P2X Receptor-Dependent Modulation of Mast Cell and Glial Cell Activities in Neuroinflammation
Abstract
1. Introduction
2. MCs and Glial Cells in Neuroinflammation
3. Expression of P2XRs and the Role of ATP and P2XR Activation in Neuroinflammation
3.1. ATP Release during Inflammation and Brain Pathology
3.2. Expression of P2XRs and The Role of ATP and P2XR Activation in Glial Cells
3.3. P2XR Expression in Astrocytes
3.4. P2XR Expression in Microglia
3.5. P2XR Expression in Oligodendrocytes
3.6. Expression of P2XRs and The Role of ATP and P2XR Activation in MCs
3.7. Expression of P2XRs: Public Gene Expression Databases
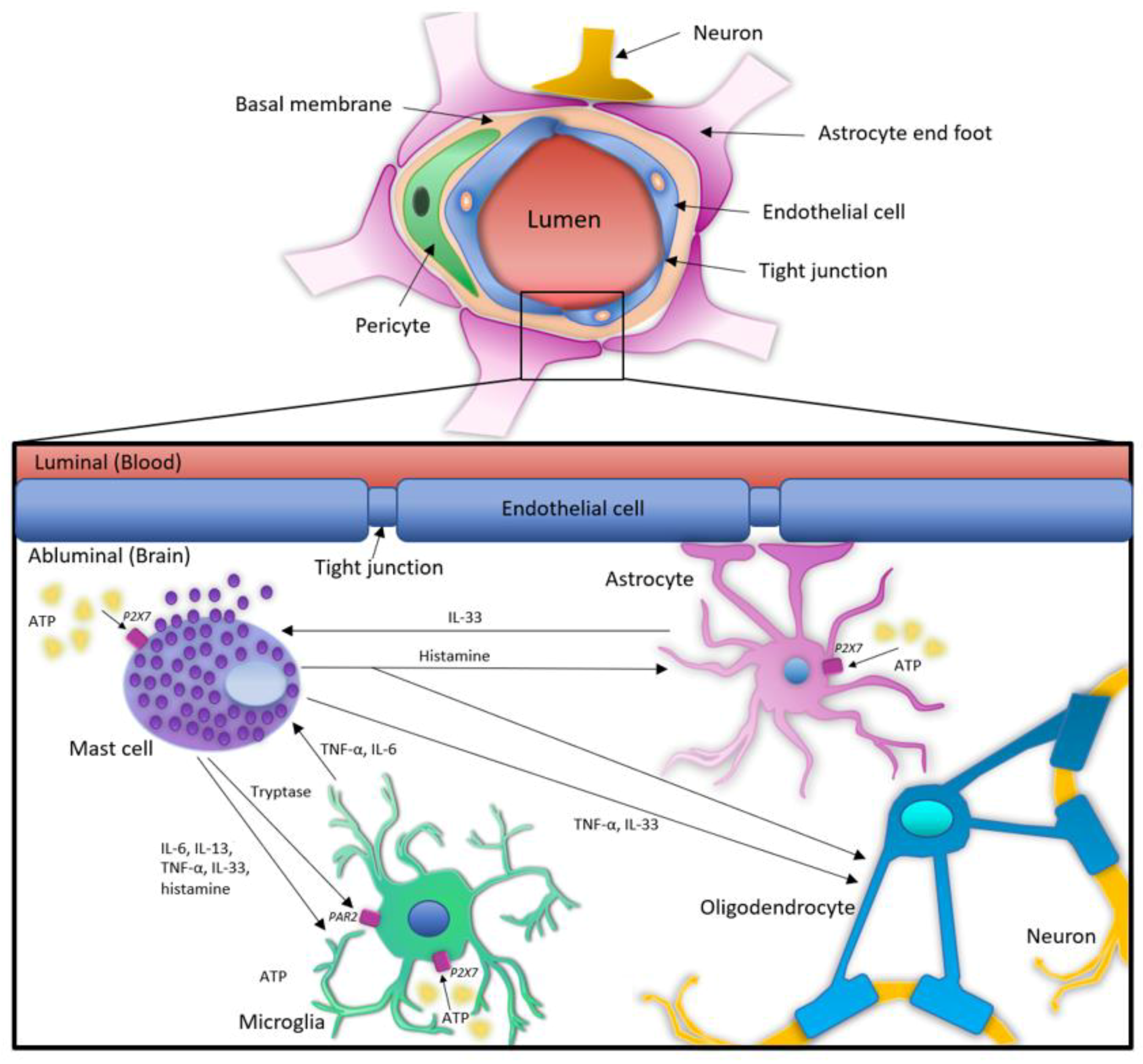
4. Interactions and Cross-Talk between MCs and Glial Cells in Neuroinflammation: The Role of ATP and P2XRs
| P2XR Induced Activities | |||
|---|---|---|---|
| Mediators | Glial Cells | MCs | References |
| Tryptase | Upregulation of P2X4 on microglia | N/A | [157] |
| PAR2 | Release of MC tryptase activates PAR2 receptor on microglia | Activation of PAR2 receptor in microglia results in TNF and IL-6 release, affecting MCs | [154,155,156] |
| TNF-α/IL-6 | Apoptosis in oligodendrocytes; glutamate release from astrocytes | Secretion of IL-13 and IL-4 from MCs, together with upregulation of TLR receptors | [9,153,158] |
| Histamine | Release of TNF-α, IL-1β and IL-6 from microglia; inhibition of TNF-α and IL-1β expression in astrocytes; negative regulation of oligodendrocytes differentiation | N/A | [160,161,162,163,164] |
| IL-33 | Promotion of microglia migration to site of CNS injury and release of pro-inflammatory mediators; inhibition of myelination by oligodendrocytes; release of IL-33 from astrocytes delays ASL disease onset and promotes microglia synapse engulfment | Functions as alarmin on MCs, affecting activation status and mediator release | [166,167,168,173,174,175] |
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | β-amyloid peptide |
| ADP | Adenosine diphosphate |
| AMP | Adenosine monophosphate |
| ALS | Amyotrophic lateral sclerosis |
| ASD | Autism spectrum disorder |
| ATP | Adenosine 5′-triphosphate |
| BBB | Blood brain barrier |
| BMMCs | Bone marrow-derived mast cells |
| CCL | Chemokine (C-C motif) ligand |
| CCR | C-C chemokine receptor type |
| CNS | Central nervous system |
| EAE | Experimental autoimmune encephalomyelitis |
| FcɛRI | High-affinity IgE receptor |
| FcγR | Fc-gamma receptor |
| IL | Interleukin |
| KO | Knockout |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MC | Mast cell |
| MS | Multiple sclerosis |
| PAR2 | Protease activated receptor 2 |
| PD | Parkinson’s disease |
| SSA | Serum amyloid A |
| TG2 | Tissue transglutaminase 2 |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor alpha |
References
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Olivera, A.; Beaven, M.A.; Metcalfe, D.D. Mast cells signal their importance in health and disease. J. Allergy Clin. Immunol. 2018, 142, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Bulle, M.J.; Msallam, R.; Gautier, G.; Launay, P.; Chen, J.; Ginhoux, F.; Bajénoff, M. Hemogenic Endothelial Fate Mapping Reveals Dual Developmental Origin of Mast Cells. Immunity 2018, 48, 1160.e5–1171.e5. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S.; Xu, J.; Zhang, X.; Han, D.; Liu, J.; Xia, M.; Yi, L.; Shen, Q.; Xu, S.; et al. Adult Connective Tissue-Resident Mast Cells Originate from Late Erythro-Myeloid Progenitors. Immunity 2018, 49, 640.e5–653.e5. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Ungerstedt, J.S.; Grootens, J.; Sander, B.; Gülen, T.; Hägglund, H.; Nilsson, G. Detection of circulating mast cells in advanced systemic mastocytosis. Leukemia 2016, 30, 1953–1956. [Google Scholar] [CrossRef]
- Méndez-Enríquez, E.; Hallgren, J. Mast Cells and Their Progenitors in Allergic Asthma. Front. Immunol. 2019, 10, 821. [Google Scholar] [CrossRef]
- Traina, G. Mast Cells in Gut and Brain and Their Potential Role as an Emerging Therapeutic Target for Neural Diseases. Front. Cell Neurosci. 2019, 13, 345. [Google Scholar] [CrossRef]
- Hendriksen, E.; van Bergeijk, D.; Oosting, R.S.; Redegeld, F.A. Mast cells in neuroinflammation and brain disorders. Neurosci. Biobehav. Rev. 2017, 79, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Maślińska, D.; Laure-Kamionowska, M.; Gujski, M.; Ciurzynska, G.; Wojtecka-Lukasik, E. Post-infectional distribution and phenotype of mast cells penetrating human brains. Inflamm. Res. 2005, 54 (Suppl. S1), S15–S16. [Google Scholar] [CrossRef]
- Joshi, A.; Page, C.E.; Damante, M.; Dye, C.N.; Haim, A.; Leuner, B.; Lenz, K.M. Sex differences in the effects of early life stress exposure on mast cells in the developing rat brain. Horm. Behav. 2019, 113, 76–84. [Google Scholar] [CrossRef]
- Tanioka, D.; Chikahisa, S.; Shimizu, N.; Shiuchi, T.; Sakai, N.; Nishino, S.; Séi, H. Intracranial mast cells contribute to the control of social behavior in male mice. Behav. Brain Res. 2021, 403, 113143. [Google Scholar] [CrossRef]
- Lenz, K.M.; Pickett, L.A.; Wright, C.L.; Davis, K.T.; Joshi, A.; McCarthy, M.M. Mast Cells in the Developing Brain Determine Adult Sexual Behavior. J. Neurosci. 2018, 38, 8044–8059. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Nugent, B.M.; Lenz, K.M. Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nat. Rev. Neurosci. 2017, 18, 471–484. [Google Scholar] [CrossRef]
- Silver, R.; Curley, J.P. Mast cells on the mind: New insights and opportunities. Trends Neurosci. 2013, 36, 513–521. [Google Scholar] [CrossRef]
- Turygin, V.V.; Babik, T.M.; Boyakov, A.A. Characteristics of mast cells in the choroid plexus of the ventricles of the human brain in aging. Neurosci. Behav. Physiol. 2005, 35, 909–911. [Google Scholar] [CrossRef] [PubMed]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Fredholm, B.B.; North, R.A.; Verkhratsky, A. The birth and postnatal development of purinergic signalling. Acta Physiol. 2010, 199, 93–147. [Google Scholar] [CrossRef]
- Wareham, K.; Vial, C.; Wykes, R.C.; Bradding, P.; Seward, E.P. Functional evidence for the expression of P2X1, P2X4 and P2X7 receptors in human lung mast cells. Br. J. Pharmacol. 2009, 157, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Wareham, K.J.; Seward, E.P. P2X7 receptors induce degranulation in human mast cells. Purinergic Signal. 2016, 12, 235–246. [Google Scholar] [CrossRef]
- Yoshida, K.; Ito, M.; Matsuoka, I. Divergent regulatory roles of extracellular ATP in the degranulation response of mouse bone marrow-derived mast cells. Int. Immunopharmacol. 2017, 43, 99–107. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Pankratov, Y.; Lalo, U.; Nedergaard, M. P2X receptors in neuroglia. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 151–161. [Google Scholar] [CrossRef]
- Martínez-Frailes, C.; Di Lauro, C.; Bianchi, C.; de Diego-García, L.; Sebastián-Serrano, Á.; Boscá, L.; Díaz-Hernández, M. Amyloid Peptide Induced Neuroinflammation Increases the P2X7 Receptor Expression in Microglial Cells, Impacting on Its Functionality. Front. Cell Neurosci. 2019, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Crabbé, M.; Van der Perren, A.; Bollaerts, I.; Kounelis, S.; Baekelandt, V.; Bormans, G.; Casteels, C.; Moons, L.; Van Laere, K. Increased P2X7 Receptor Binding Is Associated With Neuroinflammation in Acute but Not Chronic Rodent Models for Parkinson’s Disease. Front. Neurosci. 2019, 13, 799. [Google Scholar] [CrossRef]
- Domercq, M.; Matute, C. Targeting P2X4 and P2X7 receptors in multiple sclerosis. Curr. Opin. Pharmacol. 2019, 47, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.C.; Sanberg, P.R.; Cruz, L.E.; Garbuzova-Davis, S. The innate and adaptive immunological aspects in neurodegenerative diseases. J. Neuroimmunol. 2014, 269, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef]
- Liu, C.Y.; Wang, X.; Liu, C.; Zhang, H.L. Pharmacological Targeting of Microglial Activation: New Therapeutic Approach. Front. Cell Neurosci. 2019, 13, 514. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef]
- Molofsky, A.V.; Deneen, B. Astrocyte development: A Guide for the Perplexed. Glia 2015, 63, 1320–1329. [Google Scholar] [CrossRef]
- Guillamón-Vivancos, T.; Gómez-Pinedo, U.; Matías-Guiu, J. Astrocytes in neurodegenerative diseases (I): Function and molecular description. Neurologia 2015, 30, 119–129. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.W.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef] [PubMed]
- Nasrabady, S.E.; Rizvi, B.; Goldman, J.E.; Brickman, A.M. White matter changes in Alzheimer’s disease: A focus on myelin and oligodendrocytes. Acta Neuropathol. Commun. 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef]
- Tore, F.; Tuncel, N. Mast cells: Target and source of neuropeptides. Curr. Pharm. Des. 2009, 15, 3433–3445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Dong, H.; Xu, Y.; Zhang, S. Induction of Microglial Activation by Mediators Released from Mast Cells. Cell Physiol. Biochem. 2016, 38, 1520–1531. [Google Scholar] [CrossRef]
- Gupta, K.; Harvima, I.T. Mast cell-neural interactions contribute to pain and itch. Immunol. Rev. 2018, 282, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Marszalek, P.E.; Farrell, B.; Verdugo, P.; Fernandez, J.M. Kinetics of release of serotonin from isolated secretory granules. I. Amperometric detection of serotonin from electroporated granules. Biophys. J. 1997, 73, 1160–1168. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Dong, H.; Hu, Y.; Qian, Y. Bidirectional relationship of mast cells-neurovascular unit communication in neuroinflammation and its involvement in POCD. Behav. Brain Res. 2017, 322, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Mentor, S.; Thangavel, R.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Dubova, I.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Mast Cells in Stress, Pain, Blood-Brain Barrier, Neuroinflammation and Alzheimer’s Disease. Front. Cell Neurosci. 2019, 13, 54. [Google Scholar] [CrossRef]
- Tran, H.; Mittal, A.; Sagi, V.; Luk, K.; Nguyen, A.; Gupta, M.; Nguyen, J.; Lamarre, Y.; Lei, J.; Guedes, A.; et al. Mast Cells Induce Blood Brain Barrier Damage in SCD by Causing Endoplasmic Reticulum Stress in the Endothelium. Front. Cell Neurosci. 2019, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, K.M.; Dailey, C.A.; Jahn, J.L.; Rodriquez, E.; Son, N.H.; Sweedler, J.V.; Silver, R. Serotonin of mast cell origin contributes to hippocampal function. Eur. J. Neurosci. 2012, 36, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Chikahisa, S.; Kodama, T.; Soya, A.; Sagawa, Y.; Ishimaru, Y.; Séi, H.; Nishino, S. Histamine from brain resident MAST cells promotes wakefulness and modulates behavioral states. PLoS ONE 2013, 8, e78434. [Google Scholar] [CrossRef]
- Hendrix, S.; Kramer, P.; Pehl, D.; Warnke, K.; Boato, F.; Nelissen, S.; Lemmens, E.; Pejler, G.; Metz, M.; Siebenhaar, F.; et al. Mast cells protect from post-traumatic brain inflammation by the mast cell-specific chymase mouse mast cell protease-4. FASEB J. 2013, 27, 920–929. [Google Scholar] [CrossRef]
- Kulka, M.; Fukuishi, N.; Metcalfe, D.D. Human mast cells synthesize and release angiogenin, a member of the ribonuclease A (RNase A) superfamily. J. Leukoc. Biol. 2009, 86, 1217–1226. [Google Scholar] [CrossRef]
- Subramanian, V.; Crabtree, B.; Acharya, K.R. Human angiogenin is a neuroprotective factor and amyotrophic lateral sclerosis associated angiogenin variants affect neurite extension/pathfinding and survival of motor neurons. Hum. Mol. Genet. 2008, 17, 130–149. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kempuraj, D.; Iliopoulou, B.P. Mast cells, T cells, and inhibition by luteolin: Implications for the pathogenesis and treatment of multiple sclerosis. Adv. Exp. Med. Biol. 2007, 601, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Christy, A.L.; Walker, M.E.; Hessner, M.J.; Brown, M.A. Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. J. Autoimmun. 2013, 42, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Selvakumar, G.P.; Zaheer, S.; Thangavel, R.; Ahmed, M.E.; Raikwar, S.; Govindarajan, R.; Iyer, S.; Zaheer, A. Cross-Talk between Glia, Neurons and Mast Cells in Neuroinflammation Associated with Parkinson’s Disease. J. Neuroimmune Pharmacol. 2018, 13, 100–112. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.U.; Cho, J.W.; Kim, S.Y.; Shin, J.H.; Ro, J.Y. Inflammatory mediators resulting from transglutaminase 2 expressed in mast cells contribute to the development of Parkinson’s disease in a mouse model. Toxicol. Appl. Pharmacol. 2018, 358, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Ahmed, M.E.; Selvakumar, G.P.; Thangavel, R.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Burton, C.; James, D.; Zaheer, A. Mast Cell Activation, Neuroinflammation, and Tight Junction Protein Derangement in Acute Traumatic Brain Injury. Mediat. Inflamm. 2020, 2020, 4243953. [Google Scholar] [CrossRef]
- Angelini, D.F.; De Angelis, F.; Vacca, V.; Piras, E.; Parisi, C.; Nutini, M.; Spalloni, A.; Pagano, F.; Longone, P.; Battistini, L.; et al. Very Early Involvement of Innate Immunity in Peripheral Nerve Degeneration in SOD1-G93A Mice. Front. Immunol. 2020, 11, 575792. [Google Scholar] [CrossRef] [PubMed]
- Trias, E.; King, P.H.; Si, Y.; Kwon, Y.; Varela, V.; Ibarburu, S.; Kovacs, M.; Moura, I.C.; Beckman, J.S.; Hermine, O.; et al. Mast cells and neutrophils mediate peripheral motor pathway degeneration in ALS. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Miida, T.; Yamada, T.; Seino, U.; Ito, M.; Fueki, Y.; Takahashi, A.; Kosuge, K.; Soda, S.; Hanyu, O.; Obayashi, K.; et al. Serum amyloid A (SAA)-induced remodeling of CSF-HDL. Biochim. Biophys. Acta 2006, 1761, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Barbierato, M.; Borri, M.; Facci, L.; Zusso, M.; Skaper, S.D.; Giusti, P. Expression and Differential Responsiveness of Central Nervous System Glial Cell Populations to the Acute Phase Protein Serum Amyloid A. Sci. Rep. 2017, 7, 12158. [Google Scholar] [CrossRef]
- Olsson, N.; Siegbahn, A.; Nilsson, G. Serum amyloid A induces chemotaxis of human mast cells by activating a pertussis toxin-sensitive signal transduction pathway. Biochem. Biophys. Res. Commun. 1999, 254, 143–146. [Google Scholar] [CrossRef]
- Niemi, K.; Baumann, M.H.; Kovanen, P.T.; Eklund, K.K. Serum amyloid A (SAA) activates human mast cells which leads into degradation of SAA and generation of an amyloidogenic SAA fragment. Biochim. Biophys. Acta 2006, 1762, 424–430. [Google Scholar] [CrossRef][Green Version]
- Mikolajczyk, T.P.; Szczepaniak, P.; Vidler, F.; Maffia, P.; Graham, G.J.; Guzik, T.J. Role of inflammatory chemokines in hypertension. Pharmacol. Ther. 2021, 223, 107799. [Google Scholar] [CrossRef]
- Lalo, U.; Verkhratsky, A.; Pankratov, Y. Ionotropic ATP receptors in neuronal-glial communication. Semin. Cell Dev. Biol. 2011, 22, 220–228. [Google Scholar] [CrossRef]
- Cisneros-Mejorado, A.; Pérez-Samartín, A.; Gottlieb, M.; Matute, C. ATP signaling in brain: Release, excitotoxicity and potential therapeutic targets. Cell Mol. Neurobiol. 2015, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Abbracchio, M.P.; Burnstock, G.; Verkhratsky, A.; Zimmermann, H. Purinergic signalling in the nervous system: An overview. Trends Neurosci. 2009, 32, 19–29. [Google Scholar] [CrossRef]
- Merighi, S.; Poloni, T.E.; Terrazzan, A.; Moretti, E.; Gessi, S.; Ferrari, D. Alzheimer and Purinergic Signaling: Just a Matter of Inflammation? Cells 2021, 10, 1267. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Sarti, A.C.; Coutinho-Silva, R. Purinergic signaling, DAMPs, and inflammation. Am. J. Physiol. Cell Physiol. 2020, 318, C832–c835. [Google Scholar] [CrossRef]
- Rodrigues, R.J.; Tomé, A.R.; Cunha, R.A. ATP as a multi-target danger signal in the brain. Front. Neurosci. 2015, 9, 148. [Google Scholar] [CrossRef]
- Atif, M.; Alsrhani, A.; Naz, F.; Imran, M.; Imran, M.; Ullah, M.I.; Alameen, A.A.M.; Gondal, T.A.; Raza, Q. Targeting Adenosine Receptors in Neurological Diseases. Cell Reprogram. 2021, 23, 57–72. [Google Scholar] [CrossRef]
- Dosch, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP Release by Inflammatory Cells. Int. J. Mol. Sci. 2018, 19, 1222. [Google Scholar] [CrossRef]
- Dou, L.; Chen, Y.F.; Cowan, P.J.; Chen, X.P. Extracellular ATP signaling and clinical relevance. Clin. Immunol. 2018, 188, 67–73. [Google Scholar] [CrossRef]
- Burnstock, G. Introduction to Purinergic Signalling in the Brain. In Glioma Signaling. Advances in Experimental Medicine and Biology; Barańska, J., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef]
- Kopp, R.; Krautloher, A.; Ramírez-Fernández, A.; Nicke, A. P2X7 Interactions and Signaling—Making Head or Tail of It. Front. Mol. Neurosci. 2019, 12, 183. [Google Scholar] [CrossRef]
- Saul, A.; Hausmann, R.; Kless, A.; Nicke, A. Heteromeric assembly of P2X subunits. Front. Cell Neurosci. 2013, 7, 250. [Google Scholar] [CrossRef]
- Hou, Z.; Cao, J. Comparative study of the P2X gene family in animals and plants. Purinergic Signal. 2016, 12, 269–281. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Müller, C.E. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 2016, 104, 31–49. [Google Scholar] [CrossRef]
- Kong, Q.; Peterson, T.S.; Baker, O.; Stanley, E.; Camden, J.; Seye, C.I.; Erb, L.; Simonyi, A.; Wood, W.G.; Sun, G.Y.; et al. Interleukin-1beta enhances nucleotide-induced and alpha-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y(2) receptor. J. Neurochem. 2009, 109, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Ferradas, C.; Morales, J.C.; Wellmann, M.; Nualart, F.; Roncagliolo, M.; Fuenzalida, M.; Bonansco, C. Enhanced astroglial Ca2+ signaling increases excitatory synaptic strength in the epileptic brain. Glia 2015, 63, 1507–1521. [Google Scholar] [CrossRef]
- Alves, M.; Beamer, E.; Engel, T. The Metabotropic Purinergic P2Y Receptor Family as Novel Drug Target in Epilepsy. Front. Pharmacol. 2018, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, P.; Madeira, D.; Dias, L.; Simões, A.P.; Cunha, R.A.; Canas, P.M. Purinergic signaling orchestrating neuron-glia communication. Pharmacol. Res. 2020, 162, 105253. [Google Scholar] [CrossRef]
- Gao, Z.G.; Jacobson, K.A. Purinergic Signaling in Mast Cell Degranulation and Asthma. Front. Pharmacol. 2017, 8, 947. [Google Scholar] [CrossRef]
- Lalo, U.; Pankratov, Y.; Wichert, S.P.; Rossner, M.J.; North, R.A.; Kirchhoff, F.; Verkhratsky, A. P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J. Neurosci. 2008, 28, 5473–5480. [Google Scholar] [CrossRef] [PubMed]
- Lalo, U.; Palygin, O.; North, R.A.; Verkhratsky, A.; Pankratov, Y. Age-dependent remodelling of ionotropic signalling in cortical astroglia. Aging Cell 2011, 10, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kong, Y.; Wu, D.-Y.; Liu, J.-H.; Jie, W.; You, Q.-L.; Huang, L.; Hu, J.; Chu, H.-D.; Gao, F.; et al. Impaired calcium signaling in astrocytes modulates autism spectrum disorder-like behaviors in mice. Nat. Commun. 2021, 12, 3321. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Boku, S.; Okazaki, S.; Kikuyama, H.; Mizoguchi, Y.; Monji, A.; Otsuka, I.; Sora, I.; Kanazawa, T.; Hishimoto, A.; et al. ATP and repetitive electric stimulation increases leukemia inhibitory factor expression in astrocytes: A potential role for astrocytes in the action mechanism of electroconvulsive therapy. Psychiatry Clin. Neurosci. 2020, 74, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Mah, W.; Lee, S.M.; Lee, J.; Bae, J.Y.; Ju, J.S.; Lee, C.J.; Ahn, D.K.; Bae, Y.C. A role for the purinergic receptor P2X3 in astrocytes in the mechanism of craniofacial neuropathic pain. Sci. Rep. 2017, 7, 13627. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Brambilla, R.; D’Ambrosi, N.; Volonté, C.; Matteoli, M.; Verderio, C.; Abbracchio, M.P. Nucleotide-mediated calcium signaling in rat cortical astrocytes: Role of P2X and P2Y receptors. Glia 2003, 43, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Bae, J.Y.; Lee, C.J.; Bae, Y.C. Electrophysiological Evidence for Functional Astrocytic P2X(3) Receptors in the Mouse Trigeminal Caudal Nucleus. Exp. Neurobiol. 2018, 27, 88–93. [Google Scholar] [CrossRef]
- Franke, H.; Grosche, J.; Schädlich, H.; Krügel, U.; Allgaier, C.; Illes, P. P2X receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience 2001, 108, 421–429. [Google Scholar] [CrossRef]
- Ashour, F.; Deuchars, J. Electron microscopic localisation of P2X4 receptor subunit immunoreactivity to pre- and post-synaptic neuronal elements and glial processes in the dorsal vagal complex of the rat. Brain Res. 2004, 1026, 44–55. [Google Scholar] [CrossRef]
- Jabs, R.; Matthias, K.; Grote, A.; Grauer, M.; Seifert, G.; Steinhäuser, C. Lack of P2X receptor mediated currents in astrocytes and GluR type glial cells of the hippocampal CA1 region. Glia 2007, 55, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.S.; Taylor, C.W. ATP evokes Ca(2+) signals in cultured foetal human cortical astrocytes entirely through G protein-coupled P2Y receptors. J. Neurochem. 2017, 142, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Loesch, A. On P2X receptors in the brain: Microvessels. Dedicated to the memory of the late Professor Geoffrey Burnstock (1929–2020). Cell Tissue Res. 2021, 384, 577–588. [Google Scholar] [CrossRef]
- Kucher, B.M.; Neary, J.T. Bi-functional effects of ATP/P2 receptor activation on tumor necrosis factor-alpha release in lipopolysaccharide-stimulated astrocytes. J. Neurochem. 2005, 92, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Anderson, C.M.; Keung, E.C.; Chen, Y.; Chen, Y.; Swanson, R.A. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J. Neurosci. 2003, 23, 1320–1328. [Google Scholar] [CrossRef]
- Panenka, W.; Jijon, H.; Herx, L.M.; Armstrong, J.N.; Feighan, D.; Wei, T.; Yong, V.W.; Ransohoff, R.M.; MacVicar, B.A. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J. Neurosci. 2001, 21, 7135–7142. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Deussing, J.; Tang, Y.; Illes, P. Astrocytic rather than neuronal P2X7 receptors modulate the function of the tri-synaptic network in the rodent hippocampus. Brain Res. Bull. 2019, 151, 164–173. [Google Scholar] [CrossRef]
- Narcisse, L.; Scemes, E.; Zhao, Y.; Lee, S.C.; Brosnan, C.F. The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia 2005, 49, 245–258. [Google Scholar] [CrossRef]
- Martin, E.; Amar, M.; Dalle, C.; Youssef, I.; Boucher, C.; Le Duigou, C.; Brückner, M.; Prigent, A.; Sazdovitch, V.; Halle, A.; et al. New role of P2X7 receptor in an Alzheimer’s disease mouse model. Mol. Psychiatry 2019, 24, 108–125. [Google Scholar] [CrossRef]
- Gandelman, M.; Peluffo, H.; Beckman, J.S.; Cassina, P.; Barbeito, L. Extracellular ATP and the P2X7 receptor in astrocyte-mediated motor neuron death: Implications for amyotrophic lateral sclerosis. J. Neuroinflamm. 2010, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Calovi, S.; Mut-Arbona, P.; Sperlágh, B. Microglia and the Purinergic Signaling System. Neuroscience 2019, 405, 137–147. [Google Scholar] [CrossRef]
- Brautigam, V.M.; Frasier, C.; Nikodemova, M.; Watters, J.J. Purinergic receptor modulation of BV-2 microglial cell activity: Potential involvement of p38 MAP kinase and CREB. J. Neuroimmunol. 2005, 166, 113–125. [Google Scholar] [CrossRef]
- Seo, D.R.; Kim, S.Y.; Kim, K.Y.; Lee, H.G.; Moon, J.H.; Lee, J.S.; Lee, S.H.; Kim, S.U.; Lee, Y.B. Cross talk between P2 purinergic receptors modulates extracellular ATP-mediated interleukin-10 production in rat microglial cells. Exp. Mol. Med. 2008, 40, 19–26. [Google Scholar] [CrossRef]
- Lewis, N.D.; Hill, J.D.; Juchem, K.W.; Stefanopoulos, D.E.; Modis, L.K. RNA sequencing of microglia and monocyte-derived macrophages from mice with experimental autoimmune encephalomyelitis illustrates a changing phenotype with disease course. J. Neuroimmunol. 2014, 277, 26–38. [Google Scholar] [CrossRef]
- Bruttger, J.; Karram, K.; Wörtge, S.; Regen, T.; Marini, F.; Hoppmann, N.; Klein, M.; Blank, T.; Yona, S.; Wolf, Y.; et al. Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System. Immunity 2015, 43, 92–106. [Google Scholar] [CrossRef]
- Xiang, Z.; Burnstock, G. Expression of P2X receptors on rat microglial cells during early development. Glia 2005, 52, 119–126. [Google Scholar] [CrossRef]
- Janks, L.; Sharma, C.V.R.; Egan, T.M. A central role for P2X7 receptors in human microglia. J. Neuroinflamm. 2018, 15, 325. [Google Scholar] [CrossRef]
- Chiu, I.M.; Morimoto, E.T.; Goodarzi, H.; Liao, J.T.; O’Keeffe, S.; Phatnani, H.P.; Muratet, M.; Carroll, M.C.; Levy, S.; Tavazoie, S.; et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 2013, 4, 385–401. [Google Scholar] [CrossRef]
- Solga, A.C.; Pong, W.W.; Walker, J.; Wylie, T.; Magrini, V.; Apicelli, A.J.; Griffith, M.; Griffith, O.L.; Kohsaka, S.; Wu, G.F.; et al. RNA-sequencing reveals oligodendrocyte and neuronal transcripts in microglia relevant to central nervous system disease. Glia 2015, 63, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Fumagalli, M.; Pravettoni, E.; D’Ambrosi, N.; Volonte, C.; Matteoli, M.; Abbracchio, M.P.; Verderio, C. Pathophysiological roles of extracellular nucleotides in glial cells: Differential expression of purinergic receptors in resting and activated microglia. Brain Res. Brain Res. Rev. 2005, 48, 144–156. [Google Scholar] [CrossRef]
- Beggs, S.; Trang, T.; Salter, M.W. P2X4R+ microglia drive neuropathic pain. Nat. Neurosci. 2012, 15, 1068–1073. [Google Scholar] [CrossRef]
- Toyomitsu, E.; Tsuda, M.; Yamashita, T.; Tozaki-Saitoh, H.; Tanaka, Y.; Inoue, K. CCL2 promotes P2X4 receptor trafficking to the cell surface of microglia. Purinergic Signal. 2012, 8, 301–310. [Google Scholar] [CrossRef]
- Ulmann, L.; Hatcher, J.P.; Hughes, J.P.; Chaumont, S.; Green, P.J.; Conquet, F.; Buell, G.N.; Reeve, A.J.; Chessell, I.P.; Rassendren, F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J. Neurosci. 2008, 28, 11263–11268. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.A.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef]
- Zabala, A.; Vazquez-Villoldo, N.; Rissiek, B.; Gejo, J.; Martin, A.; Palomino, A.; Perez-Samartín, A.; Pulagam, K.R.; Lukowiak, M.; Capetillo-Zarate, E.; et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol. Med. 2018, 10. [Google Scholar] [CrossRef]
- Guo, L.H.; Trautmann, K.; Schluesener, H.J. Expression of P2X4 receptor by lesional activated microglia during formalin-induced inflammatory pain. J. Neuroimmunol. 2005, 163, 120–127. [Google Scholar] [CrossRef]
- Shieh, C.H.; Heinrich, A.; Serchov, T.; van Calker, D.; Biber, K. P2X7-dependent, but differentially regulated release of IL-6, CCL2, and TNF-α in cultured mouse microglia. Glia 2014, 62, 592–607. [Google Scholar] [CrossRef]
- He, Y.; Taylor, N.; Fourgeaud, L.; Bhattacharya, A. The role of microglial P2X7: Modulation of cell death and cytokine release. J. Neuroinflamm. 2017, 14, 135. [Google Scholar] [CrossRef]
- Sanz, J.M.; Chiozzi, P.; Ferrari, D.; Colaianna, M.; Idzko, M.; Falzoni, S.; Fellin, R.; Trabace, L.; Di Virgilio, F. Activation of microglia by amyloid {beta} requires P2X7 receptor expression. J. Immunol. 2009, 182, 4378–4385. [Google Scholar] [CrossRef]
- Wang, X.H.; Xie, X.; Luo, X.G.; Shang, H.; He, Z.Y. Inhibiting purinergic P2X7 receptors with the antagonist brilliant blue G is neuroprotective in an intranigral lipopolysaccharide animal model of Parkinson’s disease. Mol. Med. Rep. 2017, 15, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.E.; Roncalho, A.L.; Glaser, T.; Ulrich, H.; Wegener, G.; Joca, S. P2X7 Receptor Signaling in Stress and Depression. Int. J. Mol. Sci. 2019, 20, 2778. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosi, N.; Finocchi, P.; Apolloni, S.; Cozzolino, M.; Ferri, A.; Padovano, V.; Pietrini, G.; Carrì, M.T.; Volonté, C. The proinflammatory action of microglial P2 receptors is enhanced in SOD1 models for amyotrophic lateral sclerosis. J. Immunol. 2009, 183, 4648–4656. [Google Scholar] [CrossRef] [PubMed]
- Fabbrizio, P.; Amadio, S.; Apolloni, S.; Volonté, C. P2X7 Receptor Activation Modulates Autophagy in SOD1-G93A Mouse Microglia. Front. Cell Neurosci. 2017, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Apolloni, S.; Amadio, S.; Parisi, C.; Matteucci, A.; Potenza, R.L.; Armida, M.; Popoli, P.; D’Ambrosi, N.; Volonté, C. Spinal cord pathology is ameliorated by P2X7 antagonism in a SOD1-mutant mouse model of amyotrophic lateral sclerosis. Dis. Model. Mech. 2014, 7, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Ly, D.; Dongol, A.; Cuthbertson, P.; Guy, T.V.; Geraghty, N.J.; Sophocleous, R.A.; Sin, L.; Turner, B.J.; Watson, D.; Yerbury, J.J.; et al. The P2X7 receptor antagonist JNJ-47965567 administered thrice weekly from disease onset does not alter progression of amyotrophic lateral sclerosis in SOD1(G93A) mice. Purinergic Signal. 2020, 16, 109–122. [Google Scholar] [CrossRef]
- Agresti, C.; Meomartini, M.E.; Amadio, S.; Ambrosini, E.; Serafini, B.; Franchini, L.; Volonté, C.; Aloisi, F.; Visentin, S. Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia 2005, 50, 132–144. [Google Scholar] [CrossRef]
- Kashfi, S.; Peymani, M.; Ghaedi, K.; Baharvand, H.; Nasr-Esfahani, M.H.; Javan, M. Purinergic Receptor Expression and Potential Association with Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cell Development. Cell J. 2017, 19, 386–402. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Welsh, T.G.; Kucenas, S. Purinergic signaling in oligodendrocyte development and function. J. Neurochem. 2018, 145, 6–18. [Google Scholar] [CrossRef]
- Matute, C.; Torre, I.; Pérez-Cerdá, F.; Pérez-Samartín, A.; Alberdi, E.; Etxebarria, E.; Arranz, A.M.; Ravid, R.; Rodríguez-Antigüedad, A.; Sánchez-Gómez, M.; et al. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. 2007, 27, 9525–9533. [Google Scholar] [CrossRef]
- Morgan, J.; Alves, M.; Conte, G.; Menéndez-Méndez, A.; de Diego-Garcia, L.; de Leo, G.; Beamer, E.; Smith, J.; Nicke, A.; Engel, T. Characterization of the Expression of the ATP-Gated P2X7 Receptor Following Status Epilepticus and during Epilepsy Using a P2X7-EGFP Reporter Mouse. Neurosci. Bull. 2020, 36, 1242–1258. [Google Scholar] [CrossRef]
- Domercq, M.; Perez-Samartin, A.; Aparicio, D.; Alberdi, E.; Pampliega, O.; Matute, C. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia 2010, 58, 730–740. [Google Scholar] [CrossRef]
- Illes, P. P2X7 Receptors Amplify CNS Damage in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 5996. [Google Scholar] [CrossRef] [PubMed]
- Plum, T.; Wang, X.; Rettel, M.; Krijgsveld, J.; Feyerabend, T.B.; Rodewald, H.R. Human Mast Cell Proteome Reveals Unique Lineage, Putative Functions, and Structural Basis for Cell Ablation. Immunity 2020, 52, 404.e5–416.e5. [Google Scholar] [CrossRef]
- Bonvini, S.J.; Birrell, M.A.; Dubuis, E.; Adcock, J.J.; Wortley, M.A.; Flajolet, P.; Bradding, P.; Belvisi, M.G. Novel airway smooth muscle-mast cell interactions and a role for the TRPV4-ATP axis in non-atopic asthma. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef]
- Yoshida, K.; Tajima, M.; Nagano, T.; Obayashi, K.; Ito, M.; Yamamoto, K.; Matsuoka, I. Co-Stimulation of Purinergic P2X4 and Prostanoid EP3 Receptors Triggers Synergistic Degranulation in Murine Mast Cells. Int. J. Mol. Sci. 2019, 20, 5157. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Ito, M.A.; Sato, N.; Obayashi, K.; Yamamoto, K.; Koizumi, S.; Tanaka, S.; Furuta, K.; Matsuoka, I. Extracellular ATP Augments Antigen-Induced Murine Mast Cell Degranulation and Allergic Responses via P2X4 Receptor Activation. J. Immunol. 2020, 204, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Nurkhametova, D.; Kudryavtsev, I.; Guselnikova, V.; Serebryakova, M.; Giniatullina, R.R.; Wojciechowski, S.; Tore, F.; Rizvanov, A.; Koistinaho, J.; Malm, T.; et al. Activation of P2X7 Receptors in Peritoneal and Meningeal Mast Cells Detected by Uptake of Organic Dyes: Possible Purinergic Triggers of Neuroinflammation in Meninges. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef]
- Lundequist, A.; Pejler, G. Biological implications of preformed mast cell mediators. Cell Mol. Life Sci. 2011, 68, 965–975. [Google Scholar] [CrossRef]
- Shimokawa, C.; Kanaya, T.; Hachisuka, M.; Ishiwata, K.; Hisaeda, H.; Kurashima, Y.; Kiyono, H.; Yoshimoto, T.; Kaisho, T.; Ohno, H. Mast Cells Are Crucial for Induction of Group 2 Innate Lymphoid Cells and Clearance of Helminth Infections. Immunity 2017, 46, 863.e4–874.e4. [Google Scholar] [CrossRef]
- Motakis, E.; Guhl, S.; Ishizu, Y.; Itoh, M.; Kawaji, H.; de Hoon, M.; Lassmann, T.; Carninci, P.; Hayashizaki, Y.; Zuberbier, T.; et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 2014, 123, e58–e67. [Google Scholar] [CrossRef] [PubMed]
- Forrest, A.R.R.; Kawaji, H.; Rehli, M.; Kenneth Baillie, J.; de Hoon, M.J.L.; Haberle, V.; Lassmann, T.; Kulakovskiy, I.V.; Lizio, M.; Itoh, M.; et al. A promoter-level mammalian expression atlas. Nature 2014, 507, 462–470. [Google Scholar] [CrossRef]
- Dwyer, D.F.; Barrett, N.A.; Austen, K.F. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat. Immunol. 2016, 17, 878–887. [Google Scholar] [CrossRef]
- Ramírez-Ponce, M.P.; Sola-García, A.; Balseiro-Gómez, S.; Maldonado, M.D.; Acosta, J.; Alés, E.; Flores, J.A. Mast Cell Changes the Phenotype of Microglia via Histamine and ATP. Cell Physiol. Biochem. 2021, 55, 17–32. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, H.; Wang, F.; Zhang, J. Mast Cell Deficiency Protects Mice from Surgery-Induced Neuroinflammation. Mediat. Inflamm. 2020, 2020, 1921826. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, X.; Wang, Y.; Zhou, X.; Qian, Y.; Zhang, S. Suppression of Brain Mast Cells Degranulation Inhibits Microglial Activation and Central Nervous System Inflammation. Mol. Neurobiol. 2017, 54, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Hong, G.U.; Ro, J.Y. Signal pathways in astrocytes activated by cross-talk between of astrocytes and mast cells through CD40-CD40L. J. Neuroinflamm. 2011, 8, 25. [Google Scholar] [CrossRef]
- Medic, N.; Lorenzon, P.; Vita, F.; Trevisan, E.; Marchioli, A.; Soranzo, M.R.; Fabbretti, E.; Zabucchi, G. Mast cell adhesion induces cytoskeletal modifications and programmed cell death in oligodendrocytes. J. Neuroimmunol. 2010, 218, 57–66. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Caraffa, A.; Conti, C.; D’Ovidio, C.; Gallenga, C.E.; Tettamanti, L.; Mastrangelo, F.; Ronconi, G.; Kritas, S.K.; Conti, P. New concepts in neuroinflammation: Mast cells pro-inflammatory and anti-inflammatory cytokine mediators. J. Biol. Regul. Homeost. Agents 2018, 32, 449–454. [Google Scholar]
- Yehya, M.; Torbey, M.T. The Role of Mast Cells in Intracerebral Hemorrhage. Neurocrit. Care 2018, 28, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, A.; Matysiak, M.; Tybor, K.; Kilianek, L.; Raine, C.S.; Selmaj, K. Tumour necrosis factor-induced death of adult human oligodendrocytes is mediated by apoptosis inducing factor. Brain 2005, 128, 2675–2688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, X.; Yang, H.; Hu, G.; He, S. Mast cell tryptase induces microglia activation via protease-activated receptor 2 signaling. Cell Physiol. Biochem. 2012, 29, 931–940. [Google Scholar] [CrossRef]
- Erol, A.Y.G. The Role of Mast Cells and Neuroglia in Neuroinfectious Diseases. J. Neuroinfect. Dis. 2015, 2015. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Zhang, H.; Zhan, M.; Chen, H.; Fang, Z.; Xu, C.; Chen, H.; He, S. Induction of Mast Cell Accumulation by Tryptase via a Protease Activated Receptor-2 and ICAM-1 Dependent Mechanism. Mediat. Inflamm. 2016, 2016, 6431574. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhu, X.; Zhou, S.; Chen, Q.; Zhu, X.; Ma, X.; He, X.; Tian, M.; Shi, X. Role of mast cell activation in inducing microglial cells to release neurotrophin. J. Neurosci. Res. 2010, 88, 1348–1354. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; He, S. TNF increases expression of IL-4 and PARs in mast cells. Cell Physiol. Biochem. 2010, 26, 327–336. [Google Scholar] [CrossRef]
- Koyuncu Irmak, D.; Kilinc, E.; Tore, F. Shared Fate of Meningeal Mast Cells and Sensory Neurons in Migraine. Front. Cell Neurosci. 2019, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Santos, T.; Gonçalves, J.; Baltazar, G.; Ferreira, L.; Agasse, F.; Bernardino, L. Histamine modulates microglia function. J. Neuroinflamm. 2012, 9, 90. [Google Scholar] [CrossRef]
- Barata-Antunes, S.; Cristóvão, A.C.; Pires, J.; Rocha, S.M.; Bernardino, L. Dual role of histamine on microglia-induced neurodegeneration. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 764–769. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Qian, Q.; Wang, Y.; Dong, H.; Li, N.; Qian, Y.; Jin, W. Histamine upregulates the expression of histamine receptors and increases the neuroprotective effect of astrocytes. J. Neuroinflamm. 2018, 15, 41. [Google Scholar] [CrossRef]
- Jiang, L.; Cheng, L.; Chen, H.; Dai, H.; An, D.; Ma, Q.; Zheng, Y.; Zhang, X.; Hu, W.; Chen, Z. Histamine H2 receptor negatively regulates oligodendrocyte differentiation in neonatal hypoxic-ischemic white matter injury. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef]
- Chen, Y.; Zhen, W.; Guo, T.; Zhao, Y.; Liu, A.; Rubio, J.P.; Krull, D.; Richardson, J.C.; Lu, H.; Wang, R. Histamine Receptor 3 negatively regulates oligodendrocyte differentiation and remyelination. PLoS ONE 2017, 12, e0189380. [Google Scholar] [CrossRef]
- Kempuraj, D.; Selvakumar, G.P.; Thangavel, R.; Ahmed, M.E.; Zaheer, S.; Raikwar, S.P.; Iyer, S.S.; Bhagavan, S.M.; Beladakere-Ramaswamy, S.; Zaheer, A. Mast Cell Activation in Brain Injury, Stress, and Post-traumatic Stress Disorder and Alzheimer’s Disease Pathogenesis. Front. Neurosci. 2017, 11, 703. [Google Scholar] [CrossRef]
- Wicher, G.; Wallenquist, U.; Lei, Y.; Enoksson, M.; Li, X.; Fuchs, B.; Abu Hamdeh, S.; Marklund, N.; Hillered, L.; Nilsson, G.; et al. Interleukin-33 Promotes Recruitment of Microglia/Macrophages in Response to Traumatic Brain Injury. J. Neurotrauma 2017, 34, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Liao, X.; Lu, J.; Yao, S.; Wu, F.; Zhu, X.; Shi, D.; Wen, S.; Liu, L.; Zhou, H. IL-33/ST2 plays a critical role in endothelial cell activation and microglia-mediated neuroinflammation modulation. J. Neuroinflamm. 2018, 15, 136. [Google Scholar] [CrossRef]
- Allan, D.; Fairlie-Clarke, K.J.; Elliott, C.; Schuh, C.; Barnett, S.C.; Lassmann, H.; Linnington, C.; Jiang, H.-R. Role of IL-33 and ST2 signalling pathway in multiple sclerosis: Expression by oligodendrocytes and inhibition of myelination in central nervous system. Acta Neuropathol. Commun. 2016, 4, 75. [Google Scholar] [CrossRef]
- Skaper, S.D.; Giusti, P.; Facci, L. Microglia and mast cells: Two tracks on the road to neuroinflammation. FASEB J. 2012, 26, 3103–3117. [Google Scholar] [CrossRef]
- Dohi, E.; Choi, E.Y.; Rose, I.V.L.; Murata, A.S.; Chow, S.; Niwa, M.; Kano, S.I. Behavioral Changes in Mice Lacking Interleukin-33. eNeuro 2017, 4, ENEURO.0147-17.2017. [Google Scholar] [CrossRef] [PubMed]
- Yasuoka, S.; Kawanokuchi, J.; Parajuli, B.; Jin, S.; Doi, Y.; Noda, M.; Sonobe, Y.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Production and functions of IL-33 in the central nervous system. Brain Res. 2011, 1385, 8–17. [Google Scholar] [CrossRef]
- Reverchon, F.; Mortaud, S.; Sivoyon, M.; Maillet, I.; Laugeray, A.; Palomo, J.; Montécot, C.; Herzine, A.; Meme, S.; Meme, W.; et al. IL-33 receptor ST2 regulates the cognitive impairments associated with experimental cerebral malaria. PLoS Pathog. 2017, 13, e1006322. [Google Scholar] [CrossRef] [PubMed]
- Vainchtein, I.D.; Chin, G.; Cho, F.S.; Kelley, K.W.; Miller, J.G.; Chien, E.C.; Liddelow, S.A.; Nguyen, P.T.; Nakao-Inoue, H.; Dorman, L.C.; et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 2018, 359, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, P.; Pollari, E.; Kanninen, K.M.; Savchenko, E.; Lehtonen, Š.; Wojciechowski, S.; Pomeshchik, Y.; Van Den Bosch, L.; Goldsteins, G.; Koistinaho, J.; et al. Long-term interleukin-33 treatment delays disease onset and alleviates astrocytic activation in a transgenic mouse model of amyotrophic lateral sclerosis. IBRO Rep. 2019, 6, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.L.; Lam, C.W.K.; Tam, L.S.; Wong, C.K. IL33: Roles in Allergic Inflammation and Therapeutic Perspectives. Front. Immunol. 2019, 10, 364. [Google Scholar] [CrossRef]
| Gene | MCs | Astrocytes | Oligodendrocytes | Cell Types/Tissues with Highest RLE | |
|---|---|---|---|---|---|
| Skin (n = 4) | Cerebellum (n = 3) | Cortex (n = 3) | Precursors (n = 1) | ||
| P2X1 | 337.186 | 3.017 | 3.345 | 1.692 | 337.186 (MCs) |
| P2X2 | 0.032 | 0 | 0 | 0 | 15.242 (Seminal vesicle) |
| P2X3 | 25.120 | 67.947 | 96.481 | 51.310 | 282.548 (Smooth muscle cells) |
| P2X4 | 27.792 | 6.232 | 4.140 | 6.202 | 679.838(CD14+ monocytes) |
| P2X5 | 71.924 | 363.640 | 0.814 | 294.329 | 2237.838 (Bronchial epithelial cells) |
| P2X6 | 2.373 | 1.187 | 65.063 | 0 | 25.101(Cerebellum) |
| P2X7 | 14.628 | 0.164 | 0.324 | 0.564 | 692.939 (CD14+ monocytes) |
| Gene | MC Origin | ||||
|---|---|---|---|---|---|
| Skin (n = 3) | Peritoneal Cavity (n = 3) | Tongue (n = 3) | Oesophagus (n = 3) | Trachea (n = 3) | |
| P2X1 | 1356.95 | 2106.25 | 1715.59 | 1105.08 | 1535.27 |
| P2X2 | 77.3146 | 73.6825 | 87.4627 | 85.148 | 76.2191 |
| P2X3 | 65.3869 | 64.7405 | 61.4027 | 49.9133 | 51.1301 |
| P2X4 | 1289.72 | 3261.39 | 1656.1 | 1986.01 | 2416.66 |
| P2X5 | 165.822 | 117.41 | 146.774 | 142.33 | 143.221 |
| P2X6 | 112.974 | 97.5069 | 107.431 | 122.073 | 100.487 |
| P2X7 | 299.952 | 2413.83 | 695.205 | 871.043 | 1146.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salcman, B.; Affleck, K.; Bulfone-Paus, S. P2X Receptor-Dependent Modulation of Mast Cell and Glial Cell Activities in Neuroinflammation. Cells 2021, 10, 2282. https://doi.org/10.3390/cells10092282
Salcman B, Affleck K, Bulfone-Paus S. P2X Receptor-Dependent Modulation of Mast Cell and Glial Cell Activities in Neuroinflammation. Cells. 2021; 10(9):2282. https://doi.org/10.3390/cells10092282
Chicago/Turabian StyleSalcman, Barbora, Karen Affleck, and Silvia Bulfone-Paus. 2021. "P2X Receptor-Dependent Modulation of Mast Cell and Glial Cell Activities in Neuroinflammation" Cells 10, no. 9: 2282. https://doi.org/10.3390/cells10092282
APA StyleSalcman, B., Affleck, K., & Bulfone-Paus, S. (2021). P2X Receptor-Dependent Modulation of Mast Cell and Glial Cell Activities in Neuroinflammation. Cells, 10(9), 2282. https://doi.org/10.3390/cells10092282






