ATF3-Expressing Large-Diameter Sensory Afferents at Acute Stage as Bio-Signatures of Persistent Pain Associated with Lumbar Radiculopathy
Abstract
1. Introduction
2. Methods
2.1. Animals Handling and Preparation
2.2. Experimental Design
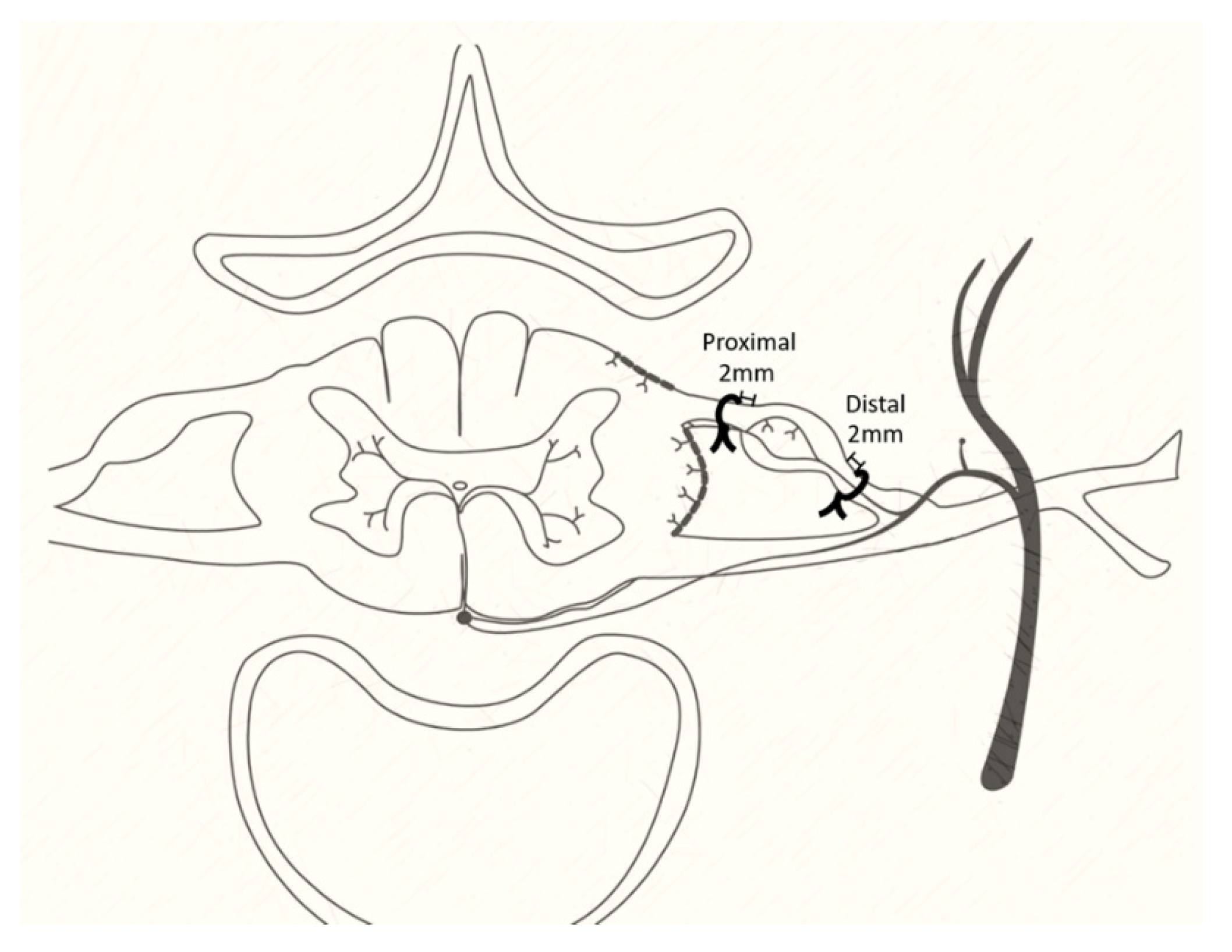
2.3. Operation
2.4. 50% Paw Withdraw Threshold (PWT)
2.5. Weight-bearing Test
2.6. Acetone Test
2.7. Immunohistochemistry
2.8. Data Sampling and Analysis
3. Results
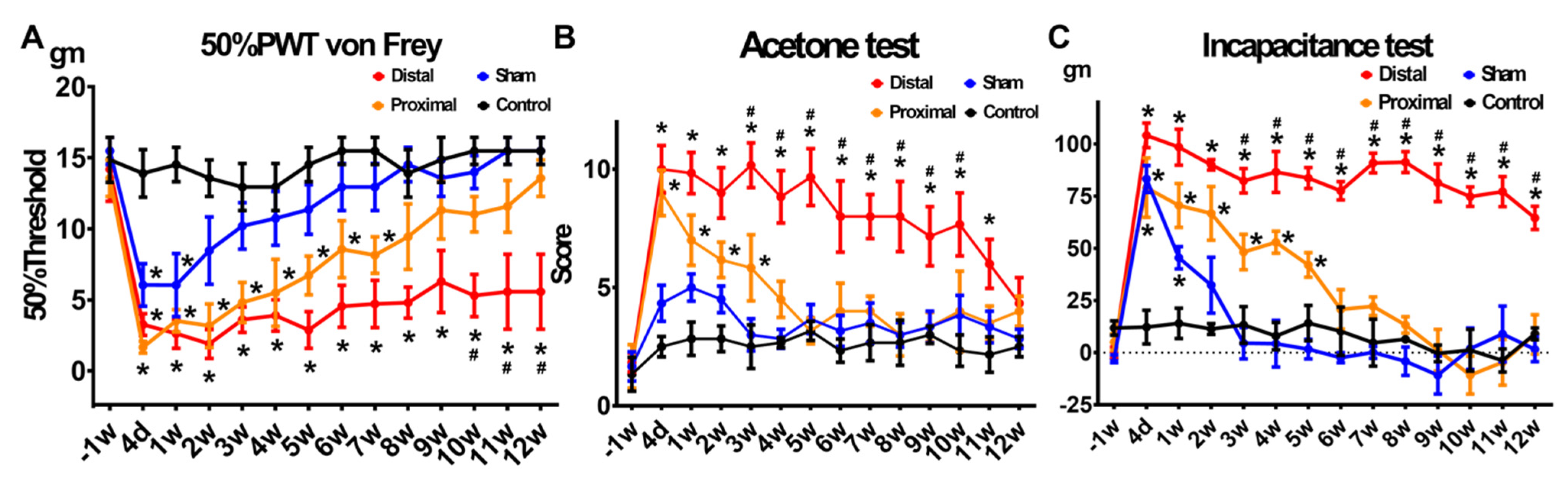
3.1. Animals with Distal Nerve Constriction Presented More Persistent Pain Behaviors Than Those with Proximal Nerve Constriction
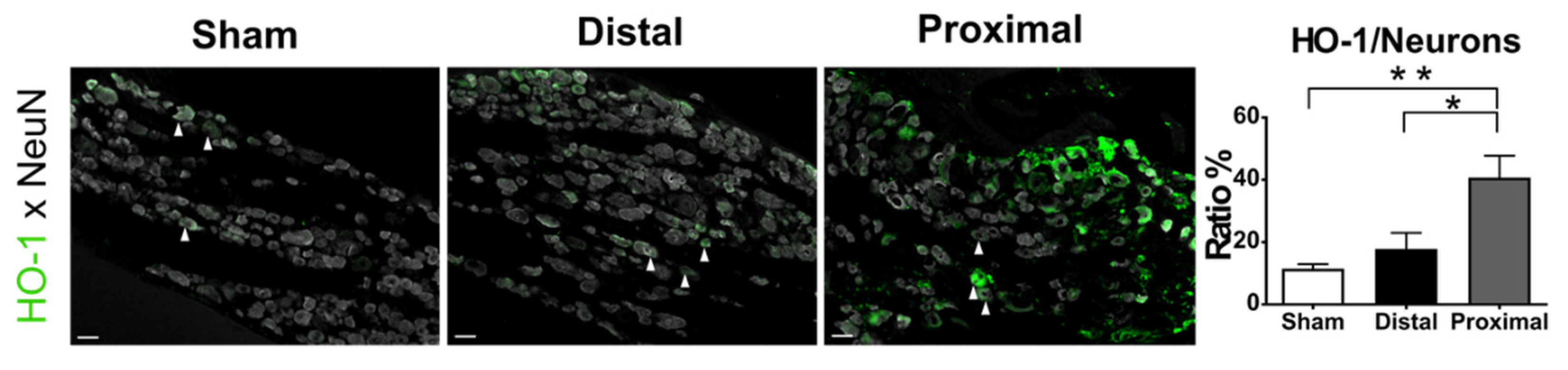
3.2. More Severe Hypoxia Occurred in the DRGs after Nerve Constriction Proximal to DRG Than Those Distal to DRG
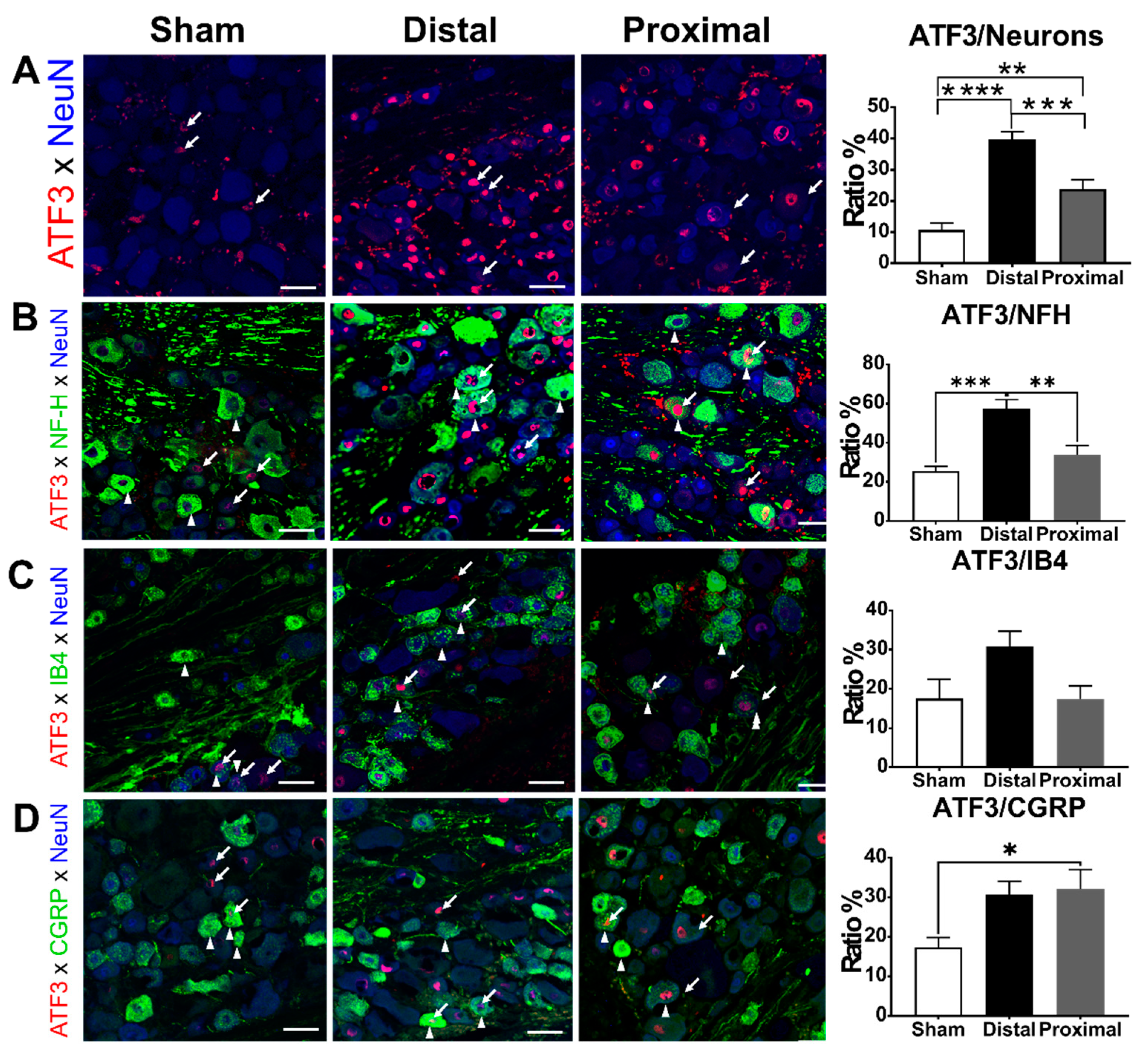
3.3. The Ratios of ATF3-Positive DRG Neurons Increased Significantly After Nerve Constriction Distal to DRG Than After Nerve Constriction Proximal to DRG
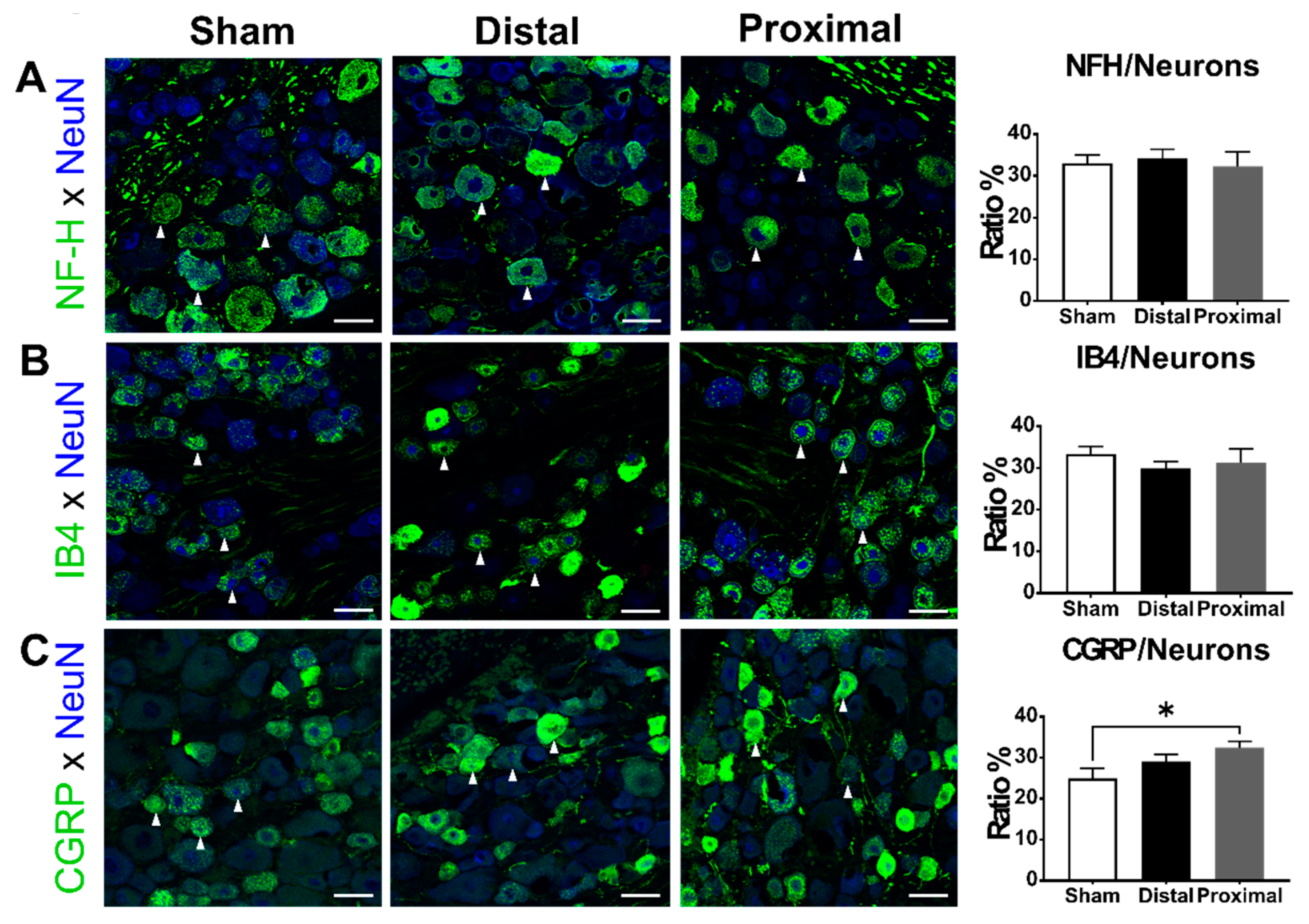
3.4. The Ratios of CGRP-Positive DRG Neurons Increased 1 Day After Proximal Nerve Constriction
4. Discussion
4.1. Possible Pathophysiology Underlying the Different Pain Phenotypes between Nerve Constriction Distal and Proximal to DRG
4.2. Differential Pathological Changes after Constriction Distal or Proximal to DRG
4.3. ATF3 Expression Related to Pain
4.4. The CGRP Expression after Peripheral Nerve Injury
4.5. Clinical Application of Large-Diameter Sensory Afferent Injury in LR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LR | lumbar radiculopathy |
| DRG | dorsal root ganglion |
| ATF3 | activating transcription factor3 |
| PFA | paraformaldehyde |
| NFH | neurofilament heavy chain |
| CGRP | calcitonin gene-related peptide |
| SD | Sprague-Dawley |
| HO-1 | hypoxyprobe-1 |
| IB4 | Isolectin B4 |
| BSA | bovine serum albumin |
| PBS | phosphate buffered saline |
| IR | immunoreactivity |
References
- Konstantinou, K.; Dunn, K.M. Sciatica: Review of epidemiological studies and prevalence estimates. Spine 2008, 33, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Vroomen, P.C.; de Krom, M.C.; Knottnerus, J.A. Predicting the outcome of sciatica at short-term follow-up. Br. J. Gen. Pr. 2002, 52, 119–123. [Google Scholar]
- Iversen, T.; Solberg, T.K.; Wilsgaard, T.; Waterloo, K.; Brox, J.I.; Ingebrigtsen, T. Outcome prediction in chronic unilateral lumbar radiculopathy: Prospective cohort study. BMC Musculoskelet Disord 2015, 16, 17. [Google Scholar] [CrossRef][Green Version]
- Nykvist, F.; Hurme, M.; Alaranta, H.; Kaitsaari, M. Severe sciatica: A 13-year follow-up of 342 patients. Eur. Spine J. 1995, 4, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Hsieh, Y.C.; Chen, Y.C.; Wang, Y.; Chen, C.C.; Chiang, Y.H. Diagnostic accuracy of standardised qualitative sensory test in the detection of lumbar lateral stenosis involving the L5 nerve root. Sci. Rep. 2017, 7, 10598. [Google Scholar] [CrossRef]
- Takiguchi, N.; Yoshida, M.; Taniguchi, W.; Hashizume, H.; Yamada, H.; Miyazaki, N.; Nishio, N.; Nakatsuka, T. Distinct degree of radiculopathy at different levels of peripheral nerve injury. Mol. Pain 2012, 8, 31. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Sekiguchi, Y.; Konno, S.; Kobayashi, H.; Homma, Y.; Kikuchi, S. Comparison of neuropathic pain and neuronal apoptosis following nerve root or spinal nerve compression. Eur. Spine J. 2009, 18, 1978–1985. [Google Scholar] [CrossRef]
- Matsuda, H.; Tsai, C.L.; Tseng, C.Y.; Noriage, A.; Tsai, T.M.; Dai, Y.C.; Jou, I.M. Neurophysiologic changes after preganglionic and postganglionic nerve-root constriction: An experimental study in the rat. Spine 2007, 32, 950–958. [Google Scholar] [CrossRef]
- Colburn, R.W.; Rickman, A.J.; DeLeo, J.A. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp. Neurol. 1999, 157, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Holm, S.; Rosenqvist, A.L.; Rydevik, B. Experimental nerve root compression. A model of acute, graded compression of the porcine cauda equina and an analysis of neural and vascular anatomy. Spine 1991, 16, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Owen, J.H.; Bridwell, K.H.; Oakley, D.M. Blood flow direction in the lumbar nerve root. Spine 1990, 15, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Xu, F.; Tang, Z.; Zhong, T.; Cao, J.; Guo, Q.; Huang, C. Distinct calcitonin gene-related peptide expression pattern in primary afferents contribute to different neuropathic symptoms following chronic constriction or crush injuries to the rat sciatic nerve. Mol. Pain 2016, 12, 1744806916681566. [Google Scholar] [CrossRef] [PubMed]
- Winkelstein, B.A.; DeLeo, J.A. Nerve root injury severity differentially modulates spinal glial activation in a rat lumbar radiculopathy model: Considerations for persistent pain. Brain Res. 2002, 956, 294–301. [Google Scholar] [CrossRef]
- Lin, J.H.; Chiang, Y.H.; Chen, C.C. Research Strategies for Pain in Lumbar Radiculopathy Focusing on Acid-Sensing Ion Channels and Their Toxins. Curr. Top. Med. Chem. 2015, 15, 617–630. [Google Scholar] [CrossRef]
- Lin, J.H.; Chen, C.C. Lumbar radiculopathy and its neurobiological basis. World J. Anesth. 2014, 3, 162–173. [Google Scholar] [CrossRef]
- Jenis, L.G.; An, H.S. Spine update. Lumbar foraminal stenosis. Spine 2000, 25, 389–394. [Google Scholar] [CrossRef]
- Hasegawa, T.; Mikawa, Y.; Watanabe, R.; An, H.S. Morphometric analysis of the lumbosacral nerve roots and dorsal root ganglia by magnetic resonance imaging. Spine 1996, 21, 1005–1009. [Google Scholar] [CrossRef]
- Kikuchi, S.; Sato, K.; Konno, S.; Hasue, M. Anatomic and radiographic study of dorsal root ganglia. Spine 1994, 19, 6–11. [Google Scholar] [CrossRef]
- Bonin, R.P.; Bories, C.; De Koninck, Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol. Pain 2014, 10, 26. [Google Scholar] [CrossRef]
- Bove, S.E.; Calcaterra, S.L.; Brooker, R.M.; Huber, C.M.; Guzman, R.E.; Juneau, P.L.; Schrier, D.J.; Kilgore, K.S. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr. Cartil. 2003, 11, 821–830. [Google Scholar] [CrossRef]
- Choi, Y.; Yoon, Y.W.; Na, H.S.; Kim, S.H.; Chung, J.M. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994, 59, 369–376. [Google Scholar]
- Gross, M.W.; Karbach, U.; Groebe, K.; Franko, A.J.; Mueller-Klieser, W. Calibration of misonidazole labeling by simultaneous measurement of oxygen tension and labeling density in multicellular spheroids. Int. J. Cancer 1995, 61, 567–573. [Google Scholar] [CrossRef]
- Song, X.J.; Vizcarra, C.; Xu, D.S.; Rupert, R.L.; Wong, Z.N. Hyperalgesia and neural excitability following injuries to central and peripheral branches of axons and somata of dorsal root ganglion neurons. J. Neurophysiol. 2003, 89, 2185–2193. [Google Scholar] [CrossRef]
- Hayashi, K.; Ozaki, N.; Kawakita, K.; Itoh, K.; Mizumura, K.; Furukawa, K.; Yasui, M.; Hori, K.; Yi, S.Q.; Yamaguchi, T.; et al. Involvement of NGF in the rat model of persistent muscle pain associated with taut band. J. Pain Off. J. Am. Pain Soc. 2011, 12, 1059–1068. [Google Scholar] [CrossRef]
- Rydevik, B.L. The effects of compression on the physiology of nerve roots. J. Manip. Physiol. Ther. 1992, 15, 62–66. [Google Scholar]
- Gladman, S.J.; Ward, R.E.; Michael-Titus, A.T.; Knight, M.M.; Priestley, J.V. The effect of mechanical strain or hypoxia on cell death in subpopulations of rat dorsal root ganglion neurons in vitro. Neuroscience 2010, 171, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.L.; Yang, Q.; Ward, R.E.; Priestley, J.V. Hypoxia-induced apoptosis in adult rat dorsal root ganglion neurons in vitro. Neuroreport 2005, 16, 89–93. [Google Scholar] [CrossRef]
- Sugawara, O.; Atsuta, Y.; Iwahara, T.; Muramoto, T.; Watakabe, M.; Takemitsu, Y. The effects of mechanical compression and hypoxia on nerve root and dorsal root ganglia. An analysis of ectopic firing using an in vitro model. Spine 1996, 21, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.G.; Chu, W.G.; Hu, S.J.; Luo, C. Characterization of Different Types of Excitability in Large Somatosensory Neurons and Its Plastic Changes in Pathological Pain States. Int. J. Mol. Sci. 2018, 19, 161. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, H.; Kondo, E.; Fukuoka, T.; Dai, Y.; Tokunaga, A.; Miki, K.; Yonenobu, K.; Ochi, T.; Noguchi, K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol. Cell Neurosci. 2000, 15, 170–182. [Google Scholar] [CrossRef]
- Kehlet, H. Perioperative analgesia to prevent chronic postmastectomy pain. Anesth. Analg. 2006, 103, 494, author reply 494-5. [Google Scholar] [CrossRef]
- Rau, K.K.; Hill, C.E.; Harrison, B.J.; Venkat, G.; Koenig, H.M.; Cook, S.B.; Rabchevsky, A.G.; Taylor, B.K.; Hai, T.; Petruska, J.C. Cutaneous tissue damage induces long-lasting nociceptive sensitization and regulation of cellular stress- and nerve injury-associated genes in sensory neurons. Exp. Neurol. 2016, 283, 413–427. [Google Scholar] [CrossRef][Green Version]
- Ogawa, N.; Kawai, H.; Terashima, T.; Kojima, H.; Oka, K.; Chan, L.; Maegawa, H. Gene therapy for neuropathic pain by silencing of TNF-alpha expression with lentiviral vectors targeting the dorsal root ganglion in mice. PLoS ONE 2014, 9, e92073. [Google Scholar] [CrossRef]
- Obata, K.; Yamanaka, H.; Fukuoka, T.; Yi, D.; Tokunaga, A.; Hashimoto, N.; Yoshikawa, H.; Noguchi, K. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain 2003, 101, 65–77. [Google Scholar] [CrossRef]
- Zheng, L.F.; Wang, R.; Xu, Y.Z.; Yi, X.N.; Zhang, J.W.; Zeng, Z.C. Calcitonin gene-related peptide dynamics in rat dorsal root ganglia and spinal cord following different sciatic nerve injuries. Brain Res. 2008, 1187, 20–32. [Google Scholar] [CrossRef]
- Dumoulin, F.L.; Raivich, G.; Streit, W.J.; Kreutzberg, G.W. Differential Regulation of Calcitonin Gene-related Peptide (CGRP) in Regenerating Rat Facial Nucleus and Dorsal Root Ganglion. Eur. J. Neurosci. 1991, 3, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Groves, M.J.; Ng, Y.W.; Ciardi, A.; Scaravilli, F. Sciatic nerve injury in the adult rat: Comparison of effects on oligosaccharide, CGRP and GAP43 immunoreactivity in primary afferents following two types of trauma. J. Neurocytol. 1996, 25, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.A.; De Vry, J.; Siegling, A.; Spreyer, P.; Denzer, D. Pharmacological sensitivity and gene expression analysis of the tibial nerve injury model of neuropathic pain. Eur. J. Pharm. 2003, 470, 17–25. [Google Scholar] [CrossRef]
- Hammond, D.L.; Ackerman, L.; Holdsworth, R.; Elzey, B. Effects of spinal nerve ligation on immunohistochemically identified neurons in the L4 and L5 dorsal root ganglia of the rat. J. Comp. Neurol. 2004, 475, 575–589. [Google Scholar] [CrossRef]
- Fukuoka, T.; Tokunaga, A.; Kondo, E.; Miki, K.; Tachibana, T.; Noguchi, K. Change in mRNAs for neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain 1998, 78, 13–26. [Google Scholar] [CrossRef]
- Miki, K.; Fukuoka, T.; Tokunaga, A.; Noguchi, K. Calcitonin gene-related peptide increase in the rat spinal dorsal horn and dorsal column nucleus following peripheral nerve injury: Up-regulation in a subpopulation of primary afferent sensory neurons. Neuroscience 1998, 82, 1243–1252. [Google Scholar] [CrossRef]
- Gardell, L.R.; Vanderah, T.W.; Gardell, S.E.; Wang, R.; Ossipov, M.H.; Lai, J.; Porreca, F. Enhanced evoked excitatory transmitter release in experimental neuropathy requires descending facilitation. J. Neurosci. 2003, 23, 8370–8379. [Google Scholar] [CrossRef]
- NASS Evidence-Based Clinical Guidelines Committee. Evidence-Based Clnical Guidlines for Multidisciplinary Spine Care: Diagnosis and Treatment of Lumbar Disc Herniation with Lumbar Radiculopathy; North American Spine Society: Burr Ridge, IL, USA, 2012. [Google Scholar]
- Xu, J.T.; Xin, W.J.; Wei, X.H.; Wu, C.Y.; Ge, Y.X.; Liu, Y.L.; Zang, Y.; Zhang, T.; Li, Y.Y.; Liu, X.G. p38 activation in uninjured primary afferent neurons and in spinal microglia contributes to the development of neuropathic pain induced by selective motor fiber injury. Exp. Neurol. 2007, 204, 355–365. [Google Scholar] [CrossRef]
- Wei, X.H.; Na, X.D.; Liao, G.J.; Chen, Q.Y.; Cui, Y.; Chen, F.Y.; Li, Y.Y.; Zang, Y.; Liu, X.G. The up-regulation of IL-6 in DRG and spinal dorsal horn contributes to neuropathic pain following L5 ventral root transection. Exp. Neurol. 2013, 241, 159–168. [Google Scholar] [CrossRef]
- He, X.H.; Zang, Y.; Chen, X.; Pang, R.P.; Xu, J.T.; Zhou, X.; Wei, X.H.; Li, Y.Y.; Xin, W.J.; Qin, Z.H.; et al. TNF-alpha contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain 2010, 151, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Chen, S.X.; Liao, G.J.; Zhu, H.Q.; Wei, X.H.; Cui, Y.; Na, X.D.; Pang, R.P.; Xin, W.J.; Zhou, L.J.; et al. Calpain-2 contributes to neuropathic pain following motor nerve injury via up-regulating interleukin-6 in DRG neurons. Brain Behav. Immun. 2015, 44, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ringkamp, M.; Hartke, T.V.; Murinson, B.B.; Campbell, J.N.; Griffin, J.W.; Meyer, R.A. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J. Neurosci. 2001, 21, RC140. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-H.; Yu, Y.-W.; Chuang, Y.-C.; Lee, C.-H.; Chen, C.-C. ATF3-Expressing Large-Diameter Sensory Afferents at Acute Stage as Bio-Signatures of Persistent Pain Associated with Lumbar Radiculopathy. Cells 2021, 10, 992. https://doi.org/10.3390/cells10050992
Lin J-H, Yu Y-W, Chuang Y-C, Lee C-H, Chen C-C. ATF3-Expressing Large-Diameter Sensory Afferents at Acute Stage as Bio-Signatures of Persistent Pain Associated with Lumbar Radiculopathy. Cells. 2021; 10(5):992. https://doi.org/10.3390/cells10050992
Chicago/Turabian StyleLin, Jiann-Her, Yu-Wen Yu, Yu-Chia Chuang, Cheng-Han Lee, and Chih-Cheng Chen. 2021. "ATF3-Expressing Large-Diameter Sensory Afferents at Acute Stage as Bio-Signatures of Persistent Pain Associated with Lumbar Radiculopathy" Cells 10, no. 5: 992. https://doi.org/10.3390/cells10050992
APA StyleLin, J.-H., Yu, Y.-W., Chuang, Y.-C., Lee, C.-H., & Chen, C.-C. (2021). ATF3-Expressing Large-Diameter Sensory Afferents at Acute Stage as Bio-Signatures of Persistent Pain Associated with Lumbar Radiculopathy. Cells, 10(5), 992. https://doi.org/10.3390/cells10050992






