Differential Tropism in Roots and Shoots of Resistant and Susceptible Cassava (Manihot esculenta Crantz) Infected by Cassava Brown Streak Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cassava Varieties, Viruses and Virus Infection
2.2. Virus Quantification
2.3. Fixation, Embedding and Sectioning
2.4. RNAscope® In Situ Hybridization
2.5. Histology Staining
2.6. Microscopy Examination
3. Results
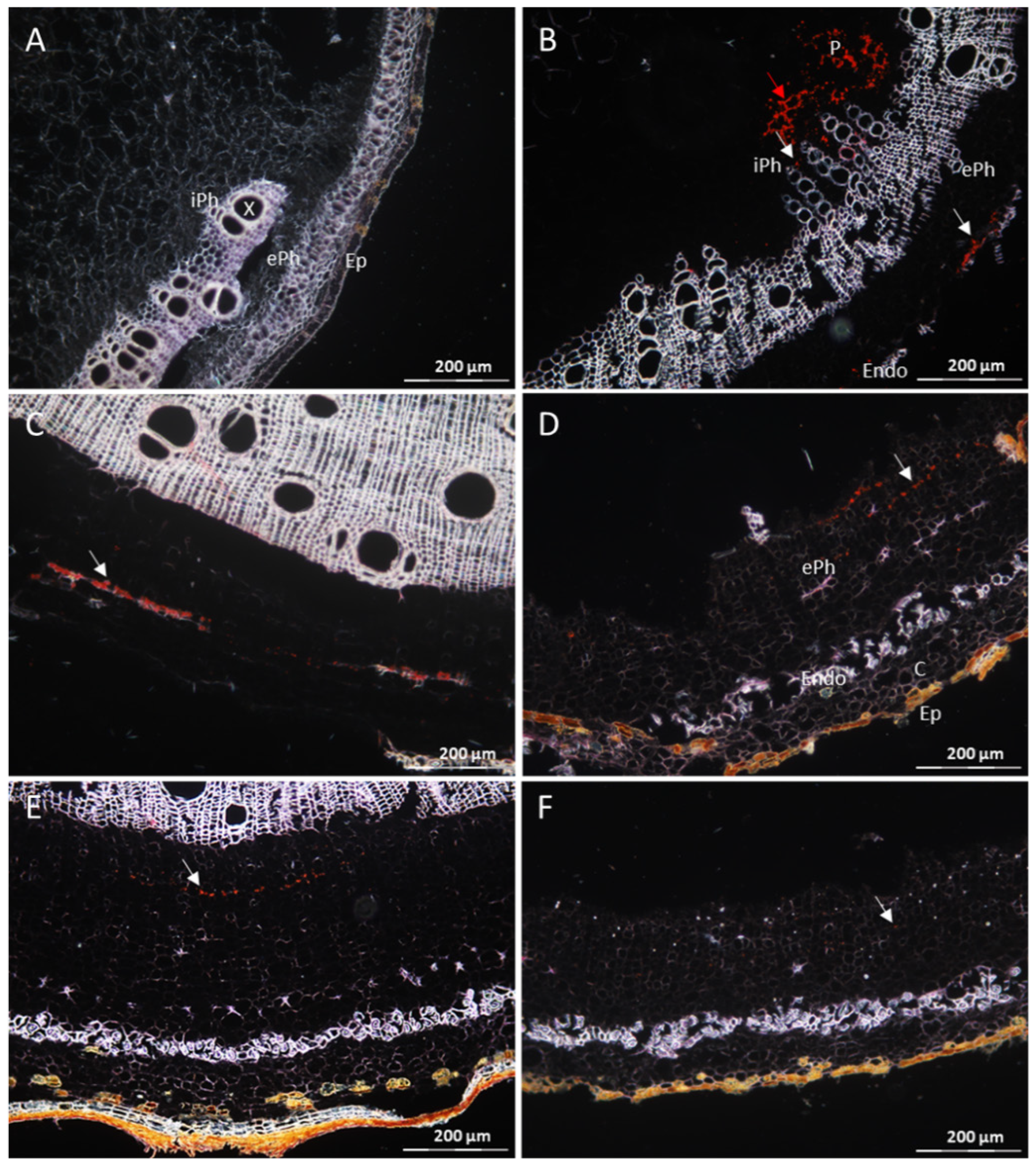
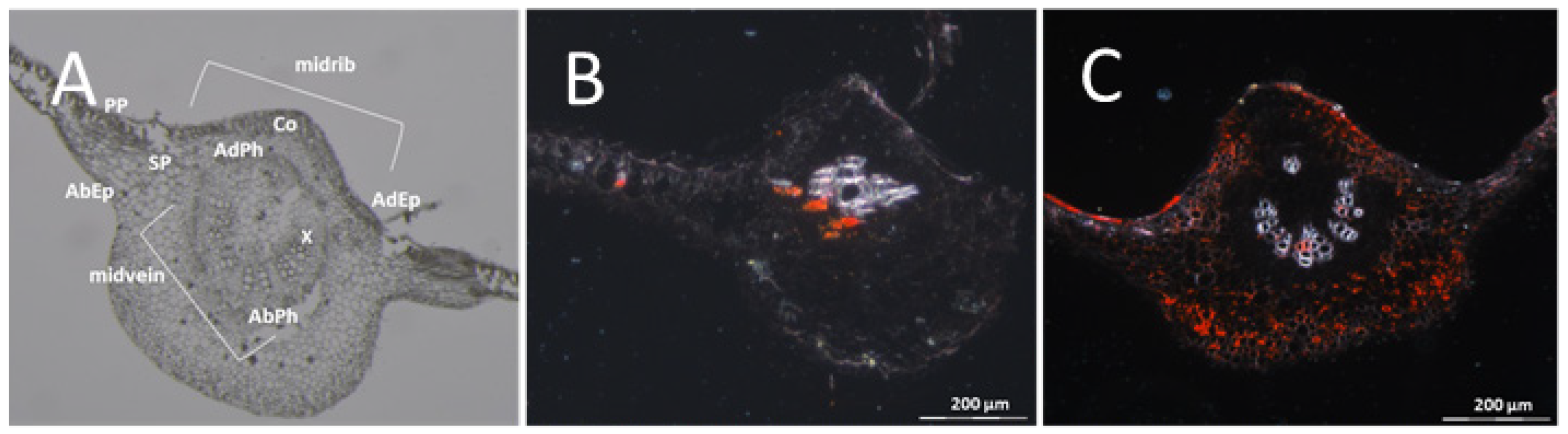
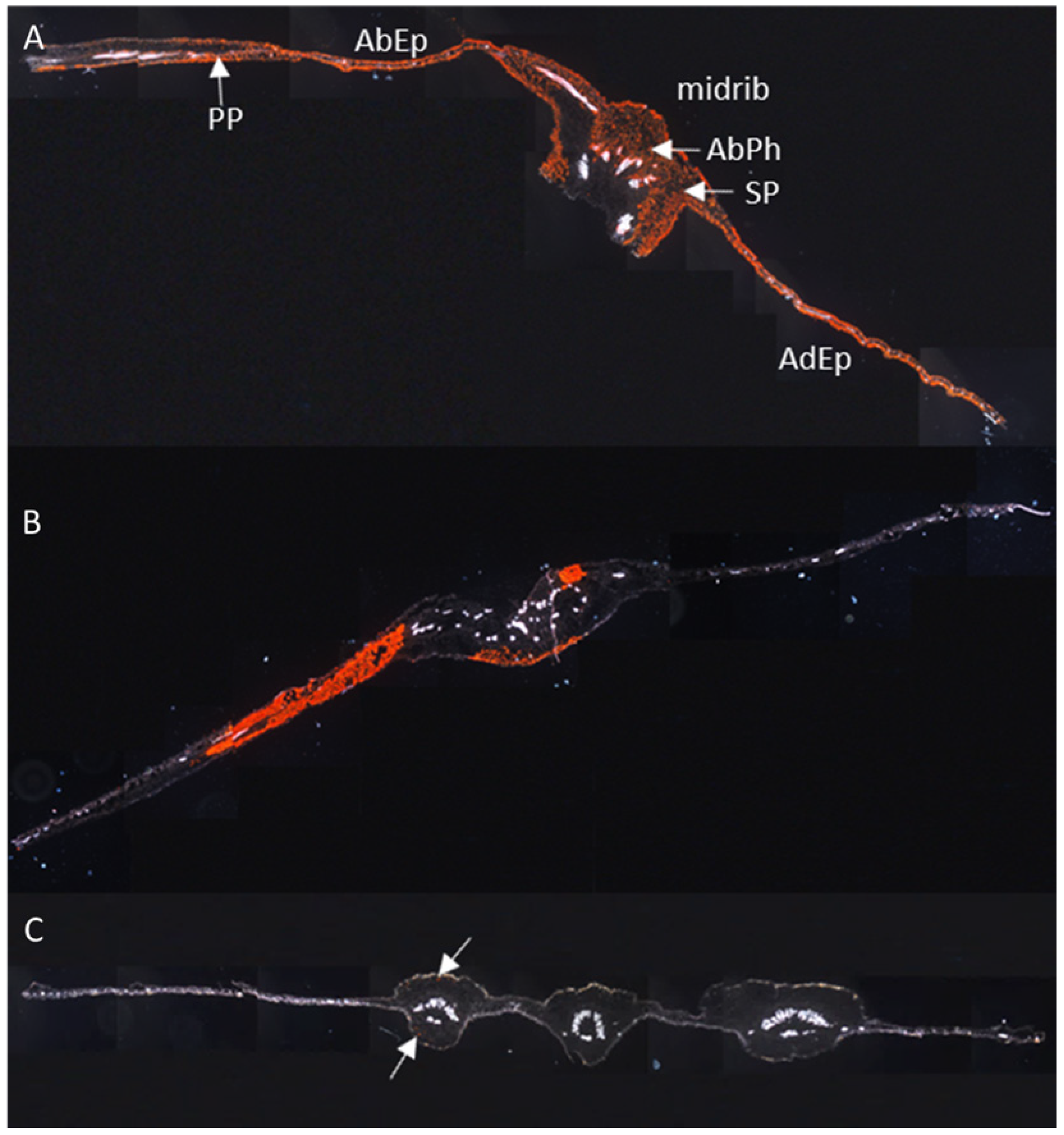
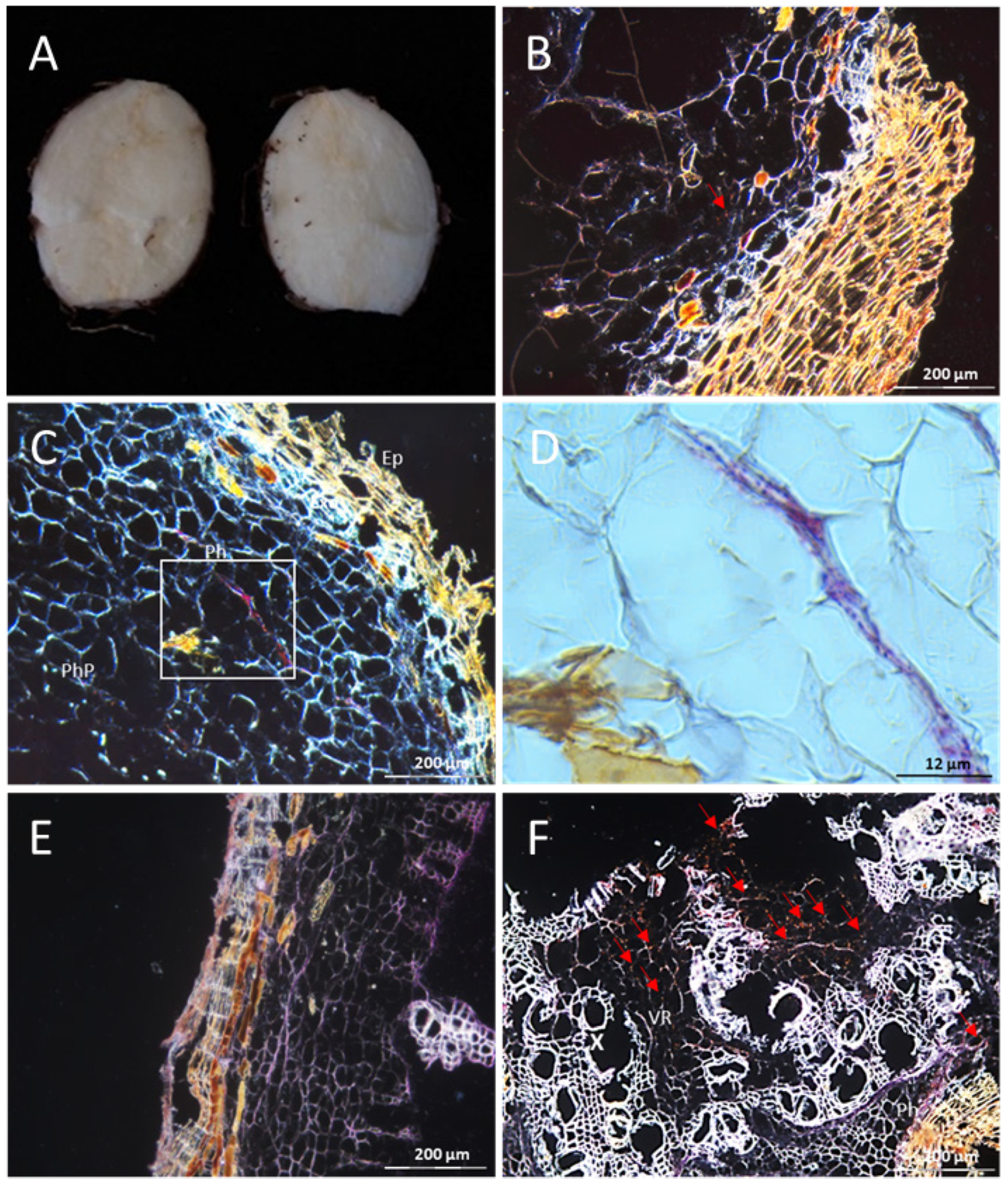
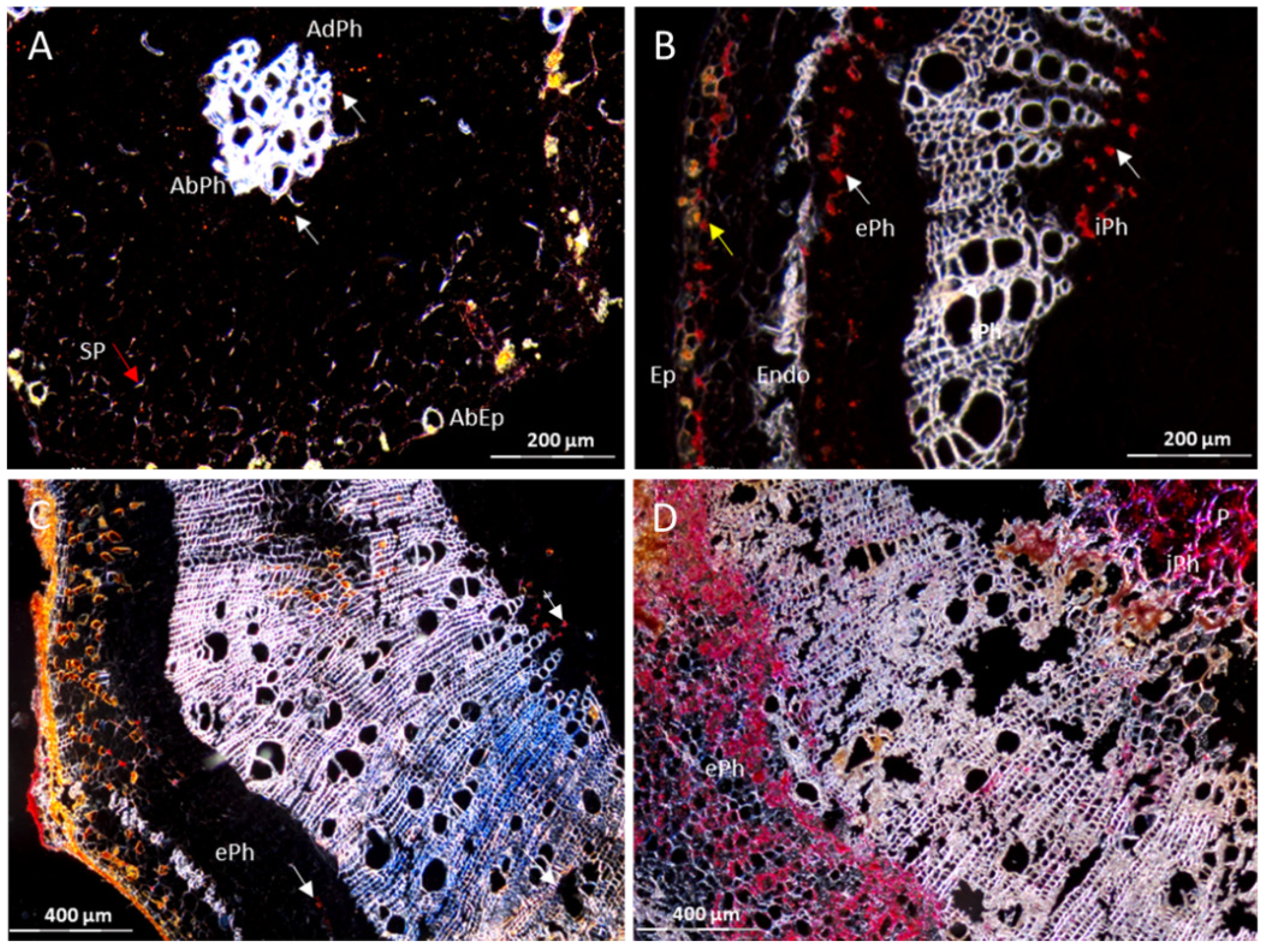
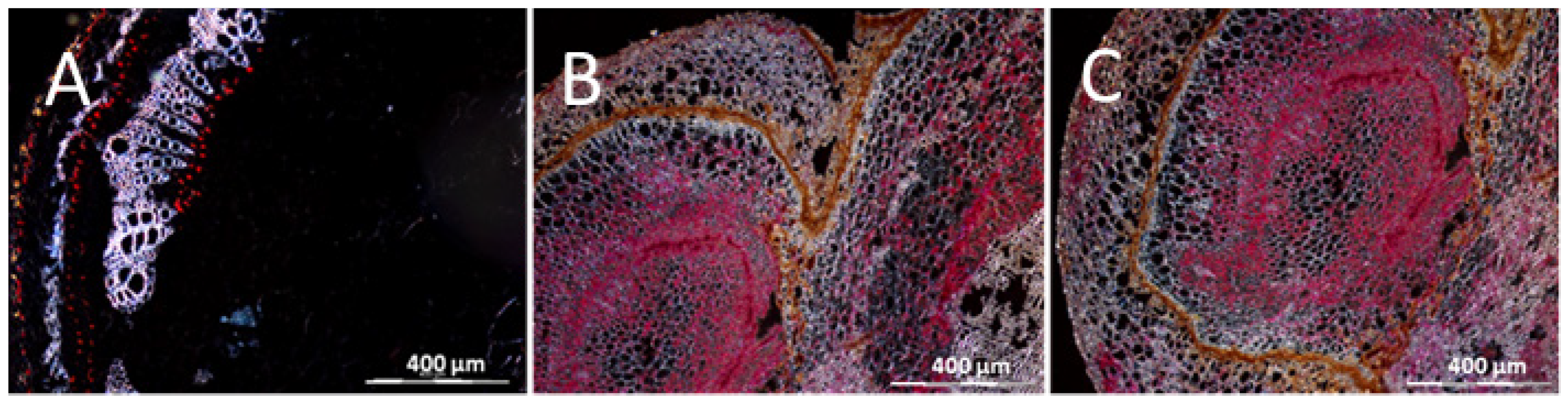
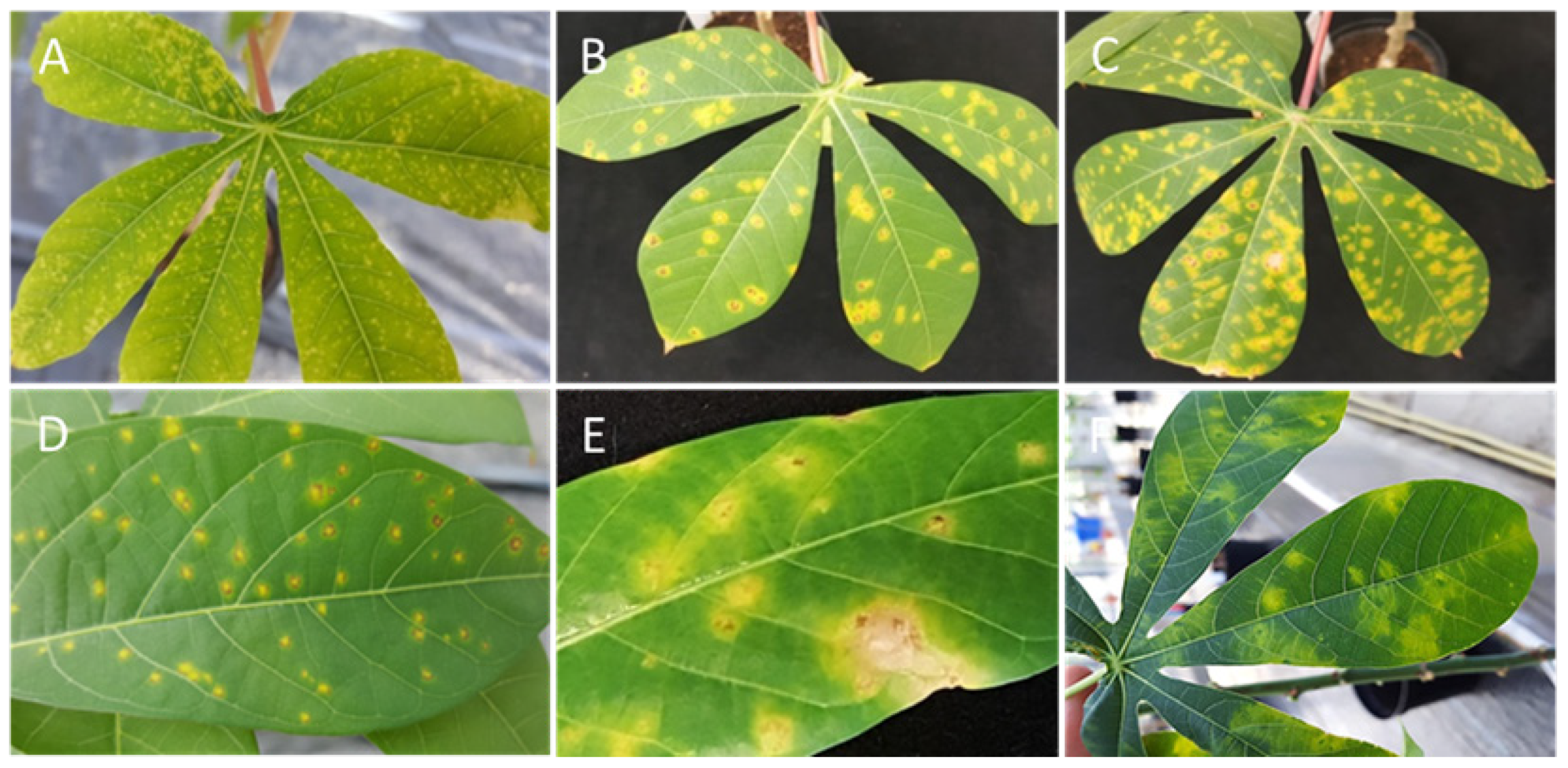
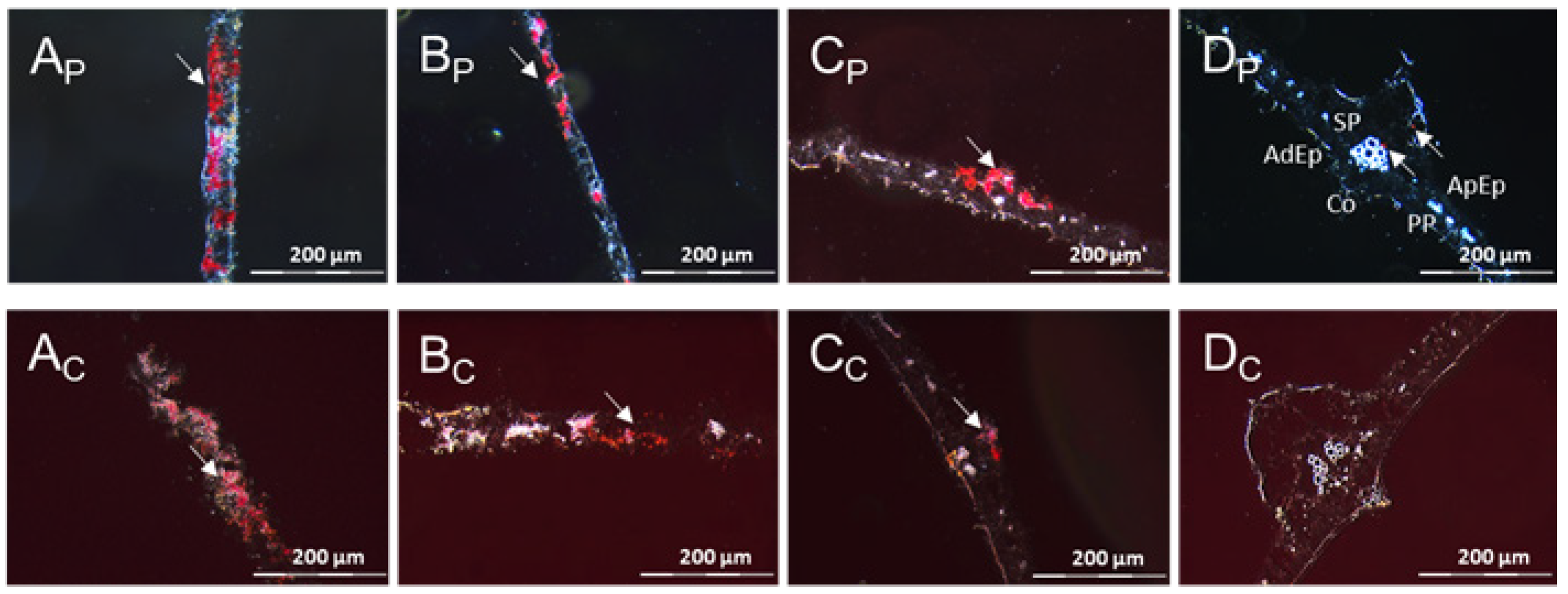
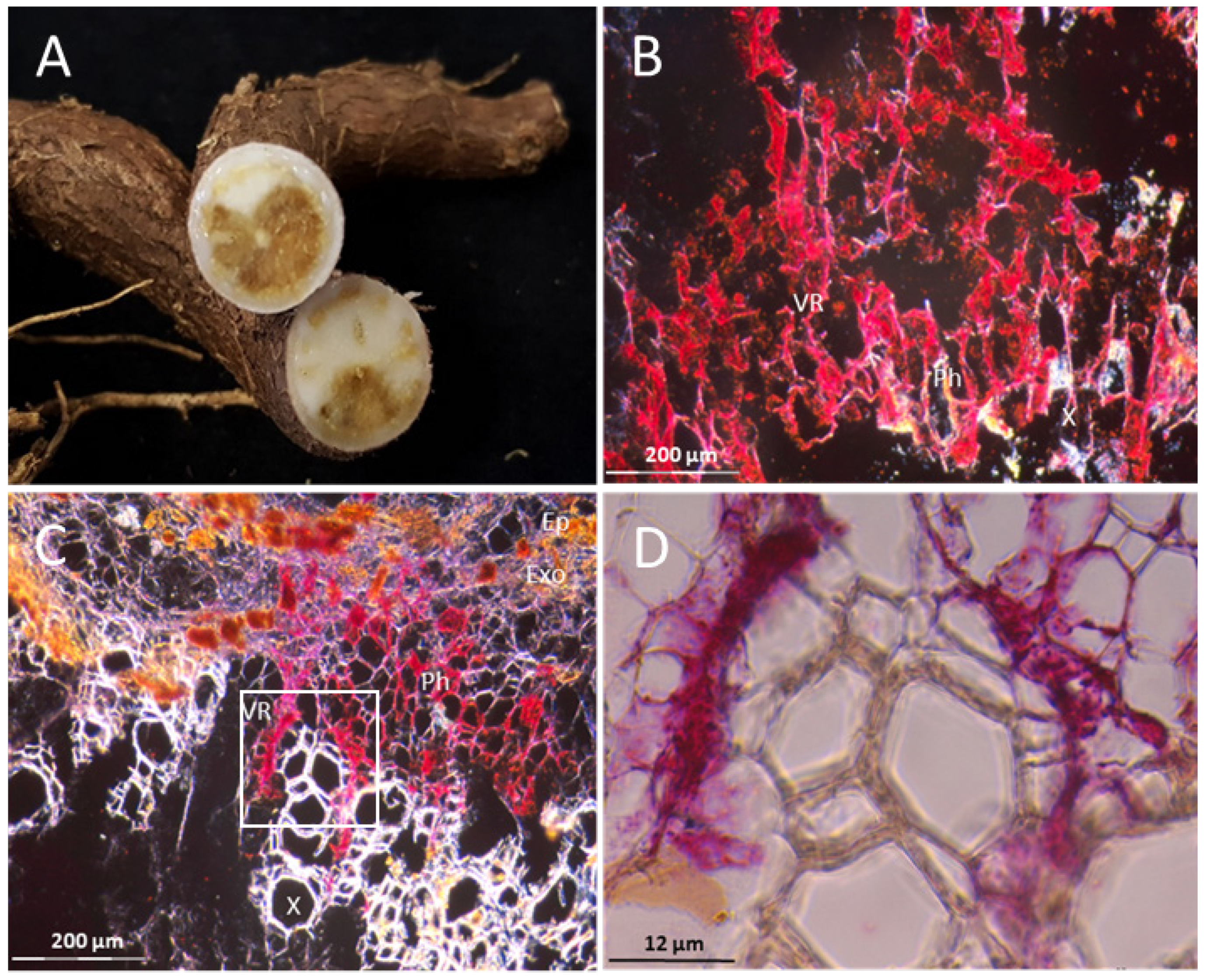
3.1. The Resistant Cassava DSC 167 Impairs Virus Replication but Not Virus Movement
3.2. The Resistant Cassava DSC 167 Restrains CBSV Replication and Confines the Virus to the Phloem
3.3. The Resistant Cassava DSC 260 Restricts Virus to the Phloem and Remains Free of Disease Symptoms
3.4. The Resistant Cassava Line DSC 167 Allows UCBSV Infection but Contains Virus around Necrotic Lesions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winter, S.; Koerbler, M.; Stein, B.; Pietruszka, A.; Paape, M.; Butgereitt, A. Analysis of Cassava brown streak viruses reveals the presence of distinct virus species causing Cassava brown streak disease in East Africa. J. Gen. Virol. 2010, 91, 1365–1372. [Google Scholar] [CrossRef]
- Kaweesi, T.; Kawuki, R.; Kyaligonza, V.; Baguma, Y.; Tusiime, G.; Ferguson, M.E. Field evaluation of selected Cassava genotypes for Cassava brown streak disease based on symptom expression and virus load. Virol. J. 2014, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Maruthi, M.N.; Bouvaine, S.; Tufan, H.A.; Mohammed, I.U.; Hillocks, R.J. Transcriptional response of virus-infected Cassava and identification of putative sources of resistance for Cassava brown streak disease. PLoS ONE 2014, 9, e96642. [Google Scholar] [CrossRef] [PubMed]
- Sheat, S.; Fuerholzner, B.; Stein, B.; Winter, S. Resistance against Cassava brown streak viruses from Africa in Cassava germplasm from South America. Front. Plant Sci. 2019, 10, 567. [Google Scholar] [CrossRef]
- Ogwok, E.; Alicai, T.; Beyene, G.; Taylor, N.; Rey, M.E.C. Distribution and accumulation of Cassava brown streak viruses within infected Cassava (Manihot esculenta) plants. Plant Pathol. 2015, 64, 1235–1246. [Google Scholar] [CrossRef]
- Kawuki, R.S.; Kaweesi, T.; Esuma, W.; Pariyo, A.; Kayondo, I.S.; Ozimati, A.; Kyaligonza, V.; Abaca, A.; Orone, J.; Tumuhimbise, R.; et al. Eleven years of breeding efforts to combat Cassava brown streak disease. Breed. Sci. 2016, 66, 560–571. [Google Scholar] [CrossRef]
- Mukiibi, D.R.; Alicai, T.; Kawuki, R.; Okao-Okuja, G.; Tairo, F.; Sseruwagi, P.; Ndunguru, J.; Ateka, E.M. Resistance of advanced Cassava breeding clones to infection by major viruses in Uganda. Crop Prot. 2019, 115, 104–112. [Google Scholar] [CrossRef]
- Ogwok, E.; Ilyas, M.; Alicai, T.; Rey, M.E.; Taylor, N.J. Comparative analysis of virus-derived small RNAs within Cassava (Manihot esculenta Crantz) infected with Cassava brown streak viruses. Virus Res. 2016, 215, 1–11. [Google Scholar] [CrossRef]
- Zaitlin, M.; Hull, R. Plant virus-host interactions. Annu. Rev. Plant Physiol. 1987, 38, 291–315. [Google Scholar] [CrossRef]
- Palukaitis, P.; Carr, J.P. Plant resistance responses to viruses. J. Plant Pathol. 2008, 90, 153–171. [Google Scholar]
- Calil, I.P.; Fontes, E.P.B. Plant immunity against viruses: Antiviral immune receptors in focus. Ann. Bot. 2016, 119, 200–723. [Google Scholar] [CrossRef]
- Incarbone, M.; Dunoyer, P. RNA silencing and its suppression: Novel insights from in planta analyses. Trends Plant Sci. 2013, 18, 382–392. [Google Scholar] [CrossRef]
- Valkonen, J. Mechanisms of resistance to viruses. Plant Prot. Sci. 2002, 38, S132–S135. [Google Scholar] [CrossRef]
- Munganyinka, E.; Margaria, P.; Sheat, S.; Ateka, E.M.; Tairo, F.; Ndunguru, J.; Winter, S. Localization of Cassava brown streak virus in Nicotiana rustica and Cassava Manihot esculenta (Crantz) using RNAscope® in situ hybridization. Virol. J. 2018, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Sheat, S.; Winter, S.; Margaria, P. Duplex in situ hybridization of virus nucleic acids in plant tissues using RNAscope®. Methods Mol. Biol. 2020, 2148, 203–215. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Andrianifahanana, M.; Lovins, K.; Dute, R.; Sikora, E.; Murphy, J.F. Pathway for phloem dependent movement of pepper mottle po-tyvirus in the stem of Capsicum anuum. Phytopathology 1997, 87, 892–898. [Google Scholar] [CrossRef]
- Hipper, C.; Brault, V.; Ziegler-Graff, V.; Revers, F. Viral and cellular factors involved in phloem transport of plant viruses. Front. Plant Sci. 2013, 4, 154. [Google Scholar] [CrossRef]
- Wan, J.; Cabanillas, D.G.; Zheng, H.; Laliberté, J.-F. Turnip mosaic virus moves systemically through both phloem and xylem as membrane-associated complexes. Plant Physiol. 2015, 167, 1374–1388. [Google Scholar] [CrossRef]
- Cheng, N.-H.; Su, C.-L.; Carter, S.A.; Nelson, R.S. Vascular invasion routes and systemic accumulation patterns of tobacco mosaic virus in Nicotiana benthamiana. Plant J. 2000, 23, 349–362. [Google Scholar] [CrossRef]
- Wilson, C.R.; Jones, R.A.C. Resistance to phloem transport of potato leafroll virus in potato plants. J. Gen. Virol. 1992, 73, 3219–3224. [Google Scholar] [CrossRef]
- Graciano-Ribeiro, D.; Hashimoto, D.; Nogueira, L.; Teodoro, D.; Miranda, S.; Nassar, N. Internal phloem in an interspecific hybrid of Cassava, an indicator of breeding value for drought resistance. Genet. Mol. Res. 2009, 8, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Nassar, N.M.A.; Abreu, L.F.A.; Teodoro, D.A.P.; Graciano-Ribeiro, D. Drought tolerant stem anatomy characteristics in Manihot esculenta (Euphorbiaceae) and a wild relative. Genet. Mol. Res. 2010, 9, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Hayden, S.M.; Hayden, W.J. Stem development, medullary bundles, and wood anatomy of Croton glandulosus Var. Septentrionalis (Euphorbiaceae). IAWA J. 1994, 15, 51–63. [Google Scholar] [CrossRef]
- Barker, H.; Harrison, B.D. Restricted distribution of potato leafroll virus antigen in resistant potato genotypes and its effect on transmission of the virus by aphids. Ann. Appl. Biol. 1986, 109, 595–604. [Google Scholar] [CrossRef]
- Guerini, M.N.; Murphy, J.F. Resistance of Capsicum annuum ‘Avelar’ to pepper mottle potyvirus and alleviation of this resistance by co-infection with cucumber mosaic cucumovirus are associated with virus movement. J. Gen. Virol. 1999, 80, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Derrick, P.M.; Barker, H. Short and long distance spread of potato leafroll luteovirus: Effects of host genes and transgenes conferring resistance to virus accumulation in potato. J. Gen. Virol. 1997, 78, 243–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gosalvez-Bernal, B.; Genoves, A.; Navarro, J.A.; Pallas, V.; Sanchez Pina, M.A. Distribution and pathway for phloem-dependent movement of Melon necrotic spot virus in melon plants. Mol. Plant Pathol. 2008, 9, 447–461. [Google Scholar] [CrossRef]
- Rajamäki, M.-L.; Valkonen, J.P. Control of nuclear and nucleolar localization of nuclear inclusion protein a of picorna-like potato virus a in Nicotiana species. Plant Cell 2009, 21, 2485–2502. [Google Scholar] [CrossRef]
- Vuorinen, A.L.; Kelloniemi, J.; Valkonen, J.P. Why do viruses need phloem for systemic invasion of plants? Plant Sci. 2011, 181, 355–363. [Google Scholar] [CrossRef]
- Develey-Rivière, M.-P.; Galiana, E. Resistance to pathogens and host developmental stage: A multifaceted relationship within the plant kingdom. New Phytol. 2007, 175, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Kus, J.V.; Zaton, K.; Sarkar, R.; Cameron, R.K. Age-related resistance in Arabidopsis is a developmentally regulated defense response to Pseudomonas syringae. Plant Cell 2002, 14, 479–490. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.-Y.; Tian, Y.-P.; Geng, C.; Yuan, X.-F.; Li, X.-D. Role of Tobacco vein banding mosaic virus 3′-UTR on virus systemic infection in tobacco. Virology 2019, 527, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Paudel, D.B.; Sanfaçon, H. Exploring the diversity of mechanisms associated with plant tolerance to virus infection. Front. Plant Sci. 2018, 9, 1575. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, K.; Hashimoto, M.; Ozeki, J.; Yamaji, Y.; Maejima, K.; Senshu, H.; Himeno, M.; Okano, Y.; Kagiwada, S.; Namba, S. Viral-induced systemic necrosis in plants involves both programmed cell death and the inhibition of viral multiplication, which are regulated by independent pathways. Mol. Plant-Microbe Interact. 2010, 23, 283–293. [Google Scholar] [CrossRef]
- Mandadi, K.K.; Scholthof, K.-B.G. Plant immune responses against viruses: How does a virus cause disease? Plant Cell 2013, 25, 1489–1505. [Google Scholar] [CrossRef]
- Kiraly, L.; Hafez, Y.M.; Fodor, J.; Kiraly, Z. Suppression of tobacco mosaic virus-induced hypersensitive-type necrotization in tobacco at high temperature is associated with downregulation of NADPH oxidase and superoxide and stimulation of dehy-droascorbate reductase. J. Gen. Virol. 2008, 89, 799–808. [Google Scholar] [CrossRef]
- Chung, B.N.; Lee, J.-H.; Kang, B.-C.; Koh, S.W.; Joa, J.H.; Choi, K.S.; Ahn, J.J. HR-mediated defense response is overcome at high temperatures in Capsicum species. Plant Pathol. J. 2018, 34, 71–77. [Google Scholar] [CrossRef]
- Chung, B.N.; Choi, K.S.; Ahn, J.J.; Joa, J.H.; Do, K.S.; Park, K.S. Effects of temperature on systemic infection and symptom ex-pression of Turnip mosaic virus in Chinese cabbage (Brassica campestris). Plant Pathol. J. 2015, 31, 363–370. [Google Scholar] [CrossRef]
- Slewinski, T.L.; Zhang, C.; Turgeon, R. Structural and functional heterogeneity in phloem loading and transport. Front. Plant Sci. 2013, 4, 244. [Google Scholar] [CrossRef]
- Aoki, K.; Suzui, N.; Fujimaki, S.; Dohmae, N.; Yonekura-Sakakibara, K.; Fujiwara, T.; Hayashi, H.; Yamaya, T.; Sakakibara, H. Destination-selective long-distance movement of phloem proteins. Plant Cell 2005, 17, 1801–1814. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheat, S.; Margaria, P.; Winter, S. Differential Tropism in Roots and Shoots of Resistant and Susceptible Cassava (Manihot esculenta Crantz) Infected by Cassava Brown Streak Viruses. Cells 2021, 10, 1221. https://doi.org/10.3390/cells10051221
Sheat S, Margaria P, Winter S. Differential Tropism in Roots and Shoots of Resistant and Susceptible Cassava (Manihot esculenta Crantz) Infected by Cassava Brown Streak Viruses. Cells. 2021; 10(5):1221. https://doi.org/10.3390/cells10051221
Chicago/Turabian StyleSheat, Samar, Paolo Margaria, and Stephan Winter. 2021. "Differential Tropism in Roots and Shoots of Resistant and Susceptible Cassava (Manihot esculenta Crantz) Infected by Cassava Brown Streak Viruses" Cells 10, no. 5: 1221. https://doi.org/10.3390/cells10051221
APA StyleSheat, S., Margaria, P., & Winter, S. (2021). Differential Tropism in Roots and Shoots of Resistant and Susceptible Cassava (Manihot esculenta Crantz) Infected by Cassava Brown Streak Viruses. Cells, 10(5), 1221. https://doi.org/10.3390/cells10051221






