Advancing Diabetic Retinopathy Research: Analysis of the Neurovascular Unit in Zebrafish
Abstract
1. Introduction
2. Zebrafish in Diabetic Retinopathy Research
2.1. The Neurovascular Unit in Mammals and in Zebrafish—Similarities and Dissimilarities
2.2. Endothelial Cells
2.2.1. Non-Genetic Zebrafish Models of Diabetic Retinopathy
2.2.2. Genetic Zebrafish Models of Diabetic Retinopathy
2.2.3. Small Molecule Testing on Zebrafish Larvae
2.3. Pericytes
2.4. Microglia
2.5. Müller Glia
2.6. Photoreceptors/Neurodegeneration
3. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Gangaputra, S.; Lovato, J.F.; Hubbard, L.; Davis, M.D.; Esser, B.A.; Ambrosius, W.T.; Chew, E.Y.; Greven, C.; Perdue, L.H.; Wong, W.T.; et al. Comparison of standardized clinical classification with fundus photograph grading for the assessment of diabetic retinopathy and diabetic macular edema severity. Retina 2013, 33, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Leasher, J.L.; Bourne, R.R.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.; Naidoo, K.; Pesudovs, K.; Price, H.; White, R.A.; Wong, T.Y.; et al. Global Estimates on the Number of People Blind or Visually Impaired by Diabetic Retinopathy: A Meta-analysis from 1990 to 2010. Diabetes Care 2016, 39, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.; Steinmetz, J.D.; Flaxman, S.; Briant, P.S.; Taylor, H.R.; Resnikoff, S.; Casson, R.J.; Abdoli, A.; Abu-Gharbieh, E.; Afshin, A.; et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e130–e143. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991, 98 (Suppl. S5), 823–833. [Google Scholar] [CrossRef]
- Stewart, J.M.; Coassin, M.; Schwartz, D.M. Diabetic Retinopathy. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., et al., Eds.; South Dartmouth: Dartmouth, MA, USA, 2000. [Google Scholar]
- UK Prospective Diabetes Study (ukpds) Group; Kohner, E.M.; Stratton, I.M.; Aldington, S.J.; Holman, R.R.; Matthews, D.R. Relationship between the severity of retinopathy and progression to photocoagulation in patients with Type 2 diabetes mellitus in the UKPDS (UKPDS 52). Diabet. Med. 2001, 18, 178–184. [Google Scholar] [CrossRef]
- Ponto, K.A.; Koenig, J.; Peto, T.; Lamparter, J.; Raum, P.; Wild, P.S.; Lackner, K.J.; Pfeiffer, N.; Mirshahi, A. Prevalence of diabetic retinopathy in screening-detected diabetes mellitus: Results from the Gutenberg Health Study (GHS). Diabetologia 2016, 59, 1913–1919. [Google Scholar] [CrossRef]
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Parng, C.; Seng, W.L.; Semino, C.; McGrath, P. Zebrafish: A Preclinical Model for Drug Screening. ASSAY Drug Dev. Technol. 2002, 1, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.L.; Spitsbergen, J.M.; Wolterbeek, A.P.M.; Woutersen, R.A. Normal Anatomy and Histology of the Adult Zebrafish. Toxicol. Pathol. 2011, 39, 759–775. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a Model for Obesity and Diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Intine, R.V.; Olsen, A.S.; Sarras, M.P., Jr. A Zebrafish Model of Diabetes Mellitus and Metabolic Memory. J. Vis. Exp. 2013, 2013, e50232. [Google Scholar] [CrossRef]
- Gleeson, M.; Connaughton, V.; Arneson, L.S. Induction of hyperglycaemia in zebrafish (Danio rerio) leads to morphological changes in the retina. Acta Diabetol. 2007, 44, 157–163. [Google Scholar] [CrossRef]
- Lawlor, N.; Khetan, S.; Ucar, D.; Stitzel, M.L. Genomics of Islet (Dys)function and Type 2 Diabetes. Trends Genet. 2017, 33, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J. The genetics of adiposity. Curr. Opin. Genet. Dev. 2018, 50, 86–95. [Google Scholar] [CrossRef]
- Lidster, K.; Readman, G.D.; Prescott, M.J.; Owen, S. International survey on the use and welfare of zebrafish Danio rerio in research. J. Fish Biol. 2017, 90, 1891–1905. [Google Scholar] [CrossRef]
- Wiggenhauser, L.M.; Kroll, J. Vascular Damage in Obesity and Diabetes: Highlighting Links Between Endothelial Dysfunction and Metabolic Disease in Zebrafish and Man. Curr. Vasc. Pharmacol. 2019, 17, 476–490. [Google Scholar] [CrossRef]
- Metea, M.R.; Newman, E.A. Signalling within the neurovascular unit in the mammalian retina. Exp. Physiol. 2007, 92, 635–640. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Feng, Y.; Busch, S.; Gretz, N.; Hoffmann, S.; Hammes, H.-P. Crosstalk in the Retinal Neurovascular Unit—Lessons for the Diabetic Retina. Exp. Clin. Endocrinol. Diabetes 2012, 120, 199–201. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.W.; Davila, J.R. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fadool, J.M.; Dowling, J.E. Zebrafish: A model system for the study of eye genetics. Prog. Retin. Eye Res. 2008, 27, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, J.; Saszik, S. The zebrafish as a model visual system. Int. J. Dev. Neurosci. 2001, 19, 621–629. [Google Scholar] [CrossRef]
- Angueyra, J.M.; Kindt, K.S. Leveraging Zebrafish to Study Retinal Degenerations. Front. Cell Dev. Biol. 2018, 6, 110. [Google Scholar] [CrossRef]
- Bibliowicz, J.; Tittle, R.K.; Gross, J.M. Toward a better understanding of human eye disease insights from the zebrafish, Danio rerio. Prog. Mol. Biol. Transl. Sci. 2011, 100, 287–330. [Google Scholar]
- Eliceiri, B.P.; Gonzalez, A.M.; Baird, A. Zebrafish Model of the Blood-Brain Barrier: Morphological and Permeability Studies. Methods Mol. Biol. 2011, 686, 371–378. [Google Scholar] [CrossRef]
- O’Brown, N.M.; Pfau, S.J.; Gu, C. Bridging barriers: A comparative look at the blood–brain barrier across organisms. Genes Dev. 2018, 32, 466–478. [Google Scholar] [CrossRef]
- Klaassen, I.; Van Noorden, C.J.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nat. Cell Biol. 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog. Retin. Eye Res. 2015, 49, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.D.; Weinstein, B.M. In Vivo Imaging of Embryonic Vascular Development Using Transgenic Zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, Y.; Cederlund, M.L.; Cottell, D.C.; Bill, B.R.; Ekker, S.C.; Torres-Vazquez, J.; Weinstein, B.M.; Hyde, D.R.; Vihtelic, T.S.; Kennedy, B.N. Genetic determinants of hyaloid and retinal vasculature in zebrafish. BMC Dev. Biol. 2007, 7, 114. [Google Scholar] [CrossRef]
- Gestri, G.; Link, B.A.; Neuhauss, S.C. The visual system of zebrafish and its use to model human ocular Diseases. Dev. Neurobiol. 2011, 72, 302–327. [Google Scholar] [CrossRef]
- Gore, A.V.; Monzo, K.; Cha, Y.R.; Pan, W.; Weinstein, B.M. Vascular Development in the Zebrafish. Cold Spring Harb. Perspect. Med. 2012, 2, a006684. [Google Scholar] [CrossRef]
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The zebrafish eye—A paradigm for investigating human ocular genetics. Eye 2017, 31, 68–86. [Google Scholar] [CrossRef]
- Fruttiger, M. Development of the retinal vasculature. Angiogenesis 2007, 10, 77–88. [Google Scholar] [CrossRef]
- Kur, J.; Newman, E.A.; Chan-Ling, T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res. 2012, 31, 377–406. [Google Scholar] [CrossRef]
- Van Dijk, H.W.; Kok, P.H.; Garvin, M.; Sonka, M.; DeVries, J.H.; Michels, R.P.; van Velthoven, M.E.; Schlingemann, R.O.; Verbraak, F.D.; Abramoff, M.D. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3404–3409. [Google Scholar] [CrossRef]
- Alvarez, Y.; Chen, K.; Reynolds, A.; Waghorne, N.; O’Connor, J.J.; Kennedy, B.N. Predominant cone photoreceptor dysfunction in a hyperglycaemic model of non-proliferative diabetic retinopathy. Dis. Model. Mech. 2010, 3, 236–245. [Google Scholar] [CrossRef]
- Cao, R.; Jensen, L.D.E.; Söll, I.; Hauptmann, G.; Cao, Y. Hypoxia-Induced Retinal Angiogenesis in Zebrafish as a Model to Study Retinopathy. PLoS ONE 2008, 3, e2748. [Google Scholar] [CrossRef] [PubMed]
- Olivares, A.M.; Althoff, K.; Chen, G.F.; Wu, S.; Morrisson, M.A.; DeAngelis, M.M.; Haider, N. Animal Models of Diabetic Retinopathy. Curr. Diabetes Rep. 2017, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.B.; D’Amore, P.; Connor, K.M. Revisiting the mouse model of oxygen-induced retinopathy. Eye Brain 2016, 8, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.S.; Sarras, M.P., Jr.; Intine, R.V. Limb regeneration is impaired in an adult zebrafish model of diabetes mellitus. Wound Repair Regen. 2010, 18, 532–542. [Google Scholar] [CrossRef]
- Benchoula, K.; Khatib, A.; Quzwain, F.M.C.; Mohamad, C.A.C.; Sulaiman, W.M.A.W.; Wahab, R.A.; Ahmed, Q.U.; Ghaffar, M.A.; Saiman, M.Z.; Alajmi, M.F.; et al. Optimization of Hyperglycemic Induction in Zebrafish and Evaluation of Its Blood Glucose Level and Metabolite Fingerprint Treated with Psychotria malayana Jack Leaf Extract. Molecules 2019, 24, 1506. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Peterson, R.T.; Yeh, J.-R.J. Methods for targeted mutagenesis in zebrafish using TALENs. Methods 2014, 69, 76–84. [Google Scholar] [CrossRef]
- Shah, A.N.; Davey, C.F.; Whitebirch, A.C.; Miller, A.C.; Moens, C.B. Rapid reverse genetic screening using CRISPR in zebrafish. Nat. Methods 2015, 12, 535–540. [Google Scholar] [CrossRef]
- Kettleborough, R.N.W.; Busch-Nentwich, E.M.; Harvey, S.A.; Dooley, C.M.; De Bruijn, E.; Van Eeden, F.; Sealy, I.; White, R.J.; Herd, C.; Nijman, I.J.; et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nat. Cell Biol. 2013, 496, 494–497. [Google Scholar] [CrossRef]
- Kimmel, R.A.; Dobler, S.; Schmitner, N.; Walsen, T.; Freudenblum, J.; Meyer, D. Diabetic pdx1-mutant zebrafish show conserved responses to nutrient overload and anti-glycemic treatment. Sci. Rep. 2015, 5, 14241. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Zang, J.; Lagali, N.; Schmitner, N.; Salvenmoser, W.; Mukwaya, A.; Neuhauss, S.C.; Jensen, L.D.; Kimmel, R.A. Photoreceptor Degeneration Accompanies Vascular Changes in a Zebrafish Model of Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2020, 61, 43. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in brain in health and disease. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1299–1343. [Google Scholar] [CrossRef]
- Wiggenhauser, L.M.; Qi, H.; Stoll, S.J.; Metzger, L.; Bennewitz, K.; Poschet, G.; Krenning, G.; Hillebrands, J.L.; Hammes, H.P.; Kroll, J. Activation of Retinal Angiogenesis in Hyperglycemic pdx1 (-/-) Zebrafish Mutants. Diabetes 2020, 69, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.; Boger, M.; Bennewitz, K.; Sticht, C.; Kopf, S.; Morgenstern, J.; Fleming, T.; Hell, R.; Yuan, Z.; Nawroth, P.P.; et al. Elevated 4-hydroxynonenal induces hyperglycaemia via Aldh3a1 loss in zebrafish and associates with diabetes progression in humans. Redox Biol. 2020, 37, 101723. [Google Scholar] [CrossRef] [PubMed]
- Lodd, E.; Wiggenhauser, L.M.; Morgenstern, J.; Fleming, T.H.; Poschet, G.; Büttner, M.; Tabler, C.T.; Wohlfart, D.P.; Nawroth, P.P.; Kroll, J. The combination of loss of glyoxalase1 and obesity results in hyperglycemia. JCI Insight 2019, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Glyoxalase in diabetes, obesity and related disorders. Semin. Cell Dev. Biol. 2011, 22, 309–317. [Google Scholar] [CrossRef]
- Salehpour, A.; Rezaei, M.; Khoradmehr, A.; Tahamtani, Y.; Tamadon, A. Which Hyperglycemic Model of Zebrafish (Danio rerio) Suites My Type 2 Diabetes Mellitus Research? A Scoring System for Available Methods. Front. Cell Dev. Biol. 2021, 9, 652061. [Google Scholar] [CrossRef] [PubMed]
- Geisler, R.; Köhler, A.; Dickmeis, T.; Strähle, U. Archiving of zebrafish lines can reduce animal experiments in biomedical research. EMBO Rep. 2017, 18, 1–2. [Google Scholar] [CrossRef]
- Cassar, S.; Adatto, I.; Freeman, J.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kim, Y.S.; Lee, Y.-R.; Kim, J.S. High glucose-induced changes in hyaloid-retinal vessels during early ocular development of zebrafish: A short-term animal model of diabetic retinopathy. Br. J. Pharmacol. 2015, 173, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Van de Venter, M.; Didloff, J.; Reddy, S.; Swanepoel, B.; Govender, S.; Dambuza, N.S.; Williams, S.; Koekemoer, T.C.; Venables, L. Wild-Type Zebrafish (Danio rerio) Larvae as a Vertebrate Model for Diabetes and Comorbidities: A Review. Animals 2020, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Kidd, K.R.; Weinstein, B.M. Fishing for novel angiogenic therapies. Br. J. Pharmacol. 2003, 140, 585–594. [Google Scholar] [CrossRef]
- Sasore, T.; Kennedy, B. Deciphering Combinations of PI3K/AKT/mTOR Pathway Drugs Augmenting Anti-Angiogenic Efficacy In Vivo. PLoS ONE 2014, 9, e105280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galvin, O.; Srivastava, A.; Carroll, O.; Kulkarni, R.; Dykes, S.; Vickers, S.; Dickinson, K.; Reynolds, A.; Kilty, C.; Redmond, G.; et al. A sustained release formulation of novel quininib-hyaluronan microneedles inhibits angiogenesis and retinal vascular permeability in vivo. J. Control. Release 2016, 233, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, R.S.; Merrigan, S.; Quigley, J.; Qi, X.; Lee, B.; Boulton, M.E.; Kennedy, B.; Seo, S.-Y.; Corson, T.W. A novel small molecule ameliorates ocular neovascularisation and synergises with anti-VEGF therapy. Sci. Rep. 2016, 6, 25509. [Google Scholar] [CrossRef] [PubMed]
- Ohnesorge, N.; Sasore, T.; Hillary, D.; Alvarez, Y.; Carey, M.; Kennedy, B.N. Orthogonal Drug Pooling Enhances Phenotype-Based Discovery of Ocular Antiangiogenic Drugs in Zebrafish Larvae. Front. Pharmacol. 2019, 10, 508. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Caporarello, N.; D’Angeli, F.; Cambria, M.T.; Candido, S.; Giallongo, C.; Salmeri, M.; Lombardo, C.; Longo, A.; Giurdanella, G.; Anfuso, C.D.; et al. Pericytes in Microvessels: From “Mural” Function to Brain and Retina Regeneration. Int. J. Mol. Sci. 2019, 20, 6351. [Google Scholar] [CrossRef]
- Leveen, P.; Pekny, M.; Gebre-Medhin, S.; Swolin, B.; Larsson, E.; Betsholtz, C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994, 8, 1875–1887. [Google Scholar] [CrossRef]
- Alarcon-Martinez, L.; Villafranca-Baughman, D.; Quintero, H.; Kacerovsky, J.B.; Dotigny, F.; Murai, K.K.; Prat, A.; Drapeau, P.; Di Polo, A. Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nat. Cell Biol. 2020, 585, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.G.; Estevez, J.J.; Liu, E.; Craig, J.E.; Finnie, J.W. Pericytes, inflammation, and diabetic retinopathy. Inflammopharmacology 2020, 28, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Pfister, F.; Przybyt, E.; Harmsen, M.C.; Hammes, H.-P. Pericytes in the eye. Pflügers Arch. Eur. J. Physiol. 2013, 465, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, L.; Moens, C.B.; Appel, B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development 2014, 141, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Fukuhara, S.; Izumi, N.; Nakajima, H.; Fukui, H.; Kelsh, R.; Mochizuki, N. Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Development 2016, 143, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.M.; Pesce, G.; Stainier, D.Y. Characterization of vascular mural cells during zebrafish development. Mech. Dev. 2009, 126, 638–649. [Google Scholar] [CrossRef]
- Ali, Z.; Mukwaya, A.; Biesemeier, A.; Ntzouni, M.; Ramsköld, D.; Giatrellis, S.; Mammadzada, P.; Cao, R.; Lennikov, A.; Marass, M.; et al. Intussusceptive Vascular Remodeling Precedes Pathological Neovascularization. Arter. Thromb. Vasc. Biol. 2019, 39, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, T.R.; Kennedy, R.M.; Carter, A.D.; Rollins, E.L.; Georgijevic, S.; Santoro, M.M. An alpha-smooth muscle actin (acta2/alphasma) zebrafish transgenic line marking vascular mural cells and visceral smooth muscle cells. PLoS ONE 2014, 9, e90590. [Google Scholar] [CrossRef]
- Caceres, L.; Prykhozhij, S.V.; Cairns, E.; Gjerde, H.; Duff, N.M.; Collett, K.; Ngo, M.; Nasrallah, G.K.; McMaster, C.R.; Litvak, M.; et al. Frizzled 4 regulates ventral blood vessel remodeling in the zebrafish retina. Dev. Dyn. 2019, 248, 1243–1256. [Google Scholar] [CrossRef]
- Dietrich, N.; Hammes, H.P. Retinal Digest Preparation: A Method to Study Diabetic Retinopathy. Methods Mol. Biol. 2012, 933, 291–302. [Google Scholar]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal microglia: Just bystander or target for therapy? Prog. Retin. Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-Y.; Green, W.R.; Tso, M.O.M. Microglial Activation in Human Diabetic Retinopathy. Arch. Ophthalmol. 2008, 126, 227–232. [Google Scholar] [CrossRef]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [PubMed]
- Arroba, A.I.; Valverde, A.M. Modulation of microglia in the retina: New insights into diabetic retinopathy. Acta Diabetol. 2017, 54, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Var, S.R.; Byrd-Jacobs, C.A. Role of Macrophages and Microglia in Zebrafish Regeneration. Int. J. Mol. Sci. 2020, 21, 4768. [Google Scholar] [CrossRef]
- Van Dyck, A.; Bollaerts, I.; Beckers, A.; Vanhunsel, S.; Glorian, N.; van Houcke, J.; van Ham, T.J.; De Groef, L.; Andries, L.; Moons, L. Müller glia–myeloid cell crosstalk accelerates optic nerve regeneration in the adult zebrafish. Glia 2021, 69, 1444–1463. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Glia of the human retina. Glia 2019, 68, 768–796. [Google Scholar] [CrossRef]
- Lundkvist, A.; Reichenbach, A.; Betsholtz, C.; Carmeliet, P.; Wolburg, H.; Pekny, M. Under stress, the absence of intermediate filaments from Muller cells in the retina has structural and functional consequences. J. Cell Sci. 2004, 117, 3481–3488. [Google Scholar] [CrossRef]
- Coughlin, B.A.; Feenstra, D.J.; Mohr, S. Müller cells and diabetic retinopathy. Vis. Res. 2017, 139, 93–100. [Google Scholar] [CrossRef]
- Goldman, D. Müller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442. [Google Scholar] [CrossRef]
- Gorsuch, R.A.; Hyde, D.R. Regulation of Müller glial dependent neuronal regeneration in the damaged adult zebrafish retina. Exp. Eye Res. 2014, 123, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.M.; Lovel, A.G.; Stenkamp, D.L. Dynamic changes in microglial and macrophage characteristics during degeneration and regeneration of the zebrafish retina. J. Neuroinflamm. 2018, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- White, D.T.; Sengupta, S.; Saxena, M.T.; Xu, Q.; Hanes, J.; Ding, D.; Ji, H.; Mumm, J.S. Immunomodulation-accelerated neuronal regeneration following selective rod photoreceptor cell ablation in the zebrafish retina. Proc. Natl. Acad. Sci. USA 2017, 114, E3719–E3728. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Castillo, H.A.; Brown, J.; Kaslin, J.; Dwyer, K.M.; Gibert, Y. High glucose levels affect retinal patterning during zebrafish embryogenesis. Sci. Rep. 2019, 9, 4121. [Google Scholar] [CrossRef]
- Kern, T.S.; Berkowitz, B.A. Photoreceptors in diabetic retinopathy. J. Diabetes Investig. 2015, 6, 371–380. [Google Scholar] [CrossRef]
- Hammes, H.P.; Federoff, H.J.; Brownlee, M. Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol. Med. 1995, 1, 527–534. [Google Scholar] [CrossRef]
- Barber, A.J.; Lieth, E.; Khin, S.; Antonetti, D.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef]
- Tanvir, Z.; Nelson, R.F.; DeCicco-Skinner, K.; Connaughton, V.P. One month of hyperglycemia alters spectral responses of the zebrafish photopic electroretinogram. Dis. Model. Mech. 2018, 11, dmm035220. [Google Scholar] [CrossRef]
- Seth, A.; Stemple, D.L.; Barroso, I. The emerging use of zebrafish to model metabolic disease. Dis. Model. Mech. 2013, 6, 1080–1088. [Google Scholar] [CrossRef]
- Carnovali, M.; Luzi, L.; Banfi, G.; Mariotti, M. Chronic hyperglycemia affects bone metabolism in adult zebrafish scale model. Endocrine 2016, 54, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Sang, S.; Leung, T. Methylglyoxal-Induced Retinal Angiogenesis in Zebrafish Embryo: A Potential Animal Model of Neovascular Retinopathy. J. Ophthalmol. 2019, 2019, 2746735. [Google Scholar] [CrossRef] [PubMed]
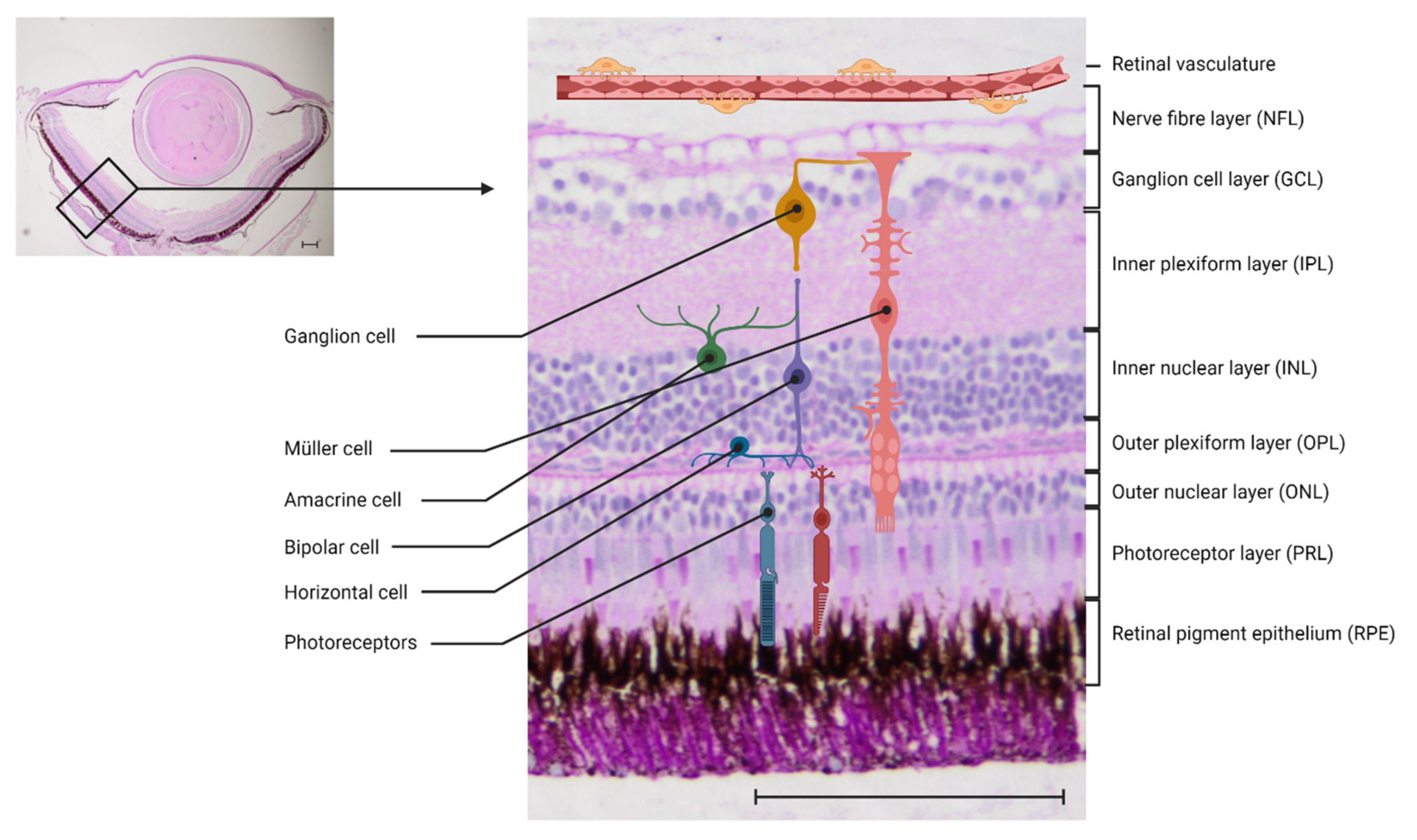
| Model | Induction | Angiogenesis | Endothelial Cell Dysfunction | Pericyte Loss | Müller Glia Activation | Photoreceptor Degeneration | Neurodegeneration |
|---|---|---|---|---|---|---|---|
| Gleeson, Connaughton et al. 2007 [16] | Exposure to alternating glucose/water solutions for 28 days (adult zf) | n.e. | n.e. | n.e. | n.e. | n.e. | +(decreased IPL thickness) |
| Cao, Jensen et al. 2008 [44] | Experimental hypoxia for up to 15 days (adult zf) | + | n.e. | n.e. | n.e. | n.e. | n.e. |
| Alvarez, Chen et al. 2010 [43] | Exposure to alternating glucose/water solutions for 30 days (adult zf) | n.e. | + (thickening of vessel basement membrane, wider tight and adherens junctions) | n.e. | + | +(abnormal retinal histology, impaired cone ERGs) | - |
| Olsen, Sarras et al. 2010 [47] | i.p. or direct caudal fin injection of STZ (adult zf) | n.e. | n.e. | n.e. | n.e. | +(decreased PRL thickness) | +(decreased IPL thickness) |
| Carnovali, Luzi et al. 2016 [102] | Exposure to 4% glucose solution for 28 days (adult zf) | n.e. | +(increased vessel diameter, aneurysm-like structure, marked fragility of the anatomical structure) | n.e. | n.e. | n.e. | n.e. |
| Jung, Kim et al. 2016 [62] | Treatment with 130 mM glucose from 3–6 days post fertilisation (zf larvae) | - | +(increased vessel diameter, irregular and discontinuous staining of ZO-1) | n.e. | n.e. | n.e. | n.e. |
| Tanvir, Nelson et al. 2018 [100] | Exposure to alternating glucose/water solutions for 28 days (adult zf) | n.e. | n.e. | n.e. | n.e. | +(impaired ERG) | +(increased IPL and OPL thickness) |
| Ali, Mukawaya et al. 2019 [78] | Experimental hypoxia for up to 15 days (adult zf) | -(however: remodelling by intussusception) | +(decrease in ZO-1 abundance, increased vessel permeability) | n.e. | n.e. | n.e. | n.e. |
| Li, Zhao et al. 2019 [103] | Incubation with 500µM methylglyoxal with or without 30 mM glucose starting at 10 hpf to 4 dpf (zf larvae) | +(MG induces an increase in vascular area and branch points) | n.e. | n.e. | n.e. | n.e. | n.e. |
| Lodd, Wiggenhauser et al. 2019 [57] | CRISPR/Cas9 generated knockout zebrafish line for glo1 + overfeeding of artemia (adult zf) | + | n.e. | n.e. | n.e. | n.e. | n.e. |
| Singh, Castillo et al. 2019 [95] | Exposure to 4 and 5% D-Glucose in a pulsatile manner from 3 hpf to 5 dpf (zf larvae, adult zf) | +(adult zf show an increased number of hyaloid blood vessel sprouts at 100 dpf after glucose treatment from 3 hpf to 5 dpf) | +(increased vessel permeability) | n.e. | (+) (reduced number of Müller glia cells) | n.e. | +(decreased IPL thickness, increased INL thickness, increased GCL thickness; decreased number of RGC) |
| Ali, Zang et al. 2020 [53] | Pdx1 mutant fish generated through the Zebrafish Mutation Project (as described by Kimmel, Dobler et al. 2015) (adult zf) | + | +(vessel constriction and stenosis, reduction of average vessel diameter, reduced ZO-1 expression, reduced GLUT1 expression, increased vessel permeability) | (+) (reduced expression of transgelin1) | +(enhance expression of GS, hypertrophic changes) | +(reduced numbers of rods and cones, impaired ERG) | +(increased nuclei in the INL, decreased nuclei in the ONL) |
| Wiggenhauser, Qi et al. 2020 [55] | CRISPR/Cas9 generated knockout line for pdx1 (zf larvae, adult zf) | +(at 6 dpf and in the adult retina) | +(increased number of endothelial cell nuclei, increased vessel permeability) | n.e. | n.e. | n.e. | n.e. |
| Lou, Boger et al. 2020 [56] | Incubation with 4-HNE (zebrafish larvae) | +(elevated vascular sprout formation) | +(increased branch diameters) | n.e. | n.e. | n.e. | n.e. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Middel, C.S.; Hammes, H.-P.; Kroll, J. Advancing Diabetic Retinopathy Research: Analysis of the Neurovascular Unit in Zebrafish. Cells 2021, 10, 1313. https://doi.org/10.3390/cells10061313
Middel CS, Hammes H-P, Kroll J. Advancing Diabetic Retinopathy Research: Analysis of the Neurovascular Unit in Zebrafish. Cells. 2021; 10(6):1313. https://doi.org/10.3390/cells10061313
Chicago/Turabian StyleMiddel, Chiara Simone, Hans-Peter Hammes, and Jens Kroll. 2021. "Advancing Diabetic Retinopathy Research: Analysis of the Neurovascular Unit in Zebrafish" Cells 10, no. 6: 1313. https://doi.org/10.3390/cells10061313
APA StyleMiddel, C. S., Hammes, H.-P., & Kroll, J. (2021). Advancing Diabetic Retinopathy Research: Analysis of the Neurovascular Unit in Zebrafish. Cells, 10(6), 1313. https://doi.org/10.3390/cells10061313





