Progenitor/Stem Cells in Vascular Remodeling during Pulmonary Arterial Hypertension
Abstract
1. Introduction
2. Endothelial Progenitor Cells
3. SMC Progenitor Cells (SPC)
4. Mechanisms for Stem/Progenitor Cell Recruitment during PH
4.1. BMP
4.2. TGF-β
4.3. FGF
4.4. Inflammatory Cytokines IL-6 and TNF-α
4.5. SDF-1/CXCR4/CXCR7 Pathway
4.6. PDGF Pathway
4.7. Wnt
4.8. Endothelin (ET-1)
4.9. Notch
4.10. Metabolism
5. Therapeutic Use of Stem/Progenitor Cells
5.1. Endothelial Progenitor Cells
5.2. MSC Therapy
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rabinovitch, M. Molecular Pathogenesis of Pulmonary Arterial Hypertension. J. Clin. Investig. 2012, 122, 4306–4313. [Google Scholar] [CrossRef]
- Long, L.; Ormiston, M.L.; Yang, X.; Southwood, M.; Gräf, S.; Machado, R.D.; Mueller, M.; Kinzel, B.; Yung, L.M.; Wilkinson, J.M.; et al. Selective Enhancement of Endothelial BMPR-II with BMP9 Reverses Pulmonary Arterial Hypertension. Nat. Med. 2015, 21, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; McLaughlin, V.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.; Escribano Subias, P.; Feldman, J.; et al. Sotatercept for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 384, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone Marrow Origin of Endothelial Progenitor Cells Responsible for Postnatal Vasculogenesis in Physiological and Pathological Neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef]
- Hur, J.; Yoon, C.-H.; Kim, H.-S.; Choi, J.-H.; Kang, H.-J.; Hwang, K.-K.; Oh, B.-H.; Lee, M.-M.; Park, Y.-B. Characterization of Two Types of Endothelial Progenitor Cells and Their Different Contributions to Neovasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 288–293. [Google Scholar] [CrossRef]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a Novel Hierarchy of Endothelial Progenitor Cells Using Human Peripheral and Umbilical Cord Blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef]
- Tura, O.; Skinner, E.M.; Barclay, G.R.; Samuel, K.; Gallagher, R.C.J.; Brittan, M.; Hadoke, P.W.F.; Newby, D.E.; Turner, M.L.; Mills, N.L. Late Outgrowth Endothelial Cells Resemble Mature Endothelial Cells and Are Not Derived from Bone Marrow. Stem Cells 2013, 31, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Yoder, M.C. Endothelial Stem and Progenitor Cells (Stem Cells): (2017 Grover Conference Series). Pulm. Circ. 2018, 8. [Google Scholar] [CrossRef]
- Sainz, J.; Al Haj Zen, A.; Caligiuri, G.; Demerens, C.; Urbain, D.; Lemitre, M.; Lafont, A. Isolation of “Side Population” Progenitor Cells from Healthy Arteries of Adult Mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 281–286. [Google Scholar] [CrossRef]
- Junhui, Z.; Xingxiang, W.; Guosheng, F.; Yunpeng, S.; Furong, Z.; Junzhu, C. Reduced Number and Activity of Circulating Endothelial Progenitor Cells in Patients with Idiopathic Pulmonary Arterial Hypertension. Respir. Med. 2008, 102, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Diller, G.-P.; van Eijl, S.; Okonko, D.O.; Howard, L.S.; Ali, O.; Thum, T.; Wort, S.J.; Bédard, E.; Gibbs, J.S.R.; Bauersachs, J.; et al. Circulating Endothelial Progenitor Cells in Patients with Eisenmenger Syndrome and Idiopathic Pulmonary Arterial Hypertension. Circulation 2008, 117, 3020–3030. [Google Scholar] [CrossRef]
- Sun, H.-X.; Li, G.-J.; Du, Z.-H.; Bing, Z.; Ji, Z.-X.; Luo, G.; Pan, S.-L. The Relationship between Endothelial Progenitor Cells and Pulmonary Arterial Hypertension in Children with Congenital Heart Disease. BMC Pediatr. 2019, 19, 502. [Google Scholar] [CrossRef] [PubMed]
- Toshner, M.; Morrell, N.W. Endothelial Progenitor Cells in Pulmonary Hypertension—Dawn of Cell-Based Therapy? Int. J. Clin. Pract. 2010, 64, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, G.; Plouffe, B.D.; Hatch, A.; von Gise, A.; Sallmon, H.; Zamanian, R.T.; Murthy, S.K. Design and Validation of an Endothelial Progenitor Cell Capture Chip and Its Application in Patients with Pulmonary Arterial Hypertension. J. Mol. Med. 2011, 89, 971. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, H.; Liu, J.; Sheng, C.; Zhang, L.; Zeng, Y. Changes of Number and Function of Late Endothelial Progenitor Cells in Peripheral Blood of COPD Patients Combined with Pulmonary Hypertension. Thorac. Cardiovasc. Surg. 2016, 64, 323–329. [Google Scholar] [CrossRef]
- Majka, S.M.; Skokan, M.; Wheeler, L.; Harral, J.; Gladson, S.; Burnham, E.; Loyd, J.E.; Stenmark, K.R.; Varella-Garcia, M.; West, J. Evidence for Cell Fusion Is Absent in Vascular Lesions Associated with Pulmonary Arterial Hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L1028–L1039. [Google Scholar] [CrossRef] [PubMed]
- Toshner, M.; Voswinckel, R.; Southwood, M.; Al-Lamki, R.; Howard, L.S.; Marchesan, D.; Yang, J.; Suntharalingam, J.; Soon, E.; Exley, A.; et al. Evidence of Dysfunction of Endothelial Progenitors in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2009, 180, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Firth, A.L.; Sacks, R.S.; Ogawa, A.; Auger, W.R.; Fedullo, P.F.; Madani, M.M.; Lin, G.Y.; Sakakibara, N.; Thistlethwaite, P.A.; et al. Identification of Putative Endothelial Progenitor Cells (CD34+ CD133+ Flk-1+) in Endarterectomized Tissue of Patients with Chronic Thromboembolic Pulmonary Hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L870–L878. [Google Scholar] [CrossRef] [PubMed]
- Asosingh, K.; Aldred, M.A.; Vasanji, A.; Drazba, J.; Sharp, J.; Farver, C.; Comhair, S.A.; Xu, W.; Licina, L.; Huang, L.; et al. Circulating Angiogenic Precursors in Idiopathic Pulmonary Arterial Hypertension. Am. J. Pathol. 2008, 172, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Farha, S.; Asosingh, K.; Xu, W.; Sharp, J.; George, D.; Comhair, S.; Park, M.; Tang, W.H.W.; Loyd, J.E.; Theil, K.; et al. Hypoxia-Inducible Factors in Human Pulmonary Arterial Hypertension: A Link to the Intrinsic Myeloid Abnormalities. Blood 2011, 117, 3485–3493. [Google Scholar] [CrossRef]
- Schiavon, M.; Fadini, G.P.; Lunardi, F.; Agostini, C.; Boscaro, E.; Calabrese, F.; Marulli, G.; Rea, F. Increased Tissue Endothelial Progenitor Cells in End-Stage Lung Diseases with Pulmonary Hypertension. J. Heart Lung Transpl. 2012, 31, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Masri, F.A.; Xu, W.; Comhair, S.A.; Asosingh, K.; Koo, M.; Vasanji, A.; Drazba, J.; Anand-Apte, B.; Erzurum, S.C. Hyperproliferative Apoptosis-Resistant Endothelial Cells in Idiopathic Pulmonary Arterial Hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L548–L554. [Google Scholar] [CrossRef] [PubMed]
- Sakao, S.; Tatsumi, K.; Voelkel, N.F. Endothelial Cells and Pulmonary Arterial Hypertension: Apoptosis, Proliferation, Interaction and Transdifferentiation. Respir. Res. 2009, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, Y.; Astudillo, O.; Jensen, L.; Reynolds, A.L.; Waghorne, N.; Brazil, D.P.; Cao, Y.; O’Connor, J.J.; Kennedy, B.N. Selective Inhibition of Retinal Angiogenesis by Targeting PI3 Kinase. PLoS ONE 2009, 4, e7867. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Kagaya, Y.; Nakano, M.; Ito, Y.; Ohta, J.; Tada, H.; Karibe, A.; Minegishi, N.; Suzuki, N.; Yamamoto, M.; et al. Important Role of Endogenous Erythropoietin System in Recruitment of Endothelial Progenitor Cells in Hypoxia-Induced Pulmonary Hypertension in Mice. Circulation 2006, 113, 1442–1450. [Google Scholar] [CrossRef]
- Marsboom, G.; Pokreisz, P.; Gheysens, O.; Vermeersch, P.; Gillijns, H.; Pellens, M.; Liu, X.; Collen, D.; Janssens, S. Sustained Endothelial Progenitor Cell Dysfunction After Chronic Hypoxia-Induced Pulmonary Hypertension. Stem Cells 2008, 26, 1017–1026. [Google Scholar] [CrossRef]
- Asosingh, K.; Farha, S.; Lichtin, A.; Graham, B.; George, D.; Aldred, M.; Hazen, S.L.; Loyd, J.; Tuder, R.; Erzurum, S.C. Pulmonary Vascular Disease in Mice Xenografted with Human BM Progenitors from Patients with Pulmonary Arterial Hypertension. Blood 2012, 120, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Bhagwani, A.R.; Farkas, D.; Harmon, B.; Authelet, K.J.; Cool, C.D.; Kolb, M.; Goncharova, E.; Yoder, M.C.; Clauss, M.; Freishtat, R.; et al. Clonally Selected Primitive Endothelial Cells Promote Occlusive Pulmonary Arteriopathy and Severe Pulmonary Hypertension in Rats Exposed to Chronic Hypoxia. Sci. Rep. 2020, 10, 1136. [Google Scholar] [CrossRef]
- Davie, N.J.; Crossno, J.T.; Frid, M.G.; Hofmeister, S.E.; Reeves, J.T.; Hyde, D.M.; Carpenter, T.C.; Brunetti, J.A.; McNiece, I.K.; Stenmark, K.R. Hypoxia-Induced Pulmonary Artery Adventitial Remodeling and Neovascularization: Contribution of Progenitor Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L668–L678. [Google Scholar] [CrossRef] [PubMed]
- Launay, J.M.; Herve, P.; Callebert, J.; Mallat, Z.; Collet, C.; Doly, S.; Belmer, A.; Diaz, S.L.; Hatia, S.; Cote, F.; et al. Serotonin 5-HT2B Receptors Are Required for Bone-Marrow Contribution to Pulmonary Arterial Hypertension. Blood 2012, 119, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Nishiwaki, T.; Sekine, A.; Nishimura, R.; Suda, R.; Urushibara, T.; Suzuki, T.; Takayanagi, S.; Terada, J.; Sakao, S.; et al. Vascular Repair by Tissue-Resident Endothelial Progenitor Cells in Endotoxin-Induced Lung Injury. Am. J. Respir. Cell. Mol. Biol. 2015, 53, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Nishiwaki, T.; Kawasaki, T.; Sekine, A.; Suda, R.; Urushibara, T.; Suzuki, T.; Takayanagi, S.; Terada, J.; Sakao, S.; et al. Hypoxia-Induced Proliferation of Tissue-Resident Endothelial Progenitor Cells in the Lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L746–L758. [Google Scholar] [CrossRef]
- Alvarez, D.F.; Huang, L.; King, J.A.; El Zarrad, M.K.; Yoder, M.C.; Stevens, T. Lung Microvascular Endothelium Is Enriched with Progenitor Cells That Exhibit Vasculogenic Capacity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L419–L430. [Google Scholar] [CrossRef]
- Nijmeh, H.; Balasubramaniam, V.; Burns, N.; Ahmad, A.; Stenmark, K.R.; Gerasimovskaya, E.V. High Proliferative Potential Endothelial Colony-Forming Cells Contribute to Hypoxia-Induced Pulmonary Artery Vasa Vasorum Neovascularization. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L661–L671. [Google Scholar] [CrossRef]
- Montani, D.; Perros, F.; Gambaryan, N.; Girerd, B.; Dorfmuller, P.; Price, L.C.; Huertas, A.; Hammad, H.; Lambrecht, B.; Simonneau, G.; et al. C-Kit-Positive Cells Accumulate in Remodeled Vessels of Idiopathic Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2011, 184, 116–123. [Google Scholar] [CrossRef]
- Duong, H.T.; Comhair, S.A.; Aldred, M.A.; Mavrakis, L.; Savasky, B.M.; Erzurum, S.C.; Asosingh, K. Pulmonary Artery Endothelium Resident Endothelial Colony-Forming Cells in Pulmonary Arterial Hypertension. Pulm. Circ. 2011, 1, 475–486. [Google Scholar] [CrossRef]
- Psaltis, P.J.; Simari, R.D. Vascular Wall Progenitor Cells in Health and Disease. Circ. Res. 2015, 116, 1392–1412. [Google Scholar] [CrossRef]
- Yoshida, T.; Owens, G.K. Molecular Determinants of Vascular Smooth Muscle Cell Diversity. Circ. Res. 2005, 96, 280–291. [Google Scholar] [CrossRef]
- Rzucidlo, E.M.; Martin, K.A.; Powell, R.J. Regulation of Vascular Smooth Muscle Cell Differentiation. J. Vasc. Surg. 2007, 45, A25–A32. [Google Scholar] [CrossRef] [PubMed]
- House, S.J.; Potier, M.; Bisaillon, J.; Singer, H.A.; Trebak, M. The Non-Excitable Smooth Muscle: Calcium Signaling and Phenotypic Switching during Vascular Disease. Pflug. Arch. Eur. J. Physiol. 2008, 456, 769–785. [Google Scholar] [CrossRef]
- Dai, Z.; Zhu, M.M.; Peng, Y.; Jin, H.; Machireddy, N.; Qian, Z.; Zhang, X.; Zhao, Y.-Y. Endothelial and Smooth Muscle Cell Interaction via FoxM1 Signaling Mediates Vascular Remodeling and Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 788–802. [Google Scholar] [CrossRef]
- Yang, X.; Long, L.; Southwood, M.; Rudarakanchana, N.; Upton, P.D.; Jeffery, T.K.; Atkinson, C.; Chen, H.; Trembath, R.C.; Morrell, N.W. Dysfunctional Smad Signaling Contributes to Abnormal Smooth Muscle Cell Proliferation in Familial Pulmonary Arterial Hypertension. Circ. Res. 2005, 96, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.Q.; Misra, A.; Rosas, I.O.; Adams, R.H.; Greif, D.M. Smooth Muscle Cell Progenitors Are Primed to Muscularize in Pulmonary Hypertension. Sci. Transl. Med. 2015, 7, 308ra159. [Google Scholar] [CrossRef]
- Sheikh, A.Q.; Lighthouse, J.K.; Greif, D.M. Recapitulation of Developing Artery Muscularization in Pulmonary Hypertension. Cell Rep. 2014, 6, 809–817. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Emerald, B.S.; Ansari, S.A. Stem Cell Fate Determination through Protein O-GlcNAcylation. J. Biol. Chem. 2021, 296, 100035. [Google Scholar] [CrossRef]
- Barron, L.; Gharib, S.A.; Duffield, J.S. Lung Pericytes and Resident Fibroblasts. Am. J. Pathol. 2016, 186, 2519–2531. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Dierick, F.; Héry, T.; Hoareau-Coudert, B.; Mougenot, N.; Monceau, V.; Claude, C.; Crisan, M.; Besson, V.; Dorfmüller, P.; Marodon, G.; et al. Resident PW1+ Progenitor Cells Participate in Vascular Remodeling During Pulmonary Arterial Hypertension. Circ. Res. 2016, 118, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Meyrick, B.; Fujiwara, K.; Reid, L. Smooth Muscle Myosin in Precursor and Mature Smooth Muscle Cells in Normal Pulmonary Arteries and the Effect of Hypoxia. Exp. Lung Res. 1981, 2, 303–313. [Google Scholar] [CrossRef]
- Patel, M.S.; Taylor, G.P.; Bharya, S.; Al-Sanna’a, N.; Adatia, I.; Chitayat, D.; Suzanne Lewis, M.E.; Human, D.G. Abnormal Pericyte Recruitment as a Cause for Pulmonary Hypertension in Adams-Oliver Syndrome. Am. J. Med. Genet. A 2004, 129A, 294–299. [Google Scholar] [CrossRef]
- Bordenave, J.; Tu, L.; Berrebeh, N.; Thuillet, R.; Cumont, A.; Le Vely, B.; Fadel, E.; Nadaud, S.; Savale, L.; Humbert, M.; et al. Lineage Tracing Reveals the Dynamic Contribution of Pericytes to the Blood Vessel Remodeling in Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Ricard, N.; Tu, L.; Le Hiress, M.; Huertas, A.; Phan, C.; Thuillet, R.; Sattler, C.; Fadel, E.; Seferian, A.; Montani, D.; et al. Increased Pericyte Coverage Mediated by Endothelial-Derived Fibroblast Growth Factor-2 and Interleukin-6 Is a Source of Smooth Muscle-like Cells in Pulmonary Hypertension. Circulation 2014, 129, 1586–1597. [Google Scholar] [CrossRef]
- Yuan, K.; Liu, Y.; Zhang, Y.; Nathan, A.; Tian, W.; Yu, J.; Sweatt, A.J.; Shamshou, E.A.; Condon, D.; Chakraborty, A.; et al. Mural Cell SDF1 Signaling Is Associated with the Pathogenesis of Pulmonary Arterial Hypertension. Am. J. Respir. Cell. Mol. Biol. 2020, 62, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.; Fessel, J.P.; Ihida-Stansbury, K.; Schmidt, E.P.; Gaskill, C.; Alvarez, D.; Graham, B.; Harrison, D.G.; Wagner, D.H., Jr.; Nozik-Grayck, E.; et al. Dysfunctional Resident Lung Mesenchymal Stem Cells Contribute to Pulmonary Microvascular Remodeling. Pulm. Circ. 2013, 3, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, K.R.; Frid, M.; Perros, F. Endothelial-to-Mesenchymal Transition: An Evolving Paradigm and a Promising Therapeutic Target in PAH. Circulation 2016, 133, 1734–1737. [Google Scholar] [CrossRef]
- Qiao, L.; Nishimura, T.; Shi, L.; Sessions, D.; Thrasher, A.; Trudell, J.R.; Berry, G.J.; Pearl, R.G.; Kao, P.N. Endothelial Fate Mapping in Mice with Pulmonary Hypertension. Circulation 2014, 129, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Good, R.B.; Gilbane, A.J.; Trinder, S.L.; Denton, C.P.; Coghlan, G.; Abraham, D.J.; Holmes, A.M. Endothelial to Mesenchymal Transition Contributes to Endothelial Dysfunction in Pulmonary Arterial Hypertension. Am. J. Pathol. 2015, 185, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Nikitopoulou, I.; Orfanos, S.E.; Kotanidou, A.; Maltabe, V.; Manitsopoulos, N.; Karras, P.; Kouklis, P.; Armaganidis, A.; Maniatis, N.A. Vascular Endothelial-Cadherin Downregulation as a Feature of Endothelial Transdifferentiation in Monocrotaline-Induced Pulmonary Hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L352–L363. [Google Scholar] [CrossRef]
- Suzuki, T.; Carrier, E.J.; Talati, M.H.; Rathinasabapathy, A.; Chen, X.; Nishimura, R.; Tada, Y.; Tatsumi, K.; West, J. Isolation and Characterization of Endothelial-to-Mesenchymal Transition Cells in Pulmonary Arterial Hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L118–L126. [Google Scholar] [CrossRef]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Péchoux, C.; Bogaard, H.J.; Dorfmüller, P.; Remy, S.; Lecerf, F.; Planté, S.; et al. Endothelial-to-Mesenchymal Transition in Pulmonary Hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Hopper, R.K.; Moonen, J.-R.A.J.; Diebold, I.; Cao, A.; Rhodes, C.J.; Tojais, N.F.; Hennigs, J.K.; Gu, M.; Wang, L.; Rabinovitch, M. In Pulmonary Arterial Hypertension, Reduced BMPR2 Promotes Endothelial-to-Mesenchymal Transition via HMGA1 and Its Target Slug. Circulation 2016, 133, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Fujita, J.; Miyake, Y.; Kawada, H.; Ando, K.; Ogawa, S.; Fukuda, K. Bone Marrow-Derived Cells Contribute to Pulmonary Vascular Remodeling in Hypoxia-Induced Pulmonary Hypertension. Chest 2005, 127, 1793–1798. [Google Scholar] [CrossRef]
- Spees, J.L.; Whitney, M.J.; Sullivan, D.E.; Lasky, J.A.; Laboy, M.; Ylostalo, J.; Prockop, D.J. Bone Marrow Progenitor Cells Contribute to Repair and Remodeling of the Lung and Heart in a Rat Model of Progressive Pulmonary Hypertension. FASEB J. 2008, 22, 1226–1236. [Google Scholar] [CrossRef]
- Sahara, M.; Sata, M.; Morita, T.; Nakamura, K.; Hirata, Y.; Nagai, R. Diverse Contribution of Bone Marrow-Derived Cells to Vascular Remodeling Associated with Pulmonary Arterial Hypertension and Arterial Neointimal Formation. Circulation 2007, 115, 509–517. [Google Scholar] [CrossRef]
- Angelini, D.J.; Su, Q.; Kolosova, I.A.; Fan, C.; Skinner, J.T.; Yamaji-Kegan, K.; Collector, M.; Sharkis, S.J.; Johns, R.A. Hypoxia-Induced Mitogenic Factor (HIMF/FIZZ1/RELMα) Recruits Bone Marrow-Derived Cells to the Murine Pulmonary Vasculature. PLoS ONE 2010, 5, e11251. [Google Scholar] [CrossRef]
- Gambaryan, N.; Perros, F.; Montani, D.; Cohen-Kaminsky, S.; Mazmanian, M.; Renaud, J.F.; Simonneau, G.; Lombet, A.; Humbert, M. Targeting of C-Kit+ Haematopoietic Progenitor Cells Prevents Hypoxic Pulmonary Hypertension. Eur. Respir. J. 2011, 37, 1392–1399. [Google Scholar] [CrossRef]
- Gambaryan, N.; Perros, F.; Montani, D.; Cohen-Kaminsky, S.; Mazmanian, G.M.; Humbert, M. Imatinib Inhibits Bone Marrow-Derived c-Kit+ Cell Mobilisation in Hypoxic Pulmonary Hypertension. Eur. Respir. J. 2010, 36, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Deng, J.; Potter, C.M.F.; Nowak, W.N.; Gu, W.; Zhang, Z.; Chen, T.; Chen, Q.; Hu, Y.; Zhou, B.; et al. Recipient C-Kit Lineage Cells Repopulate Smooth Muscle Cells of Transplant Arteriosclerosis in Mouse Models. Circ. Res. 2019, 125, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Fukumoto, Y.; Nakano, M.; Sugimura, K.; Nawata, J.; Demachi, J.; Karibe, A.; Kagaya, Y.; Ishii, N.; Sugamura, K.; et al. Statin Ameliorates Hypoxia-Induced Pulmonary Hypertension Associated with down-Regulated Stromal Cell-Derived Factor-1. Cardiovasc. Res. 2009, 81, 226–234. [Google Scholar] [CrossRef]
- Chong, S.G.; Sato, S.; Kolb, M.; Gauldie, J. Fibrocytes and Fibroblasts—Where Are We Now. Int. J. Biochem. Cell Biol. 2019, 116, 105595. [Google Scholar] [CrossRef]
- Nikam, V.S.; Nikam, S.; Sydykov, A.; Ahlbrecht, K.; Morty, R.E.; Seeger, W.; Voswinckel, R. Implication of in Vivo Circulating Fibrocytes Ablation in Experimental Pulmonary Hypertension Murine Model. Br. J. Pharmacol. 2020, 177, 2974–2990. [Google Scholar] [CrossRef]
- Phillips, R.J.; Burdick, M.D.; Hong, K.; Lutz, M.A.; Murray, L.A.; Xue, Y.Y.; Belperio, J.A.; Keane, M.P.; Strieter, R.M. Circulating Fibrocytes Traffic to the Lungs in Response to CXCL12 and Mediate Fibrosis. J. Clin. Investig. 2004, 114, 438–446. [Google Scholar] [CrossRef]
- Garibaldi, B.T.; D’Alessio, F.R.; Mock, J.R.; Files, D.C.; Chau, E.; Eto, Y.; Drummond, M.B.; Aggarwal, N.R.; Sidhaye, V.; King, L.S. Regulatory T Cells Reduce Acute Lung Injury Fibroproliferation by Decreasing Fibrocyte Recruitment. Am. J. Respir. Cell Mol. Biol. 2013, 48, 35–43. [Google Scholar] [CrossRef]
- Farkas, D.; Kraskauskas, D.; Drake, J.I.; Alhussaini, A.A.; Kraskauskiene, V.; Bogaard, H.J.; Cool, C.D.; Voelkel, N.F.; Farkas, L. CXCR4 Inhibition Ameliorates Severe Obliterative Pulmonary Hypertension and Accumulation of C-Kit+ Cells in Rats. PLoS ONE 2014, 9, e89810. [Google Scholar] [CrossRef]
- Yeager, M.E.; Nguyen, C.M.; Belchenko, D.D.; Colvin, K.L.; Takatsuki, S.; Ivy, D.D.; Stenmark, K.R. Circulating Fibrocytes Are Increased in Children and Young Adults with Pulmonary Hypertension. Eur. Respir. J. 2012, 39, 104–111. [Google Scholar] [CrossRef]
- Southgate, L.; Machado, R.D.; Gräf, S.; Morrell, N.W. Molecular Genetic Framework Underlying Pulmonary Arterial Hypertension. Nat. Rev. Cardiol. 2020, 17, 85–95. [Google Scholar] [CrossRef]
- Torihashi, S.; Hattori, T.; Hasegawa, H.; Kurahashi, M.; Ogaeri, T.; Fujimoto, T. The Expression and Crucial Roles of BMP Signaling in Development of Smooth Muscle Progenitor Cells in the Mouse Embryonic Gut. Differentiation 2009, 77, 277–289. [Google Scholar] [CrossRef]
- Applebaum, M.; Ben-Yair, R.; Kalcheim, C. Segregation of Striated and Smooth Muscle Lineages by a Notch-Dependent Regulatory Network. BMC Biol. 2014, 12, 53. [Google Scholar] [CrossRef]
- Gaskill, C.F.; Carrier, E.J.; Kropski, J.A.; Bloodworth, N.C.; Menon, S.; Foronjy, R.F.; Taketo, M.M.; Hong, C.C.; Austin, E.D.; West, J.D.; et al. Disruption of Lineage Specification in Adult Pulmonary Mesenchymal Progenitor Cells Promotes Microvascular Dysfunction. J. Clin. Investig. 2017, 127, 2262–2276. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.-G. Functions of BMP Signaling in Embryonic Stem Cell Fate Determination. Exp. Cell Res. 2013, 319, 113–119. [Google Scholar] [CrossRef]
- Ng, F.; Boucher, S.; Koh, S.; Sastry, K.S.R.; Chase, L.; Lakshmipathy, U.; Choong, C.; Yang, Z.; Vemuri, M.C.; Rao, M.S.; et al. PDGF, TGF-Beta, and FGF Signaling Is Important for Differentiation and Growth of Mesenchymal Stem Cells (MSCs): Transcriptional Profiling Can Identify Markers and Signaling Pathways Important in Differentiation of MSCs into Adipogenic, Chondrogenic, and Osteogenic Lineages. Blood 2008, 112, 295–307. [Google Scholar] [CrossRef]
- Marriott, S.; Baskir, R.S.; Gaskill, C.; Menon, S.; Carrier, E.J.; Williams, J.; Talati, M.; Helm, K.; Alford, C.E.; Kropski, J.A.; et al. ABCG2pos Lung Mesenchymal Stem Cells Are a Novel Pericyte Subpopulation That Contributes to Fibrotic Remodeling. Am. J. Physiol. Cell Physiol. 2014, 307, C684–C698. [Google Scholar] [CrossRef]
- Kurpinski, K.; Lam, H.; Chu, J.; Wang, A.; Kim, A.; Tsay, E.; Agrawal, S.; Schaffer, D.V.; Li, S. Transforming Growth Factor-Beta and Notch Signaling Mediate Stem Cell Differentiation into Smooth Muscle Cells. Stem Cells 2010, 28, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Yung, L.-M.; Nikolic, I.; Paskin-Flerlage, S.D.; Pearsall, R.S.; Kumar, R.; Yu, P.B. A Selective Transforming Growth Factor-β Ligand Trap Attenuates Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2016, 194, 1140–1151. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Feng, J.-A.; Li, P.; Xing, D.; Zhang, Y.; Serra, R.; Ambalavanan, N.; Majid-Hassan, E.; Oparil, S. Dominant Negative Mutation of the TGF-Beta Receptor Blocks Hypoxia-Induced Pulmonary Vascular Remodeling. J. Appl. Physiol. 2006, 100, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Evrard, S.M.; d’Audigier, C.; Mauge, L.; Israël-Biet, D.; Guerin, C.L.; Bieche, I.; Kovacic, J.C.; Fischer, A.-M.; Gaussem, P.; Smadja, D.M. The Profibrotic Cytokine Transforming Growth Factor-Β1 Increases Endothelial Progenitor Cell Angiogenic Properties. J. Thromb. Haemost. 2012, 10, 670–679. [Google Scholar] [CrossRef]
- Meini, S.; Giani, T.; Tascini, C. Intussusceptive Angiogenesis in Covid-19: Hypothesis on the Significance and Focus on the Possible Role of FGF2. Mol. Biol. Rep. 2020, 47, 8301–8304. [Google Scholar] [CrossRef]
- De Langhe, S.P.; Carraro, G.; Warburton, D.; Hajihosseini, M.K.; Bellusci, S. Levels of Mesenchymal FGFR2 Signaling Modulate Smooth Muscle Progenitor Cell Commitment in the Lung. Dev. Biol. 2006, 299, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Mailleux, A.A.; Kelly, R.; Veltmaat, J.M.; De Langhe, S.P.; Zaffran, S.; Thiery, J.P.; Bellusci, S. Fgf10 Expression Identifies Parabronchial Smooth Muscle Cell Progenitors and Is Required for Their Entry into the Smooth Muscle Cell Lineage. Development 2005, 132, 2157–2166. [Google Scholar] [CrossRef]
- Ramasamy, S.K.; Mailleux, A.A.; Gupte, V.V.; Mata, F.; Sala, F.G.; Veltmaat, J.M.; Del Moral, P.M.; De Langhe, S.; Parsa, S.; Kelly, L.K.; et al. Fgf10 Dosage Is Critical for the Amplification of Epithelial Cell Progenitors and for the Formation of Multiple Mesenchymal Lineages during Lung Development. Dev. Biol. 2007, 307, 237–247. [Google Scholar] [CrossRef]
- El Agha, E.; Schwind, F.; Ruppert, C.; Günther, A.; Bellusci, S.; Schermuly, R.T.; Kosanovic, D. Is the Fibroblast Growth Factor Signaling Pathway a Victim of Receptor Tyrosine Kinase Inhibition in Pulmonary Parenchymal and Vascular Remodeling? Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L248–L252. [Google Scholar] [CrossRef]
- Hashimoto-Kataoka, T.; Hosen, N.; Sonobe, T.; Arita, Y.; Yasui, T.; Masaki, T.; Minami, M.; Inagaki, T.; Miyagawa, S.; Sawa, Y.; et al. Interleukin-6/Interleukin-21 Signaling Axis Is Critical in the Pathogenesis of Pulmonary Arterial Hypertension. Proc. Natl. Acad. Sci. USA 2015, 112, E2677–E2686. [Google Scholar] [CrossRef]
- Fan, Y.; Ye, J.; Shen, F.; Zhu, Y.; Yeghiazarians, Y.; Zhu, W.; Chen, Y.; Lawton, M.T.; Young, W.L.; Yang, G.-Y. Interleukin-6 Stimulates Circulating Blood-Derived Endothelial Progenitor Cell Angiogenesis in Vitro. J. Cereb. Blood Flow Metab. 2008, 28, 90–98. [Google Scholar] [CrossRef]
- Prisco, A.R.; Hoffmann, B.R.; Kaczorowski, C.C.; McDermott-Roe, C.; Stodola, T.J.; Exner, E.C.; Greene, A.S. Tumor Necrosis Factor α Regulates Endothelial Progenitor Cell Migration via CADM1 and NF-KB. Stem Cells 2016, 34, 1922–1933. [Google Scholar] [CrossRef]
- Wong, M.M.; Chen, Y.; Margariti, A.; Winkler, B.; Campagnolo, P.; Potter, C.; Hu, Y.; Xu, Q. Macrophages Control Vascular Stem/Progenitor Cell Plasticity Through Tumor Necrosis Factor-α–Mediated Nuclear Factor-ΚB Activation. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 635–643. [Google Scholar] [CrossRef][Green Version]
- Hattori, K.; Heissig, B.; Tashiro, K.; Honjo, T.; Tateno, M.; Shieh, J.H.; Hackett, N.R.; Quitoriano, M.S.; Crystal, R.G.; Rafii, S.; et al. Plasma Elevation of Stromal Cell-Derived Factor-1 Induces Mobilization of Mature and Immature Hematopoietic Progenitor and Stem Cells. Blood 2001, 97, 3354–3360. [Google Scholar] [CrossRef]
- Bordenave, J.; Thuillet, R.; Tu, L.; Phan, C.; Cumont, A.; Marsol, C.; Huertas, A.; Savale, L.; Hibert, M.; Galzi, J.-L.; et al. Neutralization of CXCL12 Attenuates Established Pulmonary Hypertension in Rats. Cardiovasc. Res. 2020, 116, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Rafii, S.; Cao, Z.; Lis, R.; Siempos, I.I.; Chavez, D.; Shido, K.; Rabbany, S.Y.; Ding, B.-S. Platelet-Derived SDF-1 Primes the Pulmonary Capillary Vascular Niche to Drive Lung Alveolar Regeneration. Nat. Cell Biol. 2015, 17, 123–136. [Google Scholar] [CrossRef]
- Hitchon, C.; Wong, K.; Ma, G.; Reed, J.; Lyttle, D.; El-Gabalawy, H. Hypoxia-Induced Production of Stromal Cell-Derived Factor 1 (CXCL12) and Vascular Endothelial Growth Factor by Synovial Fibroblasts. Arthritis Rheumatol. 2002, 46, 2587–2597. [Google Scholar] [CrossRef]
- Martin, S.K.; Diamond, P.; Williams, S.A.; To, L.B.; Peet, D.J.; Fujii, N.; Gronthos, S.; Harris, A.L.; Zannettino, A.C.W. Hypoxia-Inducible Factor-2 Is a Novel Regulator of Aberrant CXCL12 Expression in Multiple Myeloma Plasma Cells. Haematologica 2010, 95, 776–784. [Google Scholar] [CrossRef]
- Perros, F.; Montani, D.; Dorfmuller, P.; Durand-Gasselin, I.; Tcherakian, C.; Le Pavec, J.; Mazmanian, M.; Fadel, E.; Mussot, S.; Mercier, O.; et al. Platelet-Derived Growth Factor Expression and Function in Idiopathic Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2008, 178, 81–88. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Dony, E.; Ghofrani, H.A.; Pullamsetti, S.; Savai, R.; Roth, M.; Sydykov, A.; Lai, Y.J.; Weissmann, N.; Seeger, W.; et al. Reversal of Experimental Pulmonary Hypertension by PDGF Inhibition. J. Clin. Investig. 2005, 115, 2811–2821. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Barst, R.J.; Bourge, R.C.; Feldman, J.; Frost, A.E.; Galié, N.; Gómez-Sánchez, M.A.; Grimminger, F.; Grünig, E.; Hassoun, P.M.; et al. Imatinib Mesylate as Add-on Therapy for Pulmonary Arterial Hypertension: Results of the Randomized IMPRES Study. Circulation 2013, 127, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Ten Freyhaus, H.; Berghausen, E.M.; Janssen, W.; Leuchs, M.; Zierden, M.; Murmann, K.; Klinke, A.; Vantler, M.; Caglayan, E.; Kramer, T.; et al. Genetic Ablation of PDGF-Dependent Signaling Pathways Abolishes Vascular Remodeling and Experimental Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2015. [Google Scholar] [CrossRef]
- Dahal, B.K.; Heuchel, R.; Pullamsetti, S.S.; Wilhelm, J.; Ghofrani, H.A.; Weissmann, N.; Seeger, W.; Grimminger, F.; Schermuly, R.T. Hypoxic Pulmonary Hypertension in Mice with Constitutively Active Platelet-Derived Growth Factor Receptor-β. Pulm. Circ. 2011, 1, 259–268. [Google Scholar] [CrossRef]
- Sheikh, A.Q.; Saddouk, F.Z.; Ntokou, A.; Mazurek, R.; Greif, D.M. Cell Autonomous and Non-Cell Autonomous Regulation of SMC Progenitors in Pulmonary Hypertension. Cell Rep. 2018, 23, 1152–1165. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Frank, D.B.; Kadzik, R.S.; Morley, M.P.; Rathi, K.S.; Wang, T.; Zhou, S.; Cheng, L.; Lu, M.M.; Morrisey, E.E. Hedgehog Actively Maintains Adult Lung Quiescence and Regulates Repair and Regeneration. Nature 2015, 526, 578–582. [Google Scholar] [CrossRef]
- Bostrom, H.; Willetts, K.; Pekny, M.; Leveen, P.; Lindahl, P.; Hedstrand, H.; Pekna, M.; Hellstrom, M.; Gebre-Medhin, S.; Schalling, M.; et al. PDGF-A Signaling Is a Critical Event in Lung Alveolar Myofibroblast Development and Alveogenesis. Cell 1996, 85, 863–873. [Google Scholar] [CrossRef]
- Tang, J.; Wang, H.; Huang, X.; Li, F.; Zhu, H.; Li, Y.; He, L.; Zhang, H.; Pu, W.; Liu, K.; et al. Arterial Sca1+ Vascular Stem Cells Generate De Novo Smooth Muscle for Artery Repair and Regeneration. Cell Stem Cell 2020, 26, 81–96.e4. [Google Scholar] [CrossRef]
- Baarsma, H.A.; Königshoff, M. “WNT-Er Is Coming”: WNT Signalling in Chronic Lung Diseases. Thorax 2017, 72, 746–759. [Google Scholar] [CrossRef] [PubMed]
- West, J.D.; Austin, E.D.; Gaskill, C.; Marriott, S.; Baskir, R.; Bilousova, G.; Jean, J.-C.; Hemnes, A.R.; Menon, S.; Bloodworth, N.C.; et al. Identification of a Common Wnt-Associated Genetic Signature across Multiple Cell Types in Pulmonary Arterial Hypertension. Am. J. Physiol. Cell Physiol. 2014, 307, C415–C430. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, I.; Huang, W.; Zhang, J.; Zhang, S.; Platoshyn, O.; Remillard, C.V.; Thistlethwaite, P.A.; Yuan, J.X.-J. Divergent Effects of BMP-2 on Gene Expression in Pulmonary Artery Smooth Muscle Cells from Normal Subjects and Patients with Idiopathic Pulmonary Arterial Hypertension. Exp. Lung Res. 2005, 31, 783–806. [Google Scholar] [CrossRef]
- Laumanns, I.P.; Fink, L.; Wilhelm, J.; Wolff, J.-C.; Mitnacht-Kraus, R.; Graef-Hoechst, S.; Stein, M.M.; Bohle, R.M.; Klepetko, W.; Hoda, M.A.R.; et al. The Noncanonical WNT Pathway Is Operative in Idiopathic Pulmonary Arterial Hypertension. Am. J. Respir. Cell. Mol. Biol. 2009, 40, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.D.; Ihida-Stansbury, K.; Lu, M.M.; Panettieri, R.A.; Jones, P.L.; Morrisey, E.E. Wnt Signaling Regulates Smooth Muscle Precursor Development in the Mouse Lung via a Tenascin C/PDGFR Pathway. J. Clin. Investig. 2009, 119, 2538–2549. [Google Scholar] [CrossRef]
- Karamariti, E.; Zhai, C.; Yu, B.; Qiao, L.; Wang, Z.; Potter, C.M.F.; Wong, M.M.; Simpson, R.M.L.; Zhang, Z.; Wang, X.; et al. DKK3 (Dickkopf 3) Alters Atherosclerotic Plaque Phenotype Involving Vascular Progenitor and Fibroblast Differentiation Into Smooth Muscle Cells. Arterioscler. Thromb Vasc. Biol. 2018, 38, 425–437. [Google Scholar] [CrossRef]
- Issa Bhaloo, S.; Wu, Y.; Le Bras, A.; Yu, B.; Gu, W.; Xie, Y.; Deng, J.; Wang, Z.; Zhang, Z.; Kong, D.; et al. Binding of Dickkopf-3 to CXCR7 Enhances Vascular Progenitor Cell Migration and Degradable Graft Regeneration. Circ. Res. 2018, 123, 451–466. [Google Scholar] [CrossRef]
- Summers, M.E.; Richmond, B.W.; Menon, S.; Sheridan, R.M.; Kropski, J.A.; Majka, S.A.; Taketo, M.M.; Bastarache, J.A.; West, J.D.; De Langhe, S.; et al. Resident Mesenchymal Vascular Progenitors Modulate Adaptive Angiogenesis and Pulmonary Remodeling via Regulation of Canonical Wnt Signaling. FASEB J. 2020, 34, 10267–10285. [Google Scholar] [CrossRef]
- Smadja, D.M.; Mauge, L.; Sanchez, O.; Silvestre, J.-S.; Guerin, C.; Godier, A.; Henno, P.; Gaussem, P.; Israël-Biet, D. Distinct Patterns of Circulating Endothelial Cells in Pulmonary Hypertension. Eur. Respir. J. 2010, 36, 1284–1293. [Google Scholar] [CrossRef]
- Campagnolo, P.; Tsai, T.-N.; Hong, X.; Kirton, J.P.; So, P.-W.; Margariti, A.; Di Bernardini, E.; Wong, M.M.; Hu, Y.; Stevens, M.M.; et al. C-Kit+ Progenitors Generate Vascular Cells for Tissue-Engineered Grafts through Modulation of the Wnt/Klf4 Pathway. Biomaterials 2015, 60, 53–61. [Google Scholar] [CrossRef]
- Shao, Y.; Chen, J.; Freeman, W.; Dong, L.-J.; Zhang, Z.-H.; Xu, M.; Qiu, F.; Du, Y.; Liu, J.; Li, X.-R.; et al. Canonical Wnt Signaling Promotes Neovascularization Through Determination of Endothelial Progenitor Cell Fate via Metabolic Profile Regulation. Stem Cells 2019, 37, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Hübner, K.; Grassme, K.S.; Rao, J.; Wenke, N.K.; Zimmer, C.L.; Korte, L.; Müller, K.; Sumanas, S.; Greber, B.; Herzog, W. Wnt Signaling Positively Regulates Endothelial Cell Fate Specification in the Fli1a-Positive Progenitor Population via Lef1. Dev. Biol. 2017, 430, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Motte, S.; McEntee, K.; Naeije, R. Endothelin Receptor Antagonists. Pharm. Ther. 2006, 110, 386–414. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Wang, J.; Yuan, H.; Jiao, H.; Tsai, T.-L.; Squire, M.W.; Li, W.-J. Endothelin-1 Differentially Directs Lineage Specification of Adipose- and Bone Marrow-Derived Mesenchymal Stem Cells. FASEB J. 2019, 33, 996–1007. [Google Scholar] [CrossRef]
- Tsai, T.-L.; Wang, B.; Squire, M.W.; Guo, L.-W.; Li, W.-J. Endothelial Cells Direct Human Mesenchymal Stem Cells for Osteo- and Chondro-Lineage Differentiation through Endothelin-1 and AKT Signaling. Stem Cell Res. Ther. 2015, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Pourjafar, M.; Saidijam, M.; Mansouri, K.; Malih, S.; Ranjbar Nejad, T.; Shabab, N.; Najafi, R. Cytoprotective Effects of Endothelin-1 on Mesenchymal Stem Cells: An in Vitro Study. Clin. Exp. Pharmacol. Physiol. 2016, 43, 769–776. [Google Scholar] [CrossRef]
- Soh, B.-S.; Ng, S.-Y.; Wu, H.; Buac, K.; Park, J.-H.C.; Lian, X.; Xu, J.; Foo, K.S.; Felldin, U.; He, X.; et al. Endothelin-1 Supports Clonal Derivation and Expansion of Cardiovascular Progenitors Derived from Human Embryonic Stem Cells. Nat. Commun. 2016, 7, 10774. [Google Scholar] [CrossRef]
- Kemp, S.S.; Aguera, K.N.; Cha, B.; Davis, G.E. Defining Endothelial Cell-Derived Factors That Promote Pericyte Recruitment and Capillary Network Assembly. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2632–2648. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Huang, J.; Wang, Y. Biological Significance of NOTCH Signaling Strength. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Patenaude, A.; Woerher, S.; Umlandt, P.; Wong, F.; Ibrahim, R.; Kyle, A.; Unger, S.; Fuller, M.; Parker, J.; Minchinton, A.; et al. A Novel Population of Local Pericyte Precursor Cells in Tumor Stroma That Require Notch Signaling for Differentiation. Microvasc. Res. 2015, 101, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Noseda, M.; Higginson, M.; Ly, M.; Patenaude, A.; Fuller, M.; Kyle, A.H.; Minchinton, A.I.; Puri, M.C.; Dumont, D.J.; et al. Differentiation of Vascular Smooth Muscle Cells from Local Precursors during Embryonic and Adult Arteriogenesis Requires Notch Signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 6993–6998. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Wang, W.; Peng, D.; Chiba, A.; Lagendijk, A.K.; Barske, L.; Crump, J.G.; Stainier, D.Y.R.; Lendahl, U.; Koltowska, K.; et al. Peri-Arterial Specification of Vascular Mural Cells from Naïve Mesenchyme Requires Notch Signaling. Development 2019, 146. [Google Scholar] [CrossRef]
- Mooney, C.J.; Hakimjavadi, R.; Fitzpatrick, E.; Kennedy, E.; Walls, D.; Morrow, D.; Redmond, E.M.; Cahill, P.A. Hedgehog and Resident Vascular Stem Cell Fate. Stem Cells Int. 2015, 2015, 468428. [Google Scholar] [CrossRef]
- Shih, Y.-T.; Wang, M.-C.; Yang, T.-L.; Zhou, J.; Lee, D.-Y.; Lee, P.-L.; Yet, S.-F.; Chiu, J.-J. β(2)-Integrin and Notch-1 Differentially Regulate CD34(+)CD31(+) Cell Plasticity in Vascular Niches. Cardiovasc. Res. 2012, 96, 296–307. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Leathers, R.; Makino, A.; Huang, C.; Parsa, P.; Macias, J.; Yuan, J.X.-J.; Jamieson, S.W.; Thistlethwaite, P.A. Notch3 Signaling Promotes the Development of Pulmonary Arterial Hypertension. Nat. Med. 2009, 15, 1289–1297. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, X.; Zhu, Y.; Liu, L.; Feng, W.; Pan, Y.; Zhai, C.; Ke, R.; Li, S.; Song, Y.; et al. Inhibition of Notch3 Prevents Monocrotaline-Induced Pulmonary Arterial Hypertension. Exp. Lung Res. 2015, 41, 435–443. [Google Scholar] [CrossRef]
- Steffes, L.C.; Froistad, A.A.; Andruska, A.; Boehm, M.; McGlynn, M.; Zhang, F.; Zhang, W.; Hou, D.; Tian, X.; Miquerol, L.; et al. A Notch3-Marked Subpopulation of Vascular Smooth Muscle Cells Is the Cell of Origin for Occlusive Pulmonary Vascular Lesions. Circulation 2020, 142, 1545–1561. [Google Scholar] [CrossRef] [PubMed]
- Jonigk, D.; Golpon, H.; Bockmeyer, C.L.; Maegel, L.; Hoeper, M.M.; Gottlieb, J.; Nickel, N.; Hussein, K.; Maus, U.; Lehmann, U.; et al. Plexiform Lesions in Pulmonary Arterial Hypertension Composition, Architecture, and Microenvironment. Am. J. Pathol. 2011, 179, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Dabral, S.; Tian, X.; Kojonazarov, B.; Savai, R.; Ghofrani, H.A.; Weissmann, N.; Florio, M.; Sun, J.; Jonigk, D.; Maegel, L.; et al. Notch1 Signalling Regulates Endothelial Proliferation and Apoptosis in Pulmonary Arterial Hypertension. Eur. Respir. J. 2016, 48, 1137–1149. [Google Scholar] [CrossRef]
- Xiao, Y.; Gong, D.; Wang, W. Soluble Jagged1 Inhibits Pulmonary Hypertension by Attenuating Notch Signaling. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2733–2739. [Google Scholar] [CrossRef]
- Qiao, L.; Xie, L.; Shi, K.; Zhou, T.; Hua, Y.; Liu, H. Notch Signaling Change in Pulmonary Vascular Remodeling in Rats with Pulmonary Hypertension and Its Implication for Therapeutic Intervention. PLoS ONE 2012, 7, e51514. [Google Scholar] [CrossRef]
- Coller, H.A. The Paradox of Metabolism in Quiescent Stem Cells. FEBS Lett. 2019, 593, 2817–2839. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J.P.; Hamid, R.; Wittmann, B.M.; Robinson, L.J.; Blackwell, T.; Tada, Y.; Tanabe, N.; Tatsumi, K.; Hemnes, A.R.; West, J.D. Metabolomic Analysis of Bone Morphogenetic Protein Receptor Type 2 Mutations in Human Pulmonary Endothelium Reveals Widespread Metabolic Reprogramming. Pulm. Circ. 2012, 2, 201–213. [Google Scholar] [CrossRef]
- Zhao, L.; Ashek, A.; Wang, L.; Fang, W.; Dabral, S.; Dubois, O.; Cupitt, J.; Pullamsetti, S.S.; Cotroneo, E.; Jones, H.; et al. Heterogeneity in Lung (18)FDG Uptake in Pulmonary Arterial Hypertension: Potential of Dynamic (18)FDG Positron Emission Tomography with Kinetic Analysis as a Bridging Biomarker for Pulmonary Vascular Remodeling Targeted Treatments. Circulation 2013, 128, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; El Kasmi, K.C.; Plecitá-Hlavatá, L.; Ježek, P.; Li, M.; Zhang, H.; Gupte, S.A.; Stenmark, K.R. Hallmarks of Pulmonary Hypertension: Mesenchymal and Inflammatory Cell Metabolic Reprogramming. Antioxid. Redox Signal. 2018, 28, 230–250. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ahmad, S.; Malcolm, K.C.; Miller, S.M.; Hendry-Hofer, T.; Schaack, J.B.; White, C.W. Differential Regulation of Pulmonary Vascular Cell Growth by Hypoxia-Inducible Transcription Factor-1α and Hypoxia-Inducible Transcription Factor-2α. Am. J. Respir. Cell. Mol. Biol. 2013, 49, 78–85. [Google Scholar] [CrossRef]
- Ball, M.K.; Waypa, G.B.; Mungai, P.T.; Nielsen, J.M.; Czech, L.; Dudley, V.J.; Beussink, L.; Dettman, R.W.; Berkelhamer, S.K.; Steinhorn, R.H.; et al. Regulation of Hypoxia-Induced Pulmonary Hypertension by Vascular Smooth Muscle Hypoxia-Inducible Factor-1α. Am. J. Respir. Crit. Care Med. 2014, 189, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.V.; James, J.; Rafikova, O.; Rafikov, R. Glucose-6-Phosphate Dehydrogenase Deficiency Contributes to Metabolic Abnormality and Pulmonary Hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L508–L521. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Riddle, S.R.; Frid, M.G.; El Kasmi, K.C.; McKinsey, T.A.; Sokol, R.J.; Strassheim, D.; Meyrick, B.; Yeager, M.E.; Flockton, A.R.; et al. Emergence of Fibroblasts with a Proinflammatory Epigenetically Altered Phenotype in Severe Hypoxic Pulmonary Hypertension. J. Immunol. 2011, 187, 2711–2722. [Google Scholar] [CrossRef]
- Chettimada, S.; Joshi, S.R.; Alzoubi, A.; Gebb, S.A.; McMurtry, I.F.; Gupte, R.; Gupte, S.A. Glucose-6-Phosphate Dehydrogenase Plays a Critical Role in Hypoxia-Induced CD133+ Progenitor Cells Self-Renewal and Stimulates Their Accumulation in the Lungs of Pulmonary Hypertensive Rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L545–L556. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.R.; Kitagawa, A.; Jacob, C.; Hashimoto, R.; Dhagia, V.; Ramesh, A.; Zheng, C.; Zhang, H.; Jordan, A.; Waddell, I.; et al. Hypoxic Activation of Glucose-6-Phosphate Dehydrogenase Controls the Expression of Genes Involved in the Pathogenesis of Pulmonary Hypertension through the Regulation of DNA Methylation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L773–L786. [Google Scholar] [CrossRef] [PubMed]
- Dhagia, V.; Kitagawa, A.; Jacob, C.; Zheng, C.; D’Alessandro, A.; Edwards, J.G.; Rocic, P.; Gupte, R.; Gupte, S.A. G6PD Activity Contributes to the Regulation of Histone Acetylation and Gene Expression in Smooth Muscle Cells and to the Pathogenesis of Vascular Diseases. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H999–H1016. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.W.; Tian, L.; Heresi, G.A.; Farver, C.F.; Asosingh, K.; Comhair, S.A.A.; Aulak, K.S.; Dweik, R.A. O-Linked β-N-Acetylglucosamine Transferase Directs Cell Proliferation in Idiopathic Pulmonary Arterial Hypertension. Circulation 2015, 131, 1260–1268. [Google Scholar] [CrossRef]
- Ferrer, C.M.; Lynch, T.P.; Sodi, V.L.; Falcone, J.N.; Schwab, L.P.; Peacock, D.L.; Vocadlo, D.J.; Seagroves, T.N.; Reginato, M.J. O-GlcNAcylation Regulates Cancer Metabolism and Survival Stress Signaling via Regulation of the HIF-1 Pathway. Mol. Cell 2014, 54, 820–831. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kang, M.J.; Kim, E.; Kweon, T.H.; Park, Y.S.; Ji, S.; Yang, W.H.; Yi, E.C.; Cho, J.W. O-GlcNAc Stabilizes SMAD4 by Inhibiting GSK-3β-Mediated Proteasomal Degradation. Sci. Rep. 2020, 10, 19908. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, H.S.; Kim, N.H.; Ji, S.; Cha, S.Y.; Kang, J.G.; Ota, I.; Shimada, K.; Konishi, N.; Nam, H.W.; et al. Snail1 Is Stabilized by O-GlcNAc Modification in Hyperglycaemic Condition. EMBO J. 2010, 29, 3787–3796. [Google Scholar] [CrossRef]
- Li, X.; Han, Y.; Pang, W.; Li, C.; Xie, X.; Shyy, J.Y.-J.; Zhu, Y. AMP-Activated Protein Kinase Promotes the Differentiation of Endothelial Progenitor Cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Tang, F.-Q.; Jiang, Y.-H.; Zhu, Y.; Jian, Z.; Xiao, Y.-B. AMPKα2 Deficiency Exacerbates Hypoxia-Induced Pulmonary Hypertension by Promoting Pulmonary Arterial Smooth Muscle Cell Proliferation. J. Physiol. Biochem. 2020, 76, 445–456. [Google Scholar] [CrossRef]
- Smadja, D.M.; Gaussem, P.; Mauge, L.; Israël-Biet, D.; Dignat-George, F.; Peyrard, S.; Agnoletti, G.; Vouhé, P.R.; Bonnet, D.; Lévy, M. Circulating Endothelial Cells: A New Candidate Biomarker of Irreversible Pulmonary Hypertension Secondary to Congenital Heart Disease. Circulation 2009, 119, 374–381. [Google Scholar] [CrossRef]
- Bull, T.M.; Golpon, H.; Hebbel, R.P.; Solovey, A.; Cool, C.D.; Tuder, R.M.; Geraci, M.W.; Voelkel, N.F. Circulating Endothelial Cells in Pulmonary Hypertension. Thromb. Haemost. 2003, 90, 698–703. [Google Scholar] [CrossRef]
- Foris, V.; Kovacs, G.; Tscherner, M.; Olschewski, A.; Olschewski, H. Biomarkers in Pulmonary Hypertension: What Do We Know? Chest 2013, 144, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Foris, V.; Kovacs, G.; Marsh, L.M.; Bálint, Z.; Tötsch, M.; Avian, A.; Douschan, P.; Ghanim, B.; Klepetko, W.; Olschewski, A.; et al. CD133+ Cells in Pulmonary Arterial Hypertension. Eur. Respir. J. 2016, 48, 459–469. [Google Scholar] [CrossRef]
- Pizarro, S.; García-Lucio, J.; Peinado, V.I.; Tura-Ceide, O.; Díez, M.; Blanco, I.; Sitges, M.; Petriz, J.; Torralba, Y.; Marín, P.; et al. Circulating Progenitor Cells and Vascular Dysfunction in Chronic Obstructive Pulmonary Disease. PLoS ONE 2014, 9, e106163. [Google Scholar] [CrossRef] [PubMed]
- Smits, J.; Tasev, D.; Andersen, S.; Szulcek, R.; Botros, L.; Ringgaard, S.; Andersen, A.; Vonk-Noordegraaf, A.; Koolwijk, P.; Bogaard, H.J. Blood Outgrowth and Proliferation of Endothelial Colony Forming Cells Are Related to Markers of Disease Severity in Patients with Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2018, 19, 3763. [Google Scholar] [CrossRef]
- Amabile, N.; Heiss, C.; Real, W.M.; Minasi, P.; McGlothlin, D.; Rame, E.J.; Grossman, W.; De Marco, T.; Yeghiazarians, Y. Circulating Endothelial Microparticle Levels Predict Hemodynamic Severity of Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2008, 177, 1268–1275. [Google Scholar] [CrossRef]
- Amabile, N.; Heiss, C.; Chang, V.; Angeli, F.S.; Damon, L.; Rame, E.J.; McGlothlin, D.; Grossman, W.; De Marco, T.; Yeghiazarians, Y. Increased CD62e(+) Endothelial Microparticle Levels Predict Poor Outcome in Pulmonary Hypertension Patients. J. Heart Lung Transpl. 2009, 28, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Nadaud, S.; Poirier, O.; Girerd, B.; Blanc, C.; Montani, D.; Eyries, M.; Imbert-Bismut, F.; Pacheco, A.; Vigne, J.; Tregouet, D.A.; et al. Small Platelet Microparticle Levels Are Increased in Pulmonary Arterial Hypertension. Eur. J. Clin. Investig. 2013, 43, 64–71. [Google Scholar] [CrossRef]
- Aliotta, J.M.; Pereira, M.; Amaral, A.; Sorokina, A.; Igbinoba, Z.; Hasslinger, A.; El-Bizri, R.; Rounds, S.I.; Quesenberry, P.J.; Klinger, J.R. Induction of Pulmonary Hypertensive Changes by Extracellular Vesicles from Monocrotaline-Treated Mice. Cardiovasc. Res. 2013, 100, 354–362. [Google Scholar] [CrossRef]
- Amabile, N.; Guignabert, C.; Montani, D.; Yeghiazarians, Y.; Boulanger, C.M.; Humbert, M. Cellular Microparticles in the Pathogenesis of Pulmonary Hypertension. Eur. Respir. J. 2013, 42, 272–279. [Google Scholar] [CrossRef]
- Takahashi, M.; Nakamura, T.; Toba, T.; Kajiwara, N.; Kato, H.; Shimizu, Y. Transplantation of Endothelial Progenitor Cells into the Lung to Alleviate Pulmonary Hypertension in Dogs. Tissue Eng. 2004, 10, 771–779. [Google Scholar] [CrossRef]
- Nagaya, N.; Kangawa, K.; Kanda, M.; Uematsu, M.; Horio, T.; Fukuyama, N.; Hino, J.; Harada-Shiba, M.; Okumura, H.; Tabata, Y.; et al. Hybrid Cell-Gene Therapy for Pulmonary Hypertension Based on Phagocytosing Action of Endothelial Progenitor Cells. Circulation 2003, 108, 889–895. [Google Scholar] [CrossRef]
- Zhao, Y.D.; Courtman, D.W.; Deng, Y.; Kugathasan, L.; Zhang, Q.; Stewart, D.J. Rescue of Monocrotaline-Induced Pulmonary Arterial Hypertension Using Bone Marrow-Derived Endothelial-like Progenitor Cells: Efficacy of Combined Cell and ENOS Gene Therapy in Established Disease. Circ. Res. 2005, 96, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Yip, H.-K.; Chang, L.-T.; Sun, C.-K.; Sheu, J.-J.; Chiang, C.-H.; Youssef, A.A.; Lee, F.-Y.; Wu, C.-J.; Fu, M. Autologous Transplantation of Bone Marrow-Derived Endothelial Progenitor Cells Attenuates Monocrotaline-Induced Pulmonary Arterial Hypertension in Rats. Crit. Care Med. 2008, 36, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Raoul, W.; Wagner-Ballon, O.; Saber, G.; Hulin, A.; Marcos, E.; Giraudier, S.; Vainchenker, W.; Adnot, S.; Eddahibi, S.; Maitre, B. Effects of Bone Marrow-Derived Cells on Monocrotaline- and Hypoxia-Induced Pulmonary Hypertension in Mice. Respir. Res. 2007, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Ikutomi, M.; Sahara, M.; Nakajima, T.; Minami, Y.; Morita, T.; Hirata, Y.; Komuro, I.; Nakamura, F.; Sata, M. Diverse Contribution of Bone Marrow-Derived Late-Outgrowth Endothelial Progenitor Cells to Vascular Repair under Pulmonary Arterial Hypertension and Arterial Neointimal Formation. J. Mol. Cell. Cardiol. 2015, 86, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Ormiston, M.L.; Deng, Y.; Stewart, D.J.; Courtman, D.W. Innate Immunity in the Therapeutic Actions of Endothelial Progenitor Cells in Pulmonary Hypertension. Am. J. Respir. Cell. Mol. Biol. 2010, 43, 546–554. [Google Scholar] [CrossRef]
- Lavoie, J.R.; Stewart, D.J. Genetically Modified Endothelial Progenitor Cells in the Therapy of Cardiovascular Disease and Pulmonary Hypertension. Curr. Vasc. Pharmacol. 2012, 10, 289–299. [Google Scholar] [CrossRef]
- Chen, H.; Strappe, P.; Chen, S.; Wang, L.-X. Endothelial Progenitor Cells and Pulmonary Arterial Hypertension. Heart Lung Circ. 2014, 23, 595–601. [Google Scholar] [CrossRef]
- Ahmed, L.A.; Rizk, S.M.; EL-Maraghy, S.A. Pinocembrin Ex Vivo Preconditioning Improves the Therapeutic Efficacy of Endothelial Progenitor Cells in Monocrotaline-Induced Pulmonary Hypertension in Rats. Biochem. Pharmacol. 2017, 138, 193–204. [Google Scholar] [CrossRef]
- Yen, C.-H.; Tsai, T.-H.; Leu, S.; Chen, Y.-L.; Chang, L.-T.; Chai, H.-T.; Chung, S.-Y.; Chua, S.; Tsai, C.-Y.; Chang, H.-W.; et al. Sildenafil Improves Long-Term Effect of Endothelial Progenitor Cell-Based Treatment for Monocrotaline-Induced Rat Pulmonary Arterial Hypertension. Cytotherapy 2013, 15, 209–223. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, S.; Yang, J.; Chen, B.; Sun, Z.; Ye, L.; Zhu, J.; Wang, X. Interruption of CD40 Pathway Improves Efficacy of Transplanted Endothelial Progenitor Cells in Monocrotaline Induced Pulmonary Arterial Hypertension. Cell. Physiol. Biochem. 2015, 36, 683–696. [Google Scholar] [CrossRef]
- Cao, G.; Liu, C.; Wan, Z.; Liu, K.; Sun, H.; Sun, X.; Tang, M.; Bing, W.; Wu, S.; Pang, X.; et al. Combined Hypoxia Inducible Factor-1α and Homogeneous Endothelial Progenitor Cell Therapy Attenuates Shunt Flow–Induced Pulmonary Arterial Hypertension in Rabbits. J. Thorac. Cardiovasc. Surg. 2015, 150, 621–632. [Google Scholar] [CrossRef]
- Granton, J.; Langleben, D.; Kutryk, M.B.; Camack, N.; Galipeau, J.; Courtman, D.W.; Stewart, D.J. Endothelial NO-Synthase Gene-Enhanced Progenitor Cell Therapy for Pulmonary Arterial Hypertension. Circ. Res. 2015, 117, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.L.; Maiolo, S.; Ward, R.J.; Seyfang, J.; Cockshell, M.P.; Bonder, C.S.; Reynolds, P.N. BMPR2-Expressing Bone Marrow-Derived Endothelial-like Progenitor Cells Alleviate Pulmonary Arterial Hypertension in Vivo. Respirology 2019, 24, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, E.; Bauer, N. Endothelial Extracellular Vesicles in Pulmonary Function and Disease. Curr. Top. Membr. 2018, 82, 197–256. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, P.; Goodwin, A.J.; Cook, J.A.; Halushka, P.V.; Chang, E.; Zingarelli, B.; Fan, H. Exosomes from Endothelial Progenitor Cells Improve Outcomes of the Lipopolysaccharide-Induced Acute Lung Injury. Crit. Care 2019, 23, 44. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Adamiak, M.; Mathiyalagan, P.; Kenneweg, F.; Kafert-Kasting, S.; Thum, T. Therapeutic and Diagnostic Translation of Extracellular Vesicles in Cardiovascular Diseases. Circulation 2021, 143, 1426–1449. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, M.; Suzuki, K. Mesenchymal Stem/Stromal Cell Therapy for Pulmonary Arterial Hypertension: Comprehensive Review of Preclinical Studies. J. Cardiol. 2019, 74, 304–312. [Google Scholar] [CrossRef]
- Aslam, M.; Baveja, R.; Liang, O.D.; Fernandez-Gonzalez, A.; Lee, C.; Mitsialis, S.A.; Kourembanas, S. Bone Marrow Stromal Cells Attenuate Lung Injury in a Murine Model of Neonatal Chronic Lung Disease. Am. J. Respir. Crit. Care Med. 2009, 180, 1122–1130. [Google Scholar] [CrossRef]
- Klinger, J.R.; Pereira, M.; Del Tatto, M.; Brodsky, A.S.; Wu, K.Q.; Dooner, M.S.; Borgovan, T.; Wen, S.; Goldberg, L.R.; Aliotta, J.M.; et al. Mesenchymal Stem Cell Extracellular Vesicles Reverse Sugen/Hypoxia Pulmonary Hypertension in Rats. Am. J. Respir. Cell. Mol. Biol. 2020, 62, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Ge, L.L.; Li, K.; Sun, Y.; Wang, F.; Han, Y.; Sun, C.; Wang, J.; Jiang, W.; et al. Mesenchymal Stromal Cell-Derived Exosomes Improve Pulmonary Hypertension through Inhibition of Pulmonary Vascular Remodeling. Respir. Res. 2020, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Takemiya, K.; Kai, H.; Yasukawa, H.; Tahara, N.; Kato, S.; Imaizumi, T. Mesenchymal Stem Cell-Based Prostacyclin Synthase Gene Therapy for Pulmonary Hypertension Rats. Basic Res. Cardiol. 2010, 105, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Liang, O.D.; Mitsialis, S.A.; Chang, M.S.; Vergadi, E.; Lee, C.; Aslam, M.; Fernandez-Gonzalez, A.; Liu, X.; Baveja, R.; Kourembanas, S. Mesenchymal Stromal Cells Expressing Heme Oxygenase-1 Reverse Pulmonary Hypertension. Stem Cells 2011, 29, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, K.-H.; Lim, J.; Kim, Y.-S.; Heo, J.; Choi, J.; Jeong, J.; Kim, Y.; Kim, S.W.; Oh, Y.-M.; et al. The Therapeutic Effects of Human Mesenchymal Stem Cells Primed with Sphingosine-1 Phosphate on Pulmonary Artery Hypertension. Stem Cells Dev. 2015, 24, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Han, L.; Bauer, M.; Sanada, F.; Oikonomopoulos, A.; Hosoda, T.; Unno, K.; De Almeida, P.; Leri, A.; Wu, J.C. Dissecting the Molecular Relationship among Various Cardiogenic Progenitor Cells. Circ. Res. 2013, 112, 1253–1262. [Google Scholar] [CrossRef]
- Dai, Z.; Li, M.; Wharton, J.; Zhu, M.M.; Zhao, Y.-Y. Prolyl-4 Hydroxylase 2 (PHD2) Deficiency in Endothelial Cells and Hematopoietic Cells Induces Obliterative Vascular Remodeling and Severe Pulmonary Arterial Hypertension in Mice and Humans Through Hypoxia-Inducible Factor-2α. Circulation 2016, 133, 2447–2458. [Google Scholar] [CrossRef]
- Asai, J.; Takenaka, H.; Kusano, K.F.; Ii, M.; Luedemann, C.; Curry, C.; Eaton, E.; Iwakura, A.; Tsutsumi, Y.; Hamada, H.; et al. Topical Sonic Hedgehog Gene Therapy Accelerates Wound Healing in Diabetes by Enhancing Endothelial Progenitor Cell-Mediated Microvascular Remodeling. Circulation 2006, 113, 2413–2424. [Google Scholar] [CrossRef]
- Bueno-Betí, C.; Novella, S.; Soleti, R.; Mompeón, A.; Vergori, L.; Sanchís, J.; Andriantsitohaina, R.; Martínez, M.C.; Hermenegildo, C. Microparticles Harbouring Sonic Hedgehog Morphogen Improve the Vasculogenesis Capacity of Endothelial Progenitor Cells Derived from Myocardial Infarction Patients. Cardiovasc. Res. 2019, 115, 409–418. [Google Scholar] [CrossRef]
- Moiseenko, A.; Kheirollahi, V.; Chao, C.-M.; Ahmadvand, N.; Quantius, J.; Wilhelm, J.; Herold, S.; Ahlbrecht, K.; Morty, R.E.; Rizvanov, A.A.; et al. Origin and Characterization of Alpha Smooth Muscle Actin-Positive Cells during Murine Lung Development. Stem Cells 2017, 35, 1566–1578. [Google Scholar] [CrossRef]
- Passman, J.N.; Dong, X.R.; Wu, S.P.; Maguire, C.T.; Hogan, K.A.; Bautch, V.L.; Majesky, M.W. A Sonic Hedgehog Signaling Domain in the Arterial Adventitia Supports Resident Sca1+ Smooth Muscle Progenitor Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9349–9354. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.; Eyries, M.; Montani, D.; Poirier, O.; Girerd, B.; Dorfmuller, P.; Coulet, F.; Nadaud, S.; Maugenre, S.; Guignabert, C.; et al. Genome-Wide Association Analysis Identifies a Susceptibility Locus for Pulmonary Arterial Hypertension. Nat. Genet. 2013, 45, 518–521. [Google Scholar] [CrossRef] [PubMed]
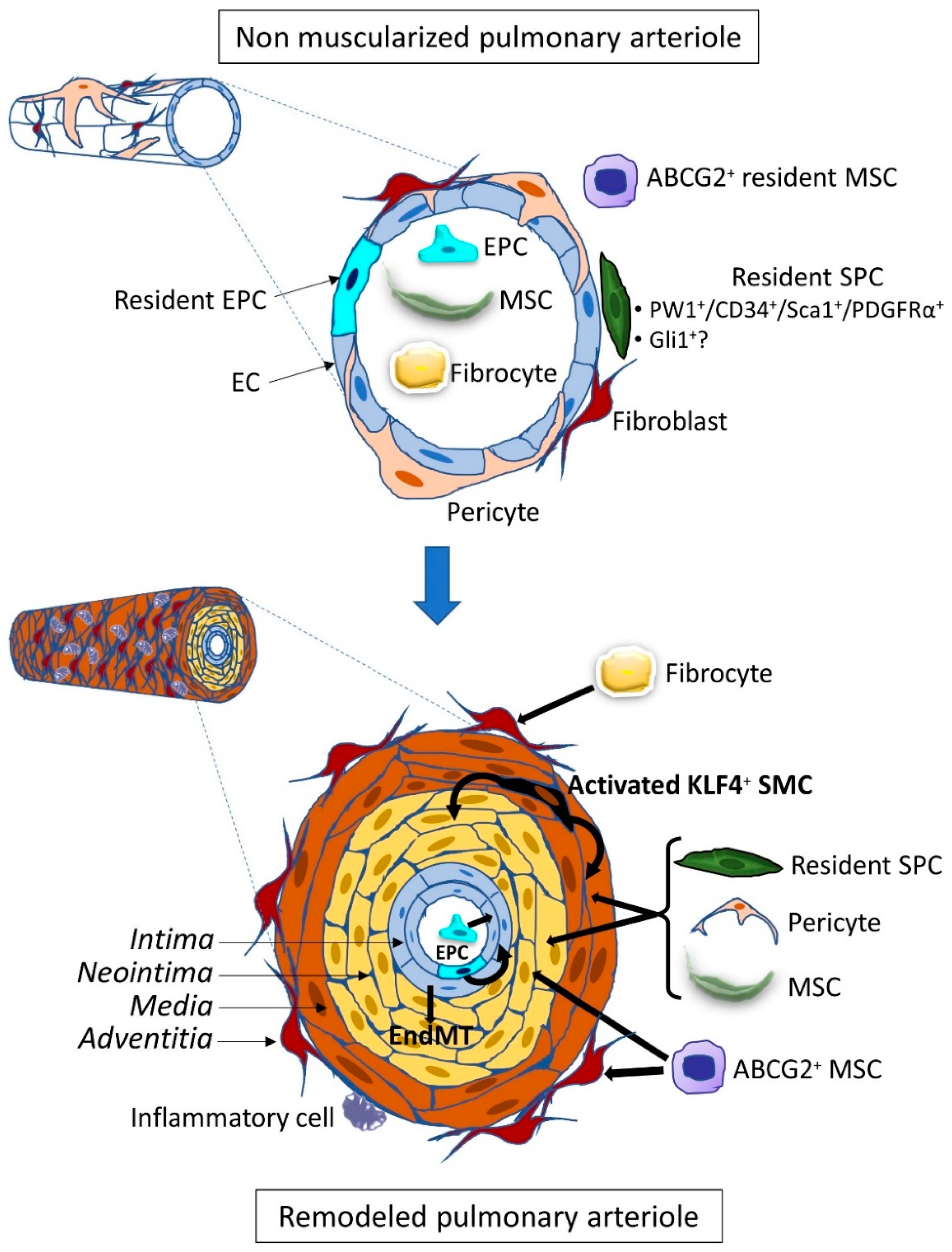
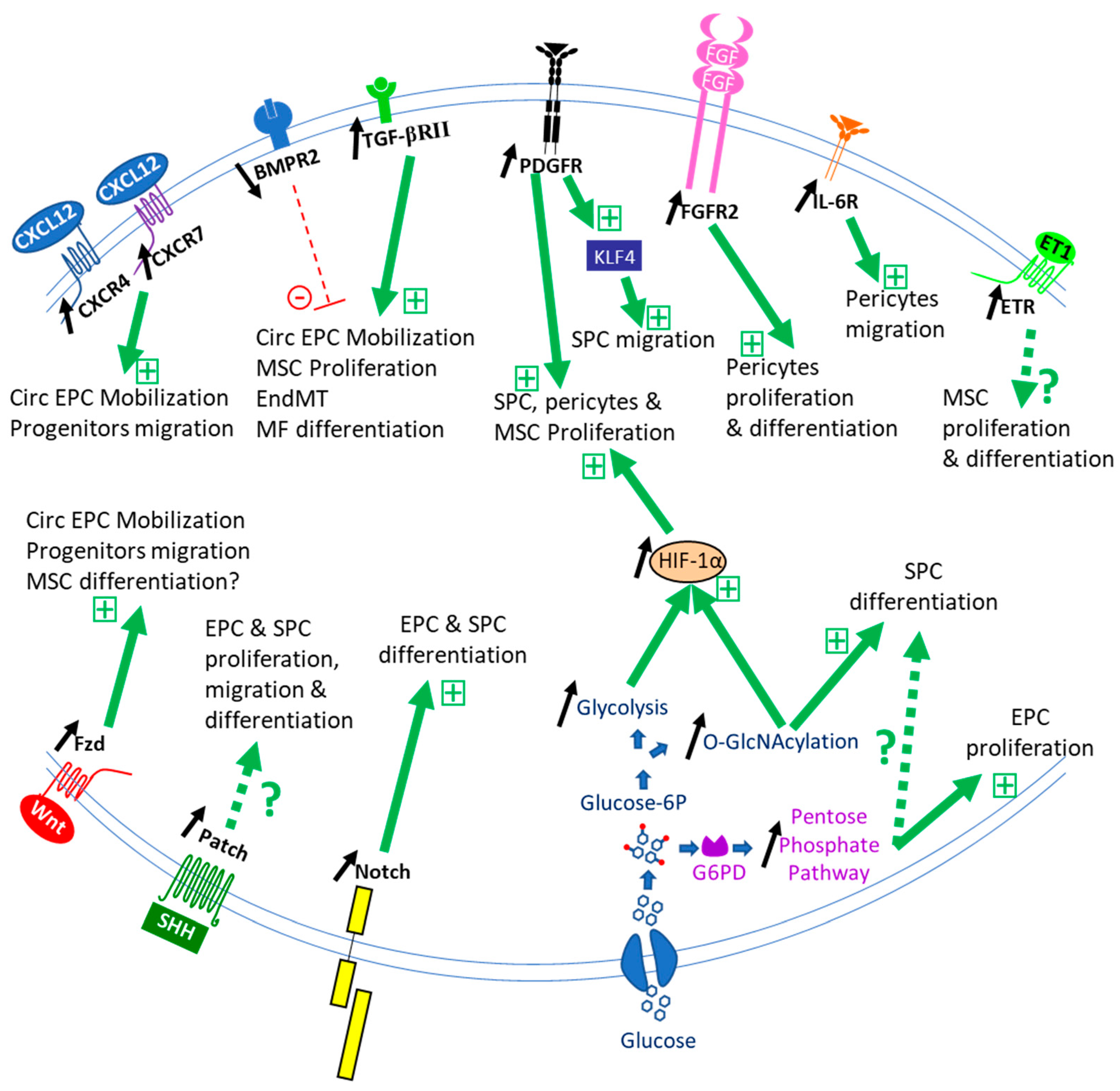

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dierick, F.; Solinc, J.; Bignard, J.; Soubrier, F.; Nadaud, S. Progenitor/Stem Cells in Vascular Remodeling during Pulmonary Arterial Hypertension. Cells 2021, 10, 1338. https://doi.org/10.3390/cells10061338
Dierick F, Solinc J, Bignard J, Soubrier F, Nadaud S. Progenitor/Stem Cells in Vascular Remodeling during Pulmonary Arterial Hypertension. Cells. 2021; 10(6):1338. https://doi.org/10.3390/cells10061338
Chicago/Turabian StyleDierick, France, Julien Solinc, Juliette Bignard, Florent Soubrier, and Sophie Nadaud. 2021. "Progenitor/Stem Cells in Vascular Remodeling during Pulmonary Arterial Hypertension" Cells 10, no. 6: 1338. https://doi.org/10.3390/cells10061338
APA StyleDierick, F., Solinc, J., Bignard, J., Soubrier, F., & Nadaud, S. (2021). Progenitor/Stem Cells in Vascular Remodeling during Pulmonary Arterial Hypertension. Cells, 10(6), 1338. https://doi.org/10.3390/cells10061338





