Age-Related Alterations in the Testicular Proteome of a Non-Human Primate
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
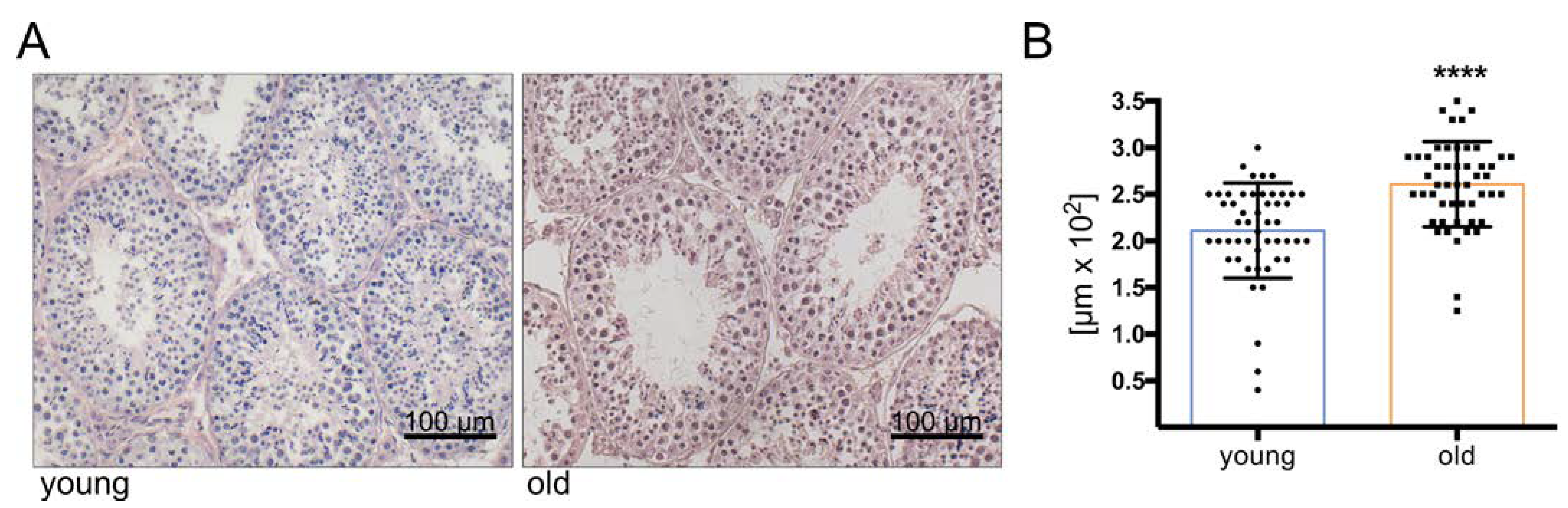
2.2. Immunohistochemistry
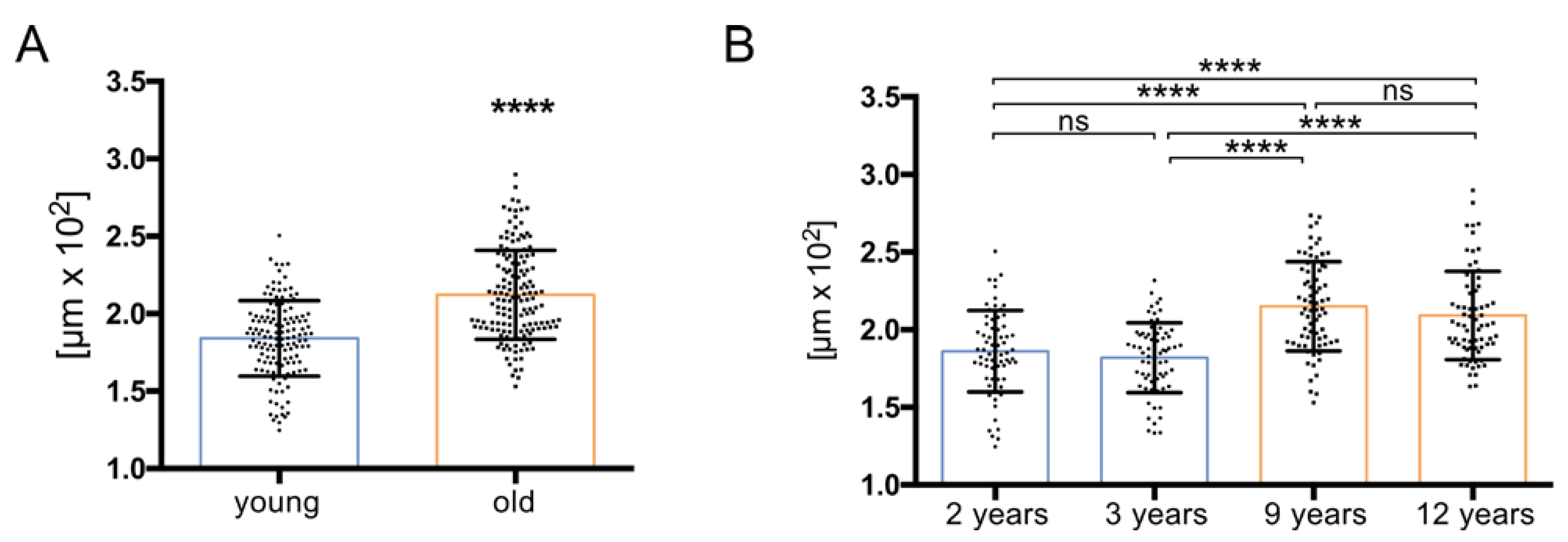
2.3. Measurement of Tubular Diameter
2.4. Sample Preparation for LC-MS/MS
2.5. LC-MS/MS Analysis
2.6. Data Analysis
3. Results
3.1. Histology Reveals Ongoing Spermatogenesis in All Samples and Slightly Increased Tubular Diameters in Old Animals
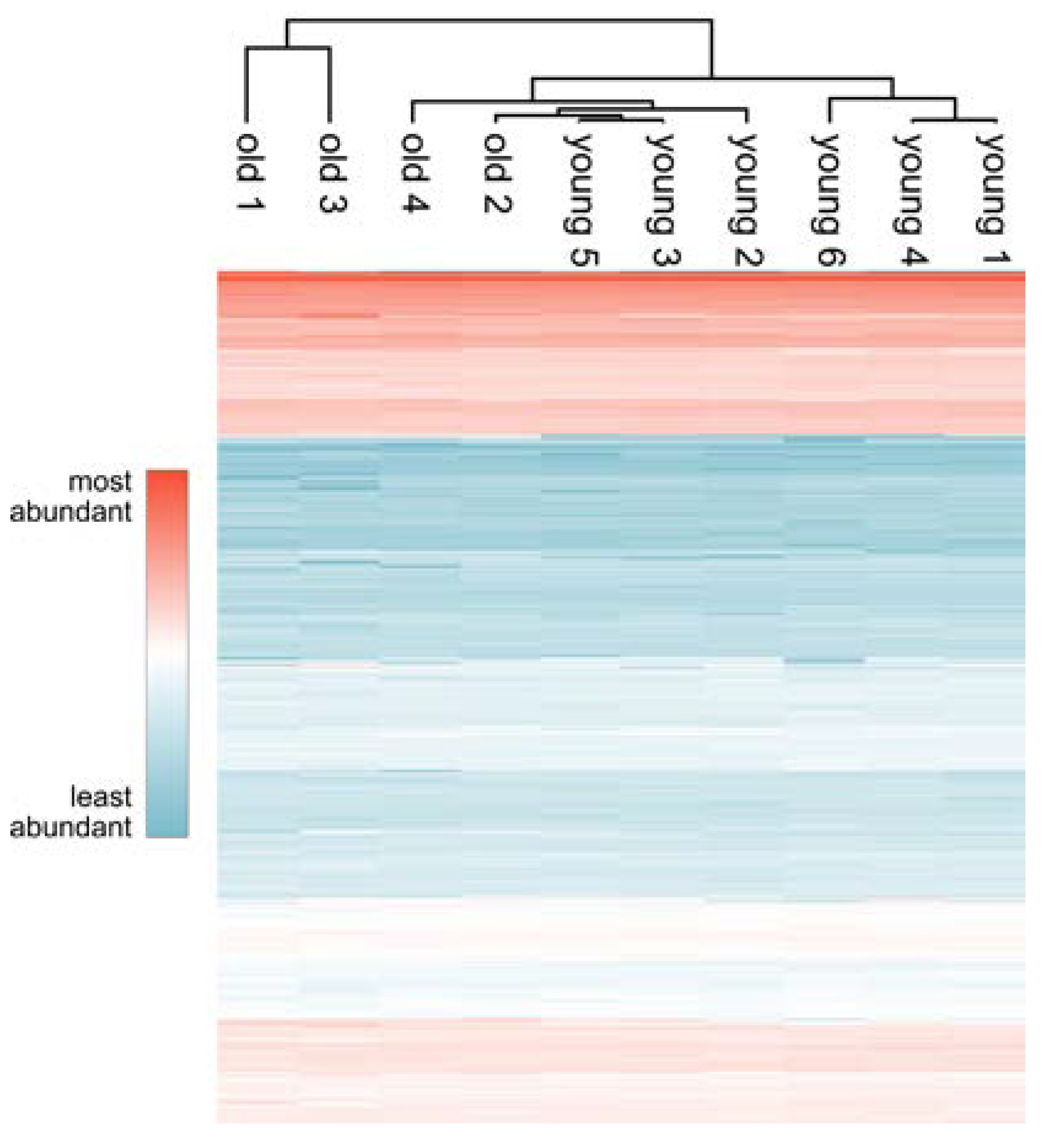
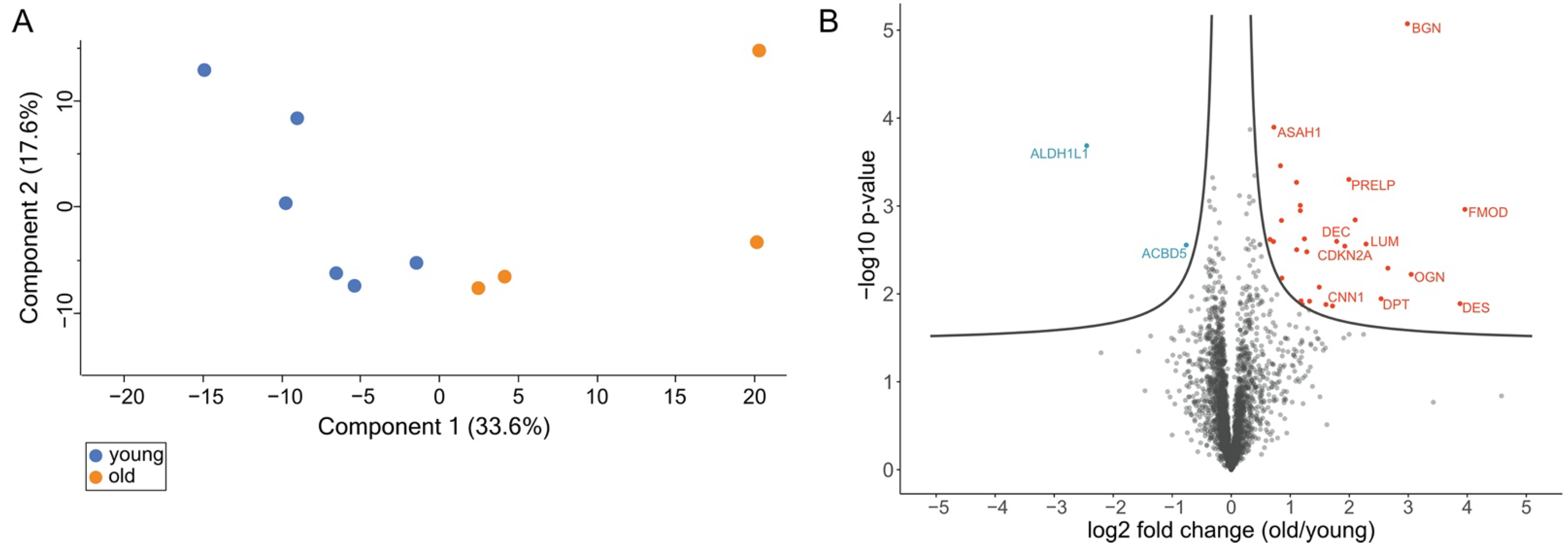
3.2. Comprehensive Proteome Analysis of Testes of Young and Old Individual Donors
3.3. Volcano Plot Analysis Reveals Significant Alterations in the Testis Proteome of Older Individuals
3.4. DAVID Analysis Reveals Clusters of Enriched Functional Terms
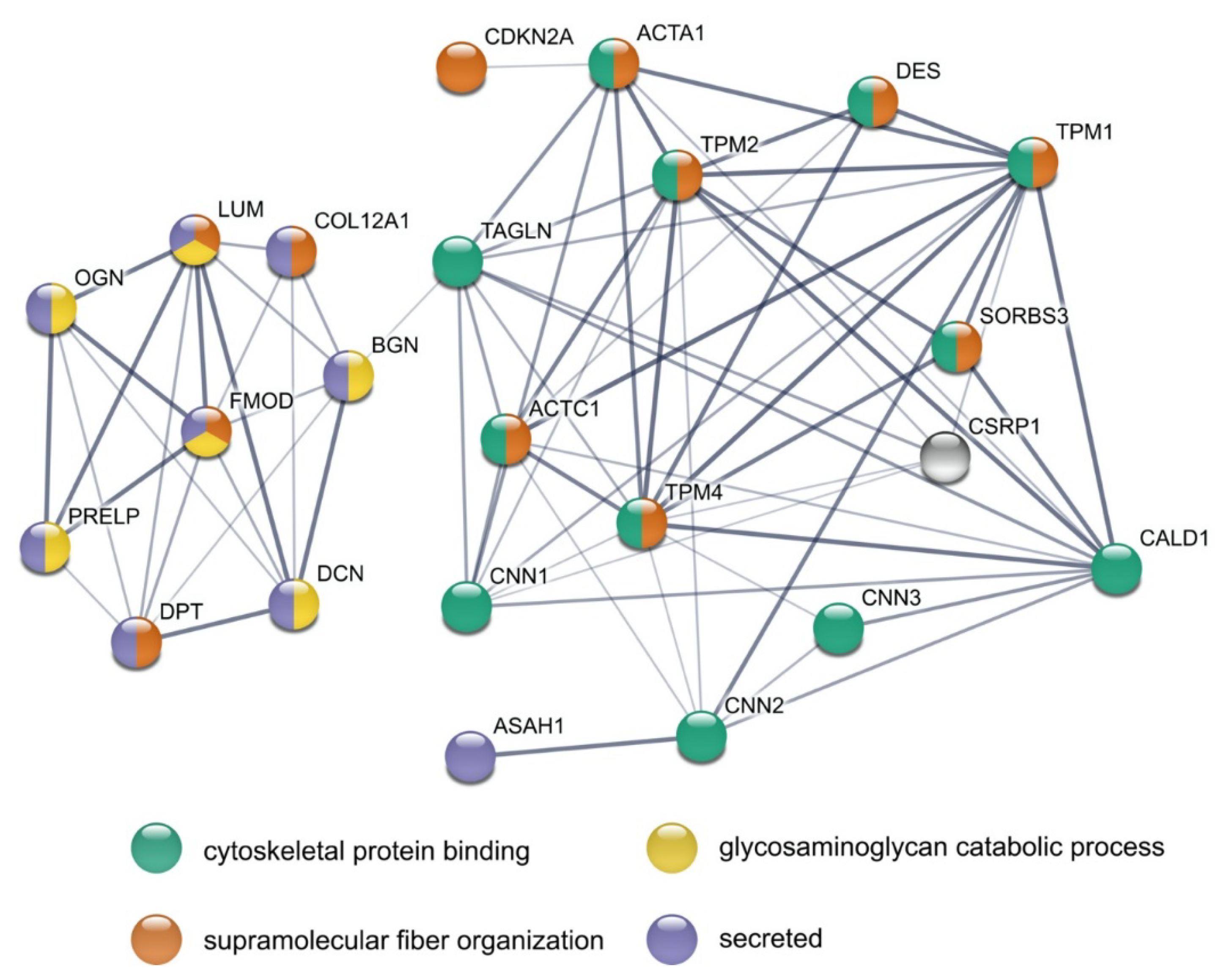
3.5. STRING Analysis Results in a Rich Interaction Network
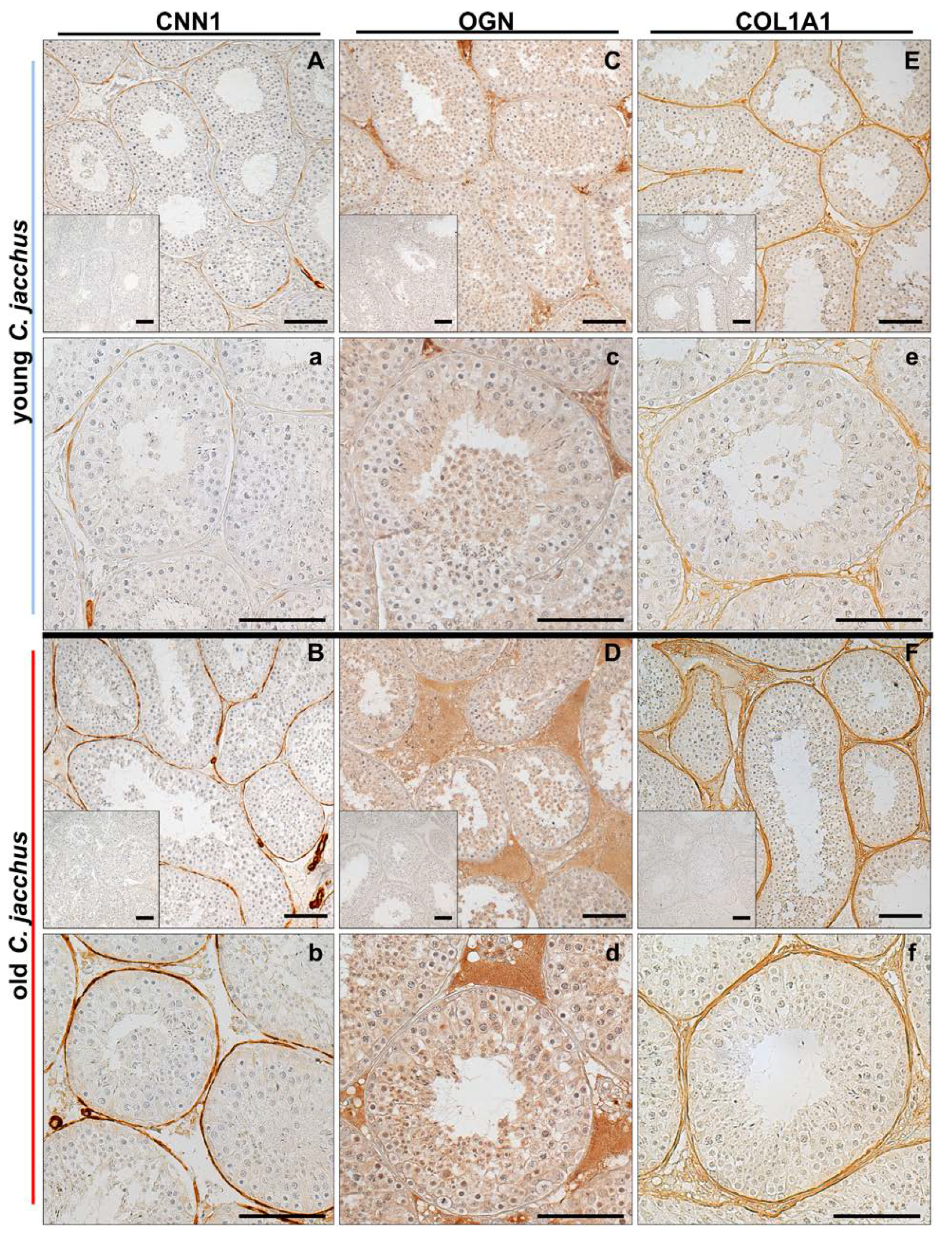
3.6. Immunostaining Illustrates the Increased Abundance of Extracellular Matrix Proteins
4. Discussion
4.1. General Remarks
4.2. Levels of Anti-Proliferative Proteins Are Increased in Testes from Older Individuals
4.3. Testis Proteomes of Aged Individuals Show a Broad Increase of Actin-Binding Proteins
4.4. Proteomic Alterations in Old Testes Point to Specific Age-Related Changes of Peritubular Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell. Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 2011, 1, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Robaire, B. Ageing of the male germ line. Nat. Rev. Urol. 2013, 10, 227–234. [Google Scholar] [CrossRef]
- Laurentino, S.; Cremers, J.F.; Horsthemke, B.; Tüttelmann, F.; Czeloth, K.; Zitzmann, M.; Pohl, E.; Rahmann, S.; Schröder, C.; Berres, S.; et al. A germ cell-specific ageing pattern in otherwise healthy men. Aging Cell 2020, 19, e13242. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Dunleavy, J.; Gemmell, N.J.; Nakagawa, S. Consistent age-dependent declines in human semen quality: A systematic review and meta-analysis. Ageing Res. Rev. 2015, 19, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Rato, L.; Sousa, M.; Alves, M.G.; Oliveira, P.F. Fertility and Sperm Quality in the Aging Male. Curr. Pharm. Des. 2017, 23, 4429–4437. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, R.; Nistal, M.; Amat, P.; Rodriguez, M.C.; Martin, A. Seminiferous Tubule Involution in Elderly Men1. Biol. Reprod. 1987, 36, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, R.; Nistal, M.; Saez, F.J.; Fraile, B. Ultrastructure of the aging human testis. J. Electron Microsc. Tech. 1991, 19, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Mularoni, V.; Esposito, V.; Di Persio, S.; Vicini, E.; Spadetta, G.; Berloco, P.; Fanelli, F.; Mezzullo, M.; Pagotto, U.; Pelusi, C.; et al. Age-related changes in human Leydig cell status. Hum. Reprod. 2020, 35, 2663–2676. [Google Scholar] [CrossRef]
- Pohl, E.; Höffken, V.; Schlatt, S.; Kliesch, S.; Gromoll, J.; Wistuba, J. Ageing in men with normal spermatogenesis alters spermatogonial dynamics and nuclear morphology in Sertoli cells. Andrology 2019, 7, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Nieschlag, E.; Lammers, U.; Freischem, C.W.; Langer, K.; Wickings, E.J. Reproductive functions in young fathers and grandfathers. J. Clin. Endocrinol. Metab. 1982, 55, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, B.; Nieschlag, E. Reproductive functions of the ageing male. Hum. Reprod. Update 2004, 10, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.-M.; Lapauw, B.; Mahmoud, A.; T’Sjoen, G.; Huhtaniemi, I.T. Aging and the Male Reproductive System. Endocr. Rev. 2019, 40, 906–972. [Google Scholar] [CrossRef]
- Santiago, J.; Silva, J.V.; Alves, M.G.; Oliveira, P.F.; Fardilha, M. Testicular Aging: An Overview of Ultrastructural, Cellular, and Molecular Alterations. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Schmid, N.; Stockl, J.B.; Flenkenthaler, F.; Dietrich, K.G.; Schwarzer, J.U.; Kohn, F.M.; Drummer, C.; Frohlich, T.; Arnold, G.J.; Behr, R.; et al. Characterization of a non-human primate model for the study of testicular peritubular cells-comparison with human testicular peritubular cells. Mol. Hum. Reprod. 2018, 24, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Stöckl, J.B.; Schmid, N.; Flenkenthaler, F.; Drummer, C.; Behr, R.; Mayerhofer, A.; Arnold, G.J.; Fröhlich, T. Proteomic Insights into Senescence of Testicular Peritubular Cells from a Nonhuman Primate Model. Cells 2020, 9, 2498. [Google Scholar] [CrossRef]
- Spinnler, K.; Frohlich, T.; Arnold, G.J.; Kunz, L.; Mayerhofer, A. Human tryptase cleaves pro-nerve growth factor (pro-NGF): Hints of local, mast cell-dependent regulation of NGF/pro-NGF action. J. Biol. Chem. 2011, 286, 31707–31713. [Google Scholar] [CrossRef]
- Mayerhofer, A. Human testicular peritubular cells: More than meets the eye. Reproduction 2013, 145, R107–R116. [Google Scholar] [CrossRef]
- Flenkenthaler, F.; Windschuttl, S.; Frohlich, T.; Schwarzer, J.U.; Mayerhofer, A.; Arnold, G.J. Secretome analysis of testicular peritubular cells: A window into the human testicular microenvironment and the spermatogonial stem cell niche in man. J. Proteome Res. 2014, 13, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Windschuttl, S.; Kampfer, C.; Mayer, C.; Flenkenthaler, F.; Frohlich, T.; Schwarzer, J.U.; Kohn, F.M.; Urbanski, H.; Arnold, G.J.; Mayerhofer, A. Human testicular peritubular cells secrete pigment epithelium-derived factor (PEDF), which may be responsible for the avascularity of the seminiferous tubules. Sci. Rep. 2015, 5, 12820. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Adam, M.; Walenta, L.; Schmid, N.; Heikela, H.; Schubert, K.; Flenkenthaler, F.; Dietrich, K.G.; Gruschka, S.; Arnold, G.J.; et al. Insights into the role of androgen receptor in human testicular peritubular cells. Andrology 2018, 6, 756–765. [Google Scholar] [CrossRef]
- Schmid, N.; Flenkenthaler, F.; Stockl, J.B.; Dietrich, K.G.; Kohn, F.M.; Schwarzer, J.U.; Kunz, L.; Luckner, M.; Wanner, G.; Arnold, G.J.; et al. Insights into replicative senescence of human testicular peritubular cells. Sci. Rep. 2019, 9, 15052. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, A. Peritubular cells of the human testis: Prostaglandin E(2) and more. Andrology 2020, 8, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Fleck, D.; Kenzler, L.; Mundt, N.; Strauch, M.; Uesaka, N.; Moosmann, R.; Bruentgens, F.; Missel, A.; Mayerhofer, A.; Merhof, D.; et al. ATP activation of peritubular cells drives testicular sperm transport. Elife 2021, 10. [Google Scholar] [CrossRef]
- Gil, J. Cellular senescence causes ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 388. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Li, L.H.; Donald, J.M.; Golub, M.S. Review on testicular development, structure, function, and regulation in common marmoset. Birth Defects Res. B Dev. Reprod. Toxicol. 2005, 74, 450–469. [Google Scholar] [CrossRef]
- Ross, C.N.; Davis, K.; Dobek, G.; Tardif, S.D. Aging Phenotypes of Common Marmosets (Callithrix jacchus). J. Aging Res. 2012, 2012, 567143. [Google Scholar] [CrossRef]
- Schell, C.; Albrecht, M.; Mayer, C.; Schwarzer, J.U.; Frungieri, M.B.; Mayerhofer, A. Exploring human testicular peritubular cells: Identification of secretory products and regulation by tumor necrosis factor-alpha. Endocrinology 2008, 149, 1678–1686. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Tusher, V.G.; Tibshirani, R.; Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 2001, 98, 5116–5121. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Casagrande, J.T.; Thomas, P.D. Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 2013, 8, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Eildermann, K.; Gromoll, J.; Behr, R. Misleading and reliable markers to differentiate between primate testis-derived multipotent stromal cells and spermatogonia in culture. Hum. Reprod. 2012, 27, 1754–1767. [Google Scholar] [CrossRef]
- Langenstroth, D.; Kossack, N.; Westernströer, B.; Wistuba, J.; Behr, R.; Gromoll, J.; Schlatt, S. Separation of somatic and germ cells is required to establish primate spermatogonial cultures. Hum. Reprod. 2014, 29, 2018–2031. [Google Scholar] [CrossRef][Green Version]
- Davidoff, M.S.; Middendorff, R.; Enikolopov, G.; Riethmacher, D.; Holstein, A.F.; Müller, D. Progenitor cells of the testosterone-producing Leydig cells revealed. J. Cell. Biol. 2004, 167, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Ivell, R.; Wade, J.D.; Anand-Ivell, R. INSL3 as a biomarker of Leydig cell functionality. Biol. Reprod. 2013, 88, 147. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.; McKinnell, C.; Kivlin, C.; Fisher, J. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003, 125, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Kossack, N.; Terwort, N.; Wistuba, J.; Ehmcke, J.; Schlatt, S.; Schöler, H.; Kliesch, S.; Gromoll, J. A combined approach facilitates the reliable detection of human spermatogonia in vitro. Hum. Reprod. 2013, 28, 3012–3025. [Google Scholar] [CrossRef]
- Mietsch, M.; Paqué, K.; Drummer, C.; Stahl-Hennig, C.; Roshani, B. The aging common marmoset’s immune system: From junior to senior. Am. J. Primatol. 2020, 82, e23128. [Google Scholar] [CrossRef]
- Moussavi, A.; Mietsch, M.; Drummer, C.; Behr, R.; Mylius, J.; Boretius, S. Cardiac MRI in common marmosets revealing age-dependency of cardiac function. Sci. Rep. 2020, 10, 10221. [Google Scholar] [CrossRef] [PubMed]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef]
- Hall, B.M.; Balan, V.; Gleiberman, A.S.; Strom, E.; Krasnov, P.; Virtuoso, L.P.; Rydkina, E.; Vujcic, S.; Balan, K.; Gitlin, I.; et al. Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging 2016, 8, 1294–1315. [Google Scholar] [CrossRef]
- Ohtani, N.; Yamakoshi, K.; Takahashi, A.; Hara, E. The p16INK4a-RB pathway: Molecular link between cellular senescence and tumor suppression. J. Med. Investig. 2004, 51, 146–153. [Google Scholar] [CrossRef]
- Ressler, S.; Bartkova, J.; Niederegger, H.; Bartek, J.; Scharffetter-Kochanek, K.; Jansen-Dürr, P.; Wlaschek, M. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 2006, 5, 379–389. [Google Scholar] [CrossRef]
- Bengtsson, E.; Mörgelin, M.; Sasaki, T.; Timpl, R.; Heinegård, D.; Aspberg, A. The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor. J. Biol. Chem. 2002, 277, 15061–15068. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Gu, J.; Niu, G.; Hu, Z.; Zhang, X.; Song, T.; Han, S.; Hong, L.; Ke, C. PRELP has prognostic value and regulates cell proliferation and migration in hepatocellular carcinoma. J. Cancer 2020, 11, 6376–6389. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, O.; Fujiwara, S. Dermatopontin, a novel player in the biology of the extracellular matrix. Connect. Tissue Res. 2006, 47, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, O.; Fujiwara, S.; Abe, M.; Sato, Y. Dermatopontin interacts with transforming growth factor beta and enhances its biological activity. Biochem. J. 1999, 337, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Tzen, C.Y.; Huang, Y.W. Cloning of murine early quiescence-1 gene: The murine counterpart of dermatopontin gene can induce and be induced by cell quiescence. Exp. Cell Res. 2004, 294, 30–38. [Google Scholar] [CrossRef]
- Cai, J.; Liu, W.; Hao, J.; Chen, M.; Li, G. Increased expression of dermatopontin and its implications for testicular dysfunction in mice. Mol. Med. Rep. 2016, 13, 2431–2438. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.J.; Wang, J.; Liu, H.F.; Zhang, X.N.; Zhan, M.; Chen, F.L. Overexpression of mimecan in human aortic smooth muscle cells inhibits cell proliferation and enhances apoptosis and migration. Exp. Med. 2015, 10, 187–192. [Google Scholar] [CrossRef][Green Version]
- Zuo, C.; Li, X.; Huang, J.; Chen, D.; Ji, K.; Yang, Y.; Xu, T.; Zhu, D.; Yan, C.; Gao, P. Osteoglycin attenuates cardiac fibrosis by suppressing cardiac myofibroblast proliferation and migration through antagonizing lysophosphatidic acid 3/matrix metalloproteinase 2/epidermal growth factor receptor signalling. Cardiovasc. Res. 2018, 114, 703–712. [Google Scholar] [CrossRef]
- Lucki, N.C.; Bandyopadhyay, S.; Wang, E.; Merrill, A.H.; Sewer, M.B. Acid ceramidase (ASAH1) is a global regulator of steroidogenic capacity and adrenocortical gene expression. Mol. Endocrinol. 2012, 26, 228–243. [Google Scholar] [CrossRef]
- Lucki, N.C.; Li, D.; Bandyopadhyay, S.; Wang, E.; Merrill, A.H.; Sewer, M.B. Acid ceramidase (ASAH1) represses steroidogenic factor 1-dependent gene transcription in H295R human adrenocortical cells by binding to the receptor. Mol. Cell Biol. 2012, 32, 4419–4431. [Google Scholar] [CrossRef]
- Li, C.M.; Park, J.H.; He, X.; Levy, B.; Chen, F.; Arai, K.; Adler, D.A.; Disteche, C.M.; Koch, J.; Sandhoff, K.; et al. The human acid ceramidase gene (ASAH): Structure, chromosomal location, mutation analysis, and expression. Genomics 1999, 62, 223–231. [Google Scholar] [CrossRef]
- Cirillo, F.; Piccoli, M.; Ghiroldi, A.; Monasky, M.M.; Rota, P.; La Rocca, P.; Tarantino, A.; D’Imperio, S.; Signorelli, P.; Pappone, C.; et al. The antithetic role of ceramide and sphingosine-1-phosphate in cardiac dysfunction. J. Cell Physiol. 2021. [Google Scholar] [CrossRef]
- Bagnjuk, K.; Stöckl, J.B.; Fröhlich, T.; Arnold, G.J.; Behr, R.; Berg, U.; Berg, D.; Kunz, L.; Bishop, C.; Xu, J.; et al. Necroptosis in primate luteolysis: A role for ceramide. Cell Death Discov. 2019, 5, 67. [Google Scholar] [CrossRef]
- Welter, H.; Kampfer, C.; Lauf, S.; Feil, R.; Schwarzer, J.U.; Kohn, F.M.; Mayerhofer, A. Partial loss of contractile marker proteins in human testicular peritubular cells in infertility patients. Andrology 2013, 1, 318–324. [Google Scholar] [CrossRef]
- Schlatt, S.; Weinbauer, G.F.; Arslan, M.; Nieschlag, E. Appearance of alpha-smooth muscle actin in peritubular cells of monkey testes is induced by androgens, modulated by follicle-stimulating hormone, and maintained after hormonal withdrawal. J. Androl. 1993, 14, 340–350. [Google Scholar] [PubMed]
- Welter, H.; Herrmann, C.; Dellweg, N.; Missel, A.; Thanisch, C.; Urbanski, H.F.; Köhn, F.-M.; Schwarzer, J.U.; Müller-Taubenberger, A.; Mayerhofer, A. The Glucocorticoid Receptor NR3C1 in Testicular Peritubular Cells is Developmentally Regulated and Linked to the Smooth Muscle-Like Cellular Phenotype. J. Clin. Med. 2020, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- North, A.J.; Gimona, M.; Cross, R.A.; Small, J.V. Calponin is localised in both the contractile apparatus and the cytoskeleton of smooth muscle cells. J. Cell Sci. 1994, 107 Pt 3, 437–444. [Google Scholar] [CrossRef]
- Liu, R.; Jin, J.P. Calponin isoforms CNN1, CNN2 and CNN3: Regulators for actin cytoskeleton functions in smooth muscle and non-muscle cells. Gene 2016, 585, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Mitarai, H.; Wada, N.; Hasegawa, D.; Yoshida, S.; Sonoda, M.; Tomokiyo, A.; Hamano, S.; Serita, S.; Mizumachi, H.; Maeda, H. Transgelin mediates transforming growth factor-β1-induced proliferation of human periodontal ligament cells. J. Periodontal. Res. 2017, 52, 984–993. [Google Scholar] [CrossRef]
- Elsafadi, M.; Manikandan, M.; Dawud, R.A.; Alajez, N.M.; Hamam, R.; Alfayez, M.; Kassem, M.; Aldahmash, A.; Mahmood, A. Transgelin is a TGFβ-inducible gene that regulates osteoblastic and adipogenic differentiation of human skeletal stem cells through actin cytoskeleston organization. Cell Death Dis. 2016, 7, e2321. [Google Scholar] [CrossRef]
- Dvorakova, M.; Nenutil, R.; Bouchal, P. Transgelins, cytoskeletal proteins implicated in different aspects of cancer development. Expert. Rev. Proteom. 2014, 11, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Eppinga, R.D.; Warren, K.S.; McCrae, K.R. Human tropomyosin isoforms in the regulation of cytoskeleton functions. Adv. Exp. Med. Biol. 2008, 644, 201–222. [Google Scholar] [CrossRef]
- Gunning, P.W.; Hardeman, E.C.; Lappalainen, P.; Mulvihill, D.P. Tropomyosin—Master regulator of actin filament function in the cytoskeleton. J. Cell Sci. 2015, 128, 2965–2974. [Google Scholar] [CrossRef] [PubMed]
- Marston, S.; Burton, D.; Copeland, O.; Fraser, I.; Gao, Y.; Hodgkinson, J.; Huber, P.; Levine, B.; el-Mezgueldi, M.; Notarianni, G. Structural interactions between actin, tropomyosin, caldesmon and calcium binding protein and the regulation of smooth muscle thin filaments. Acta Physiol. Scand. 1998, 164, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, K.Y.; Wang, Z.; Chacko, S. Inhibition of smooth muscle actomyosin ATPase by caldesmon is associated with caldesmon-induced conformational changes in tropomyosin bound to actin. Biochemistry 1995, 34, 16815–16820. [Google Scholar] [CrossRef]
- Marston, S.B.; Redwood, C.S. Inhibition of actin-tropomyosin activation of myosin MgATPase activity by the smooth muscle regulatory protein caldesmon. J. Biol. Chem. 1992, 267, 16796–16800. [Google Scholar] [CrossRef]
- Alahyan, M.; Webb, M.R.; Marston, S.B.; El-Mezgueldi, M. The mechanism of smooth muscle caldesmon-tropomyosin inhibition of the elementary steps of the actomyosin ATPase. J. Biol. Chem. 2006, 281, 19433–19448. [Google Scholar] [CrossRef]
- Yamashiro, S.; Chern, H.; Yamakita, Y.; Matsumura, F. Mutant Caldesmon lacking cdc2 phosphorylation sites delays M-phase entry and inhibits cytokinesis. Mol. Biol. Cell. 2001, 12, 239–250. [Google Scholar] [CrossRef][Green Version]
- Yokouchi, K.; Numaguchi, Y.; Kubota, R.; Ishii, M.; Imai, H.; Murakami, R.; Ogawa, Y.; Kondo, T.; Okumura, K.; Ingber, D.E.; et al. l-Caldesmon regulates proliferation and migration of vascular smooth muscle cells and inhibits neointimal formation after angioplasty. Arter. Thromb. Vasc. Biol. 2006, 26, 2231–2237. [Google Scholar] [CrossRef][Green Version]
- Hossain, M.M.; Hwang, D.Y.; Huang, Q.Q.; Sasaki, Y.; Jin, J.P. Developmentally regulated expression of calponin isoforms and the effect of h2-calponin on cell proliferation. Am. J. Physiol. Cell Physiol. 2003, 284, C156–C167. [Google Scholar] [CrossRef]
- Moazzem Hossain, M.; Wang, X.; Bergan, R.C.; Jin, J.P. Diminished expression of h2-calponin in prostate cancer cells promotes cell proliferation, migration and the dependence of cell adhesion on substrate stiffness. FEBS Open Bio 2014, 4, 627–636. [Google Scholar] [CrossRef]
- Wang, J.; Tang, C.; Yang, C.; Zheng, Q.; Hou, Y. Tropomyosin-1 Functions as a Tumor Suppressor with Respect to Cell Proliferation, Angiogenesis and Metastasis in Renal Cell Carcinoma. J. Cancer 2019, 10, 2220–2228. [Google Scholar] [CrossRef]
- Schevzov, G.; Kee, A.J.; Wang, B.; Sequeira, V.B.; Hook, J.; Coombes, J.D.; Lucas, C.A.; Stehn, J.R.; Musgrove, E.A.; Cretu, A.; et al. Regulation of cell proliferation by ERK and signal-dependent nuclear translocation of ERK is dependent on Tm5NM1-containing actin filaments. Mol. Biol. Cell 2015, 26, 2475–2490. [Google Scholar] [CrossRef]
- Wang, M.; Li, W.; Chang, G.-Q.; Ye, C.-S.; Ou, J.-S.; Li, X.-X.; Liu, Y.; Cheang, T.-Y.; Huang, X.-L.; Wang, S.-M. MicroRNA-21 Regulates Vascular Smooth Muscle Cell Function via Targeting Tropomyosin 1 in Arteriosclerosis Obliterans of Lower Extremities. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.G.; Talati, J.; Servos, G. Ultrastructure of peritubular tissue in association with tubular hyalinization in human testis. Tissue Cell 1999, 31, 90–98. [Google Scholar] [CrossRef]
- de Kretser, D.M.; Kerr, J.B.; Paulsen, C.A. The Peritubular Tissue in the Normal and Pathological Human Testis. An Ultrastructural Study. Biol. Reprod. 1975, 12, 317–324. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D.A. Mechanisms of fibrogenesis. Exp. Biol. Med. (Maywood) 2008, 233, 109–122. [Google Scholar] [CrossRef]
- Schell, C.; Albrecht, M.; Spillner, S.; Mayer, C.; Kunz, L.; Kohn, F.M.; Schwarzer, U.; Mayerhofer, A. 15-Deoxy-delta 12-14-prostaglandin-J2 induces hypertrophy and loss of contractility in human testicular peritubular cells: Implications for human male fertility. Endocrinology 2010, 151, 1257–1268. [Google Scholar] [CrossRef]
- Svensson, L.; Närlid, I.; Oldberg, A. Fibromodulin and lumican bind to the same region on collagen type I fibrils. FEBS Lett. 2000, 470, 178–182. [Google Scholar] [CrossRef]
- Chakravarti, S. Functions of lumican and fibromodulin: Lessons from knockout mice. Glycoconj. J. 2002, 19, 287–293. [Google Scholar] [CrossRef]
- Chen, S.; Birk, D.E. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013, 280, 2120–2137. [Google Scholar] [CrossRef]
- Robinson, K.A.; Sun, M.; Barnum, C.E.; Weiss, S.N.; Huegel, J.; Shetye, S.S.; Lin, L.; Saez, D.; Adams, S.M.; Iozzo, R.V.; et al. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biol. 2017, 64, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Urbanski, H.F.; Garyfallou, V.T.; Welsch, U.; Köhn, F.M.; Ullrich Schwarzer, J.; Strauss, L.; Poutanen, M.; Mayerhofer, A. High levels of the extracellular matrix proteoglycan decorin are associated with inhibition of testicular function. Int. J. Androl. 2012, 35, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Adam, M.; Glashauser, L.; Dietrich, K.; Schwarzer, J.U.; Kohn, F.M.; Strauss, L.; Welter, H.; Poutanen, M.; Mayerhofer, A. Sterile inflammation as a factor in human male infertility: Involvement of Toll like receptor 2, biglycan and peritubular cells. Sci. Rep. 2016, 6, 37128. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Schwarzer, J.U.; Köhn, F.M.; Strauss, L.; Poutanen, M.; Mayerhofer, A. Mast cell tryptase stimulates production of decorin by human testicular peritubular cells: Possible role of decorin in male infertility by interfering with growth factor signaling. Hum. Reprod. 2011, 26, 2613–2625. [Google Scholar] [CrossRef] [PubMed]


| Cell Type | Markers |
|---|---|
| Leydig cells | CYP11A1, CYP17A1, INSL3 |
| Sertoli cells | VIM, CDKN1B |
| Peritubular cells | VIM, ACTA2 |
| Germ cells | DDX4, MAGEA4, UTF1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stöckl, J.B.; Schmid, N.; Flenkenthaler, F.; Drummer, C.; Behr, R.; Mayerhofer, A.; Arnold, G.J.; Fröhlich, T. Age-Related Alterations in the Testicular Proteome of a Non-Human Primate. Cells 2021, 10, 1306. https://doi.org/10.3390/cells10061306
Stöckl JB, Schmid N, Flenkenthaler F, Drummer C, Behr R, Mayerhofer A, Arnold GJ, Fröhlich T. Age-Related Alterations in the Testicular Proteome of a Non-Human Primate. Cells. 2021; 10(6):1306. https://doi.org/10.3390/cells10061306
Chicago/Turabian StyleStöckl, Jan B., Nina Schmid, Florian Flenkenthaler, Charis Drummer, Rüdiger Behr, Artur Mayerhofer, Georg J. Arnold, and Thomas Fröhlich. 2021. "Age-Related Alterations in the Testicular Proteome of a Non-Human Primate" Cells 10, no. 6: 1306. https://doi.org/10.3390/cells10061306
APA StyleStöckl, J. B., Schmid, N., Flenkenthaler, F., Drummer, C., Behr, R., Mayerhofer, A., Arnold, G. J., & Fröhlich, T. (2021). Age-Related Alterations in the Testicular Proteome of a Non-Human Primate. Cells, 10(6), 1306. https://doi.org/10.3390/cells10061306







