Immunoproteasome Activity and Content Determine Hematopoietic Cell Sensitivity to ONX-0914 and to the Infection of Cells with Lentiviruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Cell Viability Assay
2.3. RNA Isolation and Real-Time PCR
2.4. Preparation of Lysates and Western Blotting
2.5. Preparation of Viruses
2.6. Inhibition of Proteasomes and Evaluation of Viral Infection Efficacy
2.7. Determination of Proteasome Activity
2.8. Treatment of Cells with IFN-γ
2.9. Statistical Analysis and Software
3. Results
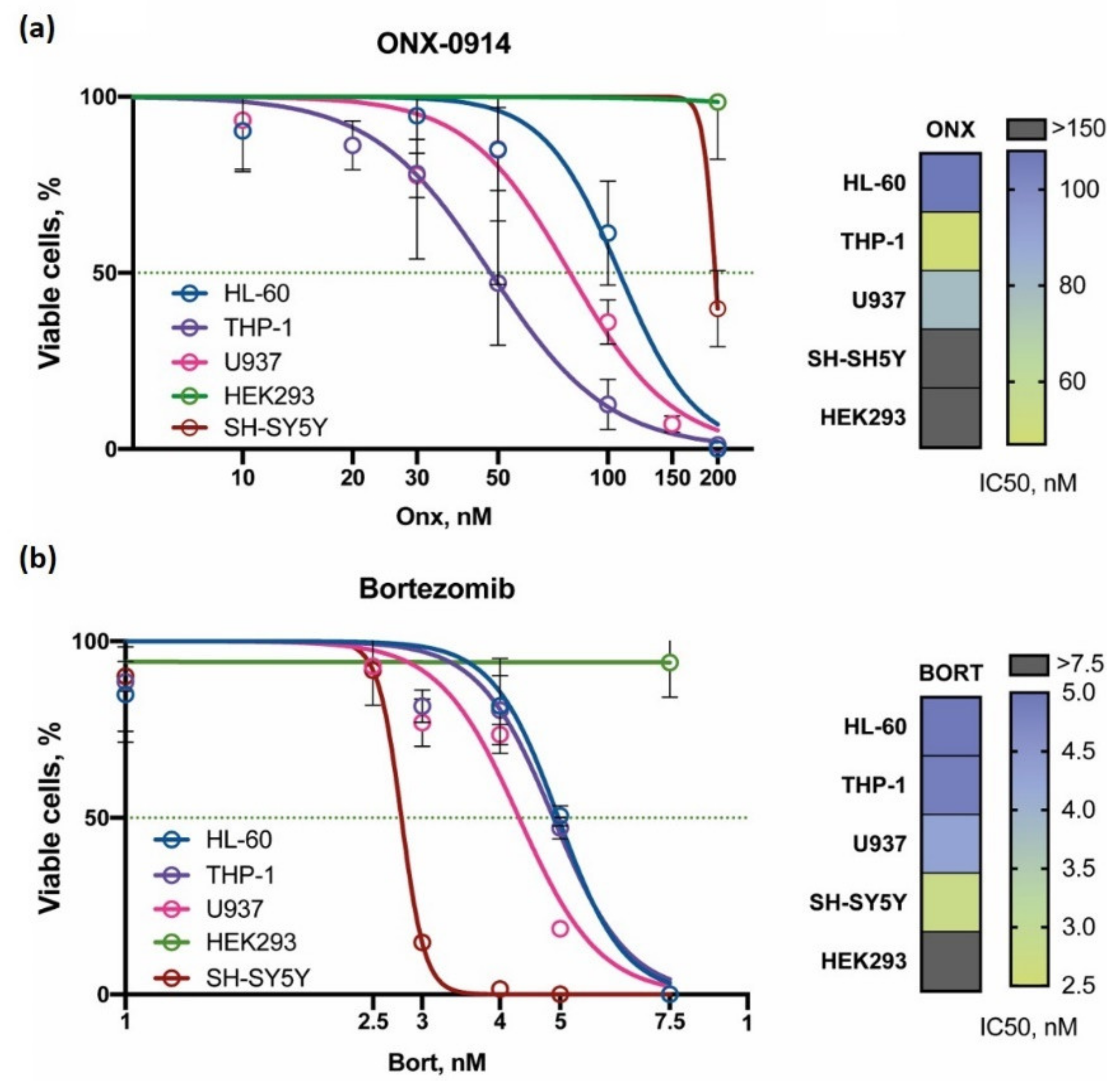
3.1. Different Cell Lines Demonstrate Different Viability Following Treatment with Proteasome Inhibitors
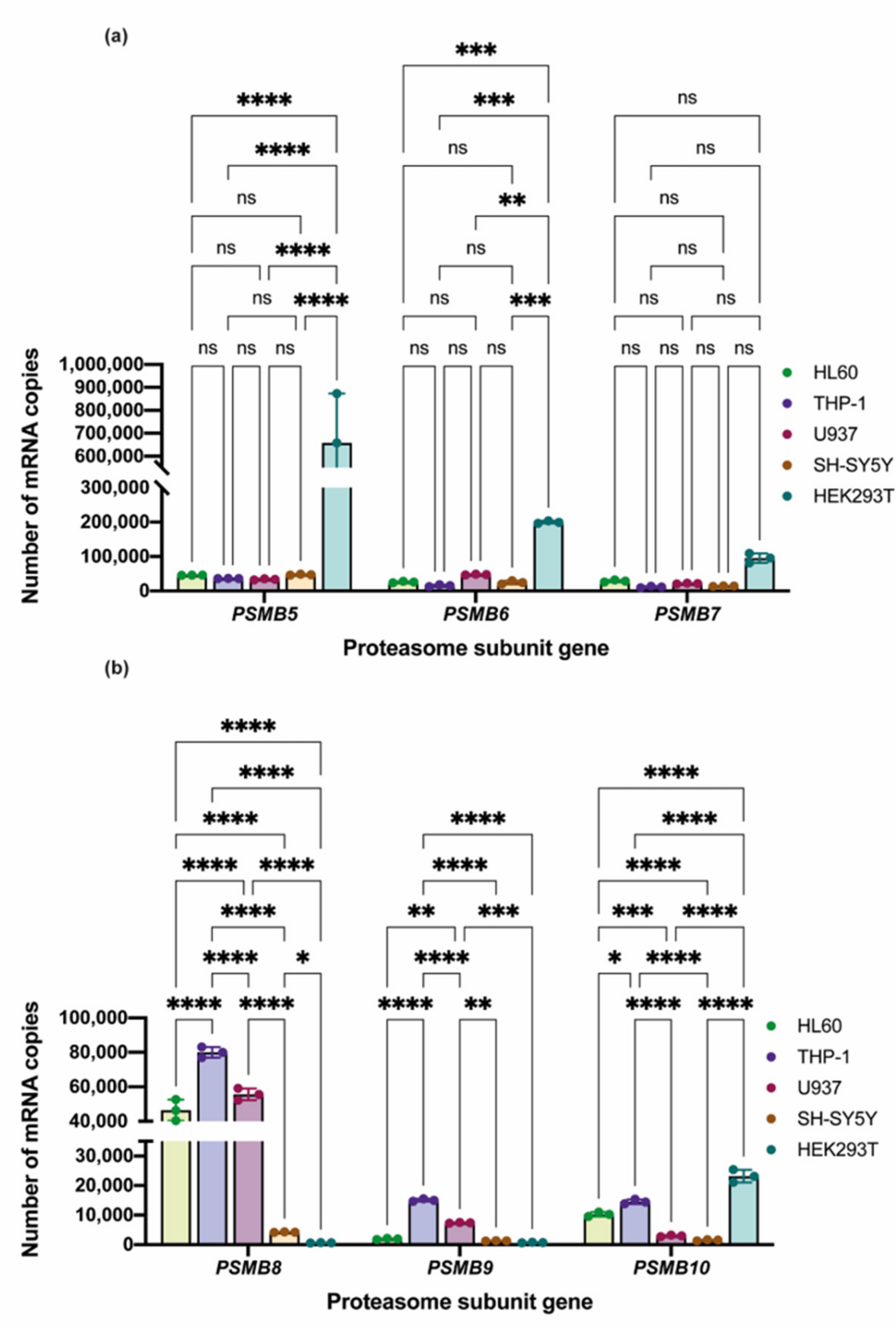
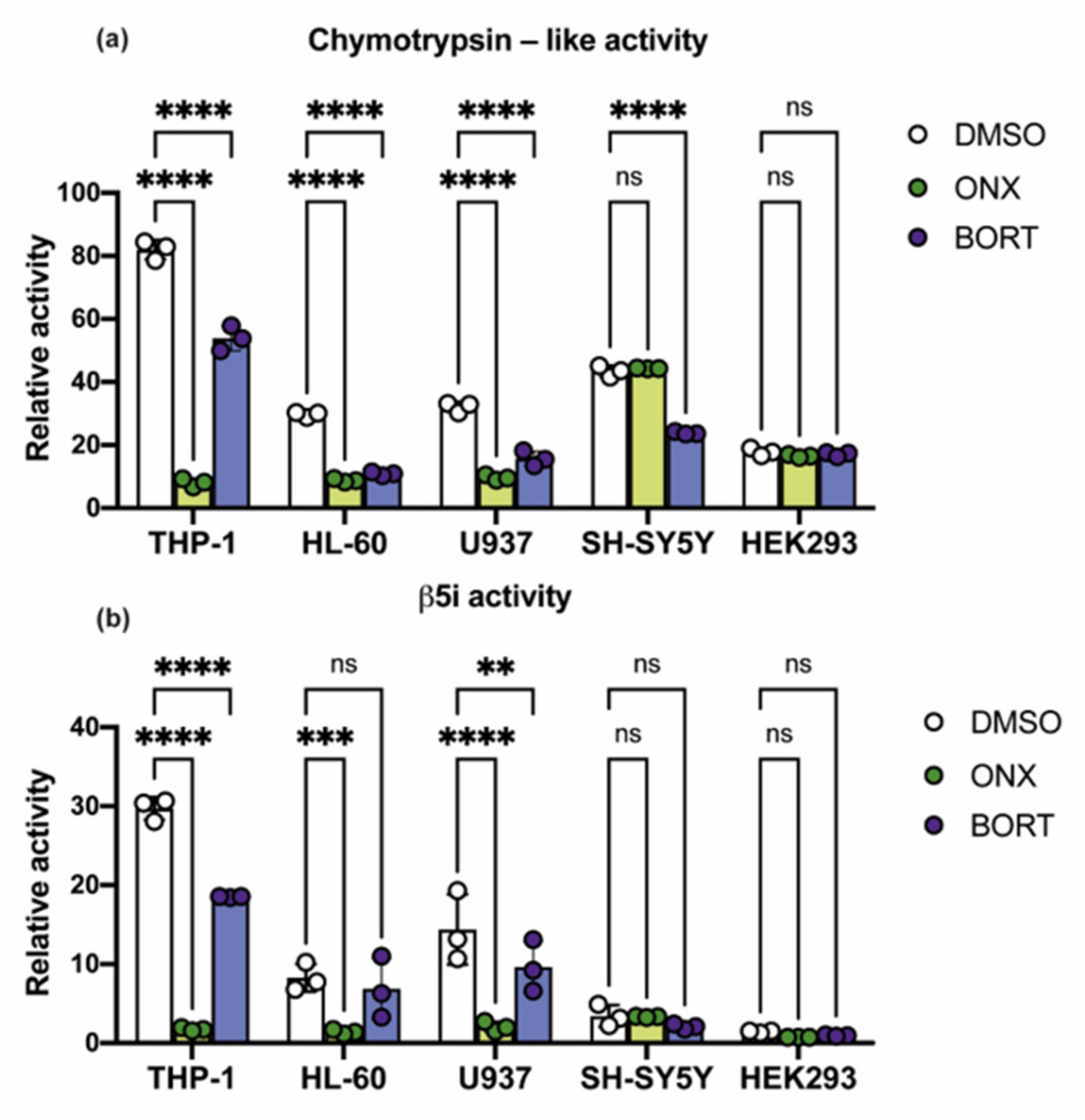
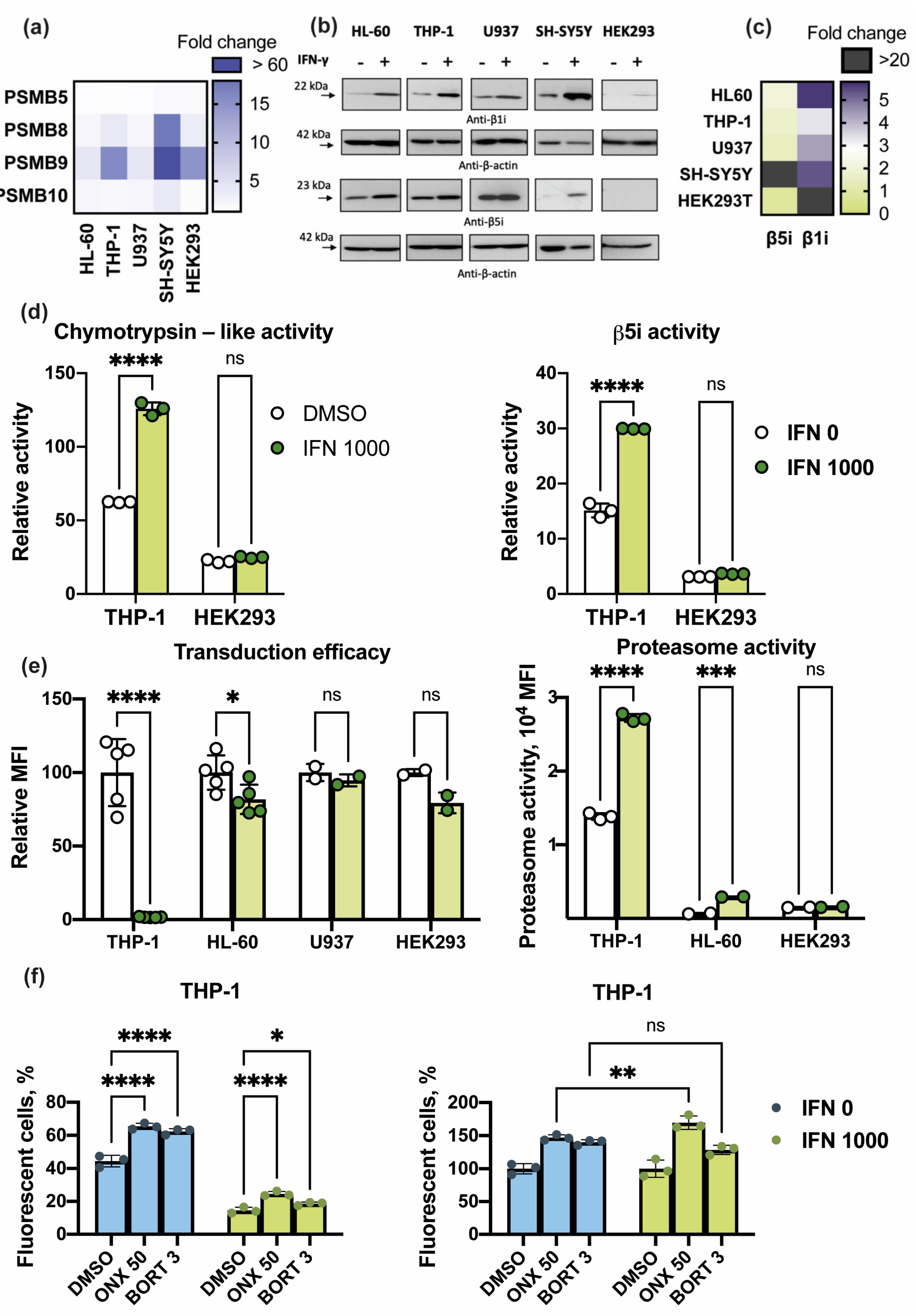
3.2. Proteasome Subunit Expression and Activity in THP-1, HL-60, U937, SH-SY5Y, and HEK 293 Cell Lines
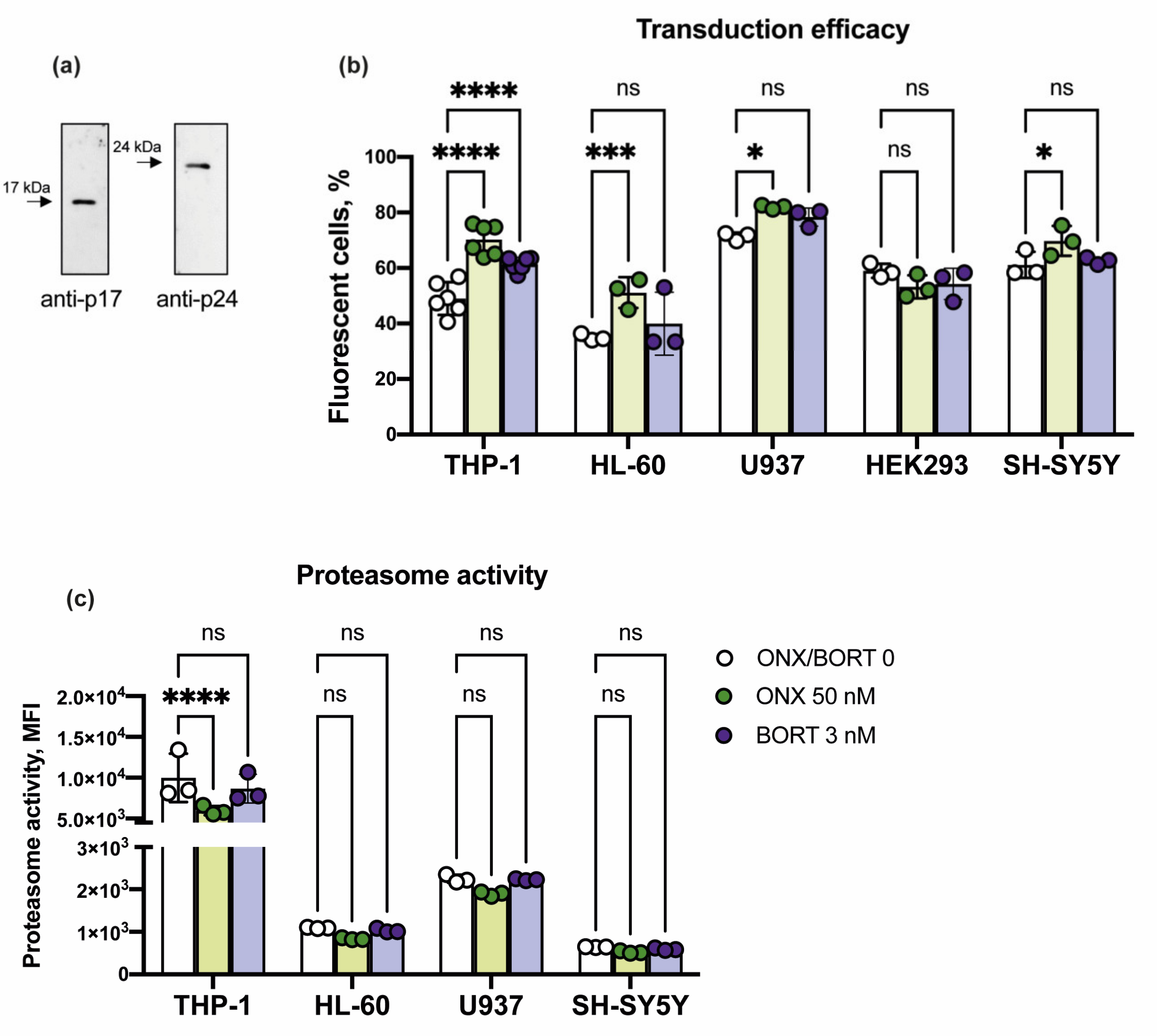
3.3. Cells with High Immunoproteasome Activity Demonstrate Enhanced Infection with Lentiviruses after Treatment with ONX-0914
3.4. IFN-γ Increases Proteasome Activity and Affects Viral Infection
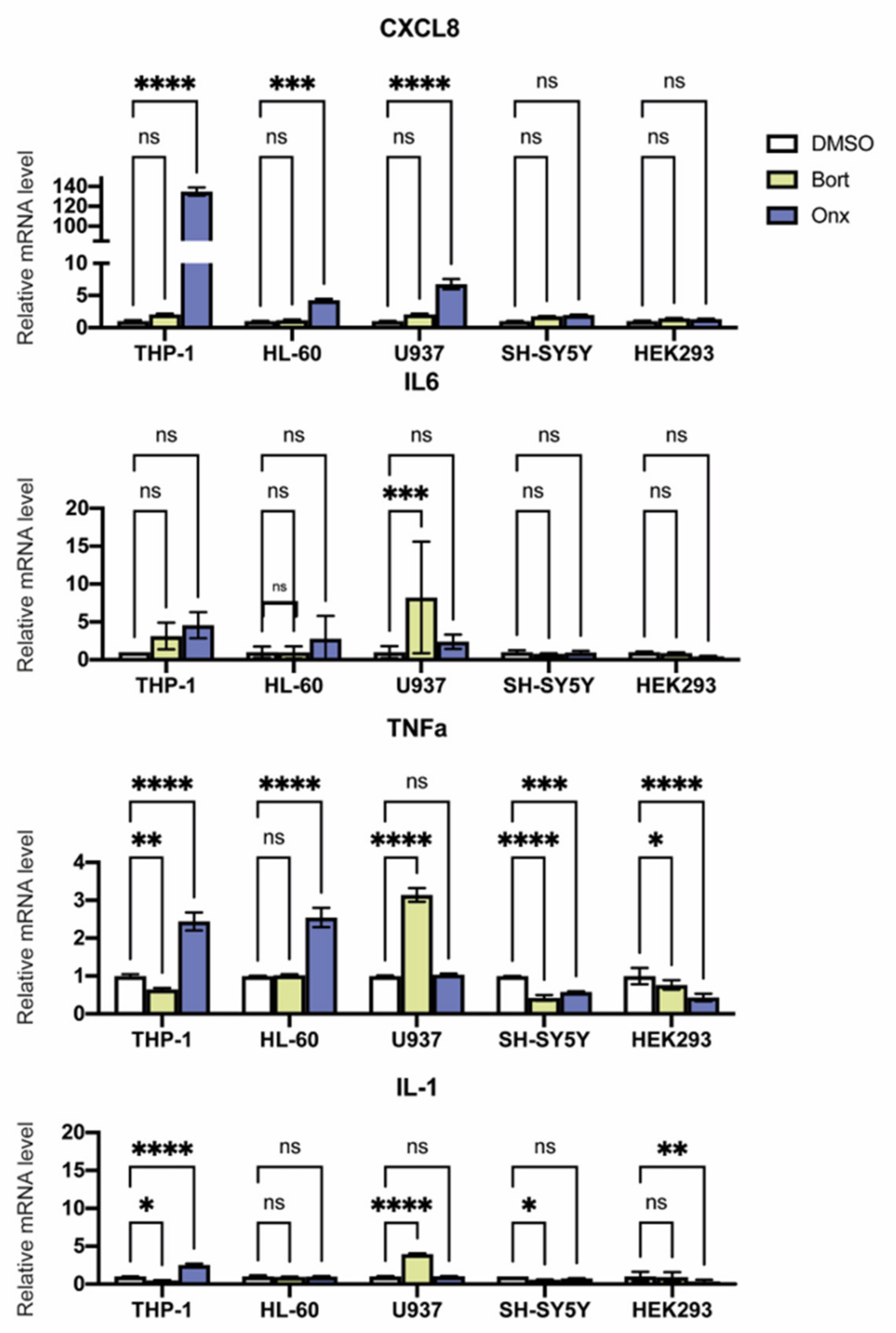
3.5. Proteasome Inhibitors Modulate Cytokine Expression in Studied Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciechanover, A.; Kwon, Y.T. Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Exp. Mol. Med. 2015, 47, e147. [Google Scholar] [CrossRef] [PubMed]
- Kisselev, A.F.; Kaganovich, D.; Goldberg, A.L. Binding of Hydrophobic Peptides to Several Non-catalytic Sites Promotes Peptide Hydrolysis by All Active Sites of 20 S Proteasomes. J. Biol. Chem. 2002, 277, 22260–22270. [Google Scholar] [CrossRef] [PubMed]
- Ferrington, D.A.; Gregerson, D.S. Immunoproteasomes: Structure, function, and antigen presentation. Prog. Mol. Biol. Transl. Sci. 2012, 109, 75–112. [Google Scholar] [CrossRef]
- Mishto, M.; Liepe, J.; Textoris-Taube, K.; Keller, C.; Henklein, P.; Weberruß, M.; Dahlmann, B.; Enenkel, C.; Voigt, A.; Kuckelkorn, U.; et al. Proteasome isoforms exhibit only quantitative differences in cleavage and epitope generation. Eur. J. Immunol. 2014, 44, 3508–3521. [Google Scholar] [CrossRef] [PubMed]
- Morozov, A.V.; Karpov, V.L. Proteasomes and Several Aspects of Their Heterogeneity Relevant to Cancer. Front. Oncol. 2019, 9, 761. [Google Scholar] [CrossRef]
- Guillaume, B.; Chapiro, J.; Stroobant, V.; Colau, D.; Van Holle, B.; Parvizi, G.; Bousquet-Dubouch, M.-P.; Théate, I.; Parmentier, N.; Eynde, B.J.V.D. Two abundant proteasome subtypes that uniquely process some antigens presented by HLA class I molecules. Proc. Natl. Acad. Sci. USA 2010, 107, 18599–18604. [Google Scholar] [CrossRef]
- Lata, S.; Mishra, R.; Banerjea, A.C. Proteasomal Degradation Machinery: Favorite Target of HIV-1 Proteins. Front. Microbiol. 2018, 9, 2738. [Google Scholar] [CrossRef]
- Schwartz, O.; Maréchal, V.; Friguet, B.; Arenzana-Seisdedos, F.; Heard, J.-M. Antiviral Activity of the Proteasome on Incoming Human Immunodeficiency Virus Type 1. J. Virol. 1998, 72, 3845–3850. [Google Scholar] [CrossRef] [PubMed]
- Schubert, U.; Ott, D.E.; Chertova, E.N.; Welker, R.; Tessmer, U.; Princiotta, M.F.; Bennink, J.R.; Kräusslich, H.-G.; Yewdell, J.W. Proteasome inhibition interferes with Gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc. Natl. Acad. Sci. USA 2000, 97, 13057–13062. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.-Y.; Lai, M.M.C. The Ubiquitin-Proteasome System Facilitates the Transfer of Murine Coronavirus from Endosome to Cytoplasm during Virus Entry. J. Virol. 2005, 79, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Anderson, J.L.; Campbell, E.M.; Joseph, A.M.; Hope, T.J. Proteasome inhibitors uncouple rhesus TRIM5 restriction of HIV-1 reverse transcription and infection. Proc. Natl. Acad. Sci. USA 2006, 103, 7465–7470. [Google Scholar] [CrossRef]
- Delboy, M.G.; Roller, D.G.; Nicola, A.V. Cellular Proteasome Activity Facilitates Herpes Simplex Virus Entry at a Postpenetration Step. J. Virol. 2008, 82, 3381–3390. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, I.; De Vries, E.; Tscherne, D.M.; García-Sastre, A.; Rottier, P.J.M.; De Haan, C.A.M. Inhibition of the Ubiquitin-Proteasome System Affects Influenza A Virus Infection at a Postfusion Step. J. Virol. 2010, 84, 9625–9631. [Google Scholar] [CrossRef] [PubMed]
- Casorla-Pérez, L.A.; López, T.; López, S.; Arias, C.F. The Ubiquitin-Proteasome System Is Necessary for Efficient Replication of Human Astrovirus. J. Virol. 2017, 92, e01809-17. [Google Scholar] [CrossRef] [PubMed]
- Luo, H. Interplay between the virus and the ubiquitin–proteasome system: Molecular mechanism of viral pathogenesis. Curr. Opin. Virol. 2016, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, L.; Tibullo, D.; Giallongo, C.; Lazzarino, G.; Tartaglia, N.; Galimberti, S.; Volti, G.L.; Palumbo, G.A.; Liso, A. Proteasome Inhibitors as a Possible Therapy for SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 3622. [Google Scholar] [CrossRef]
- McCarthy, M.K.; Weinberg, J.B. The immunoproteasome and viral infection: A complex regulator of inflammation. Front. Microbiol. 2015, 6, 21. [Google Scholar] [CrossRef]
- Morozov, A.V.; Burov, A.V.; Astakhova, T.M.; Spasskaya, D.S.; Margulis, B.A.; Karpov, V.L. Dynamics of the Functional Activity and Expression of Proteasome Subunits during Cellular Adaptation to Heat Shock. Mol. Biol. 2019, 53, 638–647. [Google Scholar] [CrossRef]
- Weber, K.; Bartsch, U.; Stocking, C.; Fehse, B. A Multicolor Panel of Novel Lentiviral “Gene Ontology” (LeGO) Vectors for Functional Gene Analysis. Mol. Ther. 2008, 16, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.R.; Westphal, M.; Ostertag, W.; Von Laer, D. Oncoretrovirus and Lentivirus Vectors Pseudotyped with Lymphocytic Choriomeningitis Virus Glycoprotein: Generation, Concentration, and Broad Host Range. J. Virol. 2002, 76, 1252–1264. [Google Scholar] [CrossRef]
- Dull, T.; Zufferey, R.; Kelly, M.; Mandel, R.J.; Nguyen, M.; Trono, D.; Naldini, L. A Third-Generation Lentivirus Vector with a Conditional Packaging System. J. Virol. 1998, 72, 8463–8471. [Google Scholar] [CrossRef]
- Prokofjeva, M.M.; Riecken, K.; Spirin, P.V.; Yanvarév, D.V.; Düsedau, A.; Ellinger, B.; Fehse, B.; Stocking, C.; Prassolov, V.S. A new system for parallel drug screening against multiple-resistant HIV mutants based on lentiviral self-inactivating (SIN) vectors and multi-colour analyses. AIDS Res. Ther. 2013, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Morozov, A.V.; Astakhova, T.M.; Garbuz, D.G.; Krasnov, G.S.; Bobkova, N.V.; Zatsepina, O.G.; Karpov, V.L.; Evgen’Ev, M.B. Interplay between recombinant Hsp70 and proteasomes: Proteasome activity modulation and ubiquitin-independent cleavage of Hsp70. Cell Stress Chaperon 2017, 22, 687–697. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.; Schuurman, K.G.; Rodenko, B.; Ovaa, H.; Berkers, C.R. Fluorescence-Based Proteasome Activity Profiling. Methods Mol. Biol. 2012, 803, 183–204. [Google Scholar] [CrossRef]
- Fabre, B.; Lambour, T.; Garrigues, L.; Ducoux-Petit, M.; Amalric, F.; Monsarrat, B.; Burlet-Schiltz, O.; Bousquet-Dubouch, M.-P. Label-Free Quantitative Proteomics Reveals the Dynamics of Proteasome Complexes Composition and Stoichiometry in a Wide Range of Human Cell Lines. J. Proteome Res. 2014, 13, 3027–3037. [Google Scholar] [CrossRef]
- Frentzel, S.; Kuhn-Hartmann, I.; Gernold, M.; Gott, P.; Seelig, A.; Kloetzel, P.-M. The major-histocompatibility-complex-encoded beta-type proteasome subunits LMP2 and LMP7. Evidence that LMP2 and LMP7 are synthesized as proproteins and that cellular levels of both mRNA and LMP-containing 20S proteasomes are differentially regulated. JBIC J. Biol. Inorg. Chem. 1993, 216, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Rouette, A.; Trofimov, A.; Haberl, D.; Boucher, G.; Lavallée, V.-P.; D’Angelo, G.; Hébert, J.; Sauvageau, G.; Lemieux, S.; Perreault, C. Expression of immunoproteasome genes is regulated by cell-intrinsic and -extrinsic factors in human cancers. Sci. Rep. 2016, 6, 34019. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, G.; Xin, B.T.; Kraus, M.; Van Der Stelt, M.; Van Der Marel, G.A.; Kisselev, A.F.; Driessen, C.; Florea, B.I.; Overkleeft, H.S. A Set of Activity-Based Probes to Visualize Human (Immuno)proteasome Activities. Angew. Chem. Int. Ed. 2015, 55, 4199–4203. [Google Scholar] [CrossRef]
- Nikolic, J.; Belot, L.; Raux, H.; Legrand, P.; Gaudin, Y.; Albertini, A.A. Structural basis for the recognition of LDL-receptor family members by VSV glycoprotein. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Aki, M.; Shimbara, N.; Takashina, M.; Akiyama, K.; Kagawa, S.; Tamura, T.; Tanahashi, N.; Yoshimura, T.; Tanaka, K.; Ichihara, A. Interferon-gamma induces different subunit organizations and functional diversity of proteasomes. J. Biochem. 1994, 115, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-M.; Wen, H.-C.; Lin, W.-W. Proteasome Inhibitors Stimulate Interleukin-8 Expression via Ras and Apoptosis Signal-Regulating Kinase-dependent Extracellular Signal-Related Kinase and c-Jun N-Terminal Kinase Activation. Am. J. Respir. Cell Mol. Biol. 2002, 27, 234–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joshi-Barve, S.; Barve, S.S.; Butt, W.; Klein, J.; McClain, C.J. Inhibition of proteasome function leads to NF-κB-independent IL-8 expression in human hepatocytes. Hepatology 2003, 38, 1178–1187. [Google Scholar] [CrossRef]
- Manna, S.; Singha, B.; Phyo, S.A.; Gatla, H.R.; Chang, T.-P.; Sanacora, S.; Ramaswami, S.; Vancurova, I. Proteasome Inhibition by Bortezomib Increases IL-8 Expression in Androgen-Independent Prostate Cancer Cells: The Role of IKKα. J. Immunol. 2013, 191, 2837–2846. [Google Scholar] [CrossRef] [PubMed]
- Bannert, N.; Kurth, R. The Evolutionary Dynamics of Human Endogenous Retroviral Families. Annu. Rev. Genom. Hum. Genet. 2006, 7, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Sweileh, W.M. Global research output on HIV/AIDS–related medication adherence from 1980 to 2017. BMC Health Serv. Res. 2018, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.; Goujon, C.; Malim, M.H. HIV-1 and interferons: Who’s interfering with whom? Nat. Rev. Genet. 2015, 13, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Mano, M.; Braga, L.; Naseem, A.; Marini, B.; Vu, D.M.; Collesi, C.; Meroni, G.; Lusic, M.; Giacca, M. Cellular TRIM33 restrains HIV-1 infection by targeting viral integrase for proteasomal degradation. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Tada, T.; Zhang, Y.; Koyama, T.; Tobiume, M.; Tsunetsugu-Yokota, Y.; Yamaoka, S.; Fujita, H.; Tokunaga, K. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins. Nat. Med. 2015, 21, 1502–1507. [Google Scholar] [CrossRef]
- Seissler, T.; Marquet, R.; Paillart, J.-C. Hijacking of the Ubiquitin/Proteasome Pathway by the HIV Auxiliary Proteins. Viruses 2017, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yu, Y.; Liu, B.; Luo, K.; Kong, W.; Mao, P. Induction of APOBEC3G Ubiquitination and Degradation by an HIV-1 Vif-Cul5-SCF Complex. Science 2003, 302, 1056–1060. [Google Scholar] [CrossRef]
- Tokarev, A.A.; Munguia, J.; Guatelli, J.C. Serine-Threonine Ubiquitination Mediates Downregulation of BST-2/Tetherin and Relief of Restricted Virion Release by HIV-1 Vpu. J. Virol. 2010, 85, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Hao, C.; Yan, J.; DeLucia, M.; Mehrens, J.; Wang, C.; Gronenborn, A.M.; Skowronski, J. HIV/Simian Immunodeficiency Virus (SIV) Accessory Virulence Factor Vpx Loads the Host Cell Restriction Factor SAMHD1 onto the E3 Ubiquitin Ligase Complex CRL4DCAF1. J. Biol. Chem. 2012, 287, 12550–12558. [Google Scholar] [CrossRef] [PubMed]
- Seeger, M.; Ferrell, K.; Frank, R.; Dubiel, W. HIV-1 Tat Inhibits the 20 S Proteasome and Its 11 S Regulator-mediated Activation. J. Biol. Chem. 1997, 272, 8145–8148. [Google Scholar] [CrossRef]
- Apcher, G.; Heink, S.; Zantopf, D.; Kloetzel, P.-M.; Schmid, H.-P.; Mayer, R.; Krüger, E. Human immunodeficiency virus-1 Tat protein interacts with distinct proteasomal α and β subunits. FEBS Lett. 2003, 553, 200–204. [Google Scholar] [CrossRef]
- Steers, N.J.; Peachman, K.K.; McClain, S.R.; Alving, C.R.; Rao, M. Human Immunodeficiency Virus Type 1 Gag p24 Alters the Composition of Immunoproteasomes and Affects Antigen Presentation. J. Virol. 2009, 83, 7049–7061. [Google Scholar] [CrossRef] [PubMed]
- Haorah, J.; Heilman, D.; Diekmann, C.; Osna, N.; Donohue, T.M.; Ghorpade, A.; Persidsky, Y. Alcohol and HIV decrease proteasome and immunoproteasome function in macrophages: Implications for impaired immune function during disease. Cell. Immunol. 2004, 229, 139–148. [Google Scholar] [CrossRef]
- Jimenez-Guardeño, J.M.; Apolonia, L.; Betancor, G.; Malim, M.H. Immunoproteasome activation enables human TRIM5α restriction of HIV-1. Nat. Microbiol. 2019, 4, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Campbell, E.M.; Wu, X.; Vandegraaff, N.; Engelman, A.; Hope, T.J. Proteasome Inhibition Reveals that a Functional Preintegration Complex Intermediate Can Be Generated during Restriction by Diverse TRIM5 Proteins. J. Virol. 2006, 80, 9754–9760. [Google Scholar] [CrossRef]
- Muchamuel, T.; Basler, M.; Aujay, M.A.; Suzuki, E.; Kalim, K.W.; Lauer, C.; Sylvain, C.; Ring, E.R.; Shields, J.; Jiang, J.; et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat. Med. 2009, 15, 781–787. [Google Scholar] [CrossRef]
- Kang, S.; Brown, H.M.; Hwang, S. Direct Antiviral Mechanisms of Interferon-Gamma. Immune Netw. 2018, 18, e33. [Google Scholar] [CrossRef]
- Miller, L.K.; Kobayashi, Y.; Chen, C.-C.; Russnak, T.A.; Ron, Y.; Dougherty, J.P. Proteasome inhibitors act as bifunctional antagonists of human immunodeficiency virus type 1 latency and replication. Retrovirology 2013, 10, 120. [Google Scholar] [CrossRef]
- Uddin, M.M.; Zou, Y.; Sharma, T.; Gatla, H.R.; Vancurova, I. Proteasome inhibition induces IKK-dependent interleukin-8 expression in triple negative breast cancer cells: Opportunity for combination therapy. PLoS ONE 2018, 13, e0201858. [Google Scholar] [CrossRef] [PubMed]
- Singha, B.; Gatla, H.R.; Manna, S.; Chang, T.-P.; Sanacora, S.; Poltoratsky, V.; Vancura, A.; Vancurova, I. Proteasome Inhibition Increases Recruitment of IκB Kinase β (IKKβ), S536P-p65, and Transcription Factor EGR1 to Interleukin-8 (IL-8) Promoter, Resulting in Increased IL-8 Production in Ovarian Cancer Cells. J. Biol. Chem. 2014, 289, 2687–2700. [Google Scholar] [CrossRef]
- Gerber, A.; Heimburg, A.; Reisenauer, A.; Wille, A.; Welte, T.; Buhling, F. Proteasome inhibitors modulate chemokine production in lung epithelial and monocytic cells. Eur. Respir. J. 2004, 24, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Liong, S.; Lim, R.; Nguyen-Ngo, C.; Barker, G.; Parkington, H.C.; Lappas, M. The immunoproteasome inhibitor ONX-0914 regulates inflammation and expression of contraction associated proteins in myometrium. Eur. J. Immunol. 2018, 48, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagapova, E.; Burov, A.; Spasskaya, D.; Lebedev, T.; Astakhova, T.; Spirin, P.; Prassolov, V.; Karpov, V.; Morozov, A. Immunoproteasome Activity and Content Determine Hematopoietic Cell Sensitivity to ONX-0914 and to the Infection of Cells with Lentiviruses. Cells 2021, 10, 1185. https://doi.org/10.3390/cells10051185
Vagapova E, Burov A, Spasskaya D, Lebedev T, Astakhova T, Spirin P, Prassolov V, Karpov V, Morozov A. Immunoproteasome Activity and Content Determine Hematopoietic Cell Sensitivity to ONX-0914 and to the Infection of Cells with Lentiviruses. Cells. 2021; 10(5):1185. https://doi.org/10.3390/cells10051185
Chicago/Turabian StyleVagapova, Elmira, Alexander Burov, Daria Spasskaya, Timofey Lebedev, Tatiana Astakhova, Pavel Spirin, Vladimir Prassolov, Vadim Karpov, and Alexey Morozov. 2021. "Immunoproteasome Activity and Content Determine Hematopoietic Cell Sensitivity to ONX-0914 and to the Infection of Cells with Lentiviruses" Cells 10, no. 5: 1185. https://doi.org/10.3390/cells10051185
APA StyleVagapova, E., Burov, A., Spasskaya, D., Lebedev, T., Astakhova, T., Spirin, P., Prassolov, V., Karpov, V., & Morozov, A. (2021). Immunoproteasome Activity and Content Determine Hematopoietic Cell Sensitivity to ONX-0914 and to the Infection of Cells with Lentiviruses. Cells, 10(5), 1185. https://doi.org/10.3390/cells10051185





