Differential use of importin-α isoforms governs cell tropism and host adaptation of influenza virus. Nat. Commun. 2011, 2, 156. [Google Scholar] [CrossRef]
- Higgs, E.S.; Gayedyu-Dennis, D.; Fisher, W.; Nason, M.; Reilly, C.; Beavogui, A.H.; Aboulhab, J.; Nordwall, J.; Lobbo, P.; Wachekwa, I.; et al. PREVAIL IV: A Randomized, Double-Blind, Two-Phase, Phase 2 Trial of Remdesivir versus Placebo for Reduction of Ebola Virus RNA in the Semen of Male Survivors. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Choopanya, K.; Martin, M.; Suntharasamai, P.; Sangkum, U.; Mock, P.A.; Leethochawalit, M.; Chiamwongpaet, S.; Kitisin, P.; Natrujirote, P.; Kittimunkong, S.; et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013, 381, 2083–2090. [Google Scholar] [CrossRef]
- Abdool Karim, S.S.; Abdool Karim, Q.; Kharsany, A.B.; Baxter, C.; Grobler, A.C.; Werner, L.; Kashuba, A.; Mansoor, L.E.; Samsunder, N.; Mindel, A.; et al. Tenofovir Gel for the Prevention of Herpes Simplex Virus Type 2 Infection. N. Engl. J. Med. 2015, 373, 530–539. [Google Scholar] [CrossRef]
- Kosugi, S.; Hasebe, M.; Entani, T.; Takayama, S.; Tomita, M.; Yanagawa, H. Design of peptide inhibitors for the importin alpha/beta nuclear import pathway by activity-based profiling. Chem. Biol. 2008, 15, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Z.; Yao, S.Y.; Veach, R.A.; Torgerson, T.R.; Hawiger, J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J. Biol. Chem. 1995, 270, 14255–14258. [Google Scholar] [CrossRef]
- Zienkiewicz, J.; Armitage, A.; Hawiger, J. Targeting nuclear import shuttles, importins/karyopherins alpha by a peptide mimicking the NFκB1/p50 nuclear localization sequence. J. Am. Heart Assoc. 2013, 2, e000386. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Y.; Fraser, J.E.; Chan, W.K.; Moreland, N.J.; Rathore, A.P.; Wang, C.; Vasudevan, S.G.; Jans, D.A. Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir. Res. 2013, 99, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef]
- Yang, S.N.Y.; Atkinson, S.C.; Fraser, J.E.; Wang, C.; Maher, B.; Roman, N.; Forwood, J.K.; Wagstaff, K.M.; Borg, N.A.; Jans, D.A. Novel flavivirus antiviral that targets the host nuclear transport importin α/β1 heterodimer. Cells 2019, 8, 281. [Google Scholar] [CrossRef]
- Yang, S.N.Y.; Atkinson, S.C.; Wang, C.; Lee, A.; Bogoyevitch, M.A.; Borg, N.A.; Jans, D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir. Res. 2020, 177, 104760. [Google Scholar] [CrossRef] [PubMed]
- Soderholm, J.F.; Bird, S.L.; Kalab, P.; Sampathkumar, Y.; Hasegawa, K.; Uehara-Bingen, M.; Weis, K.; Heald, R. Importazole, a small molecule inhibitor of the transport receptor importin-β. ACS Chem. Biol. 2011, 6, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Van der Watt, P.J.; Chi, A.; Stelma, T.; Stowell, C.; Strydom, E.; Carden, S.; Angus, L.; Hadley, K.; Lang, D.; Wei, W.; et al. Targeting the Nuclear Import Receptor Kpnβ1 as an Anticancer Therapeutic. Mol. Cancer Ther. 2016, 15, 560–573. [Google Scholar] [CrossRef] [PubMed]
- Hintersteiner, M.; Ambrus, G.; Bednenko, J.; Schmied, M.; Knox, A.J.; Meisner, N.C.; Gstach, H.; Seifert, J.M.; Singer, E.L.; Gerace, L.; et al. Identification of a small molecule inhibitor of importin β mediated nuclear import by confocal on-bead screening of tagged one-bead one-compound libraries. ACS Chem. Biol. 2010, 5, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Cansizoglu, A.E.; Lee, B.J.; Zhang, Z.C.; Fontoura, B.M.; Chook, Y.M. Structure-based design of a pathway-specific nuclear import inhibitor. Nat. Struct. Mol. Biol. 2007, 14, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Ou, X.; Li, Y.; Zou, X.; Xu, Z.; Wang, Y.; Peng, H.; Deng, Y.; Guo, Y.; Lu, M.; et al. Molecular anatomy of the subcellular localization and nuclear import mechanism of herpes simplex virus 1 UL6. Aging 2020, 12, 5751–5763. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Yoshida, M.; Fujiwara, D.; Nishikawa, M.; Horinouchi, S.; Beppu, T. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J. Biol. Chem. 1994, 269, 6320–6324. [Google Scholar] [CrossRef]
- Kudo, N.; Matsumori, N.; Taoka, H.; Fujiwara, D.; Schreiner, E.P.; Wolff, B.; Yoshida, M.; Horinouchi, S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Nat. Acad. Sci. USA 1999, 96, 9112–9117. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, M.; Tamayo, A.T.; Shacham, S.; Kauffman, M.; Lee, J.; Zhang, L.; Ou, Z.; Li, C.; Sun, L.; et al. Novel selective inhibitors of nuclear export CRM1 antagonists for therapy in mantle cell lymphoma. Exp. Hematol. 2013, 41, 67–78.e64. [Google Scholar] [CrossRef]
- Ranganathan, P.; Yu, X.; Na, C.; Santhanam, R.; Shacham, S.; Kauffman, M.; Walker, A.; Klisovic, R.; Blum, W.; Caligiuri, M.; et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood 2012, 120, 1765–1773. [Google Scholar] [CrossRef]
- Sun, Q.; Carrasco, Y.P.; Hu, Y.; Guo, X.; Mirzaei, H.; Macmillan, J.; Chook, Y.M. Nuclear export inhibition through covalent conjugation and hydrolysis of Leptomycin B by CRM1. Proc. Nat. Acad. Sci. USA 2013, 110, 1303–1308. [Google Scholar] [CrossRef]
- Lapalombella, R.; Sun, Q.; Williams, K.; Tangeman, L.; Jha, S.; Zhong, Y.; Goettl, V.; Mahoney, E.; Berglund, C.; Gupta, S.; et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood 2012, 120, 4621–4634. [Google Scholar] [CrossRef]
- Haines, J.D.; Herbin, O.; de la Hera, B.; Vidaurre, O.G.; Moy, G.A.; Sun, Q.; Fung, H.Y.; Albrecht, S.; Alexandropoulos, K.; McCauley, D.; et al. Nuclear export inhibitors avert progression in preclinical models of inflammatory demyelination. Nat. Neurosci. 2015, 18, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.T.; Landesman, Y.; Acharya, C.; Calle, Y.; Zhong, M.Y.; Cea, M.; Tannenbaum, D.; Cagnetta, A.; Reagan, M.; Munshi, A.A.; et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: Molecular mechanisms and therapeutic implications. Leukemia 2014, 28, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Newlands, E.S.; Rustin, G.J.; Brampton, M.H. Phase I trial of elactocin. Br. J. Cancer 1996, 74, 648–649. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, X.; Zhou, Q.; Burstein, E.; Yang, S.; Jia, D. Inhibiting cancer cell hallmark features through nuclear export inhibition. Signal Transduct. Target. Ther. 2016, 1, 16010. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.N.Y.; Smith, K.; Forwood, J.K.; Jans, D.A. Nuclear import inhibitor N-(4-hydroxyphenyl) retinamide targets Zika virus (ZIKV) nonstructural protein 5 to inhibit ZIKV infection. Biochem. Biophys. Res. Commun. 2017, 493, 1555–1559. [Google Scholar] [CrossRef]
- Pitts, J.D.; Li, P.C.; de Wispelaere, M.; Yang, P.L. Antiviral activity of N-(4-hydroxyphenyl) retinamide (4-HPR) against Zika virus. Antivir. Res. 2017, 147, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.E.; Watanabe, S.; Wang, C.; Chan, W.K.; Maher, B.; Lopez-Denman, A.; Hick, C.; Wagstaff, K.M.; Mackenzie, J.M.; Sexton, P.M.; et al. A nuclear transport inhibitor that modulates the unfolded protein response and provides in vivo protection against lethal dengue virus infection. J. Infect. Dis. 2014, 210, 1780–1791. [Google Scholar] [CrossRef]
- Link, J.O.; Rhee, M.S.; Tse, W.C.; Zheng, J.; Somoza, J.R.; Rowe, W.; Begley, R.; Chiu, A.; Mulato, A.; Hansen, D.; et al. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature 2020, 584, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Yant, S.R.; Mulato, A.; Hansen, D.; Tse, W.C.; Niedziela-Majka, A.; Zhang, J.R.; Stepan, G.J.; Jin, D.; Wong, M.H.; Perreira, J.M.; et al. A highly potent long-acting small-molecule HIV-1 capsid inhibitor with efficacy in a humanized mouse model. Nat. Med. 2019, 25, 1377–1384. [Google Scholar] [CrossRef]
- Mohl, G.; Liddle, N.; Nygaard, J.; Dorius, A.; Lyons, N.; Hodek, J.; Weber, J.; Michaelis, D.J.; Busath, D.D. Novel influenza inhibitors designed to target PB1 interactions with host importin RanBP5. Antivir. Res. 2019, 164, 81–90. [Google Scholar] [CrossRef]
- Tanaka, K.; Kasahara, Y.; Miyamoto, Y.; Takumi, O.; Kasai, T.; Onodera, K.; Kuwahara, M.; Oka, M.; Yoneda, Y.; Obika, S. Development of oligonucleotide-based antagonists of Ebola virus protein 24 inhibiting its interaction with karyopherin alpha 1. Org. Biomol. Chem. 2018, 16, 4456–4463. [Google Scholar] [CrossRef]
- Song, X.; Lu, L.Y.; Passioura, T.; Suga, H. Macrocyclic peptide inhibitors for the protein-protein interaction of Zaire Ebola virus protein 24 and karyopherin alpha 5. Org. Biomol. Chem. 2017, 15, 5155–5160. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, J.L.; Martinez-Chequer, J.C.; Hernandez-Celaya, M.E.; Barahona-Bustillos, E.; Andrade-Manzano, A.F. Randomized placebo-controlled evaluation of intramuscular interferon beta treatment of recurrent human papillomavirus. Obstet. Gynecol. 2001, 97, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Terenzi, F.; Saikia, P.; Sen, G.C. Interferon-inducible protein, P56, inhibits HPV DNA replication by binding to the viral protein E1. EMBO J. 2008, 27, 3311–3321. [Google Scholar] [CrossRef]
- Wu, G.; Liu, B.; Zhang, Y.; Li, J.; Arzumanyan, A.; Clayton, M.M.; Schinazi, R.F.; Wang, Z.; Goldmann, S.; Ren, Q.; et al. Preclinical characterization of GLS4, an inhibitor of hepatitis B virus core particle assembly. Antimicrob. Agents Chemother. 2013, 57, 5344–5354. [Google Scholar] [CrossRef] [PubMed]
- Weber, O.; Schlemmer, K.H.; Hartmann, E.; Hagelschuer, I.; Paessens, A.; Graef, E.; Deres, K.; Goldmann, S.; Niewoehner, U.; Stoltefuss, J.; et al. Inhibition of human hepatitis B virus (HBV) by a novel non-nucleosidic compound in a transgenic mouse model. Antivir. Res. 2002, 54, 69–78. [Google Scholar] [CrossRef]
- Brezillon, N.; Brunelle, M.N.; Massinet, H.; Giang, E.; Lamant, C.; DaSilva, L.; Berissi, S.; Belghiti, J.; Hannoun, L.; Puerstinger, G.; et al. Antiviral activity of Bay 41-4109 on hepatitis B virus in humanized Alb-uPA/SCID mice. PLoS ONE 2011, 6, e25096. [Google Scholar] [CrossRef]
- Perni, R.B.; Conway, S.C.; Ladner, S.K.; Zaifert, K.; Otto, M.J.; King, R.W. Phenylpropenamide derivatives as inhibitors of hepatitis B virus replication. Bioorg. Med. Chem. Lett. 2000, 10, 2687–2690. [Google Scholar] [CrossRef]
- King, R.W.; Ladner, S.K.; Miller, T.J.; Zaifert, K.; Perni, R.B.; Conway, S.C.; Otto, M.J. Inhibition of human hepatitis B virus replication by AT-61, a phenylpropenamide derivative, alone and in combination with (-)beta-L-2′,3′-dideoxy-3′-thiacytidine. Antimicrob. Agents Chemother. 1998, 42, 3179–3186. [Google Scholar] [CrossRef] [PubMed]
- Delaney, W.E.t.; Edwards, R.; Colledge, D.; Shaw, T.; Furman, P.; Painter, G.; Locarnini, S. Phenylpropenamide derivatives AT-61 and AT-130 inhibit replication of wild-type and lamivudine-resistant strains of hepatitis B virus in vitro. Antimicrob. Agents Chemother. 2002, 46, 3057–3060. [Google Scholar] [CrossRef] [PubMed]
- Campagna, M.R.; Liu, F.; Mao, R.; Mills, C.; Cai, D.; Guo, F.; Zhao, X.; Ye, H.; Cuconati, A.; Guo, H.; et al. Sulfamoylbenzamide derivatives inhibit the assembly of hepatitis B virus nucleocapsids. J. Virol. 2013, 87, 6931–6942. [Google Scholar] [CrossRef]
- Berke, J.M.; Dehertogh, P.; Vergauwen, K.; Van Damme, E.; Mostmans, W.; Vandyck, K.; Pauwels, F. Capsid Assembly Modulators Have a Dual Mechanism of Action in Primary Human Hepatocytes Infected with Hepatitis B Virus. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Guo, F.; Zhao, Q.; Sheraz, M.; Cheng, J.; Qi, Y.; Su, Q.; Cuconati, A.; Wei, L.; Du, Y.; Li, W.; et al. HBV core protein allosteric modulators differentially alter cccDNA biosynthesis from de novo infection and intracellular amplification pathways. PLoS Pathog. 2017, 13, e1006658. [Google Scholar] [CrossRef] [PubMed]
- Ligat, G.; Goto, K.; Verrier, E.; Baumert, T.F. Targeting Viral cccDNA for Cure of Chronic Hepatitis, B. Curr. Hepatol. Rep. 2020, 19, 235–244. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Varghese, F.S.; Kaukinen, P.; Gläsker, S.; Bespalov, M.; Hanski, L.; Wennerberg, K.; Kümmerer, B.M.; Ahola, T. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir. Res. 2016, 126, 117–124. [Google Scholar] [CrossRef]
- King, C.R.; Tessier, T.M.; Dodge, M.J.; Weinberg, J.B.; Mymryk, J.S. Inhibition of Human Adenovirus Replication by the Importin α/β1 Nuclear Import Inhibitor Ivermectin. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Bennett, S.M.; Zhao, L.; Bosard, C.; Imperiale, M.J. Role of a nuclear localization signal on the minor capsid proteins VP2 and VP3 in BKPyV nuclear entry. Virology 2015, 474, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Huang, W.; Lee, H.; van de Leemput, J.; Kane, M.A.; Han, Z. Characterization of SARS-CoV-2 proteins reveals Orf6 pathogenicity, subcellular localization, host interactions and attenuation by Selinexor. Cell Biosci. 2021, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Perwitasari, O.; Johnson, S.; Yan, X.; Howerth, E.; Shacham, S.; Landesman, Y.; Baloglu, E.; McCauley, D.; Tamir, S.; Tompkins, S.M.; et al. Verdinexor, a novel selective inhibitor of nuclear export, reduces influenza a virus replication in vitro and in vivo. J. Virol. 2014, 88, 10228–10243. [Google Scholar] [CrossRef] [PubMed]
- Perwitasari, O.; Johnson, S.; Yan, X.; Register, E.; Crabtree, J.; Gabbard, J.; Howerth, E.; Shacham, S.; Carlson, R.; Tamir, S.; et al. Antiviral Efficacy of Verdinexor In Vivo in Two Animal Models of Influenza A Virus Infection. PLoS ONE 2016, 11, e0167221. [Google Scholar] [CrossRef] [PubMed]
- López-Medina, E.; López, P.; Hurtado, I.C.; Dávalos, D.M.; Ramirez, O.; Martínez, E.; Díazgranados, J.A.; Oñate, J.M.; Chavarriaga, H.; Herrera, S.; et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: A randomized clinical trial. JAMA 2021, 325, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Elalfy, H.; Besheer, T.; El-Mesery, A.; El-Gilany, A.H.; Soliman, M.A.; Alhawarey, A.; Alegezy, M.; Elhadidy, T.; Hewidy, A.A.; Zaghloul, H.; et al. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J. Med. Virol. 2021, 93, 3176–3183. [Google Scholar] [CrossRef]
- Ahmed, S.; Karim, M.M.; Ross, A.G.; Hossain, M.S.; Clemens, J.D.; Sumiya, M.K.; Phru, C.S.; Rahman, M.; Zaman, K.; Somani, J.; et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int. J. Infect. Dis. 2021, 103, 214–216. [Google Scholar] [CrossRef]
- Gandhi, R.T.; Lynch, J.B.; del Rio, C. Mild or Moderate Covid-19. N. Engl. J. Med. 2020, 383, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Suputtamongkol, Y.; Avirutnan, P.; Mairiang, D.; Angkasekwinai, N.; Niwattayakul, K.; Yamasmith, E.; Saleh-Arong, F.A.; Songjaeng, A.; Prommool, T.; Tangthawornchaikul, N.; et al. Ivermectin accelerates circulating nonstructural protein 1 (NS1) clearance in adult dengue patients: A combined phase 2/3 randomized double-blinded placebo controlled trial. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Libraty, D.H.; Young, P.R.; Pickering, D.; Endy, T.P.; Kalayanarooj, S.; Green, S.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; Rothman, A.L. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 2002, 186, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Meng, Y.L.; Duan, S.M.; Zhan, S.B.; Guan, R.L.; Yue, T.F.; Kong, L.H.; Zhou, L.; Deng, L.H.; Huang, C.; et al. REBACIN® as a noninvasive clinical intervention for high-risk human papillomavirus persistent infection. Int. J. Cancer 2019, 145, 2712–2719. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, T.; Ming, X.; Yang, E.; Min, W.; Li, Z. REBACIN® is an optional intervention for persistent high-risk human papillomavirus infection: A retrospective analysis of 364 patients. Int. J. Gynaecol. Obstet. 2021, 152, 82–87. [Google Scholar] [CrossRef]
- Yuen, M.F.; Gane, E.J.; Kim, D.J.; Weilert, F.; Yuen Chan, H.L.; Lalezari, J.; Hwang, S.G.; Nguyen, T.; Flores, O.; Hartman, G.; et al. Antiviral Activity, Safety, and Pharmacokinetics of Capsid Assembly Modulator NVR 3-778 in Patients with Chronic HBV Infection. Gastroenterology 2019, 156, 1392–1403. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, F.; Zhu, X.; Chen, Y.; Chen, H.; Li, X.; Wu, M.; Li, C.; Liu, J.; Zhang, Y.; et al. Antiviral Activity and Pharmacokinetics of the HBV Capsid Assembly Modulator GLS4 in Patients with Chronic HBV Infection. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chaccour, C.; Casellas, A.; Blanco-Di Matteo, A.; Pineda, I.; Fernandez-Montero, A.; Ruiz-Castillo, P.; Richardson, M.A.; Rodríguez-Mateos, M.; Jordán-Iborra, C.; Brew, J.; et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine 2021, 32, 100720. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Kobayashi, A.; Taoka, A.; Watanabe-Nakayama, T.; Kikuchi, Y.; Hazawa, M.; Minamoto, T.; Fukumori, Y.; Kodera, N.; Uchihashi, T.; et al. High-speed atomic force microscopy reveals loss of nuclear pore resilience as a dying code in colorectal cancer cells. ACS Nano 2017, 11, 5567–5578. [Google Scholar] [CrossRef] [PubMed]
- Nishide, G.; Lim, K.; Mohamed, M.S.; Kobayashi, A.; Hazawa, M.; Watanabe-Nakayama, T.; Kodera, N.; Ando, T.; Wong, R.W. High-speed atomic force microscopy reveals spatiotemporal dynamics of histone protein H2A involution by DNA inchworming. J. Phys. Chem. Lett. 2021, 12, 3837–3846. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Kodera, N.; Wang, H.; Mohamed, M.S.; Hazawa, M.; Kobayashi, A.; Yoshida, T.; Hanayama, R.; Yano, S.; Ando, T.; et al. High-Speed AFM reveals molecular dynamics of human influenza A hemagglutinin and its interaction with exosomes. Nano Lett. 2020, 20, 6320–6328. [Google Scholar] [CrossRef]
- Lim, K.S.; Mohamed, M.S.; Wang, H.; Hazawa, M.; Kobayashi, A.; Voon, D.C.; Kodera, N.; Ando, T.; Wong, R.W. Direct visualization of avian influenza H5N1 hemagglutinin precursor and its conformational change by high-speed atomic force microscopy. Biochim. Biophys. Acta. Gen. Subj. 2020, 1864, 129313. [Google Scholar] [CrossRef] [PubMed]
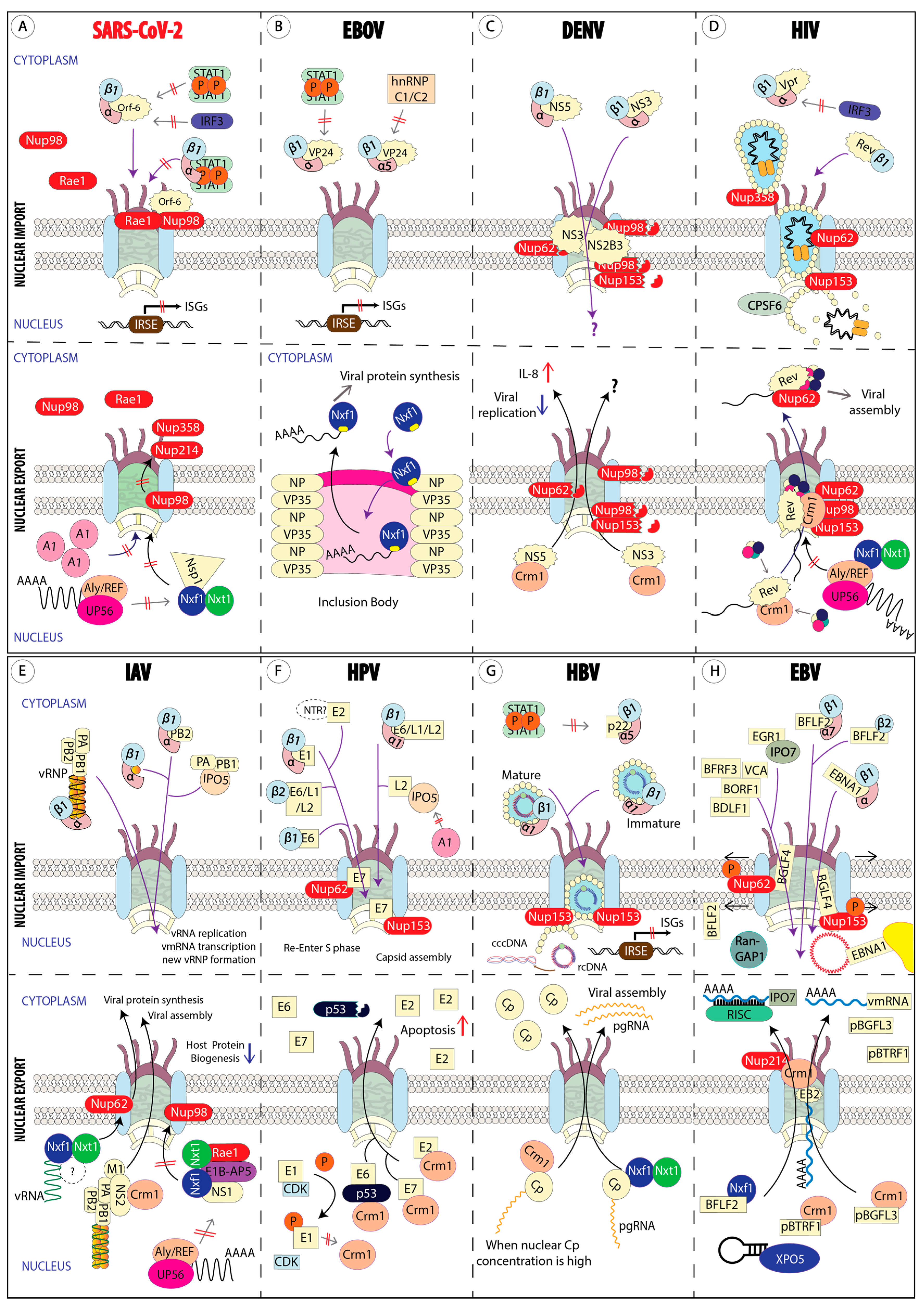
| Adaptor Protein Importin α Subfamilies | Protein Name (Importin Nomenclature) | Protein Name (Karyopherin Nomenclature) |
|---|---|---|
| α1 | Importin α1 Importin α8 | Karyopherin α2 Karyopherin α7 |
| α2 | Importin α3 Importin α4 | Karyopherin α4 Karyopherin α3 |
| α3 | Importin α5 Importin α6 Importin α7 | Karyopherin α1 Karyopherin α5 Karyopherin α6 |
| Importin β family members | Cargoes | |
| Importin β1 (Karyopherin β1) | Importin α isoforms-cNLS-bearing cargoes Snurportin 1 RIPα (RPA-interacting protein α) Importin 7 | |
| Importin β2 (Transportin-1) | hnRNP (A1, A2, and F) Ribosomal proteins TAP/Nxf1 Histones | |
| Transportin-2 | HuR (ELAV-like protein 1) | |
| Transportin-SR | SR proteins (abundant arginine/serine-rich proteins) | |
| Importin 4 (IPO4, RanBP4) | Histones and ribosomal proteins | |
| Importin 5 (IPO5, RanBP5) | Histones and ribosomal proteins | |
| Importin 7 (IPO7, RanBP7) | HIV PIC H1 histone GR (Glucocorticoid receptor) Ribosomal proteins | |
| Importin 8 (IPO8, RanBP8) | SRP19 (Signal recognition particle 19) | |
| Importin 9 (IPO9, RanBP9) | Histones and ribosomal proteins | |
| Importin 11 (IPO11, RanBP11) | UBE2E3 (Ub-conjugating enzyme) rpL12 (60S ribosomal protein L12) | |
| Crm1 (XPO1) | Cargoes with leucine-rich NES Snurportin HuR (ELAV-like protein 1) [62] Nxf3 (Nuclear RNA export factor 3) [63] LRPPRC (Leucine-rich PPR motif-containing protein) [64] Nmd3 (60S ribosomal export protein) [65] | |
| CAS (XPO2) | Importin α | |
| Exportin 4 (XPO4) | eIF-5A (Eukaryotic translation initiation factor 5A-1) | |
| Exportin 5 (XPO5) | ILF3 (Interleukin enhancer-binding factor 3) eEF1A-1 (Elongation factor 1-alpha 1) pre-miRNA, tRNA, minihelix RNA | |
| Exportin 6 (XPO6) | Profilin Actin | |
| Exportin-T (XPOT) | tRNA | |
| Importin 13 (IPO13, RanBP13), import | RBM8A (RNA-binding protein 8A) UBCE9 (SUMO-conjugating enzyme UBC9) Pax6 (Paired box protein Pax-6) | |
| Importin 13 (IPO13, RanBP13), export | eIF-4C (Eukaryotic translation initiation factor 1A, X-chromosomal) | |
| RanBP6 | Unknown | |
| RanBP16 | Unknown | |
| RanBP17 | Unknown | |
| Viral Family | Site of Replication | Virus Name | Viral Factors | Host Factors | Effect of Viral–Host Factor Interaction | References |
|---|---|---|---|---|---|---|
| Coronaviridae (+ssRNA) | Cytoplasm | SARS CoV-2 | Orf6 | Importin-α1/β1 | Blocks nuclear translocation of IRF3 to suppress IFNβ production. | [75,76,78] |
| Importin-α/β1 (isoform α1, α5) | Blocks nuclear translocation of activated STAT1/2 to suppress IFN-mediated antiviral response. | |||||
| Nup98 and Rae1 | Disrupts importin-α5/β1 docking on Nup98-Rae1 complex for nuclear import. | |||||
| Cytoplasmic accumulation of Nup98 and Rae1. | ||||||
| Nuclear accumulation of hnRNP A1. | ||||||
| Nsp1 | Nxf1 | Interrupts Nxf1 binds to mRNA export adaptors including Aly/REF and UAP56. | [83] | |||
| Reduces interaction between Nxf1 and Nups (Nup98, Nup214, Nup358). | ||||||
| SARS CoV-1 | Orf6 | Importin-α1/β1 | Connects importin-α1/β1 at ER/Golgi to sequester the receptor for activated STAT1 nuclear import. | [79,80] | ||
| Nsp1 | Nup93 | Mislocalization of Nup93 (from NE to cytoplasm). | [84] | |||
| Nucleolin | Cytoplasmic accumulation with unknown reasons. | |||||
| Orf9b | Crm1 | Nuclear export of Orf9b prevents Caspase-3-mediated apoptosis. | [85] | |||
| MERS CoV | Orf4b | Importin-α3/β1 | Blocks IFR3 and IRF7 nuclear import for IFNβ production. | [86,87] | ||
| Blocks NF-κB p65 subunit nuclear import to suppress host antiviral response. | ||||||
| Filoviridae (-ssRNA) | Cytoplasm | Zaire EBOV | VP24 | Importin-α/β1 (isoform: α5, α6, and α7) | Blocks nuclear translocation of activated STAT1 to suppress IFN-mediated antiviral response. | [91,97] |
| Importin-α5/β1 | Cytoplasmic accumulation of hnRNP C1/C2 for viral replication. | |||||
| Inclusion body (IB) | Importin-α7 | Required for IB formation. | [104] | |||
| Nucleo-protein (NP) | Nxf1 | Exports viral mRNA from IB to cytoplasm for translation | [105] | |||
| Flaviviridae (+ssRNA) | Cytoplasm | DENV | NS5 | Importin-α2/β1 (DENV serotype 2 and 3 only) | Nuclear import of NS5 has unclear functions on viral replication and pathogenesis of the disease. | [115,116] |
| Crm1 | Nuclear export of NS5 promote IL-8 production and suppress viral replication. | |||||
| NS3 | FG-Nups (Nup62, Nup153, and Nup98) | Works in concert with NS2B3 to degrade FG-Nups. | [117] | |||
| ZIKV | NS5 | Importin-α/β1 (isoform α1, α 3, α4, and α 7) | Protects NS5 from cytoplasmic degradation. | [122] | ||
| Sequesters importins α in nuclear bodies. | [123] | |||||
| NS3 | Nups (TPR, Nup153, and Nup98) | Works in concert with NS2B3 to degrade Nups. | [117] | |||
| Togaviridae (+ssRNA) | Cytoplasm | CHIKV | Capsid protein (CP) | Importin-α3/β1 | Purpose of nuclear localization of CP is unclear. | [129,130] |
| Crm1 | Mutation in CP NES blocks host nuclear import with unknown mechanisms. | |||||
| nsP2 | nil | nsP2 does not have NLS but it can enter nucleus to suppress host immunity. | [131,135] | |||
| Retroviridae (+ssRNA) | Nucleus | HIV | Viral Capsid (CA) | Nup358 and Nup62 | Nuclear import of viral capsid. | [154] |
| Nup153 and CPSF6 | Disassembles viral capsid to release PIC to nucleus. | |||||
| Vpr | Importin-α/β1 (preferably to α5, lesser extend to α1 and α4) | Inhibits IRF3 activation and to block nuclear import of IRF3 and NfκB. | [155] | |||
| Rev | Importin β1 | Rev nuclear import is needed to form viral ribonucleoprotein (vRNP) transport complex, and the complex is then exported out by Crm1. | [160,162,163,164,165] | |||
| Crm1 | ||||||
| Nups (214, Nup153, Nup98, and Nup62) | ||||||
| Orthomyxoviridae (-ssRNA) | Nucleus | Influenza A | Nucleo-protein (NP) | Importin-α/β1 (isoform: α5, α7) | vRNP nuclear import for viral replication. | [170,173] |
| PA-PB1 (RdRP subunit) | Importin 5 | Forms an import complex with PA and PB1, but also regulates PA-PB1 complex for vRNA binding. | [180,181] | |||
| PB2 (RdRP subunit) | Importin-α/β1 (isoform: α1, α5, α7) | vRNP nuclear import for viral replication. | [176,177,178] | |||
| Importin-α isoform switching for viral adaptation in different host species. | [272] | |||||
| Importin-α3/β1 | Negatively regulate PB2 polymerase activity. | [177] | ||||
| Downregulates importin-α3 expression to suppress host antiviral response by blocking NF-κB nuclear import. | [179] | |||||
| NS2 | Crm1 | Forms an export complex with vRNP, viral M1 protein. | [182,183] | |||
| NS1 | Nup98 | Downregulation of Nup98 expression to inhibit host mRNA export. | [184] | |||
| Nxf1 | Binds to FG-repeat binding site of Nxf1 to block host mRNA export. | |||||
| vRNA | Nxf1 | In severe influenza infection, vRNA binds to Nxf1 alone and interacts only with Nup62 for nuclear export. | [188] | |||
| Nup62 | ||||||
| Papillomaviridae (dsDNA) | Nucleus | HPV | High-risk E6 | Importin-α1/β1, Importin- β1, Importin-β2 | Mediates polyubiquitination of p53 for proteasome degradation independent of MDM2. | [194,196,197] |
| High-risk E7 | FG-Nups (Nup62 and Nup153) | Re-programs cell cycle to S-phase to support viral DNA amplification. | [202] | |||
| Crm1 | Nuclear export of E7. | [200] | ||||
| E1 | Importin-α/β1 (isoform: α1, α3, α5) | Phosphorylation of E1 by host ERK and/or JNK is needed to enable E1 binds importins α for nuclear import for viral replication. | [205,206,207] | |||
| CDK | Phosphorylation of E1 blocks Crm1-dependent export to retain E1 in nucleus for viral replication. | [204,208] | ||||
| E2 | Unknown import NTR | Nuclear import of E2 is needed for viral replication. | [210] | |||
| Crm1 | Cytoplasmic accumulation of high-risk E2 induces caspase-8 mediated cell apoptosis. | [209] | ||||
| L1 major capsid | Importin α1/β1 | Capsid proteins assembly in nucleus. | [211] | |||
| Importin β2 | Inhibits nuclear import of hnRNP A1. | |||||
| L2 minor capsid | Importin α1/β1 | Capsid proteins assembly in nucleus. | [212] | |||
| Importin β2 | ||||||
| Importin 5 | ||||||
| Hepadnaviridae (dsDNA) | Nucleus | HBV | Capsid protein (Cp) | Importin α1/β1 | Viral capsid binds to classical importin α/β1 to pass through NPC. Capsid disassembly starts once it interacts with Nup153. | [216,217,219] |
| Nup153 | ||||||
| Nxf1 | pgRNA export for virion production. | [220,221] | ||||
| Crm-1 | pgRNA export for virion production (when nuclear Cp concentration is high). | |||||
| p22 (HBeAg) | Importin-α5/β1 | Blocks activated STAT1 nuclear import to suppress host antiviral response. | [223] | |||
| Herpesviridae (dsDNA) | Nucleus | HSV-1 | VP1/2 | Importin-β1 | Viral capsid docking on NPC to deliver viral DNA to nucleus. | [225,226] |
| pUL25 | Nup358, Nup214 | |||||
| pUL30 | Importin-α5/β1 | Nuclear localization of pUL30 and pUL42 is needed for viral replication in host nucleus. | [230,231,232,233] | |||
| pUL42 | Importin-α/β1 (isoform: α1 and α7) | |||||
| ICP27 | Aly/REF, Nxf1 | Viral mRNA export for viral protein translation. | [237] | |||
| gM | Exportin-6 (XPO6) | Nuclear export of gM to TGN. | [241] | |||
| HCMV | pUL97 | Importin-α1/β1 | Phosphorylates CDK1 and modifies G2/M cell cycle checkpoint regulators to promote viral replication. | [244,245] | ||
| pUL79 | Importin-β2 | Function as an elongation factor of RNA polymerase II for viral gene transcription in nucleus. | [246,247] | |||
| pUL84 | Importin-α/β1 (isoform α1, α3, α4, and α5) | Initiate lytic viral DNA synthesis in nucleus. | [248,249] | |||
| Crm1 | Viral mRNA export (IRS1 transcript) | [248] | ||||
| pUL69 | UAP56/URH49, hSPT6, Nxf1 | Viral mRNA export | [253] | |||
| EBV | EBNA1 | Importin-α/β1 (isoform: α1, α5) | Nuclear EBNA1 tethers viral episomal DNA to host chromosome in latent phase. | [257] | ||
| BGLF4 | RanGAP1 | Nuclear accmulation of RanGAP1 inhibits nuclear import of cNLS-bearing cargoes. | [258] | |||
| FG-Nups (Nup62 and Nup153) | Phosphorylation of FG-Nups dilates NPC pores to facilitate nuclear import of non-NLS-bearing viral proteins. | |||||
| Microtubule | Reorganizes nucleus shape to allow nuclear import of non- NLS-bearing viral proteins. | |||||
| EGR1 | Importin-7 (IPO7) | Negatively regulates importin-7 expression to a level that is optimal for EBV-transformed cell growth. | [261] | |||
| EBV miRNA | Exportin-5 (XPO5) | |||||
| EB2 | Crm1 | Boosts EB2-mediated gene expression and mediate unspliced lytic EBV mRNA export | [263] | |||
| Nup214 | EBV mRNA export for translation. | |||||
| BFLF2 (Nuclear egress protein 1) | Ran, Importin-α7/β1, Importin-β2 | Interacts with BFRF1 (nuclear egress protein 2) at inner nuclear membrane to form nuclear egress complex (NEC). | [265,267] | |||
| Nxf1 | Interacts with Nxf1 for BFLF2 nuclear export without RNA participation | [267] | ||||
| Tegument proteins (pBTRF1 and pBGFL3) | Crm1 | Assembles with viral nucleocapsid for tegumentation in cytoplasm. | [271] |
| Types of Inhibitors | Compound | Target | Target Viruses | Types of Studies | References |
|---|---|---|---|---|---|
| Host-specific nuclear import inhibitors | Ivermectin | Importin α | SARS-CoV-2 | In vitro | [318] |
| DENV1 (EDEN-1) | In vitro and in vivo | [279] | |||
| DENV2 (EDEN-2) | In vitro and in vivo | ||||
| DENV3 (EDEN-3) | In vitro and in vivo | ||||
| DENV4 (EDEN-4) | In vitro and in vivo | ||||
| DENV2 (NGC) | In vitro and in vivo | [282] | |||
| ZIKAV (Asian/Cook Island/ 2014) | In vitro and in vivo | [282] | |||
| CHIKV-Rluc | In vitro | [319] | |||
| Adenovirus (HAdV-C5 and HAdV-B3) | In vitro and in vivo | [320] | |||
| BK polyomavirus (BKPyV) | In vitro | [321] | |||
| GW5074 | Importin α | DENV2 (NGC) | In vitro | [281] | |
| ZIKAV (Asian/Cook Island/ 2014) | In vitro | [281] | |||
| M9M | Importin β2 | HSV-1 | In vivo | [287] | |
| Host-specific nuclear export inhibitors | Selinexor | Crm1 | SARS-CoV-2 | In vitro | [322] |
| Verdinexor | Crm1 | IAV | In vitro and in vivo | [323,324] | |
| Virus-specific nuclear transport inhibitors | 4-HRP | NS5 | Flaviviruses (DENV, ZIKV) | In vitro and in vivo | [298,299,300] |
| GS-6207 and GS-CA | Capsid Protein | HIV-1 and HIV-2 | In vitro and in vivo | [301,302] | |
| Small-molecule inhibitor | PB1 | IAV | In vitro and in vivo | [303] | |
| VPKS-2 and -5 | VP24 | EBOV | In vitro | [304] | |
| High-affinity macrocyclic peptide | VP24 | EBOV | In vitro | [305] | |
| Type-I IFN | E1 | HPV | In vitro | [307] | |
| Capsid Assembly Modulator | Core/Capsid Protein | HBV | In vitro and in vivo | [308,309,310,311,312,313,314] |
| Drugs | Disease | Clinical Trial Identifier | Study Design | Phase | Dose and Duration | Main Outcomes and Measures | Results | References |
|---|---|---|---|---|---|---|---|---|
| Ivermectin | COVID-19 | NCT04405843 | Double-blind RCT: Placebo (n: 200) vs. Ivermectin (n: 200) | Phases 2 and 3 | Oral Ivermectin 300μg/kg of body weight for 5 days | Time to resolution of symptoms within a 21-day follow-up period | Ivermectin did not significantly shorten time to resolution of symptoms | [325] |
| NCT04392427 | Non-RCT: Standard Care Therapy (n: 61) vs. Combined Antiviral Therapy (n: 69) | Phase 1 | S.C.T: Paracetamol tablets (3 times/day), Zinc supplement (2 times/day), Azithromycin (case by case) | Viral clearance rate | Viral clearance rate was significantly higher in CAT compared with SCT group (CAT vs. SCT 58.1%: 0% on day 7; 73.1%: 13.7% on day 15) | [326] | ||
| C.A.T: Nitazoxanide (500mg/6 h), Ribavirin 1200mg (400mg divided doses), Ivermectin (following weight schedules) | ||||||||
| NCT04407130 | Double-blind RCT: Placebo, Ivermectin, Ivermectin + Doxycycline (n:24/group) | Phase 2 | Oral Ivermectin (12mg/day), Doxycycline (200mg on day 1, followed by 100mg/12hours) for 5 days | Viral clearance and remission of clinical symptoms (fever, cough) | Ivermectin monotherapy significantly enhanced viral clearance (Ivermectin vs. placebo: 9.7 days: 12.7 days). | [327] | ||
| No significant difference between the placebo group and Ivermectin + Doxycycline group. | ||||||||
| No significant improvement in clinical symptoms (fever, cough, sore throat) | ||||||||
| NCT04390022 | Double-blind RCT: Placebo vs. Ivermectin (n: 12/group) | Phase 2 | Oral Ivermectin (400μg/kg) for 7 days | Proportion of patients with detectable SARS-CoV-2 RNA by PCR from nasopharynx- geal swab at day 7 post-treatment. | Ivermectin did not significantly reduce viral loads, but Ivermectin-treated patients recovered faster from hyposmia/ anosmia. | [335] | ||
| Dengue Fever | NCT02045069 | Two consecutive double-blind RCT: Placebo (n:103) vs. Ivermectin: (n:100) | Phase 2 then proceeded to Phase 3 | Phase 2: 2 or 3 days of 400μg/kg/day Ivermectin. Phase 3: 3 days of 400/kg/day Ivermectin | Clinical progress and drug side effects | Ivermectin significantly improved NS1 antigenemia clearance time. The proportion of patients with detectable plasma NS1 was significantly lower in Ivermectin group compared to placebo. No significant difference in viremia clearance time between two groups | [329] | |
| GS-6207 (Lenacapavir) | HIV | NCT03739866 | Double-blind RCT: Placebo (n: 8) vs. GS-6207 (n: 32) | Phase 1 | Four GS-6207 groups in dose escalation fashion: 20, 50, 150, 450 mg, daily single subcutaneous dose for 9 days | Virus clearance assessment, drug resistant assessment | Reduction in plasma HIV RNA in a dose-dependent fashion | [301] |
| Resistant strain was found in one patient given 20mg GS-6207. Results showed a decrease in phenotypic susceptibility but no viral escape on day 9 of GS-6207 monotherapy. | ||||||||
| REBACIN® | HPV | - | Two independent, parallel RCTs: Placebo (n: 39) vs. REBACIN® (n: 40) | - | REBACIN® 0.5g per dose, every other day for 3 months except during the menstrual period | HPV viral clearance rate | Viral clearance rates: REBACIN® 61.5 and 62.5% vs. Placebo 20.0 and 12.5% | [331] |
| NVR 3-778 | HBV | NCT02112799 and NCT02401737 | Phase 1a in NCT02112799 was to evaluate the safety of NVR3-778 in health volunteers. Phase 1b RCTs of both NCT02112799 and NCT02401737 focused on high viremic chronic HBV infected patients. Placebo (n:10) vs. Treatment (n: 63) | Phase 1a and 1b | 7 treatment groups: 5 dose-escalation NVR 3-778 monotherapy groups; 1 combined group (NVR 3-778 + pegIFN); and 1 pegIFN monotherapy (placebo + pegIFN). 4 weeks treatment followed by 4 weeks post-treatment follow-up | Adverse effect assessment, virology assessment, drug resistance assessment, pharmacokinetic assessment. | Patients treated with more than 1200mg daily NVR 3-778 had lower serum HBV RNA and DNA. Synergistic effect in NVR 3-778 and pegIFN exerted strongest antiviral efficacy. No dose-related adverse effects reported. | [333] |
| NVR 3-778 monotherapy doses: 100, 200, and 400mg once daily, 600mg and 1000mg twice daily; pegIFN monotherapy: 180μg/weekly | ||||||||
| Combination therapy: NVR 3-778 600mg twice daily + pegIFN 180μg/weekly | ||||||||
| GLS4 | HBV | CTR20160068 | Double-blind RCT: Entecavir (control) vs. GLS4 + Ritonavir (n:8 per group) | Phase 1b | Control Entecavir 0.5mg; GLS4 120mg + Ritonavir 100mg; GLS4 240mg + Ritonavir 100mg for 28 days treatment | Adverse effect assessment, antiviral assessment, HBV serum markers assessment, and drug resistant assessment | 120mg GLS4 was well tolerated and exerted antiviral activity in patients with chronic HBV infection | [334] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajidah, E.S.; Lim, K.; Wong, R.W. How SARS-CoV-2 and Other Viruses Build an Invasion Route to Hijack the Host Nucleocytoplasmic Trafficking System. Cells 2021, 10, 1424. https://doi.org/10.3390/cells10061424
Sajidah ES, Lim K, Wong RW. How SARS-CoV-2 and Other Viruses Build an Invasion Route to Hijack the Host Nucleocytoplasmic Trafficking System. Cells. 2021; 10(6):1424. https://doi.org/10.3390/cells10061424
Chicago/Turabian StyleSajidah, Elma Sakinatus, Keesiang Lim, and Richard W. Wong. 2021. "How SARS-CoV-2 and Other Viruses Build an Invasion Route to Hijack the Host Nucleocytoplasmic Trafficking System" Cells 10, no. 6: 1424. https://doi.org/10.3390/cells10061424
APA StyleSajidah, E. S., Lim, K., & Wong, R. W. (2021). How SARS-CoV-2 and Other Viruses Build an Invasion Route to Hijack the Host Nucleocytoplasmic Trafficking System. Cells, 10(6), 1424. https://doi.org/10.3390/cells10061424







