Mast Cells and Skin and Breast Cancers: A Complicated and Microenvironment-Dependent Role
Abstract
1. Introduction
2. The Mast Cell—Tumor Controversy
3. Mapping the Role of Mast Cells in Cutaneous Cancers
4. The Function of Mast Cells in Malignant Breast Tumors
5. Mast Cell Key Mediators That Influence Cutaneous and Mammary Tumors
5.1. TNF Family Members
5.2. IL-6
5.3. IL-13
5.4. IL-17A
5.5. Mast Cell-Derived Chemokines
5.6. Histamine
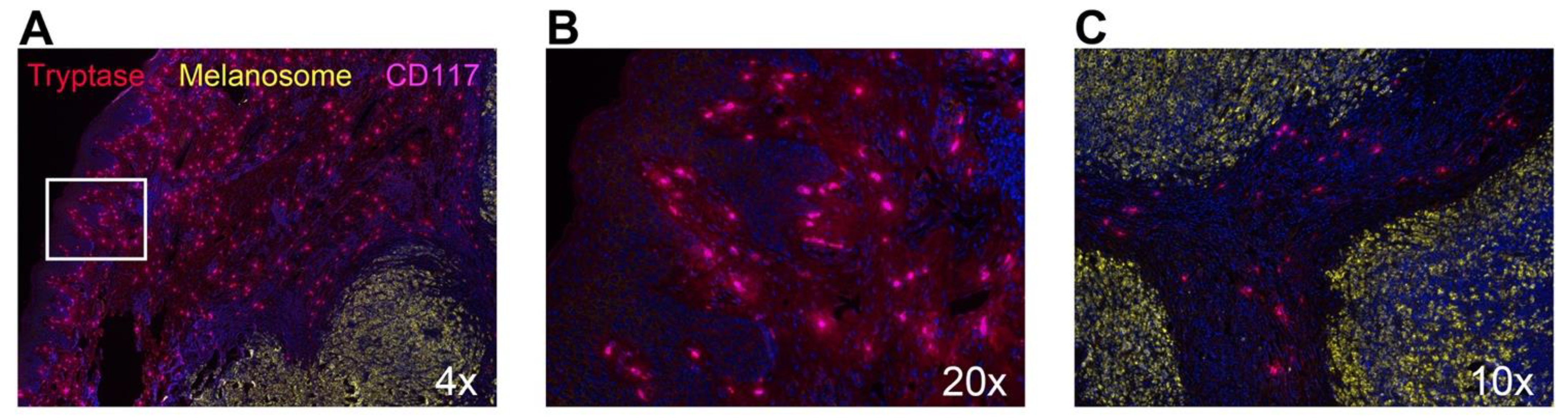
5.7. Tryptase
6. Environmental and Physiological Pathways That Differentially Program Mast Cells
6.1. Ultraviolet Irradiation
6.2. Microbiota
6.3. Hypoxia
7. Therapeutically Targeted Mast Cells Drive Anti-Tumor Responses
7.1. Mast Cells and IgE in AllergoOncology
7.2. TLR-Signaling
7.3. Virus Mediated Activation
8. Conclusions and the Future Landscape
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, J.S.; Portales-Cervantes, L.; Leong, E. Mast cell responses to viruses and pathogen products. Int. J. Mol. Sci. 2019, 20, 4241. [Google Scholar] [CrossRef]
- Marshall, J.S. Mast-cell responses to pathogens. Nat. Rev. Immunol. 2004, 4, 787–799. [Google Scholar] [CrossRef]
- McHale, C.; Mohammed, Z.; Gomez, G. Human skin-derived mast cells spontaneously secrete several angiogenesis-related factors. Front. Immunol. 2019, 10, 1445. [Google Scholar] [CrossRef]
- Starkey, J.R.; Crowle, P.K.; Taubenberger, S. Mast-cell-deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int. J. Cancer 1988, 42, 48–52. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Oh, H.-A.; Nam, S.-Y.; Han, N.-R.; Kim, Y.-S.; Kim, J.-H.; Lee, S.-J.; Kim, M.-H.; Moon, P.-D.; Kim, H.-M.; et al. The critical role of mast cell-derived hypoxia-inducible factor-1α in human and mice melanoma growth. Int. J. Cancer 2013, 132, 2492–2501. [Google Scholar] [CrossRef] [PubMed]
- Kaesler, S.; Wölbing, F.; Kempf, W.E.; Skabytska, Y.; Köberle, M.; Volz, T.; Sinnberg, T.; Amaral, T.; Möckel, S.; Yazdi, A.; et al. Targeting tumor-resident mast cells for effective anti-melanoma immune responses. JCI Insight 2019, 4, 4. [Google Scholar] [CrossRef]
- Oldford, S.A.; Marshall, J.S. Mast cells as targets for immunotherapy of solid tumors. Mol. Immunol. 2015, 63, 113–124. [Google Scholar] [CrossRef]
- Rigoni, A.; Colombo, M.; Pucillo, C. The role of mast cells in molding the tumor microenvironment. Cancer Microenviron. 2015, 8, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Wulaningsih, W.; Holmberg, L.; Garmo, H.; Karagiannis, S.N.; Ahlstedt, S.; Malmstrom, H.; Lambe, M.; Hammar, N.; Walldius, G.; Jungner, I.; et al. Investigating the association between allergen-specific immunoglobulin E, cancer risk and survival. Oncoimmunology 2016, 5, e1154250. [Google Scholar] [CrossRef]
- Schlehofer, B.; Siegmund, B.; Linseisen, J.; Schuz, J.; Rohrmann, S.; Becker, S.; Michaud, D.; Melin, B.; Bas Bueno-de-Mesquita, H.; Peeters, P.H.; et al. Primary brain tumours and specific serum immunoglobulin E: A case-control study nested in the European Prospective Investigation into Cancer and Nutrition cohort. Allergy 2011, 66, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.H.; Hsu, M.; Wiemels, J.L.; Bracci, P.M.; Zhou, M.; Patoka, J.; Reisacher, W.R.; Wang, J.; Kurtz, R.C.; Silverman, D.T.; et al. Serum immunoglobulin e and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol. Prev. Biomark. 2014, 23, 1414–1420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singer, J.; Achatz-Straussberger, G.; Bentley-Lukschal, A.; Fazekas-Singer, J.; Achatz, G.; Karagiannis, S.N.; Jensen-Jarolim, E. AllergoOncology: High innate IgE levels are decisive for the survival of cancer-bearing mice. World Allergy Organ. J. 2019, 12, 100044. [Google Scholar] [CrossRef]
- Crescioli, S.; Chiaruttini, G.; Mele, S.; Ilieva, K.M.; Pellizzari, G.; Spencer, D.I.R.; Gardner, R.A.; Lacy, K.E.; Spicer, J.F.; Tutt, A.N.J.; et al. Engineering and stable production of recombinant IgE for cancer immunotherapy and AllergoOncology. J. Allergy Clin. Immunol. 2018, 141, 1519–1523.e9. [Google Scholar] [CrossRef]
- Jensen-Jarolim, E.; Bax, H.J.; Bianchini, R.; Capron, M.; Corrigan, C.; Castells, M.; Dombrowicz, D.; Daniels-Wells, T.R.; Fazekas, J.; Fiebiger, E.; et al. AllergoOncology—the impact of allergy in oncology: EAACI position paper. Allergy 2017, 72, 866–887. [Google Scholar] [CrossRef]
- Fazekas-Singer, J.; Singer, J.; Ilieva, K.M.; Matz, M.; Herrmann, I.; Spillner, E.; Karagiannis, S.N.; Jensen-Jarolim, E. AllergoOncology: Generating a canine anticancer IgE against the epidermal growth factor receptor. J. Allergy Clin. Immunol. 2018, 142, 973–976.e11. [Google Scholar] [CrossRef]
- Takanami, I.; Takeuchi, K.; Naruke, M. Mast cell density is associated with angiogenesis and poor prognosis in pulmonary adenocarcinoma. Cancer 2000, 88, 2686–2692. [Google Scholar] [CrossRef]
- Esposito, I.; Menicagli, M.; Funel, N.; Bergmann, F.; Boggi, U.; Mosca, F.; Bevilacqua, G.; Campani, D. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J. Clin. Pathol. 2004, 57, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Norrby, K. Mast cells and angiogenesis. Apmis 2002, 110, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Raymond, W.W.; Bergers, G.; Laig-Webster, M.; Behrendtsen, O.; Werb, Z.; Caughey, G.H.; Hanahan, D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999, 13, 1382–1397. [Google Scholar] [CrossRef] [PubMed]
- Ghouse, S.M.; Polikarpova, A.; Muhandes, L.; Dudeck, J.; Tantcheva-Poór, I.; Hartmann, K.; Lesche, M.; Dahl, A.; Eming, S.; Müller, W. Although abundant in tumor tissue, mast cells have no effect on immunological micro-milieu or growth of HPV-induced or transplanted tumors. Cell Rep. 2018, 22, 27–35. [Google Scholar] [CrossRef]
- Dudeck, A.; Dudeck, J.; Scholten, J.; Petzold, A.; Surianarayanan, S.; Köhler, A.; Peschke, K.; Vöhringer, D.; Waskow, C.; Krieg, T. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 2011, 34, 973–984. [Google Scholar] [CrossRef]
- Antsiferova, M.; Martin, C.; Huber, M.; Feyerabend, T.B.; Förster, A.; Hartmann, K.; Rodewald, H.R.; Hohl, D.; Werner, S. Mast cells are dispensable for normal and activin-promoted wound healing and skin carcinogenesis. J. Immunol. 2013, 191, 6147–6155. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Metz, M.; Maurer, M. Mast cells protect from skin tumor development and limit tumor growth during cutaneous de novo carcinogenesis in a K it-dependent mouse model. Exp. Dermatol. 2014, 23, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Hatfield, J.K.; Walker, M.E.; Sayed, B.A. A game of kit and mouse: The Kit is still in the bag. Immunity 2012, 36, 891–892. [Google Scholar] [CrossRef] [PubMed]
- Rodewald, H.-R.; Feyerabend, T.B. Widespread immunological functions of mast cells: Fact or fiction? Immunity 2012, 37, 13–24. [Google Scholar] [CrossRef]
- Majorini, M.T.; Cancila, V.; Rigoni, A.; Botti, L.; Dugo, M.; Triulzi, T.; De Cecco, L.; Fontanella, E.; Jachetti, E.; Tagliabue, E.; et al. Infiltrating mast cell-mediated stimulation of estrogen receptor activity in breast cancer cells promotes the luminal phenotype. Cancer Res. 2020, 80, 2311–2324. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Ennas, M.G.; Vacca, A.; Ferreli, F.; Nico, B.; Orru, S.; Sirigu, P. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur. J. Clin. Investig. 2003, 33, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Jimi, S.; Takebayashi, S.; Kawamoto, N. Cutaneous malignant melanoma: Correlation between neovascularization and peritumor accumulation of mast cells overexpressing vascular endothelial growth factor. Hum. Pathol. 2000, 31, 955–960. [Google Scholar]
- Erkiliç, S.; Erbağci, Z. The significance of mast cells associated with basal cell carcinoma. J. Dermatol. 2001, 28, 312–315. [Google Scholar] [CrossRef]
- Siiskonen, H.; Poukka, M.; Bykachev, A.; Tyynela-Korhonen, K.; Sironen, R.; Pasonen-Seppanen, S.; Harvima, I.T. Low numbers of tryptase+ and chymase+ mast cells associated with reduced survival and advanced tumor stage in melanoma. Melanoma Res. 2015, 25, 479–485. [Google Scholar] [CrossRef]
- Stieglitz, D.; Lamm, S.; Braig, S.; Feuerer, L.; Kuphal, S.; Dietrich, P.; Arndt, S.; Echtenacher, B.; Hellerbrand, C.; Karrer, S.; et al. BMP6-induced modulation of the tumor micro-milieu. Oncogene 2019, 38, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Öhrvik, H.; Grujic, M.; Waern, I.; Gustafson, A.-M.; Ernst, N.; Roers, A.; Hartmann, K.; Pejler, G. Mast cells promote melanoma colonization of lungs. Oncotarget 2016, 7, 68990. [Google Scholar] [CrossRef]
- Oldford, S.A.; Haidl, I.D.; Howatt, M.A.; Leiva, C.A.; Johnston, B.; Marshall, J.S. A critical role for mast cells and mast cell-derived IL-6 in TLR2-mediated inhibition of tumor growth. J. Immunol. 2010, 185, 7067–7076. [Google Scholar] [CrossRef] [PubMed]
- Samoszuk, M.; Corwin, M.A. Mast cell inhibitor cromolyn increases blood clotting and hypoxia in murine breast cancer. Int. J. Cancer 2003, 107, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Marech, I.; Ammendola, M.; Sacco, R.; Capriuolo, G.S.; Patruno, R.; Rubini, R.; Luposella, M.; Zuccalà, V.; Savino, E.; Gadaleta, C.D.; et al. Serum tryptase, mast cells positive to tryptase and microvascular density evaluation in early breast cancer patients: Possible translational significance. BMC Cancer 2014, 14, 534. [Google Scholar] [CrossRef]
- Carpenco, E.; Ceauşu, R.A.; Cimpean, A.M.; Gaje, P.N.; Șaptefraţi, L.; Fulga, V.; David, V.; Raica, M. Mast Cells as an Indicator and Prognostic Marker in Molecular Subtypes of Breast Cancer. In Vivo 2019, 33, 743–748. [Google Scholar] [CrossRef]
- Glajcar, A.; Szpor, J.; Pacek, A.; Tyrak, K.E.; Chan, F.; Streb, J.; Hodorowicz-Zaniewska, D.; Okoń, K. The relationship between breast cancer molecular subtypes and mast cell populations in tumor microenvironment. Virchows Arch. 2017, 470, 505–515. [Google Scholar] [CrossRef]
- Mangia, A.; Malfettone, A.; Rossi, R.; Paradiso, A.; Ranieri, G.; Simone, G.; Resta, L. Tissue remodelling in breast cancer: Human mast cell tryptase as an initiator of myofibroblast differentiation. Histopathology 2011, 58, 1096–1106. [Google Scholar] [CrossRef]
- Ranieri, G.; Ammendola, M.; Patruno, R.; Celano, G.; Zito, F.A.; Montemurro, S.; Rella, A.; Di Lecce, V.; Gadaleta, C.D.; Battista De Sarro, G.; et al. Tryptase-positive mast cells correlate with angiogenesis in early breast cancer patients. Int. J. Oncol. 2009, 35, 115–120. [Google Scholar] [CrossRef]
- Cimpean, A.M.; Tamma, R.; Ruggieri, S.; Nico, B.; Toma, A.; Ribatti, D. Mast cells in breast cancer angiogenesis. Crit Rev. Oncol. 2017, 115, 23–26. [Google Scholar] [CrossRef]
- Ueshima, C.; Kataoka, T.R.; Hirata, M.; Furuhata, A.; Suzuki, E.; Toi, M.; Tsuruyama, T.; Okayama, Y.; Haga, H. The killer cell Ig-like receptor 2DL4 expression in human mast cells and its potential role in breast cancer invasion. Cancer Immunol. Res. 2015, 3, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, S.; Huntsman, D.; Makretsov, N.; Cheang, M.; Gilks, B.; Bajdik, C.; Gelmon, K.; Chia, S.; Hayes, M. The presence of stromal mast cells identifies a subset of invasive breast cancers with a favorable prognosis. Mod. Pathol. 2004, 17, 690–695. [Google Scholar] [CrossRef] [PubMed]
- della Rovere, F.; Granata, A.; Familiari, D.; D’Arrigo, G.; Mondello, B.; Basile, G. Mast cells in invasive ductal breast cancer: Different behavior in high and minimum hormone-receptive cancers. Anticancer Res. 2007, 27, 2465–2471. [Google Scholar] [PubMed]
- Reddy, S.M.; Reuben, A.; Barua, S.; Jiang, H.; Zhang, S.; Wang, L.; Gopalakrishnan, V.; Hudgens, C.W.; Tetzlaff, M.T.; Reuben, J.M.; et al. Poor Response to Neoadjuvant Chemotherapy Correlates with Mast Cell Infiltration in Inflammatory Breast Cancer. Cancer Immunol. Res. 2019, 7, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhu, Z.; Chen, S.; Wang, Y.; Gu, H. Mammary tumor growth and metastasis are reduced in c-Kit mutant Sash mice. Cancer Med. 2016, 5, 1292–1297. [Google Scholar] [CrossRef]
- Das Roy, L.; Curry, J.M.; Sahraei, M.; Besmer, D.M.; Kidiyoor, A.; Gruber, H.E.; Mukherjee, P. Arthritis augments breast cancer metastasis: Role of mast cells and SCF/c-Kit signaling. Breast Cancer Res. 2013, 15, R32. [Google Scholar] [CrossRef]
- Kuonen, F.; Laurent, J.; Secondini, C.; Lorusso, G.; Stehle, J.-C.; Rausch, T.; Faes-Van’t Hull, E.; Bieler, G.; Alghisi, G.-C.; Schwendener, R. Inhibition of the Kit ligand/c-Kit axis attenuates metastasis in a mouse model mimicking local breast cancer relapse after radiotherapy. Clin. Cancer Res. 2012, 18, 4365–4374. [Google Scholar] [CrossRef]
- Boesiger, J.; Tsai, M.; Maurer, M.; Yamaguchi, M.; Brown, L.F.; Claffey, K.P.; Dvorak, H.F.; Galli, S.J. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E–dependent upregulation of Fcε receptor I expression. J. Exp. Med. 1998, 188, 1135–1145. [Google Scholar] [CrossRef]
- Abdel-Majid, R.M.; Marshall, J.S. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J. Immunol. 2004, 172, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Skobe, M.; Hawighorst, T.; Jackson, D.G.; Prevo, R.; Janes, L.; Velasco, P.; Riccardi, L.; Alitalo, K.; Claffey, K.; Detmar, M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001, 7, 192–198. [Google Scholar] [CrossRef]
- Detoraki, A.; Staiano, R.I.; Granata, F.; Giannattasio, G.; Prevete, N.; de Paulis, A.; Ribatti, D.; Genovese, A.; Triggiani, M.; Marone, G. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J. Allergy Clin. Immunol. 2009, 123, 1142–1149.e5. [Google Scholar] [CrossRef]
- Sammarco, G.; Varricchi, G.; Ferraro, V.; Ammendola, M.; De Fazio, M.; Altomare, D.F.; Luposella, M.; Maltese, L.; Currò, G.; Marone, G. Mast cells, angiogenesis and lymphangiogenesis in human gastric cancer. Int. J. Mol. Sci. 2019, 20, 2106. [Google Scholar] [CrossRef]
- Olynych, T.J.; Jakeman, D.L.; Marshall, J.S. Fungal zymosan induces leukotriene production by human mast cells through a dectin-1–dependent mechanism. J. Allergy Clin. Immunol. 2006, 118, 837–843. [Google Scholar] [CrossRef]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Bissonnette, E.Y.; Befus, A.D. Anti-inflammatory effect of β2-agonists: Inhibition of TNF-α release from human mast cells. J. Allergy Clin. Immunol. 1997, 100, 825–831. [Google Scholar] [CrossRef]
- Benyon, R.C.; Bissonnette, E.; Befus, A. Tumor necrosis factor-alpha dependent cytotoxicity of human skin mast cells is enhanced by anti-IgE antibodies. J. Immunol. 1991, 147, 2253–2258. [Google Scholar]
- Dudeck, J.; Kotrba, J.; Immler, R.; Hoffmann, A.; Voss, M.; Alexaki, V.I.; Morton, L.; Jahn, S.R.; Katsoulis-Dimitriou, K.; Winzer, S. Directional mast cell degranulation of tumor necrosis factor into blood vessels primes neutrophil extravasation. Immunity 2021, 54, 468–483.e5. [Google Scholar] [CrossRef] [PubMed]
- Jawdat, D.M.; Rowden, G.; Marshall, J.S. Mast cells have a pivotal role in TNF-independent lymph node hypertrophy and the mobilization of Langerhans cells in response to bacterial peptidoglycan. J. Immunol. 2006, 177, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Dudeck, J.; Ghouse, S.M.; Lehmann, C.H.; Hoppe, A.; Schubert, N.; Nedospasov, S.A.; Dudziak, D.; Dudeck, A. Mast-cell-derived TNF amplifies CD8+ dendritic cell functionality and CD8+ T cell priming. Cell Rep. 2015, 13, 399–411. [Google Scholar] [CrossRef]
- Shelburne, C.P.; Nakano, H.; St John, A.L.; Chan, C.; McLachlan, J.B.; Gunn, M.D.; Staats, H.F.; Abraham, S.N. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe 2009, 6, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, R.; Ikeda, T.; Ross, E.; Abraham, S.N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature 1996, 381, 77–80. [Google Scholar] [CrossRef]
- Nakae, S.; Suto, H.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc. Natl. Acad. Sci. USA 2005, 102, 6467–6472. [Google Scholar] [CrossRef]
- Gri, G.; Piconese, S.; Frossi, B.; Manfroi, V.; Merluzzi, S.; Tripodo, C.; Viola, A.; Odom, S.; Rivera, J.; Colombo, M.P. CD4+ CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity 2008, 29, 771–781. [Google Scholar] [CrossRef]
- Piconese, S.; Gri, G.; Tripodo, C.; Musio, S.; Gorzanelli, A.; Frossi, B.; Pedotti, R.; Pucillo, C.E.; Colombo, M.P. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood 2009, 114, 2639–2648. [Google Scholar] [CrossRef]
- Nakae, S.; Suto, H.; Iikura, M.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast cells enhance T cell activation: Importance of mast cell costimulatory molecules and secreted TNF. J. Immunol. 2006, 176, 2238–2248. [Google Scholar] [CrossRef]
- Vosskuhl, K.; Greten, T.F.; Manns, M.P.; Korangy, F.; Wedemeyer, J. Lipopolysaccharide-mediated mast cell activation induces IFN-gamma secretion by NK cells. J. Immunol. 2010, 185, 119–125. [Google Scholar] [CrossRef]
- Berent-Maoz, B.; Salemi, S.; Mankuta, D.; Simon, H.-U.; Levi-Schaffer, F. Human mast cells express intracellular TRAIL. Cell. Immunol. 2010, 262, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Palm, A.-K.E.; Garcia-Faroldi, G.; Lundberg, M.; Pejler, G.; Kleinau, S. Activated mast cells promote differentiation of B cells into effector cells. Sci. Rep. 2016, 6, 20531. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Ho, W.J.; Mohan, A.; Shah, Y.; Vithayathil, T.; Leatherman, J.; Dennison, L.; Zaidi, N.; Ganguly, S.; Woolman, S. Effects of B cell–activating factor on tumor immunity. JCI Insight 2020, 5, 5. [Google Scholar] [CrossRef]
- Pelekanou, V.; Notas, G.; Athanasouli, P.; Alexakis, K.; Kiagiadaki, F.; Peroulis, N.; Kalyvianaki, K.; Kampouri, E.; Polioudaki, H.; Theodoropoulos, P. BCMA (TNFRSF17) induces APRIL and BAFF mediated breast cancer cell stemness. Front. Oncol. 2018, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Trauzold, A.; Siegmund, D.; Schniewind, B.; Sipos, B.; Egberts, J.; Zorenkov, D.; Emme, D.; Röder, C.; Kalthoff, H.; Wajant, H. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene 2006, 25, 7434–7439. [Google Scholar] [CrossRef]
- Griffith, T.S.; Chin, W.A.; Jackson, G.C.; Lynch, D.H.; Kubin, M.Z. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J. Immunol. 1998, 161, 2833–2840. [Google Scholar]
- Leal-Berumen, I.; Conlon, P.; Marshall, J.S. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J. Immunol. 1994, 152, 5468–5476. [Google Scholar] [PubMed]
- George, D.J.; Halabi, S.; Shepard, T.F.; Sanford, B.; Vogelzang, N.J.; Small, E.J.; Kantoff, P.W. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: Results from cancer and leukemia group B 9480. Clin. Cancer Res. 2005, 11, 1815–1820. [Google Scholar] [CrossRef]
- Bachelot, T.; Ray-Coquard, I.; Menetrier-Caux, C.; Rastkha, M.; Duc, A.; Blay, J. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br. J. Cancer 2003, 88, 1721–1726. [Google Scholar] [CrossRef]
- Hashwah, H.; Bertram, K.; Stirm, K.; Stelling, A.; Wu, C.T.; Kasser, S.; Manz, M.G.; Theocharides, A.P.; Tzankov, A.; Müller, A. The IL-6 signaling complex is a critical driver, negative prognostic factor, and therapeutic target in diffuse large B-cell lymphoma. EMBO Mol. Med. 2019, 11, e10576. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, E.; Rose-John, S.; Gerhartz, C.; Müllberg, J.; Stoyan, T.; Yasukawa, K.; Heinrich, P.C.; Graeve, L. Identification of a region within the cytoplasmic domain of the interleukin-6 (IL-6) signal transducer gp130 important for ligand-induced endocytosis of the IL-6 receptor. J. Biol. Chem. 1994, 269, 19014–19020. [Google Scholar] [CrossRef]
- Dawicki, W.; Jawdat, D.W.; Xu, N.; Marshall, J.S. Mast cells, histamine, and IL-6 regulate the selective influx of dendritic cell subsets into an inflamed lymph node. J. Immunol. 2010, 184, 2116–2123. [Google Scholar] [CrossRef]
- Eissmann, M.F.; Dijkstra, C.; Jarnicki, A.; Phesse, T.; Brunnberg, J.; Poh, A.R.; Etemadi, N.; Tsantikos, E.; Thiem, S.; Huntington, N.D. IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Fujisawa, T.; Joshi, B.H.; Puri, R.K. IL-13 regulates cancer invasion and metastasis through IL-13Rα2 via ERK/AP-1 pathway in mouse model of human ovarian cancer. Int. J. Cancer 2012, 131, 344–356. [Google Scholar] [CrossRef]
- Perales-Puchalt, A.; Svoronos, N.; Villarreal, D.O.; Zankharia, U.; Reuschel, E.; Wojtak, K.; Payne, K.K.; Duperret, E.K.; Muthumani, K.; Conejo-Garcia, J.R. IL-33 delays metastatic peritoneal cancer progression inducing an allergic microenvironment. Oncoimmunology 2019, 8, e1515058. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Yoshimatsu, Y.; Tomizawa, T.; Kunita, A.; Takayama, R.; Morikawa, T.; Komura, D.; Takahashi, K.; Oshima, T.; Sato, M. Interleukin-13 receptor α2 is a novel marker and potential therapeutic target for human melanoma. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.P.; Wang, G.Y.; Arima, K.; Guan, S.P.; Waters, M.R.; Cavenee, W.K.; Pan, E.; Aliwarga, E.; Chong, S.T.; Kok, C.Y. Interleukin-13 receptor alpha 2 cooperates with EGFRvIII signaling to promote glioblastoma multiforme. Nat. Commun. 2017, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Choi, J.E.; Bae, Y.K. Interleukin-13 receptor alpha 2 expression in tumor cells is associated with reduced disease-free survival in patients with luminal subtype invasive breast cancer. Tumor Biol. 2018, 40, 1010428318783657. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Hollins, F.; Woodman, L.; Yang, W.; Monk, P.; May, R.; Bradding, P.; Brightling, C. Mast cells express IL-13Rα1: IL-13 promotes human lung mast cell proliferation and FcɛRI expression. Allergy 2006, 61, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Noordenbos, T.; Blijdorp, I.; Chen, S.; Stap, J.; Mul, E.; Cañete, J.D.; Lubberts, E.; Yeremenko, N.; Baeten, D. Human mast cells capture, store, and release bioactive, exogenous IL-17A. J. Leukoc. Biol. 2016, 100, 453–462. [Google Scholar] [CrossRef]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef]
- Kenna, T.J.; Brown, M.A. The role of IL-17-secreting mast cells in inflammatory joint disease. Nat. Rev. Rheumatol. 2013, 9, 375–379. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Huang, T.-H.; Liu, C.-L.; Chen, H.-S.; Lee, M.-H.; Chen, H.-W.; Shen, C.-R. Locally targeting the IL-17/IL-17RA axis reduced tumor growth in a murine B16F10 melanoma model. Hum. Gene Ther. 2019, 30, 273–285. [Google Scholar] [CrossRef]
- Roy, L.D.; Pathangey, L.B.; Tinder, T.L.; Schettini, J.L.; Gruber, H.E.; Mukherjee, P. Breast cancer-associated metastasis is significantly increased in a model of autoimmune arthritis. Breast Cancer Res. 2009, 11, R56. [Google Scholar]
- Wang, L.; Yi, T.; Zhang, W.; Pardoll, D.M.; Yu, H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010, 70, 10112–10120. [Google Scholar] [CrossRef]
- Lee, M.-H.; Chang, J.T.-C.; Liao, C.-T.; Chen, Y.-S.; Kuo, M.-L.; Shen, C.-R. Interleukin 17 and peripheral IL-17-expressing T cells are negatively correlated with the overall survival of head and neck cancer patients. Oncotarget 2018, 9, 9825. [Google Scholar] [CrossRef]
- Chen, W.C.; Lai, Y.H.; Chen, H.Y.; Guo, H.R.; Su, I.J.; Chen, H.H. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology 2013, 63, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Pappu, R.; Ramirez-Carrozzi, V.; Sambandam, A. The interleukin-17 cytokine family: Critical players in host defence and inflammatory diseases. Immunology 2011, 134, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Nakajima, H.; Suzuki, K.; Kagami, S.-i.; Hirose, K.; Suto, A.; Saito, Y.; Iwamoto, I. Mast cells produce interleukin-25 upon FcεRI-mediated activation. BloodJ. Am. Soc. Hematol. 2003, 101, 3594–3596. [Google Scholar] [CrossRef] [PubMed]
- Vilgelm, A.E.; Richmond, A. Chemokines modulate immune surveillance in tumorigenesis, metastasis, and response to immunotherapy. Front. Immunol. 2019, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Hirasawa, N.; Ohtsu, H.; Watanabe, T.; Ohuchi, K. Defective angiogenesis in the inflammatory granulation tissue in histidine decarboxylase–deficient mice but not in mast cell–deficient mice. J. Exp. Med. 2002, 195, 973–982. [Google Scholar] [CrossRef]
- Yang, X.D.; Ai, W.; Asfaha, S.; Bhagat, G.; Friedman, R.A.; Jin, G.; Park, H.; Shykind, B.; Diacovo, T.G.; Falus, A.; et al. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nat. Med. 2011, 17, 87–95. [Google Scholar] [CrossRef]
- Jawdat, D.M.; Albert, E.J.; Rowden, G.; Haidl, I.D.; Marshall, J.S. IgE-mediated mast cell activation induces Langerhans cell migration in vivo. J. Immunol. 2004, 173, 5275–5282. [Google Scholar] [CrossRef]
- Fritz, I.; Wagner, P.; Bottai, M.; Eriksson, H.; Ingvar, C.; Krakowski, I.; Nielsen, K.; Olsson, H. Desloratadine and loratadine use associated with improved melanoma survival. Allergy 2020, 75, 2096–2099. [Google Scholar] [CrossRef]
- Martin, R.K.; Saleem, S.J.; Folgosa, L.; Zellner, H.B.; Damle, S.R.; Nguyen, G.K.; Ryan, J.J.; Bear, H.D.; Irani, A.M.; Conrad, D.H. Mast cell histamine promotes the immunoregulatory activity of myeloid-derived suppressor cells. J. Leukoc. Biol 2014, 96, 151–159. [Google Scholar] [CrossRef]
- Vila-Leahey, A.; Oldford, S.A.; Marignani, P.A.; Wang, J.; Haidl, I.D.; Marshall, J.S. Ranitidine modifies myeloid cell populations and inhibits breast tumor development and spread in mice. Oncoimmunology 2016, 5, e1151591. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.; Vila-Leahey, A.; Pessoa, A.C.; Oldford, S.; Marignani, P.A.; Marshall, J.S. Ranitidine Inhibition of Breast Tumor Growth Is B Cell Dependent and Associated With an Enhanced Antitumor Antibody Response. Front. Immunol. 2018, 9, 1894. [Google Scholar] [CrossRef]
- Blair, R.J.; Meng, H.; Marchese, M.J.; Ren, S.; Schwartz, L.B.; Tonnesen, M.G.; Gruber, B.L. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J. Clin. Investig. 1997, 99, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Rabelo Melo, F.; Santosh Martin, S.; Sommerhoff, C.P.; Pejler, G. Exosome-mediated uptake of mast cell tryptase into the nucleus of melanoma cells: A novel axis for regulating tumor cell proliferation and gene expression. Cell Death Dis. 2019, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Grujic, M.; Hellman, L.; Gustafson, A.M.; Akula, S.; Melo, F.R.; Pejler, G. Protective role of mouse mast cell tryptase Mcpt6 in melanoma. Pigment. Cell Melanoma Res. 2020, 33, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Grujic, M.; Paivandy, A.; Gustafson, A.M.; Thomsen, A.R.; Ohrvik, H.; Pejler, G. The combined action of mast cell chymase, tryptase and carboxypeptidase A3 protects against melanoma colonization of the lung. Oncotarget 2017, 8, 25066–25079. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.J. Ultraviolet B irradiation of skin induces mast cell degranulation and release of tumour necrosis factor-alpha. Immunol. Cell Biol. 1995, 73, 226–233. [Google Scholar] [CrossRef]
- Bald, T.; Quast, T.; Landsberg, J.; Rogava, M.; Glodde, N.; Lopez-Ramos, D.; Kohlmeyer, J.; Riesenberg, S.; van den Boorn-Konijnenberg, D.; Hömig-Hölzel, C.; et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 2014, 507, 109–113. [Google Scholar] [CrossRef]
- Bernard, J.J.; Cowing-Zitron, C.; Nakatsuji, T.; Muehleisen, B.; Muto, J.; Borkowski, A.W.; Martinez, L.; Greidinger, E.L.; Yu, B.D.; Gallo, R.L. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat. Med. 2012, 18, 1286–1290. [Google Scholar] [CrossRef]
- Dejean, E.; Foisseau, M.; Lagarrigue, F.; Lamant, L.; Prade, N.; Marfak, A.; Delsol, G.; Giuriato, S.; Gaits-Iacovoni, F.; Meggetto, F. ALK+ ALCLs induce cutaneous, HMGB-1–dependent IL-8/CXCL8 production by keratinocytes through NF-κB activation. Blood J. Am. Soc. Hematol. 2012, 119, 4698–4707. [Google Scholar] [CrossRef] [PubMed]
- Endoh, I.; Di Girolamo, N.; Hampartzoumian, T.; Cameron, B.; Geczy, C.L.; Tedla, N. Ultraviolet B irradiation selectively increases the production of interleukin-8 in human cord blood-derived mast cells. Clin. Exp. Immunol. 2007, 148, 161–167. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, J.D.; Lin, T.J.; Marshall, J.S. Toll-like receptor 4-mediated activation of murine mast cells. J. Leukoc. Biol. 2001, 70, 977–984. [Google Scholar]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Singh, A.; Willems, E.; Singh, A.; Hafeez, B.B.; Ong, I.M.; Mehta, S.L.; Verma, A.K. Ultraviolet radiation-induced tumor necrosis factor alpha, which is linked to the development of cutaneous SCC, modulates differential epidermal microRNAs expression. Oncotarget 2016, 7, 17945–17956. [Google Scholar] [CrossRef]
- Fu, X.-Q.; Liu, B.; Wang, Y.-P.; Li, J.-K.; Zhu, P.-L.; Li, T.; Tse, K.-W.; Chou, J.-Y.; Yin, C.-L.; Bai, J.-X. Activation of STAT3 is a key event in TLR4 signaling-mediated melanoma progression. Cell Death Dis. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Lewis, W.; Simanyi, E.; Li, H.; Thompson, C.A.; Nasti, T.H.; Jaleel, T.; Xu, H.; Yusuf, N. Regulation of ultraviolet radiation induced cutaneous photoimmunosuppression by toll-like receptor-4. Arch. Biochem. Biophys. 2011, 508, 171–177. [Google Scholar] [CrossRef]
- Quist, S.R.; Wiswedel, I.; Quist, J.; Gollnick, H.P. Kinetic Profile of Inflammation Markers in Human Skin In vivo Following Exposure to Ultraviolet B Indicates Synchronic Release of Cytokines and Prostanoids. Acta Derm. Venereol. 2016, 96, 910–916. [Google Scholar] [CrossRef]
- Dawes, J.M.; Calvo, M.; Perkins, J.R.; Paterson, K.J.; Kiesewetter, H.; Hobbs, C.; Kaan, T.K.; Orengo, C.; Bennett, D.L.; McMahon, S.B. CXCL5 mediates UVB irradiation–induced pain. Sci. Transl. Med. 2011, 3, 90ra60. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, Y.; Lee, D.H.; Seo, J.; Cho, K.; Eun, H.; Chung, J.H. Acute exposure of human skin to ultraviolet or infrared radiation or heat stimuli increases mast cell numbers and tryptase expression in human skin in vivo. Br. J. Dermatol. 2009, 160, 393–402. [Google Scholar] [CrossRef]
- Rigby, C.; Deep, G.; Jain, A.; Orlicky, D.J.; Agarwal, C.; Agarwal, R. Silibinin inhibits ultraviolet B radiation-induced mast cells recruitment and bone morphogenetic protein 2 expression in the skin at early stages in Ptch(+/−) mouse model of basal cell carcinoma. Mol. Carcinog. 2019, 58, 1260–1271. [Google Scholar] [CrossRef]
- Hart, P.H.; Grimbaldeston, M.A.; Swift, G.J.; Hosszu, E.K.; Finlay-Jones, J.J. A critical role for dermal mast cells in cis-urocanic acid-induced systemic suppression of contact hypersensitivity responses in mice. Photochem. Photobiol. 1999, 70, 807–812. [Google Scholar] [CrossRef]
- Pham, D.L.; Lim, K.M.; Joo, K.M.; Park, H.S.; Leung, D.Y.M.; Ye, Y.M. Increased cis-to-trans urocanic acid ratio in the skin of chronic spontaneous urticaria patients. Sci. Rep. 2017, 7, 1318. [Google Scholar] [CrossRef]
- Walterscheid, J.P.; Nghiem, D.X.; Kazimi, N.; Nutt, L.K.; McConkey, D.J.; Norval, M.; Ullrich, S.E. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 17420–17425. [Google Scholar] [CrossRef] [PubMed]
- Wille, J.J.; Kydonieus, A.F.; Murphy, G.F. cis-Urocanic Acid Induces Mast Cell Degranulation and Release of Preformed TNF--α: A Possible Mechanism Linking UVB and cis-Urocanic Acid to Immunosuppression of Contact Hypersensitivity. Ski. Pharmacol. Physiol. 1999, 12, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.N.; Beaugie, C.; O’Sullivan, C.; Leighton, S.; Halliday, G.M. The immune-modulating cytokine and endogenous Alarmin interleukin-33 is upregulated in skin exposed to inflammatory UVB radiation. Am. J. Pathol. 2011, 179, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdi, Z.; Smith, D.E.; Comeau, M.R.; Delespesse, G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J. Immunol. 2007, 179, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Wang, Z.; Franke, K.; Guhl, S.; Artuc, M.; Zuberbier, T. Yin-yang of IL-33 in human skin mast cells: Reduced degranulation, but augmented histamine synthesis through p38 activation. J. Investig. Dermatol. 2019, 139, 1516–1525.e3. [Google Scholar] [CrossRef]
- Ocana, J.A.; Romer, E.; Sahu, R.; Pawelzik, S.-C.; FitzGerald, G.A.; Kaplan, M.H.; Travers, J.B. Platelet-Activating Factor–Induced Reduction in Contact Hypersensitivity Responses Is Mediated by Mast Cells via Cyclooxygenase-2–Dependent Mechanisms. J. Immunol. 2018, 200, 4004–4011. [Google Scholar] [CrossRef]
- Byrne, S.N.; Limón-Flores, A.Y.; Ullrich, S.E. Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J. Immunol. 2008, 180, 4648–4655. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Salinas, R.; Chen, L.; Chávez-Blanco, A.D.; Limón-Flores, A.Y.; Ma, Y.; Ullrich, S.E. An essential role for platelet-activating factor in activating mast cell migration following ultraviolet irradiation. J. Leukoc. Biol. 2014, 95, 139–148. [Google Scholar] [CrossRef]
- Schwarz, A.; Noordegraaf, M.; Maeda, A.; Torii, K.; Clausen, B.E.; Schwarz, T. Langerhans cells are required for UVR-induced immunosuppression. J. Investig. Dermatol. 2010, 130, 1419–1427. [Google Scholar] [CrossRef]
- Markle, J.G.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; Von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- de la Cuesta-Zuluaga, J.; Kelley, S.T.; Chen, Y.; Escobar, J.S.; Mueller, N.T.; Ley, R.E.; McDonald, D.; Huang, S.; Swafford, A.D.; Knight, R. Age-and sex-dependent patterns of gut microbial diversity in human adults. Msystems 2019, 4, e00261-19. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, J.J.; Myeong, N.R.; Kim, T.; Kim, D.; An, S.; Kim, H.; Park, T.; Jang, S.I.; Yeon, J.H.; et al. Segregation of age-related skin microbiome characteristics by functionality. Sci. Rep. 2019, 9, 16748. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Kong, H.H.; Renaud, G.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Wolfsberg, T.G.; Turner, M.L.; Segre, J.A. A diversity profile of the human skin microbiota. Genome Res. 2008, 18, 1043–1050. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, J.D.; Olynych, T.J.; Maher, L.H.; Marshall, J.S. Cutting edge: Distinct Toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J. Immunol. 2003, 170, 1625–1629. [Google Scholar] [CrossRef]
- Okayama, Y. Mast cell-derived cytokine expression induced via Fc receptors and Toll-like receptors. Chem Immunol. Allergy 2005, 87, 101–110. [Google Scholar] [CrossRef]
- Kubo, Y.; Fukuishi, N.; Yoshioka, M.; Kawasoe, Y.; Iriguchi, S.; Imajo, N.; Yasui, Y.; Matsui, N.; Akagi, M. Bacterial components regulate the expression of Toll-like receptor 4 on human mast cells. Inflamm. Res. 2007, 56, 70–75. [Google Scholar] [CrossRef]
- Rossini, A.; Rumio, C.; Sfondrini, L.; Tagliabue, E.; Morelli, D.; Miceli, R.; Mariani, L.; Palazzo, M.; Ménard, S.; Balsari, A. Influence of antibiotic treatment on breast carcinoma development in proto-neu transgenic mice. Cancer Res. 2006, 66, 6219–6224. [Google Scholar] [CrossRef] [PubMed]
- Patra, V.; Wagner, K.; Arulampalam, V.; Wolf, P. Skin microbiome modulates the effect of ultraviolet radiation on cellular response and immune function. iScience 2019, 15, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V.; Hoey, C.; Liu, L.Y.; Lalonde, E.; Ray, J.; Livingstone, J.; Lesurf, R.; Shiah, Y.-J.; Vujcic, T.; Huang, X. Molecular landmarks of tumor hypoxia across cancer types. Nat. Genet. 2019, 51, 308–318. [Google Scholar] [CrossRef]
- Ramírez-Moreno, I.G.; Ibarra-Sánchez, A.; Castillo-Arellano, J.I.; Blank, U.; González-Espinosa, C. Mast Cells Localize in Hypoxic Zones of Tumors and Secrete CCL-2 under Hypoxia through Activation of L-Type Calcium Channels. J. Immunol. 2020, 204, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G. Nucleotide-and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2008, 1783, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Ngiow, S.F.; Madore, J.; Reinhardt, J.; Landsberg, J.; Chitsazan, A.; Rautela, J.; Bald, T.; Barkauskas, D.S.; Ahern, E. Targeting adenosine in BRAF-mutant melanoma reduces tumor growth and metastasis. Cancer Res. 2017, 77, 4684–4696. [Google Scholar] [CrossRef]
- Loi, S.; Pommey, S.; Haibe-Kains, B.; Beavis, P.A.; Darcy, P.K.; Smyth, M.J.; Stagg, J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 11091–11096. [Google Scholar] [CrossRef]
- Turcotte, M.; Allard, D.; Mittal, D.; Bareche, Y.; Buisseret, L.; José, V.; Pommey, S.; Delisle, V.; Loi, S.; Joensuu, H. CD73 promotes resistance to HER2/ErbB2 antibody therapy. Cancer Res. 2017, 77, 5652–5663. [Google Scholar] [CrossRef]
- Marquardt, D.; Gruber, H.; Wasserman, S. Adenosine release from stimulated mast cells. Proc. Natl. Acad. Sci. USA 1984, 81, 6192–6196. [Google Scholar] [CrossRef]
- Rudich, N.; Ravid, K.; Sagi-Eisenberg, R. Mast cell adenosine receptors function: A focus on the a3 adenosine receptor and inflammation. Front. Immunol. 2012, 3, 134. [Google Scholar] [CrossRef]
- Gao, Z.-G.; Jacobson, K.A. Purinergic signaling in mast cell degranulation and asthma. Front. Pharmacol. 2017, 8, 947. [Google Scholar] [CrossRef]
- Feoktistov, I.; Ryzhov, S.; Goldstein, A.E.; Biaggioni, I. Mast cell–mediated stimulation of angiogenesis: Cooperative interaction between A2B and A3 adenosine receptors. Circ. Res. 2003, 92, 485–492. [Google Scholar] [CrossRef]
- Tilley, S.L.; Tsai, M.; Williams, C.M.; Wang, Z.-S.; Erikson, C.J.; Galli, S.J.; Koller, B.H. Identification of A3 receptor-and mast cell-dependent and-independent components of adenosine-mediated airway responsiveness in mice. J. Immunol. 2003, 171, 331–337. [Google Scholar] [CrossRef]
- Ryzhov, S.; Novitskiy, S.V.; Goldstein, A.E.; Biktasova, A.; Blackburn, M.R.; Biaggioni, I.; Dikov, M.M.; Feoktistov, I. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+ Gr1+ cells. J. Immunol. 2011, 187, 6120–6129. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Kini, R.; Ohta, A.; Subramanian, M.; Madasu, M.; Sitkovsky, M. The development and immunosuppressive functions of CD4+ CD25+ FoxP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 2012, 3, 190. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Meineke, V. Radiation-induced mast cell mediators differentially modulate chemokine release from dermal fibroblasts. J. Derm. Sci. 2011, 61, 199–205. [Google Scholar] [CrossRef]
- Soule, B.P.; Brown, J.M.; Kushnir-Sukhov, N.M.; Simone, N.L.; Mitchell, J.B.; Metcalfe, D.D. Effects of gamma radiation on FcepsilonRI and TLR-mediated mast cell activation. J. Immunol. 2007, 179, 3276–3286. [Google Scholar] [CrossRef] [PubMed]
- Norrby, K.; Abok, K.; Adamson, P.; Forsberg, B. Radiation effects on mast cells: Secretory ability, histamine release and recovery, and cell number. Acta Pathol. Microbiol. Immunol. Scand. A 1984, 92, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Palaniyandi, S.; Tomei, E.; Li, Z.; Conrad, D.H.; Zhu, X. CD23-dependent transcytosis of IgE and immune complex across the polarized human respiratory epithelial cells. J. Immunol. 2011, 186, 3484–3496. [Google Scholar] [CrossRef]
- Shiver, J.W.; Henkart, P.A. A noncytotoxic mast cell tumor line exhibits potent IgE-dependent cytotoxicity after transfection with the cytolysin/perforin gene. Cell 1991, 64, 1175–1181. [Google Scholar] [CrossRef]
- Shiver, J.W.; Su, L.; Henkart, P.A. Cytotoxicity with target DNA breakdown by rat basophilic leukemia cells expressing both cytolysin and granzyme A. Cell 1992, 71, 315–322. [Google Scholar] [CrossRef]
- Jensen-Jarolim, E.; Bax, H.J.; Bianchini, R.; Crescioli, S.; Daniels-Wells, T.R.; Dombrowicz, D.; Fiebiger, E.; Gould, H.J.; Irshad, S.; Janda, J.; et al. AllergoOncology: Opposite outcomes of immune tolerance in allergy and cancer. Allergy 2018, 73, 328–340. [Google Scholar] [CrossRef]
- Jensen-Jarolim, E.; Achatz, G.; Turner, M.C.; Karagiannis, S.; Legrand, F.; Capron, M.; Penichet, M.L.; Rodriguez, J.A.; Siccardi, A.G.; Vangelista, L.; et al. AllergoOncology: The role of IgE-mediated allergy in cancer. Allergy 2008, 63, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Della Valle, L.; Gatta, A.; Farinelli, A.; Scarano, G.; Lumaca, A.; Tinari, N.; Cipollone, F.; Paganelli, R.; Di Gioacchino, M. Allergooncology: An expanding research area. J. Biol. Regul. Homeost Agents 2020, 34, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.A.; Brini, A.T.; Yenagi, V.A.; Ferreira, L.M.; Achatz-Straussberger, G.; Ambrosi, A.; Sanvito, F.; Soprana, E.; Van Anken, E.; Achatz, G. Cutting edge: IgE plays an active role in tumor immunosurveillance in mice. J. Immunol. 2016, 197, 2583–2588. [Google Scholar] [CrossRef]
- Karagiannis, P.; Singer, J.; Hunt, J.; Gan, S.K.; Rudman, S.M.; Mechtcheriakova, D.; Knittelfelder, R.; Daniels, T.R.; Hobson, P.S.; Beavil, A.J. Characterisation of an engineered trastuzumab IgE antibody and effector cell mechanisms targeting HER2/neu-positive tumour cells. Cancer Immunol. Immunother. 2009, 58, 915–930. [Google Scholar] [CrossRef]
- Plotkin, J.D.; Elias, M.G.; Fereydouni, M.; Daniels-Wells, T.R.; Dellinger, A.L.; Penichet, M.L.; Kepley, C.L. Human Mast Cells From Adipose Tissue Target and Induce Apoptosis of Breast Cancer Cells. Front. Immunol. 2019, 10, 138. [Google Scholar] [CrossRef]
- Teo, P.Z.; Utz, P.J.; Mollick, J.A. Using the allergic immune system to target cancer: Activity of IgE antibodies specific for human CD20 and MUC1. Cancer Immunol. Immunother. 2012, 61, 2295–2309. [Google Scholar] [CrossRef]
- Jiménez-Andrade, G.Y.; Ibarra-Sánchez, A.; González, D.; Lamas, M.; González-Espinosa, C. Immunoglobulin E induces VEGF production in mast cells and potentiates their pro-tumorigenic actions through a Fyn kinase-dependent mechanism. J. Hematol. Oncol. 2013, 6, 1–14. [Google Scholar] [CrossRef]
- Josephs, D.; Nakamura, M.; Bax, H.; Dodev, T.; Muirhead, G.; Saul, L.; Karagiannis, P.; Ilieva, K.; Crescioli, S.; Gazinska, P. An immunologically relevant rodent model demonstrates safety of therapy using a tumour-specific IgE. Allergy 2018, 73, 2328–2341. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Martínez-Maza, O.; Penichet, M.L. Animal models for IgE-meditated cancer immunotherapy. Cancer Immunol. Immunother. 2012, 61, 1535–1546. [Google Scholar] [CrossRef]
- Yasinska, I.M.; Sakhnevych, S.S.; Pavlova, L.; Teo Hansen Selno, A.; Teuscher Abeleira, A.M.; Benlaouer, O.; Goncalves Silva, I.; Mosimann, M.; Varani, L.; Bardelli, M.; et al. The Tim-3-Galectin-9 Pathway and Its Regulatory Mechanisms in Human Breast Cancer. Front. Immunol. 2019, 10, 1594. [Google Scholar] [CrossRef]
- Kojima, R.; Ohno, T.; Iikura, M.; Niki, T.; Hirashima, M.; Iwaya, K.; Tsuda, H.; Nonoyama, S.; Matsuda, A.; Saito, H.; et al. Galectin-9 enhances cytokine secretion, but suppresses survival and degranulation, in human mast cell line. PLoS ONE 2014, 9, e086106. [Google Scholar] [CrossRef]
- Groot Kormelink, T.; Powe, D.G.; Kuijpers, S.A.; Abudukelimu, A.; Fens, M.H.; Pieters, E.H.; Kassing van der Ven, W.W.; Habashy, H.O.; Ellis, I.O.; Blokhuis, B.R.; et al. Immunoglobulin free light chains are biomarkers of poor prognosis in basal-like breast cancer and are potential targets in tumor-associated inflammation. Oncotarget 2014, 5, 3159–3167. [Google Scholar] [CrossRef][Green Version]
- Drobits, B.; Holcmann, M.; Amberg, N.; Swiecki, M.; Grundtner, R.; Hammer, M.; Colonna, M.; Sibilia, M. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J. Clin. Investig. 2012, 122, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Oyama, S.; Funasaka, Y.; Tsuchiya, S.i.; Kawana, S.; Saeki, H. Increased number of mast cells in the dermis in actinic keratosis lesions effectively treated with imiquimod. J. Dermatol. 2017, 44, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Tang, R.; Luo, M. Oncolytic virotherapy, alone or in combination with immune checkpoint inhibitors, for advanced melanoma: A systematic review and meta-analysis. Int. Immunopharmacol. 2020, 78, 106050. [Google Scholar] [CrossRef] [PubMed]
- Lalu, M.; Leung, G.J.; Dong, Y.Y.; Montroy, J.; Butler, C.; Auer, R.C.; Fergusson, D.A. Mapping the preclinical to clinical evidence and development trajectory of the oncolytic virus talimogene laherparepvec (T-VEC): A systematic review. BMJ Open 2019, 9, e029475. [Google Scholar] [CrossRef]
- Lawler, S.E.; Speranza, M.C.; Cho, C.F.; Chiocca, E.A. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017, 3, 841–849. [Google Scholar] [CrossRef]
- McAlpine, S.M.; Issekutz, T.B.; Marshall, J.S. Virus stimulation of human mast cells results in the recruitment of CD56+ T cells by a mechanism dependent on CCR5 ligands. FASEB J. 2012, 26, 1280–1289. [Google Scholar] [CrossRef]
- Burke, S.M.; Issekutz, T.B.; Mohan, K.; Lee, P.W.; Shmulevitz, M.; Marshall, J.S. Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism. Blood J. Am. Soc. Hematol. 2008, 111, 5467–5476. [Google Scholar] [CrossRef]
- Portales-Cervantes, L.; Dawod, B.; Marshall, J.S. Mast Cells and Natural Killer Cells-A Potentially Critical Interaction. Viruses 2019, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Portales-Cervantes, L.; Haidl, I.D.; Lee, P.W.; Marshall, J.S. Virus-Infected Human Mast Cells Enhance Natural Killer Cell Functions. J. Innate Immun. 2017, 9, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Oldford, S.A.; Salsman, S.P.; Portales-Cervantes, L.; Alyazidi, R.; Anderson, R.; Haidl, I.D.; Marshall, J.S. Interferon α2 and interferon γ induce the degranulation independent production of VEGF-A and IL-1 receptor antagonist and other mediators from human mast cells. Immun. Inflamm. Dis. 2018, 6, 176–189. [Google Scholar] [CrossRef]
- Mantri, C.K.; St John, A.L. Immune synapses between mast cells and gammadelta T cells limit viral infection. J. Clin. Investig. 2019, 129, 1094–1108. [Google Scholar] [CrossRef]
- Guerin, M.V.; Finisguerra, V.; Van den Eynde, B.J.; Bercovici, N.; Trautmann, A. Preclinical murine tumor models: A structural and functional perspective. Elife 2020, 9, e50740. [Google Scholar] [CrossRef] [PubMed]
- Dudeck, J.; Medyukhina, A.; Fröbel, J.; Svensson, C.-M.; Kotrba, J.; Gerlach, M.; Gradtke, A.-C.; Schröder, B.; Speier, S.; Figge, M.T. Mast cells acquire MHCII from dendritic cells during skin inflammation. J. Exp. Med. 2017, 214, 3791–3811. [Google Scholar] [CrossRef] [PubMed]
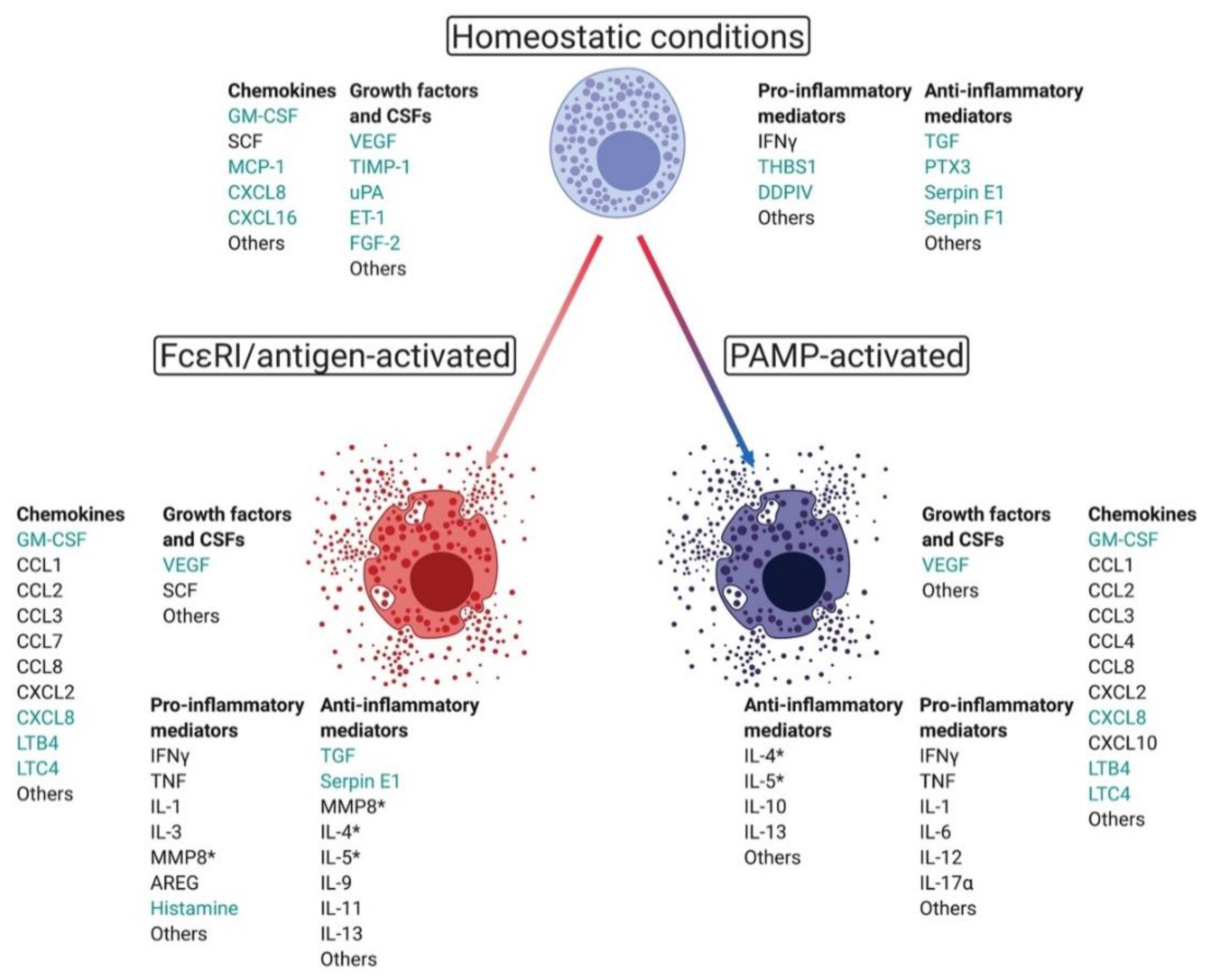
| Cancer Type | Tumor Model | Mouse Strain | MC-Deficiency Model | MC-Modulator | Role | Publication |
|---|---|---|---|---|---|---|
| Melanoma | B16-F10 (S.c.) | C57BL/6 | KitW/KitW-v (c-Kit-dependent) | None | Pro-tumor | Starkey et al., 1988 [4] |
| Melanoma | B16-F10 (S.c.) | C57BL/6 | KitW-sh/KitW-sh (c-Kit-dependent) | None | Pro-tumor | Oldford et al., 2010 [33] |
| Melanoma | B16-F10 (S.c.) | C57BL/6 | Mcpt5-Cre+R-DTA+ (c-Kit-independent) | None | No contribution | Öhrvik et al., 2016 [32] |
| Melanoma | B16-F10 (I.d.) | C57BL/6 | Mcpt5-Cre+R-DTA+ (c-Kit-independent) | None | No contribution | Ghouse et al., 2018 [20] |
| Melanoma | Tg(GRM1)Epv (Spontaneous) | C57BL/6 | Cre-Master (c-Kit-independent) | None | Anti-tumor | Stieglitz et al., 2019 [31] |
| Skin carcinogenesis | DMBA/TPA (Topical) | C57BL/6 | Cre-Master (c-Kit-independent) | None | No contribution | Antsiferova et al., 2013 [22] |
| Skin carcinogenesis | DMBA/TPA (Topical) | WBB6F1 | KitW/KitW-v (c-Kit-dependent) | None | Anti-tumor | Siebenhaar et al., 2014 [23] |
| Squamous cell carcinoma | K14-HPV16 (Spontaneous) | FVB/n | Mcpt5-Cre+R-DTA+ (c-Kit-independent) | None | No contribution | Ghouse et al., 2018 [20] |
| Squamous cell carcinoma | K14-HPV16 (Spontaneous) | FVB/n | KitW/KitW-v (c-Kit-dependent) | None | Pro-tumor | Coussens et al., 1999 [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanes, M.R.; Giacomantonio, C.A.; Marshall, J.S. Mast Cells and Skin and Breast Cancers: A Complicated and Microenvironment-Dependent Role. Cells 2021, 10, 986. https://doi.org/10.3390/cells10050986
Hanes MR, Giacomantonio CA, Marshall JS. Mast Cells and Skin and Breast Cancers: A Complicated and Microenvironment-Dependent Role. Cells. 2021; 10(5):986. https://doi.org/10.3390/cells10050986
Chicago/Turabian StyleHanes, Mark R., Carman A. Giacomantonio, and Jean S. Marshall. 2021. "Mast Cells and Skin and Breast Cancers: A Complicated and Microenvironment-Dependent Role" Cells 10, no. 5: 986. https://doi.org/10.3390/cells10050986
APA StyleHanes, M. R., Giacomantonio, C. A., & Marshall, J. S. (2021). Mast Cells and Skin and Breast Cancers: A Complicated and Microenvironment-Dependent Role. Cells, 10(5), 986. https://doi.org/10.3390/cells10050986






