Complex Roles of Neutrophils during Arboviral Infections
Abstract
1. Introduction
2. Mosquitoes
3. Zika Virus
4. Dengue Virus
5. West Nile Virus
6. Alphaviruses
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leliefeld, P.H.C.; Koenderman, L.; Pillay, J. How neutrophils shape adaptive immune responses. Front. Immunol. 2015, 6, 471. [Google Scholar] [CrossRef] [PubMed]
- Geerdink, R.J.; Pillay, J.; Meyaard, L.; Bont, L. Neutrophils in respiratory syncytial virus infection: A target for asthma prevention. J. Allergy Clin. Immunol. 2015, 136, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.; Yang, F.; Xu, Y.; Feng, C.; Zhao, Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun. Signal. 2019, 17, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, M.; Lau, L.T.; Lu, J.; Gao, Z.; Liu, J.; Yu, A.C.H.; Cao, Q.; Ye, J.; McNutt, M.A.; et al. Neutrophils may be a vehicle for viral replication and dissemination in human h5n1 avian influenza. Clin. Infect. Dis. 2008, 47, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Kong, K.F.; Dai, J.; Qian, F.; Zhang, L.; Brown, C.R.; Fikrig, E.; Montgomery, R.R. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J. Infect. Dis. 2010, 202, 1804–1812. [Google Scholar] [CrossRef]
- Elliott, R.M. Orthobunyaviruses: Recent genetic and structural insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Solomon, T. Pathogenic flaviviruses. Lancet 2008, 371, 500–509. [Google Scholar] [CrossRef]
- Powers, A.M.; Brault, A.C.; Shirako, Y.; Strauss, E.G.; Kang, W.; Strauss, J.H.; Weaver, S.C. Evolutionary relationships and systematics of the alphaviruses. J. Virol. 2001, 75, 10118–10131. [Google Scholar] [CrossRef]
- Durbin, A.P.; Mayer, S.V.; Rossi, S.L.; Amaya-Larios, I.Y.; Ramos-Castaneda, J.; Eong Ooi, E.; Jane Cardosa, M.; Munoz-Jordan, J.L.; Tesh, R.B.; Messer, W.B.; et al. Emergence potential of sylvatic dengue virus type 4 in the urban transmission cycle is restrained by vaccination and homotypic immunity. Virology 2013, 439, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.S.; Higgs, S.; Horne, K.M.E.; Vanlandingham, D.L. Flavivirus-Mosquito interactions. Viruses 2014, 6, 4703–4730. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, L.; Scott, T.W.; Gubler, D.J. Consequences of the expanding global distribution of aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 2010, 4, e646. [Google Scholar] [CrossRef]
- Miller, M.J.; Loaiza, J.R. Geographic expansion of the invasive mosquito aedes albopictus across Panama—Implications for control of dengue and chikungunya viruses. Plos Negl. Trop. Dis. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Guagliardo, S.A.; Barboza, J.L.; Morrison, A.C.; Astete, H.; Vazquez-Prokopec, G.; Kitron, U. Patterns of geographic expansion of aedes aegypti in the Peruvian Amazon. PLoS Negl. Trop. Dis. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Nieto, L.M.; Maciá, A.; Perotti, M.A.; Berón, C.M. Geographical limits of the southeastern distribution of aedes aegypti (diptera, culicidae) in Argentina. Plos Negl. Trop. Dis. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, J.S.; Williams, D.T. The zoonotic flaviviruses of southern, South-Eastern and eastern Asia, and Australasia: The potential for emergent viruses. Zoonoses Public Health 2009, 56, 338–356. [Google Scholar] [CrossRef]
- Gabriel, C.; Her, Z.; Ng, L.F.P. Neutrophils: Neglected players in viral diseases. DNA Cell Biol. 2013, 32, 665–675. [Google Scholar] [CrossRef]
- Cox, J.; Mota, J.; Sukupolvi-Petty, S.; Diamond, M.S.; Rico-Hesse, R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J. Virol. 2012, 86, 7637–7649. [Google Scholar] [CrossRef]
- Edwards, J.F.; Higgs, S.; Beaty, B.J. Mosquito feeding-induced enhancement of cache valley virus (Bunyaviridae) infection in mice. J. Med. Entomol. 1998, 35, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Limesand, K.H.; Higgs, S.; Pearson, L.D.; Beaty, B.J. Potentiation of vesicular stomatitis New Jersey virus infection in mice by mosquito saliva. Parasite Immunol. 2000, 22, 461–467. [Google Scholar] [CrossRef]
- Schneider, B.S.; Soong, L.; Girard, Y.A.; Campbell, G.; Mason, P.; Higgs, S. Potentiation of West Nile encephalitis by mosquito feeding. Viral Immunol. 2006, 19, 74–82. [Google Scholar] [CrossRef]
- Dessens, J.T.; Nuttall, P.A. Mx1-based resistance to thogoto virus in A2G mice is bypassed in tick-mediated virus delivery. J. Virol. 1998, 72, 8362–8364. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef]
- WHO. A Global Brief on Vector-Borne Diseases; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- WHO. WHO Factsheet: Vector-Borne Diseases, Factsheet #387; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Luplertlop, N.; Surasombatpattana, P.; Patramool, S.; Dumas, E.; Wasinpiyamongkol, L.; Saune, L.; Hamel, R.; Bernard, E.; Sereno, D.; Thomas, F.; et al. Induction of a peptide with activity against a broad spectrum of pathogens in the aedes aegypti salivary gland, following infection with dengue virus. PLoS Pathog. 2011, 7, e1001252. [Google Scholar] [CrossRef]
- Salazar, M.I.; Richardson, J.H.; Sánchez-Vargas, I.; Olson, K.E.; Beaty, B.J. Dengue virus type 2: Replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007, 7, 9. [Google Scholar] [CrossRef]
- Vazeille, M.; Mousson, L.; Martin, E.; Failloux, A.-B. Orally co-infected aedes albopictus from La Reunion Island, Indian Ocean, can deliver both dengue and chikungunya infectious viral particles in their saliva. PLoS Negl. Trop. Dis. 2010, 4, e706. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.A.; Nuckols, J.; McGee, C.E.; Huang, Y.J.S.; Vanlandingham, D.L.; Tesh, R.B.; Higgs, S. In vivo imaging of chikungunya virus in mice and aedes mosquitoes using a Renilla luciferase clone. Vector Borne Zoonotic Dis. 2011, 11, 1471–1477. [Google Scholar] [CrossRef]
- Heath, W.R.; Carbone, F.R. The skin-resident and migratory immune system in steady state and memory: Innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 2013, 14, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.C.; Francischetti, I.M.B. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003, 48, 73–88. [Google Scholar] [CrossRef]
- Schneider, B.S.; Higgs, S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 400–408. [Google Scholar] [CrossRef]
- Olson, K.E.; Blair, C.D. Arbovirus-mosquito interactions: RNAi pathway. Curr. Opin. Virol. 2015, 15, 119–126. [Google Scholar] [CrossRef]
- Ahlers, L.R.H.; Goodman, A.G. The immune responses of the animal hosts of West Nile virus: A comparison of insects, birds, and mammals. Front. Cell. Infect. Microbiol. 2018, 8, 96. [Google Scholar] [CrossRef]
- Colpitts, T.M.; Cox, J.; Vanlandingham, D.L.; Feitosa, F.M.; Cheng, G.; Kurscheid, S.; Wang, P.; Krishnan, M.N.; Higgs, S.; Fikrig, E. Alterations in the Aedes aegypti Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses. PLoS Pathog. 2011, 7, e1002189. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, R.; Scott, T.W. Apoptosis in mosquito midgut epithelia associated with West Nile virus infection. Apoptosis 2006, 11, 1643–1651. [Google Scholar] [CrossRef]
- Girard, Y.A.; Schneider, B.S.; McGee, C.E.; Wen, J.; Han, V.C.; Popov, V.; Mason, P.W.; Higgs, S. Salivary gland morphology and virus transmission during long-term cytopathologic west Nile virus infection in Culex mosquitoes. Am. J. Trop. Med. Hyg. 2007, 76, 118–128. [Google Scholar] [CrossRef]
- Patramool, S.; Choumet, V.; Surasombatpattana, P.; Sabatier, L.; Thomas, F.; Thongrungkiat, S.; Rabilloud, T.; Boulanger, N.; Biron, D.G.; Missé, D. Update on the proteomics of major arthropod vectors of human and animal pathogens. Proteomics 2012, 12, 3510–3523. [Google Scholar] [CrossRef]
- Briant, L.; Desprès, P.; Choumet, V.; Missé, D. Role of skin immune cells on the host susceptibility to mosquito-borne viruses. Virology 2014, 464–465, 26–32. [Google Scholar] [CrossRef]
- Le Coupanec, A.; Babin, D.; Fiette, L.; Jouvion, G.; Ave, P.; Misse, D.; Bouloy, M.; Choumet, V. Aedes Mosquito Saliva Modulates Rift Valley Fever Virus Pathogenicity. PLoS Negl. Trop. Dis. 2013, 7, e2237. [Google Scholar] [CrossRef]
- Schneider, B.S.; Soong, L.; Coffey, L.L.; Stevenson, H.L.; McGee, C.E.; Higgs, S. Aedes aegypti saliva alters leukocyte recruitment and cytokine signaling by antigen-presenting cells during West Nile Virus Infection. PLoS ONE 2010, 5, e11704. [Google Scholar] [CrossRef]
- Styer, L.M.; Lim, P.-Y.; Louie, K.L.; Albright, R.G.; Kramer, L.D.; Bernard, K.A. Mosquito saliva causes enhancement of West Nile virus infection in mice. J. Virol. 2011, 85, 1517–1527. [Google Scholar] [CrossRef]
- Surasombatpattana, P.; Ekchariyawat, P.; Hamel, R.; Patramool, S.; Thongrungkiat, S.; Denizot, M.; Delaunay, P.; Thomas, F.; Luplertlop, N.; Yssel, H.; et al. Aedes aegypti saliva contains a prominent 34-kDa protein that strongly enhances dengue virus replication in human keratinocytes. J. Investig. Derm. 2014, 134, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, S.; Higgs, S.; Ziegler, S.; Vanlandingham, D.; Tesh, R.; Wikel, S. Host immune response to mosquito-transmitted chikungunya virus differs from that elicited by needle inoculated virus. PLoS ONE 2010, 5, e12137. [Google Scholar] [CrossRef]
- McCracken, M.K.; Christofferson, R.C.; Chisenhall, D.M.; Mores, C.N. Analysis of early dengue virus infection in mice as modulated by aedes aegypti probing. J. Virol. 2014, 88, 1881–1889. [Google Scholar] [CrossRef][Green Version]
- Pingen, M.; Bryden, S.R.; Pondeville, E.; Schnettler, E.; Kohl, A.; Merits, A.; Fazakerley, J.K.; Graham, G.J.; McKimmie, C.S. Host inflammatory response to mosquito bites enhances the severity of arbovirus infection. Immunity 2016, 44, 1455–1469. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.S.; Soong, L.; Zeidner, N.S.; Higgs, S. Aedes aegypti salivary gland extracts modulate anti-viral and T H1/TH2 cytokine responses to sindbis virus infection. Viral Immunol. 2004, 17, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Styer, L.M.; Bernard, K.A.; Kramer, L.D. Enhanced early West Nile virus infection in young chickens infected by mosquito bite: Effect of viral dose. Am. J. Trop. Med. Hyg. 2006, 75, 337–345. [Google Scholar] [CrossRef]
- Vogt, M.B.; Lahon, A.; Arya, R.P.; Kneubehl, A.R.; Spencer Clinton, J.L.; Paust, S.; Rico-Hesse, R. Mosquito saliva alone has profound effects on the human immune system. PLoS Negl. Trop. Dis. 2018, 12, e0006439. [Google Scholar] [CrossRef]
- Choumet, V.; Attout, T.; Chartier, L.; Khun, H.; Sautereau, J.; Robbe-Vincent, A.; Brey, P.; Huerre, M.; Bain, O. Visualizing non infectious and infectious anopheles gambiae blood feedings in naive and saliva-immunized mice. PLoS ONE 2012, 7, e50464. [Google Scholar] [CrossRef]
- Demeure, C.E.; Brahimi, K.; Hacini, F.; Marchand, F.; Péronet, R.; Huerre, M.; St.-Mezard, P.; Nicolas, J.-F.; Brey, P.; Delespesse, G.; et al. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J. Immunol. 2005, 174, 3932–3940. [Google Scholar] [CrossRef]
- Owhashi, M.; Harada, M.; Suguri, S.; Ohmae, H.; Ishii, A. The role of saliva of Anopheles stephensi in inflammatory response: Identification of a high molecular weight neutrophil chemotactic factor. Parasitol. Res. 2001, 87, 376–382. [Google Scholar] [CrossRef]
- Ferguson, M.C.; Saul, S.; Fragkoudis, R.; Weisheit, S.; Cox, J.; Patabendige, A.; Sherwood, K.; Watson, M.; Merits, A.; Fazakerley, J.K. Ability of the encephalitic arbovirus semliki forest virus to cross the blood-brain barrier is determined by the charge of the E2 glycoprotein. J. Virol. 2015, 89, 7536–7549. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Andres, J.; Rani, S.; Varjak, M.; Chase-Topping, M.E.; Beck, M.H.; Ferguson, M.C.; Schnettler, E.; Fragkoudis, R.; Barry, G.; Merits, A.; et al. Phenoloxidase activity acts as a mosquito innate immune response against infection with semliki forest virus. PLoS Pathog. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D. Zika virus infection in man. Trans. R. Soc. Trop. Med. Hyg. 1964, 58, 335–338. [Google Scholar] [CrossRef]
- Bearcroft, W. Zika virus infection experimentally induced in a human volunteer. Trans. R. Soc. Trop. Med. Hyg. 1956, 50, 442–448. [Google Scholar] [CrossRef]
- Bogoch, I.I.; Brady, O.J.; Kraemer, M.U.G.; German, M.; Creatore, M.I.; Kulkarni, M.A.; Brownstein, J.S.; Mekaru, S.R.; Hay, S.I.; Groot, E.; et al. Anticipating the international spread of Zika virus from Brazil. Lancet 2016, 387, 335–336. [Google Scholar] [CrossRef]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.L.; Horovitz, D.D.G.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.R.; Neri, J.I.; de Neto, J.M.P.; Wanderley, H.Y.C.; et al. Possible association between zika virus infection and microcephaly—Brazil. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.V.; Maia, M.; Bravo-Filho, V.; Góis, A.L.; Belfort, R. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016, 387, 228. [Google Scholar] [CrossRef]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastãre, S.; Valour, F.; Baudouin, L.; Mallet, H.P.; Musso, D.; Ghawche, F. Zika virus infection complicated by guillain-barré syndrome—Case report, French Polynesia, December. Eurosurveillance 2014, 19. [Google Scholar] [CrossRef]
- Ioos, S.; Mallet, H.P.; Leparc Goffart, I.; Gauthier, V.; Cardoso, T.; Herida, M. Current Zika virus epidemiology and recent epidemics. Med. Mal. Infect. 2014, 44, 302–307. [Google Scholar] [CrossRef]
- Hastings, A.K.; Uraki, R.; Gaitsch, H.; Dhaliwal, K.; Stanley, S.; Sproch, H.; Williamson, E.; MacNeil, T.; Marin-Lopez, A.; Hwang, J.; et al. Aedes aegypti NeSt1 protein enhances zika virus pathogenesis by activating neutrophils. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Zukor, K.; Wang, H.; Siddharthan, V.; Julander, J.G.; Morrey, J.D. Zika virus-induced acute myelitis and motor deficits in adult interferon αβ/γ receptor knockout mice. J. Neurovirol. 2018, 24, 273–290. [Google Scholar] [CrossRef]
- WHO. Handbook for Clinical Management of Dengue; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Wilder-Smith, A.; Schwartz, E. Dengue in travelers. N. Engl. J. Med. 2005, 353, 924–932. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, west Nile and dengue viruses. Nat. Med. 2004, 10, S98–S109. [Google Scholar] [CrossRef]
- Thein, T.L.; Lye, D.C.; Leo, Y.S.; Wong, J.G.X.; Hao, Y.; Wilder-Smith, A. Short report: Severe neutropenia in dengue patients: Prevalence and significance. Am. J. Trop. Med. Hyg. 2014, 90, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Shourick, J.; Dinh, A.; Matt, M.; Salomon, J.; Davido, B. Severe neutropenia revealing a rare presentation of dengue fever: A case report. BMC Res. Notes 2017, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Screaton, G.; Mongkolsapaya, J.; Yacoub, S.; Roberts, C. New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol. 2015, 15, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.T.; Lynn, D.J.; Henn, M.; Birren, B.W.; Lennon, N.J.; Le, P.T.; Duong, K.T.H.; Nguyen, T.T.H.; Mai, L.N.; Farrar, J.J.; et al. The early whole-blood transcriptional signature of dengue virus and features associated with progression to dengue shock syndrome in vietnamese children and young adults. J. Virol. 2010, 84, 12982–12994. [Google Scholar] [CrossRef]
- Kunder, M.; Lakshmaiah, V.; Moideen Kutty, A.V. Plasma neutrophil elastase, α1-antitrypsin, α2-macroglobulin and neutrophil elastase–α1-antitrypsin complex levels in patients with dengue fever. Indian J. Clin. Biochem. 2018, 33, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Yost, C.C.; Schwertz, H.; Cody, M.J.; Wallace, J.A.; Campbell, R.A.; Vieira-De-Abreu, A.; Araujo, C.V.; Schubert, S.; Harris, E.S.; Rowley, J.W.; et al. Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. J. Clin. Investig. 2016, 126, 3783–3798. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science. 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra20. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, E.; Yalavarthi, S.; Berthier, C.C.; Hodgin, J.B.; Khandpur, R.; Lin, A.M.; Rubin, C.J.; Zhao, W.; Olsen, S.H.; Klinker, M.; et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011, 187, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, A.; Furnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef]
- Opasawatchai, A.; Amornsupawat, P.; Jiravejchakul, N.; Chan-in, W.; Spoerk, N.J.; Manopwisedjaroen, K.; Singhasivanon, P.; Yingtaweesak, T.; Suraamornkul, S.; Mongkolsapaya, J.; et al. Neutrophil activation and early features of NET formation are associated with dengue virus infection in human. Front. Immunol. 2019, 9, 3007. [Google Scholar] [CrossRef]
- Lacy, P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98. [Google Scholar] [CrossRef]
- Yoon, J.; Terada, A.; Kita, H. CD66b regulates adhesion and activation of human eosinophils. J. Immunol. 2007, 179, 8454–8462. [Google Scholar] [CrossRef]
- Lien, T.S.; Sun, D.S.; Hung, S.C.; Wu, W.S.; Chang, H.H. Dengue virus envelope protein domain III induces nlrp3 inflammasome-dependent NETosis-mediated inflammation in mice. Front. Immunol. 2021, 12, 618577. [Google Scholar] [CrossRef]
- Ogura, H.; Kawasaki, T.; Tanaka, H.; Koh, T.; Tanaka, R.; Ozeki, Y.; Hosotsubo, H.; Kuwagata, Y.; Shimazu, T.; Sugimoto, H. Activated platelets enhance microparticle formation and platelet-leukocyte interaction in severe trauma and sepsis. J. Trauma. 2001, 50, 801–809. [Google Scholar] [CrossRef]
- Wu, M.F.; Chen, S.T.; Hsieh, S.L. Distinct regulation of dengue virus-induced inflammasome activation in human macrophage subsets. J. Biomed. Sci. 2013, 20, 36. [Google Scholar] [CrossRef]
- Wu, M.F.; Chen, S.T.; Yang, A.H.; Lin, W.W.; Lin, Y.L.; Chen, N.J.; Tsai, I.S.; Li, L.; Hsieh, S.L. CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages. Blood 2013, 121, 95–106. [Google Scholar] [CrossRef]
- Hottz, E.D.; Lopes, J.F.; Freitas, C.; Valls-De-Souza, R.; Oliveira, M.F.; Bozza, M.T.; Da Poian, A.T.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, F.A.; et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 2013, 122, 3405–3414. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Sung, P.S.; Huang, T.F.; Hsieh, S.L. Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Huang, Y.L.; Chen, S.T.; Liu, R.S.; Chen, Y.H.; Lin, C.Y.; Huang, C.H.; Shu, P.Y.; Liao, C.L.; Hsieh, S.L. CLEC5A is critical for dengue virus-induced osteoclast activation and bone homeostasis. J. Mol. Med. 2016, 94, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Lin, Y.L.; Huang, M.T.; Wu, M.F.; Cheng, S.C.; Lei, H.Y.; Lee, C.K.; Chiou, T.W.; Wong, C.H.; Hsieh, S.L. CLEC5A is critical for dengue-virus-induced lethal disease. Nature 2008, 453, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. The continuing spread of West Nile virus in the Western Hemisphere. Clin. Infect. Dis. 2007, 45, 1039–1046. [Google Scholar] [CrossRef]
- Shrestha, B.; Samuel, M.A.; Diamond, M.S. CD8+ T cells require perforin to clear west nile virus from infected neurons. J. Virol. 2006, 80, 119–129. [Google Scholar] [CrossRef]
- Bai, F.; Town, T.; Qian, F.; Wang, P.; Kamanaka, M.; Connolly, T.M.; Gate, D.; Montgomery, R.R.; Flavell, R.A.; Fikrig, E. IL-10 signaling blockade controls murine West Nile virus infection. PLoS Pathog. 2009, 5. [Google Scholar] [CrossRef]
- Town, T.; Bai, F.; Wang, T.; Kaplan, A.T.; Qian, F.; Montgomery, R.R.; Anderson, J.F.; Flavell, R.A.; Fikrig, E. Toll-like receptor 7 mitigates lethal west Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity 2009, 30, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Rawal, A.; Gavin, P.J.; Sturgis, C.D. Cerebrospinal fluid cytology in seasonal epidemic West Nile Virus meningo-encephalitis. Diagn. Cytopathol. 2006, 34, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Tyler, K.L.; Pape, J.; Goody, R.J.; Corkill, M.; Kleinschmidt-DeMasters, B.K. CSF findings in 250 patients with serologically confirmed West Nile virus meningitis and encephalitis. Neurology 2006, 66, 361–365. [Google Scholar] [CrossRef]
- Baxter, V.K.; Heise, M.T. Genetic control of alphavirus pathogenesis. Mamm. Genome 2018, 29, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Hollidge, B.S.; González-Scarano, F.; Soldan, S.S. Arboviral encephalitides: Transmission, emergence, and pathogenesis. J. Neuroimmune Pharmacol. 2010, 5, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Steele, K.; Reed, D.; Glass, P. Medical aspects of biological warfare. In Alphavirus Encephalitides; Office of the Surgeon General, US Army Medical Department Center and School, Borden Institute: Washington, DC, USA, 2007; pp. 241–270. [Google Scholar]
- Harley, D.; Sleigh, A.; Ritchie, S. Ross river virus transmission, infection, and disease: A cross-disciplinary review. Clin. Microbiol. Rev. 2001, 14, 909–932. [Google Scholar] [CrossRef]
- Williams, M.C.; Woodall, J.P.; Gillett, J.D. O’Nyong-Nyong fever: An epidemic virus disease in East Africa. Trans. R. Soc. Trop. Med. Hyg. 1965, 59, 186–197. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef]
- Chikungunya Fever in EU/EEA. Available online: https://www.ecdc.europa.eu/en/chikungunya/threats-and-outbreaks/chikungunya-fever-eueea (accessed on 28 March 2021).
- Roth, A.; Hoy, D.; Horwood, P.F.; Ropa, B.; Hancock, T.; Guillaumot, L.; Rickart, K.; Frison, P.; Pavlin, B.; Souares, Y. Preparedness for threat of chikungunya in the pacific. Emerg. Infect. Dis. 2014, 20, e130696. [Google Scholar] [CrossRef]
- Grandadam, M.; Caro, V.; Plumet, S.; Thiberge, J.M.; Souarès, Y.; Failloux, A.B.; Tolou, H.J.; Budelot, M.; Cosserat, D.; Leparc-Goffart, I.; et al. Chikungunya virus, Southeastern France. Emerg. Infect. Dis. 2011, 17, 910–913. [Google Scholar] [CrossRef]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. Infection with chikungunya virus in Italy: An outbreak in a temperate region. Lancet 2007, 370, 1840–1846. [Google Scholar] [CrossRef]
- Greenlee, J.E. The equine encephalitides. In Handbook of Clinical Neurology; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 123, pp. 417–432. [Google Scholar]
- Hatanpaa, K.J.; Kim, J.H. Neuropathology of viral infections. In Handbook of Clinical Neurology; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 123, pp. 193–214. [Google Scholar]
- Staples, J.E.; Breiman, R.F.; Powers, A.M. Chikungunya fever: An epidemiological review of a re-emerging infectious disease. Clin. Infect. Dis. 2009, 49, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Sissoko, D.; Malvy, D.; Ezzedine, K.; Renault, P.; Moscetti, F.; Ledrans, M.; Pierre, V. Post-epidemic Chikungunya disease on reunion island: Course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl. Trop. Dis. 2009, 3, 389. [Google Scholar] [CrossRef] [PubMed]
- Borgherini, G.; Poubeau, P.; Jossaume, A.; Gouix, A.; Cotte, L.; Michault, A.; Arvin-Berod, C.; Paganin, F. Persistent arthralgia associated with chikungunya virus: A study of 88 adult patients on reunion island. Clin. Infect. Dis. 2008, 47, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, S.; Pouderoux, N.; Pistone, T.; Filleul, L.; Receveur, M.-C.; Sissoko, D.; Ezzedine, K.; Malvy, D. Factors associated with persistence of arthralgia among chikungunya virus-infected travellers: Report of 42 French cases. J. Clin. Virol. 2010, 47, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Parola, P.; Grandadam, M.; Fourcade, S.; Oliver, M.; Brouqui, P.; Hance, P.; Kraemer, P.; Mohamed, A.A.; de Lamballerie, X.; et al. Chikungunya infection. Medicine 2007, 86, 123–137. [Google Scholar] [CrossRef]
- Kularatne, S.A.M.; Weerasinghe, S.C.; Gihan, C.; Wickramasinghe, S.; Dharmarathne, S.; Abeyrathna, A.; Jayalath, T. Epidemiology, clinical manifestations, and long-term outcomes of a major outbreak of Chikungunya in a Hamlet in Sri Lanka, in 2007: A longitudinal cohort study. J. Trop. Med. 2012, 2012, 639178. [Google Scholar] [CrossRef]
- Harley, D.; Bossingham, D.; Purdie, D.M.; Pandeya, N.; Sleigh, A.C. Ross river virus disease in tropical Queensland: Evolution of rheumatic manifestations in an inception cohort followed for six months. Med. J. Aust. 2002, 177, 352–355. [Google Scholar] [CrossRef]
- Mylonas, A.D.; Brown, A.M.; Carthew, T.L.; Purdie, D.M.; Pandeya, N.; Collins, L.G.; Suhrbier, A.; McGrath, B.; Reymond, E.J.; Vecchio, P.C.; et al. Natural history of Ross River virus-induced epidemic polyarthritis. Med. J. Aust. 2002, 177, 356–360. [Google Scholar] [CrossRef]
- Stoermer, K.A.; Burrack, A.; Oko, L.; Montgomery, S.A.; Borst, L.B.; Gill, R.G.; Morrison, T.E. Genetic ablation of arginase 1 in macrophages and neutrophils enhances clearance of an arthritogenic alphavirus. J. Immunol. 2012, 189, 4047–4059. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Munder, M. Arginase: An emerging key player in the mammalian immune system: REVIEW. Br. J. Pharmacol. 2009, 158, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.C.; Ochoa, A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol. Rev. 2008, 222, 180–191. [Google Scholar] [CrossRef]
- Silva, L.A.; Dermody, T.S. Chikungunya virus: Epidemiology, replication, disease mechanisms, and prospective intervention strategies. J. Clin. Investig. 2017, 127, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Wang, S.-Z.; Dowling, K.; Forsyth, K. Leucocyte populations in respiratory syncytial virus-induced bronchiolitis. J. Paediatr. Child Health 2001, 37, 146–151. [Google Scholar] [CrossRef]
- Wojtasiak, M.; Pickett, D.L.; Tate, M.D.; Londrigan, S.L.; Bedoui, S.; Brooks, A.G.; Reading, P.C. Depletion of Gr-1+, but not Ly6G+, immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. J. Gen. Virol. 2010, 91, 2158–2166. [Google Scholar] [CrossRef]
- Agraz-Cibrian, J.M.; Giraldo, D.M.; Mary, F.M.; Urcuqui-Inchima, S. Understanding the molecular mechanisms of NETs and their role in antiviral innate immunity. Virus Res. 2017, 228, 124–133. [Google Scholar] [CrossRef]
- Palha, N.; Guivel-Benhassine, F.; Briolat, V.; Lutfalla, G.; Sourisseau, M.; Ellett, F.; Wang, C.-H.; Lieschke, G.J.; Herbomel, P.; Schwartz, O.; et al. Real-time whole-body visualization of chikungunya virus infection and host interferon response in zebrafish. PLoS Pathog. 2013, 9, e1003619. [Google Scholar] [CrossRef]
- Chang, A.Y.; Martins, K.A.O.; Encinales, L.; Reid, S.P.; Acuña, M.; Encinales, C.; Matranga, C.B.; Pacheco, N.; Cure, C.; Shukla, B.; et al. Chikungunya arthritis mechanisms in the Americas. Arthritis Rheumatol. 2018, 70, 585–593. [Google Scholar] [CrossRef]
- Lin, T.; Geng, T.; Harrison, A.G.; Yang, D.; Vella, A.T.; Fikrig, E.; Wang, P. CXCL10 signaling contributes to the pathogenesis of arthritogenic alphaviruses. Viruses 2020, 12, 1252. [Google Scholar] [CrossRef]
- Hiroki, C.H.; Toller-Kawahisa, J.E.; Fumagalli, M.J.; Colon, D.F.; Figueiredo, L.T.M.; Fonseca, B.A.L.D.; Franca, R.F.O.; Cunha, F.Q. Neutrophil extracellular traps effectively control acute chikungunya virus infection. Front. Immunol. 2020, 10. [Google Scholar] [CrossRef]
- Raftery, M.J.; Lalwani, P.; Krautkrämer, E.; Peters, T.; Scharffetter-Kochanek, K.; Krüger, R.; Hofmann, J.; Seeger, K.; Krüger, D.H.; Schönrich, G. β2 integrin mediates hantavirus-induced release of neutrophil extracellular traps. J. Exp. Med. 2014, 211, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Cortjens, B.; de Boer, O.J.; de Jong, R.; Antonis, A.F.; Sabogal Piñeros, Y.S.; Lutter, R.; van Woensel, J.B.; Bem, R.A. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 2016, 238, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Apel, F.; Zychlinsky, A.; Kenny, E.F. The role of neutrophil extracellular traps in rheumatic diseases. Nat. Rev. Rheumatol. 2018, 14, 467–475. [Google Scholar] [CrossRef]
- Poo, Y.S.; Nakaya, H.; Gardner, J.; Larcher, T.; Schroder, W.A.; Le, T.T.; Major, L.D.; Suhrbier, A. CCR2 deficiency promotes exacerbated chronic erosive neutrophil-dominated chikungunya virus arthritis. J. Virol. 2014, 88, 6862–6872. [Google Scholar] [CrossRef]
- Quinones, M.P.; Ahuja, S.K.; Jimenez, F.; Schaefer, J.; Garavito, E.; Rao, A.; Chenaux, G.; Reddick, R.L.; Kuziel, W.A.; Ahuja, S.S. Experimental arthritis in CC chemokine receptor 2–null mice closely mimics severe human rheumatoid arthritis. J. Clin. Investig. 2004, 113, 856–866. [Google Scholar] [CrossRef]
- Sawanobori, Y.; Ueha, S.; Kurachi, M.; Shimaoka, T.; Talmadge, J.E.; Abe, J.; Shono, Y.; Kitabatake, M.; Kakimi, K.; Mukaida, N.; et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 2008, 111, 5457–5466. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Baba, T.; Yamagishi, M.; Kawano, M.; Mukaida, N. The role of a chemokine receptor, CCR2, in suppressing the development of arthritis in IL-1 receptor antagonist-deficient mice. Inflamm. Regen. 2012, 32, 124–131. [Google Scholar] [CrossRef][Green Version]
- Montgomery, R.R.; Booth, C.J.; Wang, X.; Blaho, V.A.; Malawista, S.E.; Brown, C.R. Recruitment of macrophages and polymorphonuclear leukocytes in lyme carditis. Infect. Immun. 2007, 75, 613–620. [Google Scholar] [CrossRef]
- Eyles, J.L.; Hickey, M.J.; Norman, M.U.; Croker, B.A.; Roberts, A.W.; Drake, S.F.; James, W.G.; Metcalf, D.; Campbell, I.K.; Wicks, I.P. A key role for G-CSF induced neutrophil production and trafficking during inflammatory arthritis. Blood 2008, 112, 5193–5201. [Google Scholar] [CrossRef]
- Sadik, C.D.; Kim, N.D.; Iwakura, Y.; Luster, A.D. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcγR signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E3177–E3185. [Google Scholar] [CrossRef] [PubMed]
- Ajuebor, M.; Das, A.; Virag, L.; Flower, R.; Szabo, C.; Perretti, M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: Evidence for an inhibitory loop involving endogenous IL-10. J. Immunol. 1999, 162, 1685–1691. [Google Scholar] [PubMed]
- Lotfi, R.; Herzog, G.I.; DeMarco, R.A.; Beer-Stolz, D.; Lee, J.J.; Rubartelli, A.; Schrezenmeier, H.; Lotze, M.T. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J. Immunol. 2009, 183, 5023–5031. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, R.R.; Tarrant, T.K.; Vallanat, C.T.; Quintero-Matthews, T.; Weeks, M.F.; Esserman, D.A.; Clark, J.; Di Padova, F.; Patel, D.D.; Fong, A.M.; et al. Enhanced Th17-cell responses render CCR2-deficient mice more susceptible for autoimmune arthritis. PLoS ONE 2011, 6, e25833. [Google Scholar] [CrossRef] [PubMed]
- Rudd, P.A.; Wilson, J.; Gardner, J.; Larcher, T.; Babarit, C.; Le, T.T.; Anraku, I.; Kumagai, Y.; Loo, Y.-M.; Gale, M.; et al. Interferon response factors 3 and 7 protect against chikungunya virus hemorrhagic fever and shock. J. Virol. 2012, 86, 9888–9898. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.K.; Reynoso, G.V.; Winkler, E.S.; Mack, M.; Diamond, M.S.; Hickman, H.D.; Morrison, T.E. MyD88-dependent influx of monocytes and neutrophils impairs lymph node B cell responses to chikungunya virus infection via Irf5, Nos2 and Nox2. PLoS Pathog. 2020, 16, e1008292. [Google Scholar] [CrossRef]
- Hawman, D.W.; Fox, J.M.; Ashbrook, A.W.; May, N.A.; Schroeder, K.M.S.; Torres, R.M.; Crowe, J.E.; Dermody, T.S.; Diamond, M.S.; Morrison, T.E. Pathogenic chikungunya virus evades B cell responses to establish persistence. Cell Rep. 2016, 16, 1326–1338. [Google Scholar] [CrossRef]
- McCarthy, M.K.; Davenport, B.J.; Reynoso, G.V.; Lucas, E.D.; May, N.A.; Elmore, S.A.; Tamburini, B.A.; Hickman, H.D.; Morrison, T.E. Chikungunya virus impairs draining lymph node function by inhibiting HEV-mediated lymphocyte recruitment. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Cook, L.E.; Locke, M.C.; Young, A.R.; Monte, K.; Hedberg, M.L.; Shimak, R.M.; Sheehan, K.C.F.; Veis, D.J.; Diamond, M.S.; Lenschow, D.J. Distinct roles of interferon alpha and beta in controlling chikungunya virus replication and modulating neutrophil-mediated inflammation. J. Virol. 2019, 94. [Google Scholar] [CrossRef]
- Lee, P.Y.; Wang, J.-X.; Parisini, E.; Dascher, C.C.; Nigrovic, P.A. Ly6 family proteins in neutrophil biology. J. Leukoc. Biol. 2013, 94, 585–594. [Google Scholar] [CrossRef] [PubMed]
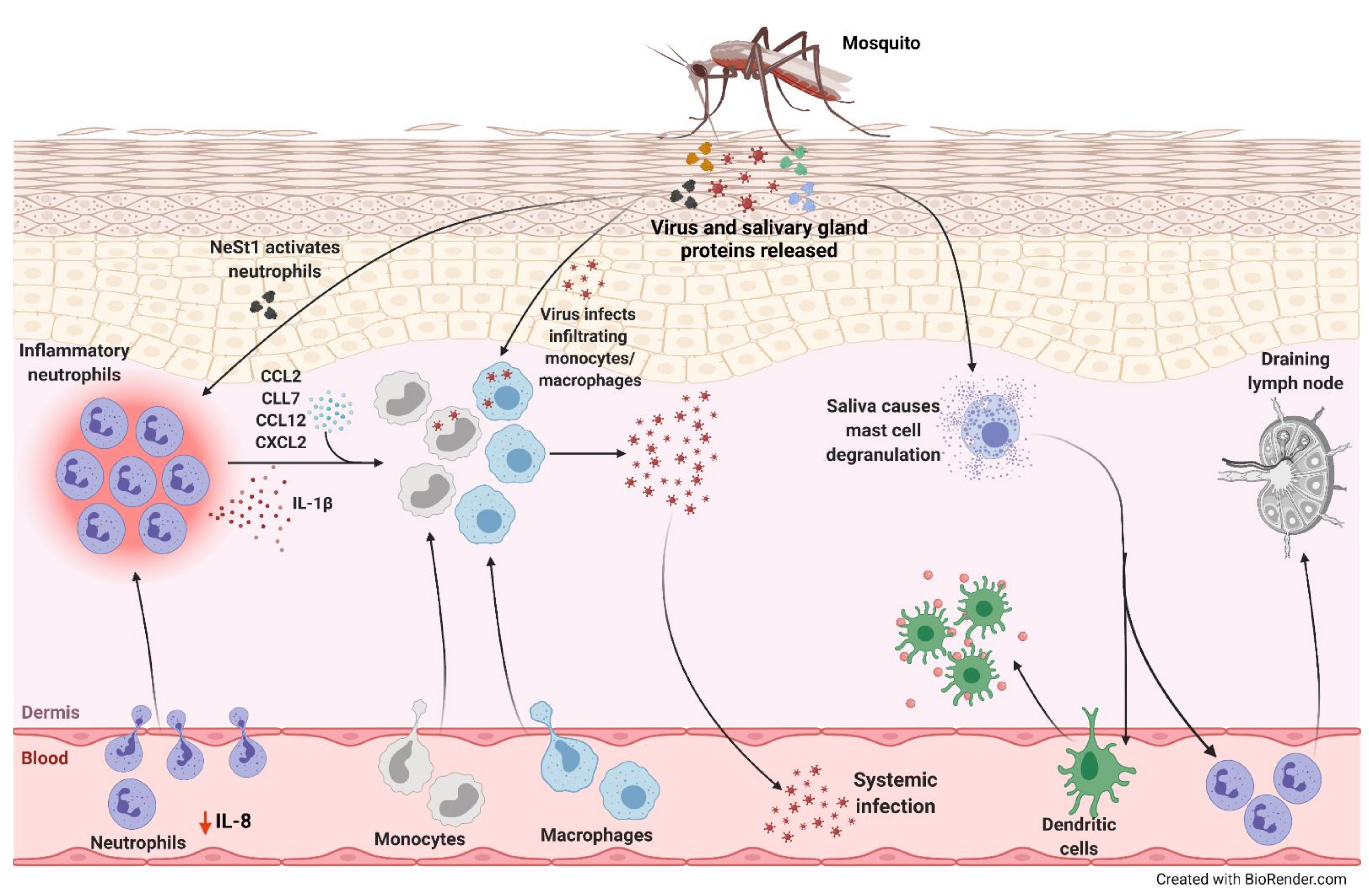
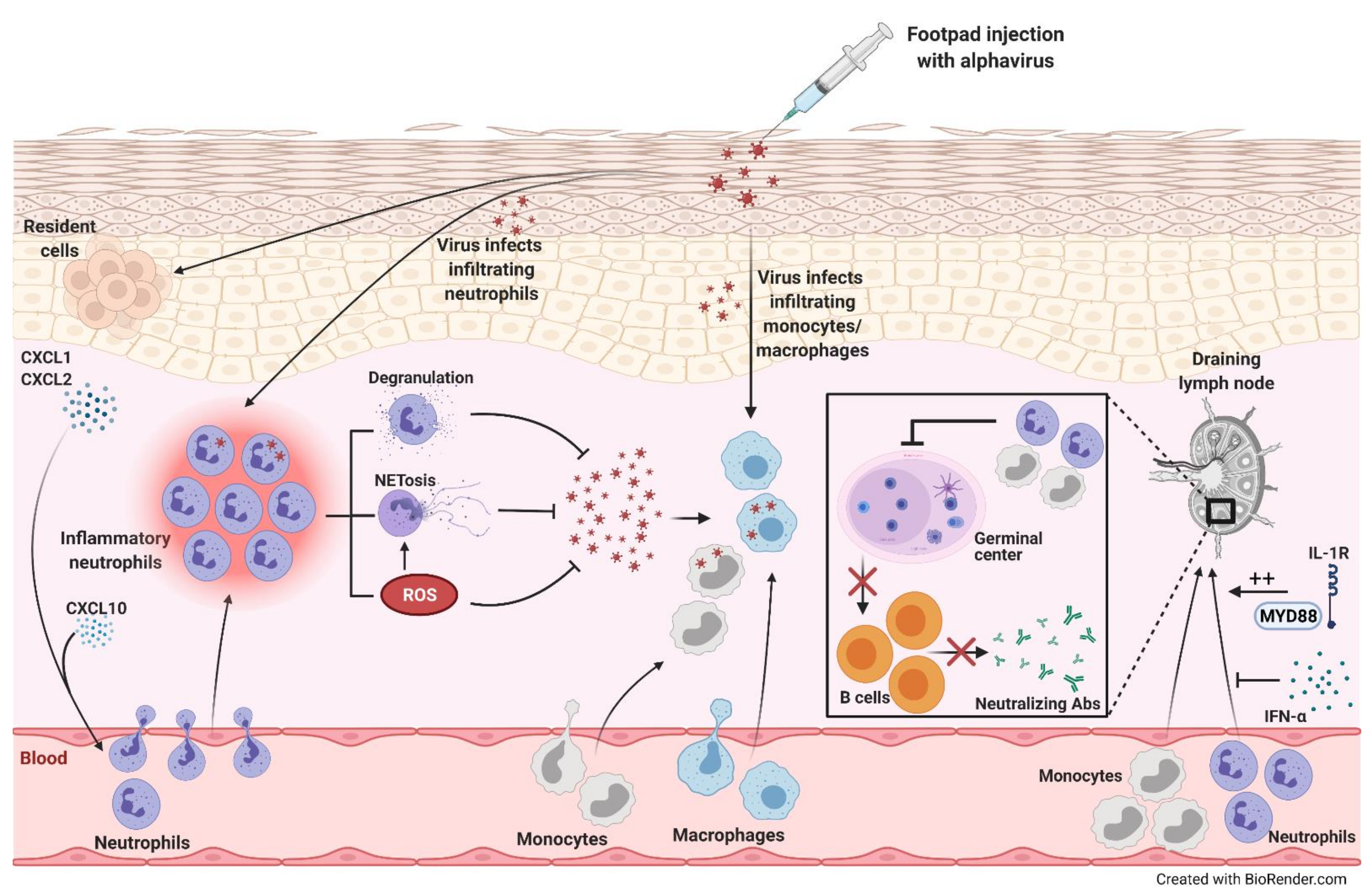
| Arbovirus | Beneficial Roles of Neutrophils | Detrimental Roles of Neutrophils | Replication in Neutrophils |
|---|---|---|---|
| Zika virus (ZIKV) | • In AG129 IFN-α/β receptor knockout mice, ZIKV induced neutrophil infiltration, which inversely correlated with virus-induced paresis protecting mice from motor deficits [63] | • Neutrophil-stimulating factor 1 (NeSt1) in mosquito saliva activated neutrophils, inducing IL-1β, CXCL2 and CCL2 expression, which augmented early viral infection in mice [62] | |
| Dengue virus (DENV) | Clinical studies:
| ||
| West Nile virus (WNV) | • Neutrophil depletion after infection increased viremia and death rate in mice [5] | • Neutrophil depletion before infection reduced viral burden and enhanced survival in mice [5] | Yes [5] |
| Semliki Forest virus (SFV) | Mice: • At later stages of infection, neutrophils were required to resolve the infection and decrease mortality [46] | Mice:
| |
| Ross River virus (RRV) | • Deletion of Arg1 in macrophages and neutrophils enhanced viral clearance and improved skeletal muscle tissue pathology in late stages of infection in mice, with no effect in the acute phase of infection [118] | ||
| Chikungunya virus (CHIKV) | Zebrafish: • Neutrophils served as a major source of type I interferon for eliminating the virus and alleviating disease [126] Mice: | Mice:
| |
| O’nyong nyong virus (ONNV) | • CXCL10−/− mice had reduced influx of macrophages and neutrophils, which was associated with decreased viral loads and foot swelling [128] | Yes [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muralidharan, A.; Reid, S.P. Complex Roles of Neutrophils during Arboviral Infections. Cells 2021, 10, 1324. https://doi.org/10.3390/cells10061324
Muralidharan A, Reid SP. Complex Roles of Neutrophils during Arboviral Infections. Cells. 2021; 10(6):1324. https://doi.org/10.3390/cells10061324
Chicago/Turabian StyleMuralidharan, Abenaya, and St Patrick Reid. 2021. "Complex Roles of Neutrophils during Arboviral Infections" Cells 10, no. 6: 1324. https://doi.org/10.3390/cells10061324
APA StyleMuralidharan, A., & Reid, S. P. (2021). Complex Roles of Neutrophils during Arboviral Infections. Cells, 10(6), 1324. https://doi.org/10.3390/cells10061324







