Hallmarks of Testicular Aging: The Challenge of Anti-Inflammatory and Antioxidant Therapies Using Natural and/or Pharmacological Compounds to Improve the Physiopathological Status of the Aged Male Gonad
Abstract
1. Introduction
2. Testicular Aging
2.1. General Aspects of Age-Dependent Alterations in the Human Testis
2.2. Animal Models for the Study of Testicular Aging: What We Know So Far
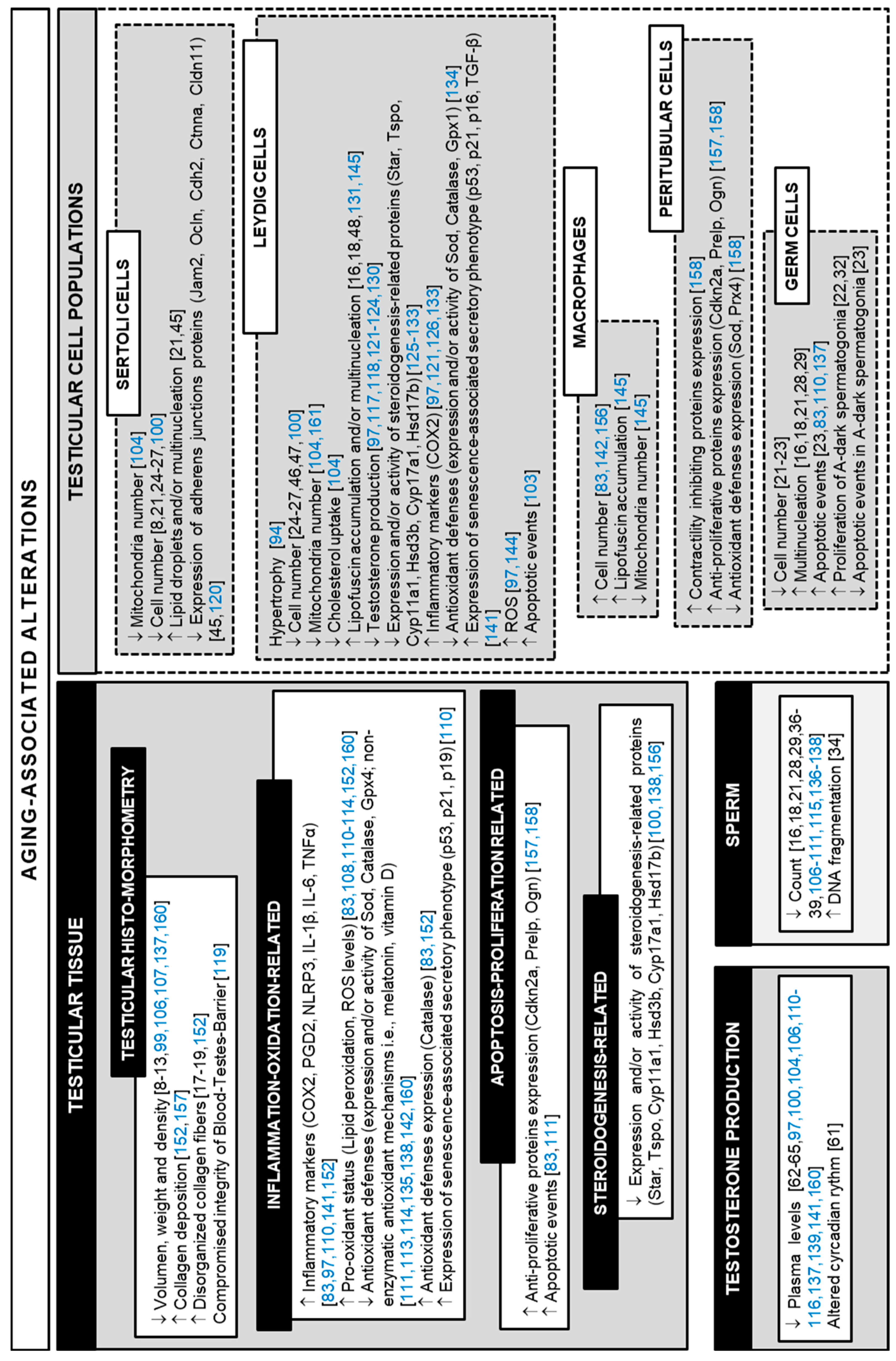
3. Interventions to Reverse Aging-Related Testicular Alterations
3.1. Common Anti-Inflammatory Drugs
3.2. Common Antioxidant Compounds
3.3. Nutrient-Sensing Pathway Inhibition
3.4. Senolytics and Senomorphics
3.5. Herbs and Nutraceuticals from Traditional Oriental Medicine
3.6. Probiotics, Prebiotics, and Synbiotics

4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hermann, M.; Untergasser, H.; Rumpold, H.; Berger, P. Aging of the male reproductive system. Exp. Gerontol. 2000, 35, 1267–1279. [Google Scholar] [CrossRef]
- Frungieri, M.B.; Calandra, R.S.; Bartke, A.; Matzkin, M.E. Ageing and inflammation in the male reproductive tract. Andrologia 2018, 50, e13034. [Google Scholar] [CrossRef]
- Frungieri, M.B.; Calandra, R.S.; Bartke, A.; Matzkin, M.E. Male and female gonadal ageing: Its impact on health span and life span. Mech. Ageing. Dev. 2021, 197, 111519. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Miquel, J. An update of the oxidation-inflammation theory of aging: The involvement of the immune system in oxi-inflamm-aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef]
- Pataky, M.W.; Young, W.F.; Nair, K.S. Hormonal and Metabolic Changes of Aging and the Influence of Lifestyle Modifications. Mayo Clin. Proc. 2021, 96, 788–814. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Rastrelli, G.; Maseroli, E.; Forti, G.; Maggi, M. Sexual function of the ageing male. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 581–601. [Google Scholar] [CrossRef]
- Ni Lochlainn, M.; Kenny, R.A. Sexual activity and aging. J. Am. Med. Dir. Assoc. 2013, 14, 565–572. [Google Scholar] [CrossRef]
- Johnson, L.; Petty, C.S.; Neaves, W.B. Influence of age on sperm production and testicular weights in men. J. Reprod. Fertil. 1984, 70, 211–218. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Staraj, S. Testicular Size: The Effects of Aging, Malnutrition, and Illness. J. Androl. 1985, 6, 144–151. [Google Scholar] [CrossRef]
- Paniagua, R.; Martín, A.; Nistal, M.; Amat, P. Testicular involution in elderly men: Comparison of histologic quantitative studies with hormone patterns. Fertil. Steril. 1987, 47, 671–679. [Google Scholar] [CrossRef]
- Arenas, M.I.; Bethencourt, F.R.; Fraile, B.; Paniagua, R. Immunocytochemical and quantitative study of the tunica albuginea testis in young and ageing men. Histochem. Cell Biol. 1997, 107, 469–477. [Google Scholar] [CrossRef]
- Yang, H.; Chryssikos, T.; Houseni, H.; Alzeair, S.; Sansovini, M.; Iruvuri, S.; Torigian, D.A.; Zhuang, H.; Dadparvar, S.; Basu, S.; et al. The effects of aging on testicular volume and glucose metabolism: An investigation with ultrasonography and FDG-PET. Mol. Imaging Biol. 2011, 13, 391–398. [Google Scholar] [CrossRef]
- Lasiene, K.; Gasiliunas, D.; Juodziukyniene, N.; Dabuzinskiene, A.; Vitkus, A.; Zilaitiene, B. Age-related morphological peculiarities of human testes. Folia Morphol. 2021, 80, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Sasano, N.; Ichijo, S. Vascular patterns of the human testis with special reference to its senile changes. Tohoku J. Exp. Med. 1969, 99, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Regadera, J.; Nistal, M.; Paniagua, R. Testis, epididymis, and spermatic cord in elderly men. Correlation of angiographic and histologic studies with systemic arteriosclerosis. Arch. Pathol. Lab. Med. 1985, 109, 663–667. [Google Scholar]
- Nistal, M.; González-Peramato, P.; Serrano, A. Testicular Changes in Elderly Men. In Clues in the Diagnosis of Non-tumoral Testicular Pathology, 1st ed.; Nistal, M., González-Peramato, P., Serrano, A., Eds.; Sringer International Publishing: Cham, Switzerland, 2017; pp. 349–361. [Google Scholar]
- Xi, Y.P.; Nette, E.G.; King, D.W.; Rosen, M. Age-related changes in normal human basement membrane. Mech. Ageing Dev. 1982, 19, 315–324. [Google Scholar] [CrossRef]
- Paniagua, R.; Nistal, M.; Sáez, F.J.; Fraile, B. Ultrastructure of the aging human testis. J. Electron. Microsc. Tech. 1991, 19, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Pop, O.T.; Cotoi, C.G.; Pleşea, I.E.; Gherghiceanu, M.; Enache, S.D.; Mandache, E.; Hortopan, G.; Pleşea, R.M. Histological and ultrastructural analysis of the seminiferous tubule wall in ageing testis. Rom. J. Morphol. Embryol. 2011, 52, 241–248. [Google Scholar] [PubMed]
- Johnson, L.; Petty, C.S.; Neaves, W.B. Age-related variation in seminiferous tubules in men. A stereologic evaluation. J. Androl. 1986, 7, 316–322. [Google Scholar] [CrossRef]
- Paniagua, R.; Nistal, M.; Amat, P.; Rodríguez, M.C.; Martín, A. Seminiferous Tubule Involution in Elderly Men. Biol. Reprod. 1987, 36, 939–947. [Google Scholar] [CrossRef]
- Kimura, M.; Itoh, N.; Takagi, S.; Sasao, T.; Takahashi, A.; Masumori, N.; Tsukamoto, T. Balance of apoptosis and proliferation of germ cells related to spermatogenesis in aged men. J. Androl. 2003, 24, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhu, W.J.; Li, J.; Chen, Q.J.; Liang, W.B.; Gu, Y.Q. Quantitative histological analysis and ultrastructure of the aging human testis. Int. Urol. Nephrol. 2014, 46, 879–885. [Google Scholar] [CrossRef]
- Petersen, P.M.; Pakkenberg, B. Stereological quantitation of Leydig and Sertoli cells in the testis from young and old men. Image Anal. Stereol. 2000, 19, 215–218. [Google Scholar] [CrossRef][Green Version]
- Xia, Y.; Zhu, W.J.; Hao, S.F.; Liang, W.B.; Li, J. Stereological analysis of age-related changes of testicular peritubular cells in men. Arch. Gerontol. Geriatr. 2012, 55, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.P.; Seierøe, K.; Pakkenberg, B. The total number of Leydig and Sertoli cells in the testes of men across various age groups—A stereological study. J. Anat. 2015, 226, 175–179. [Google Scholar] [CrossRef]
- Mularoni, V.; Esposito, V.; Di Persio, S.; Vicini, E.; Spadetta, G.; Berloco, P.; Fanelli, F.; Mezzullo, M.; Pagotto, U.; Pelusi, C.; et al. Age-related changes in human Leydig cell status. Hum. Reprod. 2020, 35, 2663–2676. [Google Scholar] [CrossRef]
- Nistal, M.; Codesal, J.; Paniagua, R. Multinucleate spermatids in aging human testes. Arch. Androl. 1986, 16, 125–129. [Google Scholar] [CrossRef]
- Miething, A. Multinuclearity of germ cells in the senescent human testis originates from a process of cell-cell fusion. J. Submicrosc. Cytol. Pathol. 1995, 27, 105–113. [Google Scholar]
- Holstein, A.F.; Schulze, W.; Davidoff, M. Understanding spermatogenesis is a prerequisite for treatment. Reprod. Biol. Endocrinol. 2003, 1, 107. [Google Scholar] [CrossRef]
- Amann, R.P. The cycle of the seminiferous epithelium in humans: A need to revisit? J. Androl. 2008, 29, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Pohl, E.; Höffken, V.; Schlatt, S.; Kliesch, S.; Gromoll, J.; Wistuba, J. Ageing in men with normal spermatogenesis alters spermatogonial dynamics and nuclear morphology in Sertoli cells. Andrology 2019, 7, 827–839. [Google Scholar] [CrossRef]
- Yatsenko, A.N.; Turek, P.J. Reproductive genetics and the aging male. J. Assist. Reprod. Genet. 2018, 35, 933–941. [Google Scholar] [CrossRef]
- Pohl, E.; Gromoll, J.; Wistuba, J.; Laurentino, S. Healthy ageing and spermatogenesis. Reproduction 2021, 161, R89–R101. [Google Scholar] [CrossRef]
- Wu, F.C. Male hypogonadism—Current concepts and trends. Clin. Obstet. Gynaecol. 1985, 12, 531–555. [Google Scholar] [CrossRef]
- Schwartz, D.; Mayaux, M.J.; Spira, A.; Moscato, M.L.; Jouannet, P.; Czyglik, F.; David, G. Semen characteristics as a function of age in 833 fertile men. Fertil. Steril. 1983, 39, 530–535. [Google Scholar] [CrossRef]
- Kidd, S.A.; Eskenazi, B.; Wyrobek, A.J. Effects of male age on semen quality and fertility: A review of the literature. Fertil. Steril. 2001, 75, 237–248. [Google Scholar] [CrossRef]
- Hellstrom, W.G.J.; Overstreet, J.W.; Sikka, S.C.; Denne, J.; Ahuja, S.; Hoover, A.M.; Sides, G.D.; Cordell, W.H.; Harrison, L.M.; Whitaker, J.S. Semen and sperm reference ranges for men 45 years of age and older. J. Androl. 2006, 27, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Paoli, D.; Pecora, G.; Pallotti, F.; Faja, F.; Pelloni, M.; Lenzi, A.; Lombardo, F. Cytological and molecular aspects of the ageing sperm. Hum. Reprod. 2019, 34, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Cocuzza, M.; Athayde, K.S.; Agarwal, A.; Sharma, R.; Pagani, R.; Lucon, A.M.; Srougi, M.; Hallak, J. Age-related increase of reactive oxygen species in neat semen in healthy fertile men. Urology 2008, 71, 490–494. [Google Scholar] [CrossRef]
- Desai, N.; Sabanegh, E., Jr.; Kim, T.; Agarwal, A. Free radical theory of aging: Implications in male infertility. Urology 2010, 75, 14–19. [Google Scholar] [CrossRef]
- de Lamirande, E.; Jiang, H.; Zini, A.; Kodama, H.; Gagnon, C. Reactive oxygen species and sperm physiology. Rev. Reprod. 1997, 2, 48–54. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Sikka, S.C. Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front. Biosci. 1996, 1, e78–e86. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, R.; Amat, P.; Nistal, M.; Martin, A. Ultrastructural changes in Sertoli cells in ageing humans. Int. J. Androl. 1985, 8, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Kaler, L.W.; Neaves, W.B. Attrition of the human Leydig cell population with advancing age. Anat. Rec. 1978, 192, 513–518. [Google Scholar] [CrossRef]
- Neaves, W.B.; Johnson, L.; Porter, J.C.; Parker, C.R., Jr.; Petty, C.S. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J. Clin. Endocrinol. Metab. 1984, 59, 756–763. [Google Scholar] [CrossRef]
- Kothari, L.K.; Gupta, A.S. Effect of ageing on the volume, structure and total Leydig cell content of the human testis. Int. J. Fertil. 1974, 19, 140–146. [Google Scholar]
- Porta, E.A. Pigments in aging: An overview. Ann. N. Y. Acad. Sci. 2002, 959, 57–65. [Google Scholar] [CrossRef]
- Terman, A.; Brunk, U.T. Oxidative stress, accumulation of biological ‘garbage’, and aging. Antioxid. Redox Signal. 2006, 8, 197–204. [Google Scholar] [CrossRef]
- Double, K.L.; Dedov, V.N.; Fedorow, H.; Kettle, E.; Halliday, G.M.; Garner, B.; Brunk, U.T. The comparative biology of neuromelanin and lipofuscin in the human brain. Cell. Mol. Life Sci. 2008, 65, 1669–1682. [Google Scholar] [CrossRef]
- Reeg, S.; Grune, T. Protein Oxidation in Aging: Does It Play a Role in Aging Progression? Antioxid. Redox Signal. 2015, 23, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Moreno-García, A.; Kun, A.; Calero, O.; Medina, M.; Calero, M. An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration. Front. Neurosci. 2018, 12, 464. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, O.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Krabbe, K.S.; Pedersen, M.; Bruunsgaard, H. Inflammatory mediators in the elderly. Exp. Gerontol. 2004, 39, 687–699. [Google Scholar] [CrossRef]
- Maggio, M.; Basaria, S.; Ceda, G.P.; Ble, A.; Ling, S.M.; Bandinelli, S.; Valenti, G.; Ferrucci, L. The relationship between testosterone and molecular markers of inflammation in older men. J. Endocrinol. Investig. 2005, 28, 116–119. [Google Scholar]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Madeo, F.; Tavernarakis, N.; Kroemer, G. Can autophagy promote longevity? Nat. Cell. Biol. 2010, 12, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Brugh, V.M., 3rd; Matschke, H.M.; Lipshultz, L.I. Male factor infertility. Endocrinol. Metab. Clin. N. Am. 2003, 32, 689–707. [Google Scholar] [CrossRef]
- Biasizzo, M.; Kopitar-Jerala, N. Interplay between NLRP3 Inflammasome and Autophagy. Front. Immunol. 2020, 11, 591803. [Google Scholar] [CrossRef]
- Bremner, W.J.; Vitiello, M.V.; Prinz, P.N. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J. Clin. Endocrinol. Metab. 1983, 56, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Stearns, E.L.; MacDonnell, J.A.; Kaufman, B.J.; Padua, R.; Lucman, T.S.; Winter, J.S.; Faiman, C. Declining testicular function with age. Hormonal and clinical correlates. Am. J. Med. 1974, 57, 761–766. [Google Scholar] [CrossRef]
- Baker, H.W.; Burger, H.G.; de Kretser, D.M.; Hudson, B.; O’Connor, S.; Wang, C.; Mirovics, A.; Court, J.; Dunlop, M.; Rennie, G.C. Changes in the pituitary-testicular system with age. Clin. Endocrinol. 1976, 5, 349–372. [Google Scholar] [CrossRef]
- Vermeulen, A.; Rubens, R.; Verdonck, L. Testosterone secretion and metabolism in male senescence. J. Clin. Endocrinol. Metab. 1972, 34, 730–735. [Google Scholar] [CrossRef]
- Yeap, B.B.; Manning, L.; Chubb, S.A.P.; Handelsman, D.J.; Almeida, O.P.; Hankey, G.J.; Flicker, L. Progressive impairment of testicular endocrine function in ageing men: Testosterone and dihydrotestosterone decrease, and luteinizing hormone increases, in men transitioning from the 8th to 9th decades of life. Clin. Endocrinol. 2018, 88, 88–95. [Google Scholar] [CrossRef]
- Harman, S.M.; Tsitouras, P.D. Reproductive hormones in aging men. I. Measurement of sex steroids, basal luteinizing hormone, and Leydig cell response to human chorionic gonadotropin. J. Clin. Endocrinol. Metab. 1980, 51, 35–40. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Goemaere, S.; El-Garem, Y.; Van Pottelbergh, I.; Comhaire, F.H.; Kaufman, J.M. Testicular volume in relation to hormonal indices of gonadal function in community-dwelling elderly men. J. Clin. Endocrinol. Metab. 2003, 88, 179–184. [Google Scholar] [CrossRef]
- Almeida, S.; Rato, L.; Sousa, M.; Alves, M.G.; Oliveira, P.F. Fertility and Sperm Quality in the Aging Male. Curr. Pharm. Des. 2017, 23, 4429–4437. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.M.; Vermeulen, A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr. Rev. 2005, 26, 833–876. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.A. Aging across the tree of life: The importance of a comparative perspective for the use of animal models in aging. Biochim. Biophys. Acta Mol. Basis. Dis. 2018, 1864 Pt A, 2680–2689. [Google Scholar] [CrossRef]
- Liang, H.; Masoro, E.J.; Nelson, J.F.; Strong, R.; McMahan, C.A.; Richardson, A. Genetic mouse models of extended lifespan. Exp. Gerontol. 2003, 38, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Vanhooren, V.; Libert, C. The mouse as a model organism in aging research: Usefulness, pitfalls and possibilities. Ageing Res. Rev. 2013, 12, 8–21. [Google Scholar] [CrossRef]
- Colman, R.J. Non-human primates as a model for aging. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt A, 2733–2741. [Google Scholar] [CrossRef]
- Hook, M.; Roy, S.; Williams, E.G.; Bou Sleiman, M.; Mozhui, K.; Nelson, J.F.; Lu, L.; Auwerx, J.; Williams, R.W. Genetic cartography of longevity in humans and mice: Current landscape and horizons. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt A, 2718–2732. [Google Scholar] [CrossRef] [PubMed]
- Folgueras, A.R.; Freitas-Rodríguez, S.; Velasco, G.; López-Otín, C. Mouse Models to Disentangle the Hallmarks of Human Aging. Circ. Res. 2018, 123, 905–924. [Google Scholar] [CrossRef] [PubMed]
- Taormina, G.; Ferrante, F.; Vieni, S.; Grassi, N.; Russo, A.; Mirisola, M.G. Longevity: Lesson from Model Organisms. Genes 2019, 10, 518. [Google Scholar] [CrossRef]
- Guevara-Aguirre, J.; Balasubramanian, P.; Guevara-Aguirre, M.; Wei, M.; Madia, F.; Cheng, C.W.; Hwang, D.; Martin-Montalvo, A.; Saavedra, J.; Ingles, S.; et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 2011, 3, 70ra13. [Google Scholar] [CrossRef]
- Aguiar-Oliveira, M.H.; Bartke, A. Growth Hormone Deficiency: Health and Longevity. Endocr. Rev. 2019, 40, 575–601. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A.; Sun, L.Y.; Longo, V. Somatotropic signaling: Trade-offs between growth, reproductive development, and longevity. Physiol. Rev. 2013, 93, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A.; List, E.O.; Kopchick, J.J. The somatotropic axis and aging: Benefits of endocrine defects. Growth Horm. IGF Res. 2016, 27, 41–45. [Google Scholar] [CrossRef]
- Bartke, A.; Cecim, M.; Tang, K.; Steger, R.W.; Chandrashekar, V.; Turyn, D. Neuroendocrine and reproductive consequences of overexpression of growth hormone in transgenic mice. Proc. Soc. Exp. Biol. Med. 1994, 206, 345–359. [Google Scholar] [CrossRef]
- Carrero, D.; Soria-Valles, C.; López-Otín, C. Hallmarks of progeroid syndromes: Lessons from mice and reprogrammed cells. Dis. Model. Mech. 2016, 9, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Matzkin, M.E.; Miquet, J.G.; Fang, Y.; Hill, C.M.; Turyn, D.; Calandra, R.S.; Bartke, A.; Frungieri, M.B. Alterations in oxidative, inflammatory and apoptotic events in short-lived and long-lived mice testes. Aging 2016, 8, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Spong, A.; Swindell, W.R.; Fang, Y.; Hill, C.; Huber, J.A.; Boehm, J.D.; Westbrook, R.; Salvatori, R.; Bartke, A. Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. ELife 2013, 2, e01098. [Google Scholar] [CrossRef] [PubMed]
- Flurkey, K.; Papaconstantinou, J.; Miller, R.A.; Harrison, D.E. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. USA 2001, 98, 6736–6741. [Google Scholar] [CrossRef]
- Coschigano, K.T.; Holland, A.N.; Riders, M.E.; List, E.O.; Flyvbjerg, A.; Kopchick, J.J. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 2003, 144, 3799–3810. [Google Scholar] [CrossRef]
- List, E.O.; Sackmann-Sala, L.; Berryman, D.E.; Funk, K.; Kelder, B.; Gosney, E.S.; Okada, S.; Ding, J.; Cruz-Topete, D.; Kopchick, J.J. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocr. Rev. 2011, 32, 356–386. [Google Scholar] [CrossRef]
- Hauck, S.J.; Hunter, W.S.; Danilovich, N.; Kopchick, J.J.; Bartke, A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp. Biol. Med. 2001, 226, 552–558. [Google Scholar] [CrossRef]
- Hsieh, C.C.; DeFord, J.H.; Flurkey, K.; Harrison, D.E.; Papaconstantinou, J. Effects of the Pit1 mutation on the insulin signaling pathway: Implications on the longevity of the long-lived Snell dwarf mouse. Mech. Ageing Dev. 2002, 123, 1245–1255. [Google Scholar] [CrossRef]
- Bartke, A.; Brown-Borg, H. Life extension in the dwarf mouse. Curr. Top. Dev. Biol. 2004, 63, 189–225. [Google Scholar]
- Vergara, M.; Smith-Wheelock, M.; Harper, J.M.; Sigler, R.; Miller, R.A. Hormone-treated snell dwarf mice regain fertility but remain long lived and disease resistant. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 1244–1250. [Google Scholar] [CrossRef]
- Chandrashekar, V.; Dawson, C.R.; Martin, E.R.; Rocha, J.S.; Bartke, A.; Kopchick, J.J. Age-related alterations in pituitary and testicular functions in long-lived growth hormone receptor gene-disrupted mice. Endocrinology 2007, 148, 6019–6025. [Google Scholar] [CrossRef]
- Bartlett, J.M.; Charlton, H.M.; Robinson, I.C.; Nieschlag, E. Pubertal development and testicular function in the male growth hormone-deficient rat. J. Endocrinol. 1990, 126, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, K.; Sluczanowska-Glabowska, S.; Kucia, M.; Bartke, A.; Laszczynska, M.; Ratajczak, M.Z. Histological changes of testes in growth hormone transgenic mice with high plasma level of GH and insulin-like growth factor-1. Folia Histochem. Cytobiol. 2015, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Vaish, V.; Feng, M.; Field, K.; Chatzistamou, I.; Shim, M. Transgenic expression of cyclooxygenase-2 (COX2) causes premature aging phenotypes in mice. Aging 2016, 8, 2392–2406. [Google Scholar] [CrossRef]
- Takeda, T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem. Res. 2009, 34, 639–659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Qu, Y.; Wang, L.; Geng, D.; Chen, W.; Li, L.; Tian, Y.; Chang, S.; Zhao, C.; et al. The roles of p38 MAPK → COX2 and NF-κB → COX2 signal pathways in age-related testosterone reduction. Sci. Rep. 2019, 9, 10556. [Google Scholar] [CrossRef]
- Silva, A.; Socorro, S.; Hurtado de Llera, A.; Vaz, C.V.; Correia, S.; Maia, C.J. Overexpression of regucalcin mitigates the ageing-related changes in oxidative stress and sperm quality. Theriogenology 2020, 157, 472–482. [Google Scholar] [CrossRef]
- Chen, Y.F.; Kao, C.H.; Chen, Y.T.; Wang, C.H.; Wu, C.Y.; Tsai, C.Y.; Liu, F.C.; Yang, C.W.; Wei, Y.H.; Hsu, M.T.; et al. Cisd2 deficiency drives premature aging and causes mitochondria-mediated defects in mice. Genes Dev. 2009, 23, 1183–1194. [Google Scholar] [CrossRef]
- Curley, M.; Milne, L.; Smith, S.; Jørgensen, A.; Frederiksen, H.; Hadoke, P.; Potter, P.; Smith, L.B. A young testicular microenvironment protects Leydig cells against age-related dysfunction in a mouse model of premature aging. FASEB J. 2019, 33, 978–995. [Google Scholar] [CrossRef]
- Linkermann, A.; Green, D.R. Necroptosis. N. Engl. J. Med. 2014, 370, 455–465. [Google Scholar] [CrossRef]
- Meng, L.; Jin, W.; Wang, X. RIP3-mediated necrotic cell death accelerates systematic inflammation and mortality. Proc. Natl. Acad. Sci. USA 2015, 112, 11007–11012. [Google Scholar] [CrossRef]
- Li, D.; Meng, L.; Xu, T.; Su, Y.; Liu, X.; Zhang, Z.; Wang, X. RIPK1-RIPK3-MLKL-dependent necrosis promotes the aging of mouse male reproductive system. Elife 2017, 6, e27692. [Google Scholar] [CrossRef]
- Gao, F.; Li, G.; Liu, C.; Gao, H.; Wang, H.; Liu, W.; Chen, M.; Shang, Y.; Wang, L.; Shi, J.; et al. Autophagy regulates testosterone synthesis by facilitating cholesterol uptake in Leydig cells. J. Cell Biol. 2018, 217, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Bao, M.; Li, D.; Li, Y.M. Advanced glycation in D-galactose induced mouse aging model. Mech. Ageing Dev. 1999, 108, 239–251. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Oroojan, A.A.; Heidari, H. Effects of exendin-4 on male reproductive parameters of d-galactose induced aging mouse model. World J. Mens Health 2014, 32, 176–183. [Google Scholar] [CrossRef]
- Jeremy, M.; Gurusubramanian, G.; Roy, V.K. Vitamin D3 regulates apoptosis and proliferation in the testis of D-galactose-induced aged rat model. Sci. Rep. 2019, 9, 14103. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Chen, B.H.; Chiang, H.S.; Chen, C.W.; Chen, M.F.; Ke, C.C.; Wang, Y.Y.; Lin, W.N.; Wang, C.C.; Lin, Y.H. Optimizing a Male Reproductive Aging Mouse Model by D-Galactose Injection. Int. J. Mol. Sci. 2016, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Yanar, K.; Simsek, B.; Cebe, T.; Sitar, M.E.; Belce, A.; Cakatay, U. Galactose-induced Aging Model in Rat Testicular Tissue. J. Coll. Physicians Surg. Pak. 2018, 28, 501–504. [Google Scholar] [CrossRef]
- Wang, Z.L.; Chen, L.B.; Qiu, Z.; Chen, X.B.; Liu, Y.; Li, J.; Wang, L.; Wang, Y.P. Ginsenoside Rg1 ameliorates testicular senescence changes in D-gal-induced aging mice via anti-inflammatory and antioxidative mechanisms. Mol. Med. Rep. 2018, 17, 6269–6276. [Google Scholar]
- Li, L.; Chen, B.; An, T.; Zhang, H.; Xia, B.; Li, R.; Zhu, R.; Tian, Y.; Wang, L.; Zhao, D.; et al. BaZiBuShen alleviates altered testicular morphology and spermatogenesis and modulates Sirt6/P53 and Sirt6/NF-κB pathways in aging mice induced by D-galactose and NaNO2. J. Ethnopharmacol. 2021, 271, 113810. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, T.; Liu, S.; Chen, Y. Effects of bone marrow mesenchymal stem cells on ovarian and testicular function in aging Sprague-Dawley rats induced by D-galactose. Cell Cycle 2020, 19, 2340–2350. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Harbi, M.S.; Al-Hazaa, M.A. Neurological Alterations and Testicular Damages in Aging Induced by D-Galactose and Neuro and Testicular Protective Effects of Combinations of Chitosan Nanoparticles, Resveratrol and Quercetin in Male Mice. Coatings 2021, 11, 435. [Google Scholar] [CrossRef]
- Jeremy, M.; Gurusubramanian, G.; Roy, V.K. Vitamin D3 mediated regulation of steroidogenesis mitigates testicular activity in an aged rat model. J. Steroid Biochem. Mol. Biol. 2019, 190, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Leung, A.; Sinha-Hikim, A.P. Reproductive aging in the male brown-Norway rat: A model for the human. Endocrinology 1993, 133, 2773–2781. [Google Scholar] [CrossRef] [PubMed]
- Zirkin, B.R.; Santulli, R.; Strandberg, J.D.; Wright, W.W.; Ewing, L.L. Testicular steroidogenesis in the aging brown Norway rat. J. Androl. 1993, 14, 118–123. [Google Scholar] [PubMed]
- Chen, H.; Hardy, M.P.; Huhtaniemi, I.; Zirkin, B.R. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J. Androl. 1994, 15, 551–557. [Google Scholar]
- Chen, H.; Huhtaniemi, I.; Zirkin, B.R. Depletion and repopulation of Leydig cells in the testes of aging brown Norway rats. Endocrinology 1996, 137, 3447–3452. [Google Scholar] [CrossRef]
- Levy, S.; Serre, V.; Hermo, L.; Robaire, B. The effects of aging on the seminiferous epithelium and the blood-testis barrier of the Brown Norway rat. J. Androl. 1999, 20, 356–365. [Google Scholar]
- Paul, C.; Robaire, B. Impaired function of the blood-testis barrier during aging is preceded by a decline in cell adhesion proteins and GTPases. PLoS ONE 2013, 8, e84354. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.L.; Dyson, M.T.; Eimerl, S.; Orly, J.; Hutson, J.C.; Stocco, D.M. Cyclooxygenase-2 regulation of the age-related decline in testosterone biosynthesis. Endocrinology 2005, 146, 4202–4208. [Google Scholar] [CrossRef]
- Beattie, M.C.; Adekola, L.; Papadopoulos, V.; Chen, H.; Zirkin, B.R. Leydig cell aging and hypogonadism. Exp. Gerontol. 2015, 68, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, F.W.; Chen, H.; Allegretti, J.; Zirkin, B.R. Does age-associated reduced Leydig cell testosterone production in Brown Norway rats result from under-stimulation by luteinizing hormone? J. Androl. 1998, 19, 625–630. [Google Scholar]
- Chen, H.; Cangello, D.; Benson, S.; Folmer, J.; Zhu, H.; Trush, M.A.; Zirkin, B.R. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: Relationship to reduced steroidogenic function? Exp. Gerontol. 2001, 36, 1361–1373. [Google Scholar] [CrossRef]
- Luo, L.; Chen, H.; Zirkin, B.R. Are Leydig cell steroidogenic enzymes differentially regulated with aging? J. Androl. 1996, 17, 509–515. [Google Scholar] [PubMed]
- Syntin, P.; Chen, H.; Zirkin, B.R.; Robaire, B. Gene expression in Brown Norway rat Leydig cells: Effects of age and of age-related germ cell loss. Endocrinology 2001, 142, 5277–5285. [Google Scholar] [CrossRef]
- Luo, L.; Chen, H.; Zirkin, B.R. Leydig cell aging: Steroidogenic acute regulatory protein (StAR) and cholesterol side-chain cleavage enzyme. J. Androl. 2001, 22, 149–156. [Google Scholar]
- Luo, L.; Chen, H.; Zirkin, B.R. Temporal relationships among testosterone production, steroidogenic acute regulatory protein (StAR), and P450 side-chain cleavage enzyme (P450scc) during Leydig cell aging. J. Androl. 2005, 26, 25–31. [Google Scholar]
- Culty, M.; Luo, L.; Yao, Z.X.; Chen, H.; Papadopoulos, V.; Zirkin, B.R. Cholesterol transport, peripheral benzodiazepine receptor, and steroidogenesis in aging Leydig cells. J. Androl. 2002, 23, 439–447. [Google Scholar]
- Li, W.R.; Chen, L.; Chang, Z.J.; Xin, H.; Liu, T.; Zhang, Y.Q.; Li, G.Y.; Zhou, F.; Gong, Y.Q.; Gao, Z.Z.; et al. Autophagic deficiency is related to steroidogenic decline in aged rat Leydig cells. Asian J. Androl. 2011, 13, 881–888. [Google Scholar] [CrossRef]
- Wang, F.F.; Wang, Q.; Chen, Y.; Lin, Q.; Gao, H.B.; Zhang, P. Chronic stress induces ageing-associated degeneration in rat Leydig cells. Asian J. Androl. 2012, 14, 643–648. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.; Ge, R.; Lian, Q.; Papadopoulos, V.; Zirkin, B.R. Steroidogenic fate of the Leydig cells that repopulate the testes of young and aged Brown Norway rats after elimination of the preexisting Leydig cells. Exp. Gerontol. 2015, 72, 8–15. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction 2017, 154, R111–R122. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Leers-Sucheta, S.; Azhar, S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J. Steroid Biochem. Mol. Biol. 2004, 88, 61–67. [Google Scholar] [CrossRef]
- Luo, L.; Chen, H.; Trush, M.A.; Show, M.D.; Anway, M.D.; Zirkin, B.R. Aging and the brown Norway rat leydig cell antioxidant defense system. J. Androl. 2006, 27, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Pearl, C.A. Alterations in the estrogen environment of the testis contribute to declining sperm production in aging rats. Syst. Biol. Reprod. Med. 2014, 60, 89–97. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, N.; Liu, Z.; Wang, T.; Yuan, C.; He, Y.; Dun, Y.; Zhou, Z.; Yuan, D.; Zhang, C. Protective effect of Wuzi Yanzong recipe on testicular dysfunction through inhibition of germ cell apoptosis in ageing rats via endoplasmic reticulum stress. Andrologia 2019, 51, e13181. [Google Scholar] [CrossRef]
- Kopalli, S.R.; Cha, K.M.; Lee, S.H.; Hwang, S.Y.; Lee, Y.J.; Koppula, S.; Kim, S.K. Cordycepin, an Active Constituent of Nutrient Powerhouse and Potential Medicinal Mushroom Cordyceps militaris Linn., Ameliorates Age-Related Testicular Dysfunction in Rats. Nutrients 2019, 11, 906. [Google Scholar] [CrossRef]
- Salomon, T.B.; Hackenhaar, F.S.; Almeida, A.C.; Schüller, A.K.; Gil Alabarse, P.V.; Ehrenbrink, G.; Benfato, M.S. Oxidative stress in testis of animals during aging with and without reproductive activity. Exp. Gerontol. 2013, 48, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, C.; Kar, F.; Kanbak, G. Reproductive Effects of Nicotinamide on Testicular Function and Structure in Old Male Rats: Oxidative, Apoptotic, Hormonal, and Morphological Analyses. Reprod. Sci. 2021, 28, 3352–3360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xie, Y.; Chen, H.; Lv, L.; Yao, J.; Zhang, M.; Xia, K.; Feng, X.; Li, Y.; Liang, X.; et al. FOXO4-DRI alleviates age-related testosterone secretion insufficiency by targeting senescent Leydig cells in aged mice. Aging 2020, 12, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wei, W.; Xie, F.; Zhu, X.; Zheng, L.; Lv, Z. Steroidogenesis decline accompanied with reduced antioxidation and endoplasmic reticulum stress in mice testes during ageing. Andrologia 2018, 50, e12816. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jin, S.; Guo, J.; Kombairaju, P.; Biswal, S.; Zirkin, B.R. Knockout of the transcription factor Nrf2: Effects on testosterone production by aging mouse Leydig cells. Mol. Cell. Endocrinol. 2015, 409, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Fan, K.; Chen, S.; Yao, Q.; Zeng, R.; Hong, Z.; Peng, L.; Shao, Y.; Yao, B. Role of peroxiredoxin 2 in H2O2-induced oxidative stress of primary Leydig cells. Mol. Med. Rep. 2016, 13, 4807–4813. [Google Scholar] [CrossRef]
- Giannessi, F.; Giambelluca, M.A.; Scavuzzo, M.C.; Ruffoli, R. Ultrastructure of testicular macrophages in aging mice. J. Morphol. 2005, 263, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K. Marmoset models commonly used in biomedical research. Comp. Med. 2003, 53, 383–392. [Google Scholar]
- Tardif, S.D.; Mansfield, K.G.; Ratnam, R.; Ross, C.N.; Ziegler, T.E. The marmoset as a model of aging and age-related diseases. ILAR J. 2011, 52, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.A. Nonhuman primate models in biogerontology. Exp. Gerontol. 2000, 35, 533–541. [Google Scholar] [CrossRef]
- Lankau, E.W.; Turner, P.V.; Mullan, R.J.; Galland, G.G. Use of nonhuman primates in research in North America. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 278–282. [Google Scholar]
- Buffenstein, R. Negligible senescence in the longest living rodent, the naked mole-rat: Insights from a successfully aging species. J. Comp. Physiol. B 2008, 178, 439–445. [Google Scholar] [CrossRef]
- Edrey, Y.H.; Hanes, M.; Pinto, M.; Mele, J.; Buffenstein, R. Successful aging and sustained good health in the naked mole rat: A long-lived mammalian model for biogerontology and biomedical research. ILAR J. 2011, 52, 41–53. [Google Scholar] [CrossRef]
- Matzkin, M.E.; Valchi, P.; Riviere, E.; Rossi, S.P.; Tavalieri, Y.E.; Muñoz de Toro, M.M.; Mayerhofer, A.; Bartke, A.; Calandra, R.S.; Frungieri, M.B. Aging in the Syrian hamster testis: Inflammatory-oxidative status and the impact of photoperiod. Exp. Gerontol. 2019, 124, 110649. [Google Scholar] [CrossRef]
- Braude, S.; Holtze, S.; Begall, S.; Brenmoehl, J.; Burda, H.; Dammann, P.; Del Marmol, D.; Gorshkova, E.; Henning, Y.; Hoeflich, A.; et al. Surprisingly long survival of premature conclusions about naked mole-rat biology. Biol. Rev. 2021, 96, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Garcia Montero, A.; Vole, C.; Burda, H.; Malkemper, E.P.; Holtze, S.; Morhart, M.; Saragusty, J.; Hildebrandt, T.B.; Begall, S. Non-Breeding Eusocial Mole-Rats Produce Viable Sperm--Spermiogram and Functional Testicular Morphology of Fukomys anselli. PLoS ONE 2016, 11, e0150112. [Google Scholar] [CrossRef]
- van der Horst, G.; Kotzé, S.H.; O’Riain, M.J.; Maree, L. Testicular Structure and Spermatogenesis in the Naked Mole-Rat is Unique (Degenerate) and Atypical Compared to Other Mammals. Front. Cell. Dev. Biol. 2019, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Tardif, S.D.; Araujo, A.; Arruda, M.F.; French, J.A.; Sousa, M.B.; Yamamoto, M.E. Primate Reproductive Aging. Cross-Taxon Perspectives. In Interdiciplinary Topics in Gerontology, 1st ed.; Atsalis, S., Margulis, S.W., Hof, P.R., Eds.; Karger: Basel, Switzerland, 2008; Volume 36, pp. 29–48. [Google Scholar]
- Stöckl, J.B.; Schmid, N.; Flenkenthaler, F.; Drummer, C.; Behr, R.; Mayerhofer, A.; Arnold, G.J.; Fröhlich, T. Age-Related Alterations in the Testicular Proteome of a Non-Human Primate. Cells 2021, 10, 1306. [Google Scholar] [CrossRef]
- Stöckl, J.B.; Schmid, N.; Flenkenthaler, F.; Drummer, C.; Behr, R.; Mayerhofer, A.; Arnold, G.J.; Fröhlich, T. Proteomic Insights into Senescence of Testicular Peritubular Cells from a Nonhuman Primate Model. Cells 2020, 9, 2498. [Google Scholar] [CrossRef]
- Li, R.; Miao, J.; Fan, Z.; Song, S.; Kong, I.K.; Wang, Y.; Wang, Z. Production of Genetically Engineered Golden Syrian Hamsters by Pronuclear Injection of the CRISPR/Cas9 Complex. J. Vis. Exp. 2018, 131, 56263. [Google Scholar] [CrossRef]
- Mukherjee, A.; Haldar, C. Melatonin membrane receptor (MT1R) expression and nitro-oxidative stress in testis of golden hamster, Mesocricetus auratus: An age-dependent study. Exp. Gerontol. 2015, 69, 211–220. [Google Scholar] [CrossRef]
- Beltrán-Frutos, E.; Seco-Rovira, V.; Ferrer, C.; Madrid, J.F.; Sáez, F.J.; Canteras, M.; Pastor, L.M. Cellular changes in the hamster testicular interstitium with ageing and after exposure to short photoperiod. Reprod. Fertil. Dev. 2016, 28, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Gonzalez-Calvar, S.I.; Parborell, F.; Albrecht, M.; Mayerhofer, A.; Calandra, R.S. Cyclooxygenase-2 and prostaglandin F2 alpha in Syrian hamster Leydig cells: Inhibitory role on luteinizing hormone/human chorionic gonadotropin-stimulated testosterone production. Endocrinology 2006, 147, 4476–4485. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Calandra, R.S.; Mayerhofer, A.; Matzkin, M.E. Cyclooxygenase and prostaglandins in somatic cell populations of the testis. Reproduction 2015, 149, R169–R180. [Google Scholar] [CrossRef]
- Albert, O.; Desdoits-Lethimonier, C.; Lesné, L.; Legrand, A.; Guillé, F.; Bensalah, K.; Dejucq-Rainsford, N.; Jégou, B. Paracetamol, aspirin and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum. Reprod. 2013, 28, 1890–1898. [Google Scholar] [CrossRef]
- Kristensen, D.M.; Mazaud-Guittot, S.; Gaudriault, P.; Lesné, L.; Serrano, T.; Main, K.M.; Jégou, B. Analgesic use—Prevalence, biomonitoring and endocrine and reproductive effects. Nat. Rev. Endocrinol. 2016, 12, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, D.M.; Desdoits-Lethimonier, C.; Mackey, A.L.; Dalgaard, M.D.; De Masi, F.; Munkbøl, C.H.; Styrishave, B.; Antignac, J.P.; Le Bizec, B.; Platel, C.; et al. Ibuprofen alters human testicular physiology to produce a state of compensated hypogonadism. Proc. Natl. Acad. Sci. USA 2018, 115, E715–E724. [Google Scholar] [CrossRef] [PubMed]
- Rey-Ares, V.; Rossi, S.P.; Dietrich, K.G.; Köhn, F.M.; Schwarzer, J.U.; Welter, H.; Mayerhofer, A. Prostaglandin E2 (PGE2) is a testicular peritubular cell-derived factor involved in human testicular homeostasis. Mol. Cell. Endocrinol. 2018, 473, 217–224. [Google Scholar] [CrossRef]
- Martin-Hidalgo, D.; Bragado, M.J.; Batista, A.R.; Oliveira, P.F.; Alves, M.G. Antioxidants and Male Fertility: From Molecular Studies to Clinical Evidence. Antioxidants 2019, 8, 89. [Google Scholar] [CrossRef]
- Tvrdá, E.; Benko, F.; Slanina, T.; du Plessis, S.S. The Role of Selected Natural Biomolecules in Sperm Production and Functionality. Molecules 2021, 26, 5196. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R. Central and peripheral actions of melatonin on reproduction in seasonal and continuous breeding mammals. Gen. Comp. Endocrinol. 2021, 300, 113620. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Calandra, R.S.; Rossi, S.P. Local Actions of Melatonin in Somatic Cells of the Testis. Int. J. Mol. Sci. 2017, 18, 1170. [Google Scholar] [CrossRef]
- Muratoğlu, S.; Akarca Dizakar, O.S.; Keskin Aktan, A.; Ömeroğlu, S.; Akbulut, K.G. The protective role of melatonin and curcumin in the testis of young and aged rats. Andrologia 2019, 51, e13203. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, K.G.; Gonul, B.; Akbulut, H. The role of melatonin on gastric mucosal cell proliferation and telomerase activity in ageing. J. Pineal Res. 2009, 47, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Mayerhofer, A.; Zitta, K.; Pignataro, O.P.; Calandra, R.S.; Gonzalez-Calvar, S.I. Direct effect of melatonin on Syrian hamster testes: Melatonin subtype 1a receptors, inhibition of androgen production, and interaction with the local corticotropin-releasing hormone system. Endocrinology 2005, 146, 1541–1552. [Google Scholar] [CrossRef]
- Rossi, S.P.; Matzkin, M.E.; Terradas, C.; Ponzio, R.; Puigdomenech, E.; Levalle, O.; Calandra, R.S.; Frungieri, M.B. New insights into melatonin/CRH signaling in hamster Leydig cells. Gen. Comp. Endocrinol. 2012, 178, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.K.; Ray, A.K. Role of light in the mediation of acute effects of a single afternoon melatonin injection on steroidogenic activity of testis in the rat. J. Biosci. 2000, 25, 253–256. [Google Scholar] [CrossRef]
- Luo, F.; Sandhu, A.F.; Rungratanawanich, W.; Williams, G.E.; Akbar, M.; Zhou, S.; Song, B.J.; Wang, X. Melatonin and Autophagy in Aging-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 7174. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, C.Q.; Zeng, L.; Cheng, L.; Ma, L.; Zhang, M.; Zhang, Y.Z. Melatonin regulates the cross-talk between autophagy and apoptosis by SIRT3 in testicular Leydig cells. Biochem. Biophys. Res. Commun. 2021, 555, 182–189. [Google Scholar] [CrossRef]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef]
- Dong, Q.; Bergendahl, M.; Huhtaniemi, I.; Handelsman, D.J. Effect of undernutrition on pulsatile luteinizing hormone (LH) secretion in castrate and intact male rats using an ultrasensitive immunofluorometric LH assay. Endocrinology 1994, 135, 745–750. [Google Scholar] [CrossRef]
- Young, K.A.; Zirkin, B.R.; Nelson, R.J. Testicular regression in response to food restriction and short photoperiod in white-footed mice (Peromyscus leucopus) is mediated by apoptosis. Biol. Reprod. 2000, 62, 347–354. [Google Scholar] [CrossRef]
- Rocha, J.S.; Bonkowski, M.S.; França, L.R.; Bartke, A. Mild calorie restriction does not affect testosterone levels and testicular gene expression in mutant mice. Exp. Biol. Med. 2007, 232, 1050–1063. [Google Scholar] [CrossRef]
- Rocha, J.S.; Bonkowski, M.S.; Masternak, M.M.; França, L.R.; Bartke, A. Effects of adult onset mild calorie restriction on weight of reproductive organs, plasma parameters and gene expression in male mice. Anim. Reprod. 2012, 9, 40–51. [Google Scholar] [PubMed]
- Chen, H.; Irizarry, R.A.; Luo, L.; Zirkin, B.R. Leydig cell gene expression: Effects of age and caloric restriction. Exp. Gerontol. 2004, 39, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Markaki, M.; Tavernarakis, N. Metabolic control by target of rapamycin and autophagy during ageing—A mini-review. Gerontology 2013, 59, 340–348. [Google Scholar] [CrossRef]
- Oliveira, P.F.; Cheng, C.Y.; Alves, M.G. Emerging Role for Mammalian Target of Rapamycin in Male Fertility. Trends Endocrinol. Metab. 2017, 28, 165–167. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef]
- Wilkinson, J.E.; Burmeister, L.; Brooks, S.V.; Chan, C.C.; Friedline, S.; Harrison, D.E.; Hejtmancik, J.F.; Nadon, N.; Strong, R.; Wood, L.K.; et al. Rapamycin slows aging in mice. Aging Cell 2012, 11, 675–682. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Rapamycin extends life- and health span because it slows aging. Aging 2013, 5, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A. Growth hormone and aging. Rev. Endocr. Metab. Disord. 2021, 22, 71–80. [Google Scholar] [CrossRef]
- Xu, H.; Shen, L.; Chen, X.; Ding, Y.; He, J.; Zhu, J.; Wang, Y.; Liu, X. mTOR/P70S6K promotes spermatogonia proliferation and spermatogenesis in Sprague Dawley rats. Reprod. Biomed. Online 2016, 32, 207–217. [Google Scholar] [CrossRef]
- Busada, J.T.; Niedenberger, B.A.; Velte, E.K.; Keiper, B.D.; Geyer, C.B. Mammalian target of rapamycin complex 1 (mTORC1) is required for mouse spermatogonial differentiation in vivo. Dev. Biol. 2015, 407, 90–102. [Google Scholar] [CrossRef]
- Ross, C.N.; Salmon, A.B. Aging research using the common marmoset: Focus on aging interventions. Nutr. Healthy Aging 2019, 5, 97–109. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R.; et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012, 35, 1364–1379. [Google Scholar] [CrossRef]
- Glossmann, H.H.; Lutz, O.M.D. Metformin and Aging: A Review. Gerontology 2019, 65, 581–590. [Google Scholar] [CrossRef]
- Martin-Montalvo, A.; Mercken, E.M.; Mitchell, S.J.; Palacios, H.H.; Mote, P.L.; Scheibye-Knudsen, M.; Gomes, A.P.; Ward, T.M.; Minor, R.K.; Blouin, M.J.; et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013, 4, 2192. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. The Effect of Metformin on Male Reproductive Function and Prostate: An Updated Review. World J. Mens Health 2021, 39, e15. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- von Kobbe, C. Targeting senescent cells: Approaches, opportunities, challenges. Aging 2019, 11, 12844. [Google Scholar] [CrossRef]
- Martin, L.J.; Touaibia, M. Improvement of Testicular Steroidogenesis Using Flavonoids and Isoflavonoids for Prevention of Late-Onset Male Hypogonadism. Antioxidants 2020, 9, 237. [Google Scholar] [CrossRef]
- Couture, R.; Mora, N.; Al Bittar, S.; Najih, M.; Touaibia, M.; Martin, L.J. Luteolin modulates gene expression related to steroidogenesis, apoptosis, and stress response in rat LC540 tumor Leydig cells. Cell Biol. Toxicol. 2020, 36, 31–49. [Google Scholar] [CrossRef]
- Li, W.; Pandey, A.K.; Yin, X.; Chen, J.J.; Stocco, D.M.; Grammas, P.; Wang, X. Effects of apigenin on steroidogenesis and steroidogenic acute regulatory gene expression in mouse Leydig cells. J. Nutr. Biochem. 2011, 22, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The Role of Resveratrol in Mammalian Reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef]
- Goel, A.; Jhurani, S.; Aggarwal, B.B. Multi-targeted therapy by curcumin: How spicy is it? Mol. Nutr. Food Res. 2008, 52, 1010–1030. [Google Scholar] [CrossRef]
- Martins, R.V.L.; Silva, A.M.S.; Duarte, A.P.; Socorro, S.; Correia, S.; Maia, C.J. Natural Products as Protective Agents for Male Fertility. BioChem 2021, 1, 122–147. [Google Scholar] [CrossRef]
- Baar, M.P.; Brandt, R.; Putavet, D.A.; Klein, J.; Derks, K.; Bourgeois, B.; Stryeck, S.; Rijksen, Y.; van Willigenburg, H.; Feijtel, D.A.; et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 2017, 169, 132–147.e16. [Google Scholar] [CrossRef]
- Ng, T.B.; Wang, H.X. Pharmacological actions of Cordyceps, a prized folk medicine. J. Pharm. Pharmacol. 2005, 57, 1509–1519. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, G.; Wang, Q. Chemical constituents in the roots of Lycium chinense Mill. Zhongguo Zhong Yao Za Zhi 1996, 21, 675–676. [Google Scholar]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium Barbarum: A Traditional Chinese Herb and A Promising Anti-Aging Agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Weng, W.; Gao, R.; Liu, Y. New Insights for Cellular and Molecular Mechanisms of Aging and Aging-Related Diseases: Herbal Medicine as Potential Therapeutic Approach. Oxidative Med. Cell. Longev. 2019, 2019, 4598167. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.C.; Jeon, S.H.; Guan Qun, Z.; Bashraheel, F.; Choi, S.W.; Kim, S.J.; Bae, W.J.; Cho, H.J.; Ha, U.S.; Hong, S.H.; et al. Lycium chinense Mill improves hypogonadism via anti-oxidative stress and anti-apoptotic effect in old aged rat model. Aging Male 2020, 23, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Makarova, M.N.; Pozharitskaya, O.N.; Shikov, A.N.; Tesakova, S.V.; Makarov, V.G.; Tikhonov, V.P. Effect of lipid-based suspension of Epimedium koreanum Nakai extract on sexual behavior in rats. J. Ethnopharmacol. 2007, 114, 412–416. [Google Scholar] [CrossRef]
- Zhao, H.; You, X.; Chen, Q.; Yang, S.; Ma, Q.; He, Y.; Liu, C.; Dun, Y.; Wu, J.; Zhang, C.; et al. Icariin Improves Age-Related Testicular Dysfunction by Alleviating Sertoli Cell Injury via Upregulation of the ERα/Nrf2-Signaling Pathway. Front. Pharmacol. 2020, 11, 677. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Yang, Q.T. The testosterone mimetic properties of icariin. Asian J. Androl. 2006, 8, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hao, J.; Yang, Q.; Li, G. Effects of icariin on reproductive functions in male rats. Molecules 2014, 19, 9502–9514. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.Z.; Wei, Y.C.; Zhang, G.W.; Sun, L.J. Effect of total flavonoids of herba euphorbiae humifusae on the expression of telomerase activity in aged mice. West China. J. Pharm. Sci. 2011, 2, 189–190. [Google Scholar]
- Kara, H.; Orem, A.; Yulug, E.; Yucesan, F.B.; Kerimoglu, G.; Yaman, S.O.; Bodur, A.; Turedi, S.; Alasalvar, C. Hazelnut consumption improves testicular antioxidant function and semen quality in young and old male rats. Food Chem. 2019, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ale, E.C.; Binetti, A.G. Role of Probiotics, Prebiotics, and Synbiotics in the Elderly: Insights into Their Applications. Front. Microbiol. 2021, 12, 631254. [Google Scholar] [CrossRef]
- Poutahidis, T.; Springer, A.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS ONE 2014, 9, e84877. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matzkin, M.E.; Calandra, R.S.; Rossi, S.P.; Bartke, A.; Frungieri, M.B. Hallmarks of Testicular Aging: The Challenge of Anti-Inflammatory and Antioxidant Therapies Using Natural and/or Pharmacological Compounds to Improve the Physiopathological Status of the Aged Male Gonad. Cells 2021, 10, 3114. https://doi.org/10.3390/cells10113114
Matzkin ME, Calandra RS, Rossi SP, Bartke A, Frungieri MB. Hallmarks of Testicular Aging: The Challenge of Anti-Inflammatory and Antioxidant Therapies Using Natural and/or Pharmacological Compounds to Improve the Physiopathological Status of the Aged Male Gonad. Cells. 2021; 10(11):3114. https://doi.org/10.3390/cells10113114
Chicago/Turabian StyleMatzkin, María Eugenia, Ricardo Saúl Calandra, Soledad Paola Rossi, Andrzej Bartke, and Mónica Beatriz Frungieri. 2021. "Hallmarks of Testicular Aging: The Challenge of Anti-Inflammatory and Antioxidant Therapies Using Natural and/or Pharmacological Compounds to Improve the Physiopathological Status of the Aged Male Gonad" Cells 10, no. 11: 3114. https://doi.org/10.3390/cells10113114
APA StyleMatzkin, M. E., Calandra, R. S., Rossi, S. P., Bartke, A., & Frungieri, M. B. (2021). Hallmarks of Testicular Aging: The Challenge of Anti-Inflammatory and Antioxidant Therapies Using Natural and/or Pharmacological Compounds to Improve the Physiopathological Status of the Aged Male Gonad. Cells, 10(11), 3114. https://doi.org/10.3390/cells10113114






