CD4+ T Cells in Chronic Hepatitis B and T Cell-Directed Immunotherapy
Abstract
1. Introduction
2. CD4+ T Cells in Viral Infections
3. CD4+ T Cells in Acute Hepatitis B
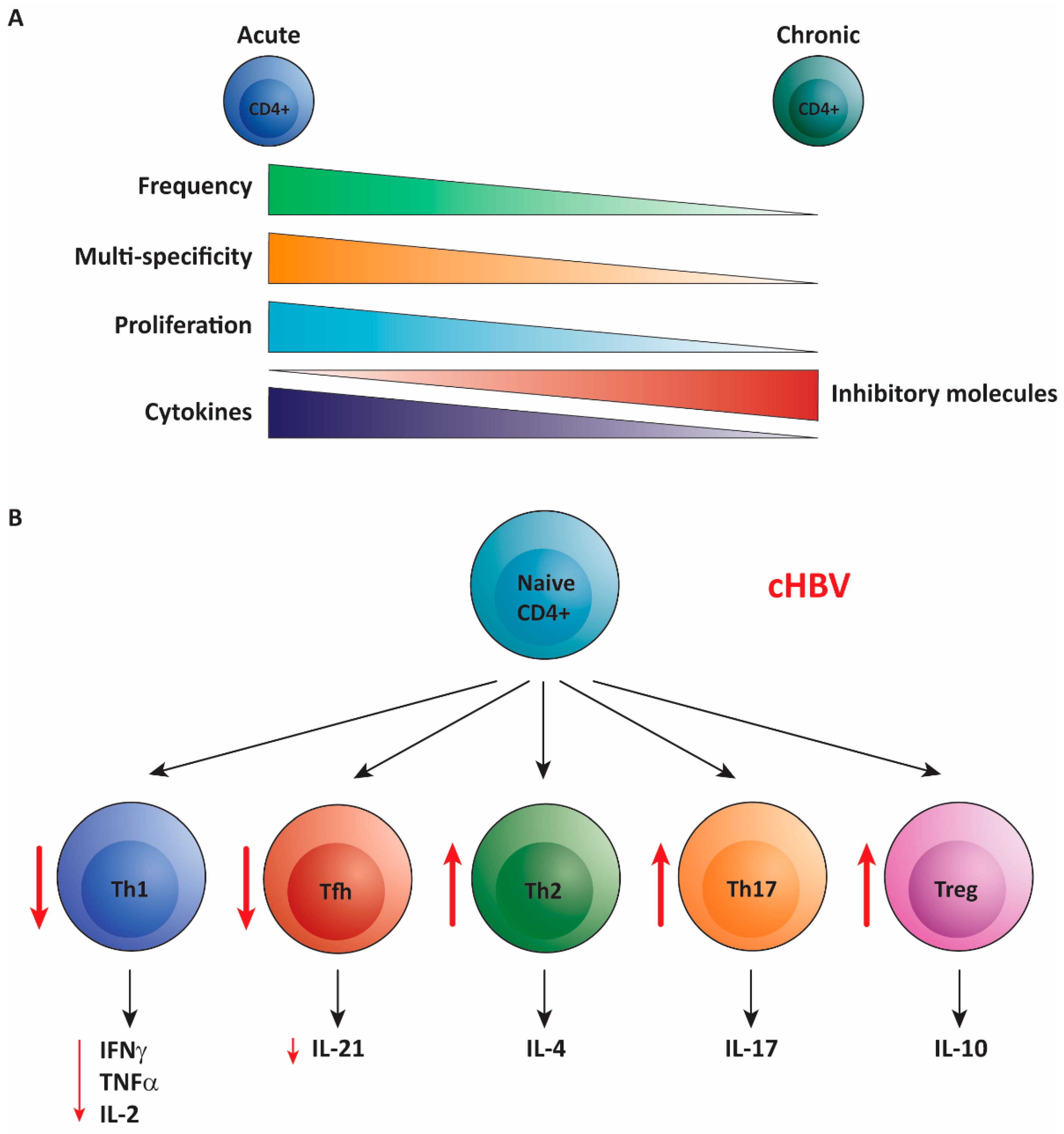
4. CD4+ T Cells in Chronic Hepatitis B
5. CD4+ T Cells in Past Clinical Studies Testing T Cell-Directed Therapies
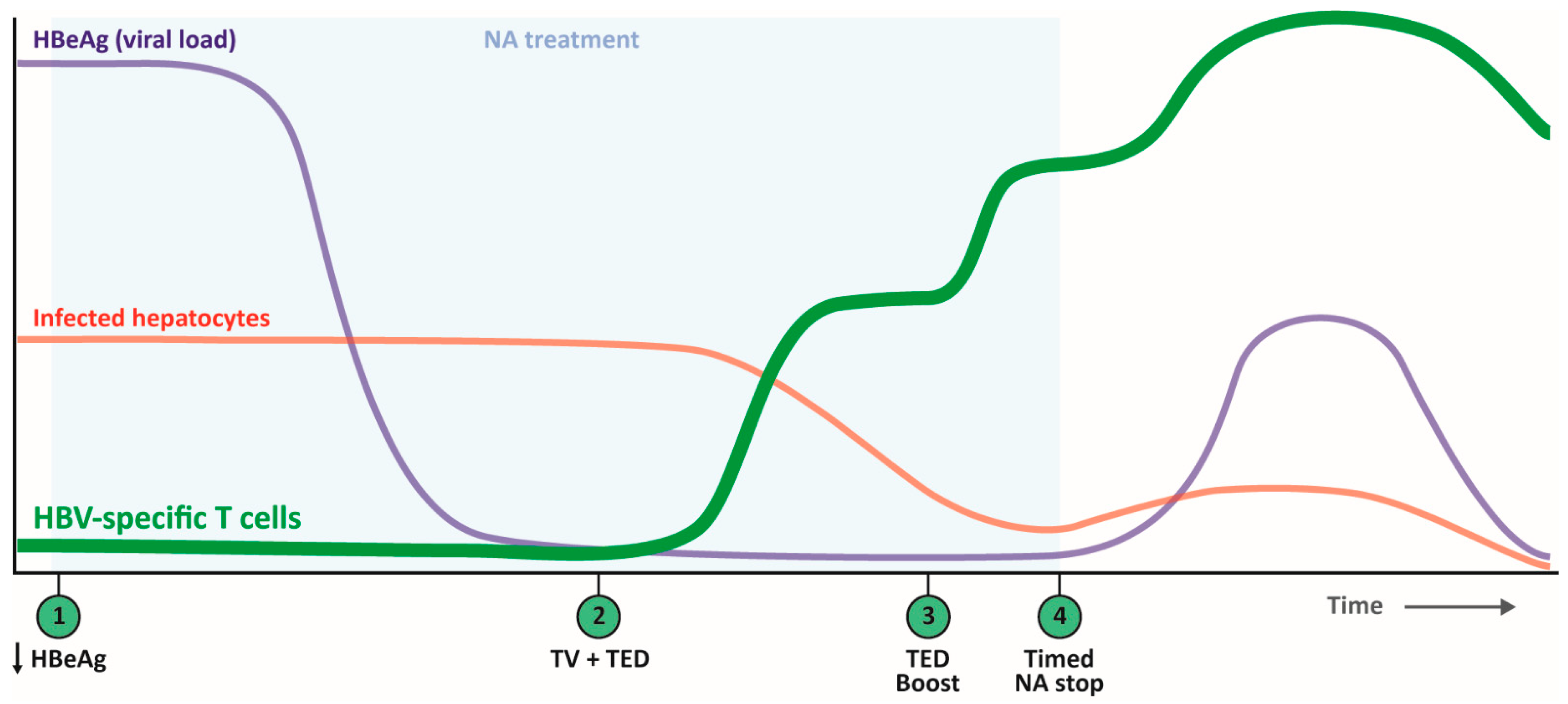
6. Harnessing CD4+ T Cells with Future Therapeutic Vaccination
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Revill, P.A.; Chisari, F.V.; Block, J.M.; Dandri, M.; Gehring, A.J.; Guo, H.; Hu, J.; Kramvis, A.; Lampertico, P.; Janssen, H.L.A.; et al. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol. Hepatol. 2019, 4, 545–558. [Google Scholar] [CrossRef]
- Ilan, Y.; Nagler, A.; Adler, R.; Tur-Kaspa, R.; Slavin, S.; Shouval, D. Ablation of persistent hepatitis B by bone marrow transplantation from a hepatitis B-immune donor. Gastroenterology 1993, 104, 1818–1821. [Google Scholar] [CrossRef]
- Lau, G.K.; Lok, A.S.; Liang, R.H.; Lai, C.L.; Chiu, E.K.; Lau, Y.L.; Lam, S.K. Clearance of hepatitis B surface antigen after bone marrow transplantation: Role of adoptive immunity transfer. Hepatology 1997, 25, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.K.; Suri, D.; Liang, R.; Rigopoulou, E.I.; Thomas, M.G.; Mullerova, I.; Nanji, A.; Yuen, S.T.; Williams, R.; Naoumov, N.V. Resolution of chronic hepatitis B and anti-HBs seroconversion in humans by adoptive transfer of immunity to hepatitis B core antigen. Gastroenterology 2002, 122, 614–624. [Google Scholar] [CrossRef]
- Loggi, E.; Bihl, F.; Chisholm, J.V., 3rd; Biselli, M.; Bontadini, A.; Vitale, G.; Ercolani, G.; Grazi, G.L.; Pinna, A.D.; Bernardi, M.; et al. Anti-HBs re-seroconversion after liver transplantation in a patient with past HBV infection receiving a HBsAg positive graft. J. Hepatol. 2009, 50, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Thimme, R.; Wieland, S.; Steiger, C.; Ghrayeb, J.; Reimann, K.A.; Purcell, R.H.; Chisari, F.V. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 2003, 77, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Penna, A.; Artini, M.; Cavalli, A.; Levrero, M.; Bertoletti, A.; Pilli, M.; Chisari, F.V.; Rehermann, B.; Del Prete, G.; Fiaccadori, F.; et al. Long-lasting memory T cell responses following self-limited acute hepatitis B. J. Clin. Investig. 1996, 98, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.; Penna, A.; Bertoletti, A.; Valli, A.; Antoni, A.D.; Giuberti, T.; Cavalli, A.; Petit, M.A.; Fiaccadori, F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J. Immunol. 1990, 145, 3442–3449. [Google Scholar] [PubMed]
- Penna, A.; Chisari, F.V.; Bertoletti, A.; Missale, G.; Fowler, P.; Giuberti, T.; Fiaccadori, F.; Ferrari, C. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J. Exp. Med. 1991, 174, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Bertoletti, A.; Ferrari, C.; Fiaccadori, F.; Penna, A.; Margolskee, R.; Schlicht, H.J.; Fowler, P.; Guilhot, S.; Chisari, F.V. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc. Natl. Acad. Sci. USA 1991, 88, 10445–10449. [Google Scholar] [CrossRef]
- Jung, M.C.; Stemler, M.; Weimer, T.; Spengler, U.; Döhrmann, J.; Hoffmann, R.; Eichenlaub, D.; Eisenburg, J.; Paumgartner, G.; Riethmüller, G.; et al. Immune response of peripheral blood mononuclear cells to HBx-antigen of hepatitis B virus. Hepatology 1991, 13, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Nayersina, R.; Fowler, P.; Guilhot, S.; Missale, G.; Cerny, A.; Schlicht, H.J.; Vitiello, A.; Chesnut, R.; Person, J.L.; Redeker, A.G.; et al. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J. Immunol. 1993, 150, 4659–4671. [Google Scholar]
- Rehermann, B.; Fowler, P.; Sidney, J.; Person, J.; Redeker, A.; Brown, M.; Moss, B.; Sette, A.; Chisari, F.V. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J. Exp. Med. 1995, 181, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Boni, C.; Laccabue, D.; Lampertico, P.; Giuberti, T.; Viganò, M.; Schivazappa, S.; Alfieri, A.; Pesci, M.; Gaeta, G.B.; Brancaccio, G.; et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology 2012, 143, 963–973 e969. [Google Scholar] [CrossRef]
- Desmond, C.P.; Bartholomeusz, A.; Gaudieri, S.; Revill, P.A.; Lewin, S.R. A systematic review of T-cell epitopes in hepatitis B virus: Identification, genotypic variation and relevance to antiviral therapeutics. Antivir. Ther. 2008, 13, 161–175. [Google Scholar]
- Chisari, F.V. Cytotoxic T cells and viral hepatitis. J. Clin. Investig. 1997, 99, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Thursz, M.R.; Thomas, H.C.; Greenwood, B.M.; Hill, A.V. Heterozygote advantage for HLA class-II type in hepatitis B virus infection. Nat. Genet. 1997, 17, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, R.C.; Robidoux, M.P.; Schwarz, T.; Heydmann, L.; Cheney, J.A.; Kvistad, D.; Aneja, J.; Melgaço, J.G.; Fernandes, C.A.; Chung, R.T.; et al. Phenotype and function of HBV-specific T cells is determined by the targeted epitope in addition to the stage of infection. Gut 2019, 68, 893–904. [Google Scholar] [CrossRef]
- Jansen, D.T.; Dou, Y.; de Wilde, J.W.; Woltman, A.M.; Buschow, S.I. Designing the next-generation therapeutic vaccines to cure chronic hepatitis B: Focus on antigen presentation, vaccine properties and effect measures. Clin. Transl. Immunol. 2021, 10, e1232. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bao, M.; Ge, J.; Ren, S.; Zhou, T.; Qi, F.; Pu, X.; Dou, J. Research progress of therapeutic vaccines for treating chronic hepatitis B. Hum. Vaccin. Immunother. 2017, 13, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Maini, M.K.; Pallett, L.J. Defective T-cell immunity in hepatitis B virus infection: Why therapeutic vaccination needs a helping hand. Lancet Gastroenterol. Hepatol. 2018, 3, 192–202. [Google Scholar] [CrossRef]
- Swain, S.L.; McKinstry, K.K.; Strutt, T.M. Expanding roles for CD4⁺ T cells in immunity to viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Bevan, M.J. Helping the CD8(+) T-cell response. Nat. Rev. Immunol. 2004, 4, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.R.; Carbone, F.R.; Karamalis, F.; Flavell, R.A.; Miller, J.F.; Heath, W.R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 1998, 393, 478–480. [Google Scholar] [CrossRef]
- Schoenberger, S.P.; Toes, R.E.; van der Voort, E.I.; Offringa, R.; Melief, C.J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 1998, 393, 480–483. [Google Scholar] [CrossRef]
- Ridge, J.P.; Di Rosa, F.; Matzinger, P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 1998, 393, 474–478. [Google Scholar] [CrossRef]
- Eickhoff, S.; Brewitz, A.; Gerner, M.Y.; Klauschen, F.; Komander, K.; Hemmi, H.; Garbi, N.; Kaisho, T.; Germain, R.N.; Kastenmüller, W. Robust Anti-viral Immunity Requires Multiple Distinct T Cell-Dendritic Cell Interactions. Cell 2015, 162, 1322–1337. [Google Scholar] [CrossRef]
- Hor, J.L.; Whitney, P.G.; Zaid, A.; Brooks, A.G.; Heath, W.R.; Mueller, S.N. Spatiotemporally Distinct Interactions with Dendritic Cell Subsets Facilitates CD4+ and CD8+ T Cell Activation to Localized Viral Infection. Immunity 2015, 43, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, D.; Kugler, D.G.; Sher, A. IL-10 production by CD4+ effector T cells: A mechanism for self-regulation. Mucosal. Immunol. 2010, 3, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.A.; Schreiber, R.D. The molecular cell biology of interferon-gamma and its receptor. Annu. Rev. Immunol. 1993, 11, 571–611. [Google Scholar] [CrossRef]
- Graham, M.B.; Braciale, V.L.; Braciale, T.J. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J. Exp. Med. 1994, 180, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Arens, R.; Wang, P.; Sidney, J.; Loewendorf, A.; Sette, A.; Schoenberger, S.P.; Peters, B.; Benedict, C.A. Cutting edge: Murine cytomegalovirus induces a polyfunctional CD4 T cell response. J. Immunol. 2008, 180, 6472–6476. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Veiga-Parga, T.; Rajasagi, N.K.; Reddy, P.B.; Sehrawat, S.; Sharma, S.; Rouse, B.T. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J. Immunol. 2011, 187, 1919–1930. [Google Scholar] [CrossRef]
- Alwan, W.H.; Kozlowska, W.J.; Openshaw, P.J. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 1994, 179, 81–89. [Google Scholar] [CrossRef]
- Moran, T.M.; Isobe, H.; Fernandez-Sesma, A.; Schulman, J.L. Interleukin-4 causes delayed virus clearance in influenza virus-infected mice. J. Virol. 1996, 70, 5230–5235. [Google Scholar] [CrossRef]
- Coutelier, J.P.; van der Logt, J.T.; Heessen, F.W.; Warnier, G.; Van Snick, J. IgG2a restriction of murine antibodies elicited by viral infections. J. Exp. Med. 1987, 165, 64–69. [Google Scholar] [CrossRef]
- Maloy, K.J.; Burkhart, C.; Junt, T.M.; Odermatt, B.; Oxenius, A.; Piali, L.; Zinkernagel, R.M.; Hengartner, H. CD4(+) T cell subsets during virus infection. Protective capacity depends on effector cytokine secretion and on migratory capability. J. Exp. Med. 2000, 191, 2159–2170. [Google Scholar] [CrossRef]
- Hou, W.; Kang, H.S.; Kim, B.S. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J. Exp. Med. 2009, 206, 313–328. [Google Scholar] [CrossRef]
- Ye, P.; Rodriguez, F.H.; Kanaly, S.; Stocking, K.L.; Schurr, J.; Schwarzenberger, P.; Oliver, P.; Huang, W.; Zhang, P.; Zhang, J.; et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001, 194, 519–527. [Google Scholar] [CrossRef]
- Hegazy, A.N.; Peine, M.; Helmstetter, C.; Panse, I.; Fröhlich, A.; Bergthaler, A.; Flatz, L.; Pinschewer, D.D.; Radbruch, A.; Löhning, M. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity 2010, 32, 116–128. [Google Scholar] [CrossRef]
- Lee, Y.K.; Turner, H.; Maynard, C.L.; Oliver, J.R.; Chen, D.; Elson, C.O.; Weaver, C.T. Late developmental plasticity in the T helper 17 lineage. Immunity 2009, 30, 92–107. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Paul, W.E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 2010, 327, 1098–1102. [Google Scholar] [CrossRef]
- Zhou, L.; Chong, M.M.; Littman, D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef]
- Asabe, S.; Wieland, S.F.; Chattopadhyay, P.K.; Roederer, M.; Engle, R.E.; Purcell, R.H.; Chisari, F.V. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J. Virol. 2009, 83, 9652–9662. [Google Scholar] [CrossRef]
- Trautmann, T.; Kozik, J.H.; Carambia, A.; Richter, K.; Lischke, T.; Schwinge, D.; Mittrücker, H.W.; Lohse, A.W.; Oxenius, A.; Wiegard, C.; et al. CD4+ T-cell help is required for effective CD8+ T cell-mediated resolution of acute viral hepatitis in mice. PLoS ONE 2014, 9, e86348. [Google Scholar]
- Yan, Z.H.; Fan, Y.; Wang, X.H.; Mao, Q.; Deng, G.H.; Wang, Y.M. Relationship between HLA-DR gene polymorphisms and outcomes of hepatitis B viral infections: A meta-analysis. World J. Gastroenterol. 2012, 18, 3119–3128. [Google Scholar] [CrossRef]
- Matsuura, K.; Isogawa, M.; Tanaka, Y. Host genetic variants influencing the clinical course of hepatitis B virus infection. J. Med. Virol. 2016, 88, 371–379. [Google Scholar] [CrossRef]
- Brouwer, W.P.; Sonneveld, M.J.; Tabak, F.; Simon, K.; Cakaloglu, Y.; Akarca, U.S.; Zeuzem, S.; Ferenci, P.; Heathcote, J.E.; de Knegt, R.J.; et al. Polymorphisms of HLA-DP are associated with response to peginterferon in Caucasian patients with chronic hepatitis B. Aliment. Pharmacol. Ther. 2014, 40, 811–818. [Google Scholar] [CrossRef]
- Webster, G.J.; Reignat, S.; Maini, M.K.; Whalley, S.A.; Ogg, G.S.; King, A.; Brown, D.; Amlot, P.L.; Williams, R.; Vergani, D.; et al. Incubation phase of acute hepatitis B in man: Dynamic of cellular immune mechanisms. Hepatology 2000, 32, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C. HBV and the immune response. Liver Int. 2015, 35 (Suppl. 1), S121–S128. [Google Scholar] [CrossRef]
- Mizukoshi, E.; Sidney, J.; Livingston, B.; Ghany, M.; Hoofnagle, J.H.; Sette, A.; Rehermann, B. Cellular immune responses to the hepatitis B virus polymerase. J. Immunol. 2004, 173, 5863–5871. [Google Scholar] [CrossRef]
- Penna, A.; Del Prete, G.; Cavalli, A.; Bertoletti, A.; D’Elios, M.M.; Sorrentino, R.; D’Amato, M.; Boni, C.; Pilli, M.; Fiaccadori, F.; et al. Predominant T-helper 1 cytokine profile of hepatitis B virus nucleocapsid-specific T cells in acute self-limited hepatitis B. Hepatology 1997, 25, 1022–1027. [Google Scholar] [CrossRef]
- Szkaradkiewicz, A.; Jopek, A.; Wysocki, J.; Grzymislawski, M.; Malecka, I.; Woźniak, A. HBcAg-specific cytokine production by CD4 T lymphocytes of children with acute and chronic hepatitis B. Virus Res. 2003, 97, 127–133. [Google Scholar] [CrossRef]
- Morell, A.; Roth-Wicky, B.; Skvaril, F. Immunoglobulin G subclass restriction of antibodies against hepatitis B surface antigen. Infect. Immun. 1983, 39, 565–568. [Google Scholar] [CrossRef]
- Jung, M.C.; Diepolder, H.M.; Spengler, U.; Wierenga, E.A.; Zachoval, R.; Hoffmann, R.M.; Eichenlaub, D.; Frösner, G.; Will, H.; Pape, G.R. Activation of a heterogeneous hepatitis B (HB) core and e antigen-specific CD4+ T-cell population during seroconversion to anti-HBe and anti-HBs in hepatitis B virus infection. J. Virol. 1995, 69, 3358–3368. [Google Scholar] [CrossRef]
- Park, J.J.; Wong, D.K.; Wahed, A.S.; Lee, W.M.; Feld, J.J.; Terrault, N.; Khalili, M.; Sterling, R.K.; Kowdley, K.V.; Bzowej, N.; et al. Hepatitis B Virus--Specific and Global T-Cell Dysfunction in Chronic Hepatitis B. Gastroenterology 2016, 150, 684–695 e685. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Zhang, L.; Zhu, Q.; Chen, C.; Bao, J.; Chen, Y. CD4(+) T cell exhaustion revealed by high PD-1 and LAG-3 expression and the loss of helper T cell function in chronic hepatitis B. BMC Immunol. 2019, 20, 27. [Google Scholar] [CrossRef]
- Ge, J.; Wang, K.; Meng, Q.H.; Qi, Z.X.; Meng, F.L.; Fan, Y.C. Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J. Clin. Immunol. 2010, 30, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gao, W.; Zhang, L.; Wang, J.; Chen, M.; Peng, M.; Ren, H.; Hu, P. Th17/Treg imbalance and increased interleukin-21 are associated with liver injury in patients with chronic severe hepatitis B. Int. Immunopharmacol. 2017, 46, 48–55. [Google Scholar] [CrossRef]
- Boni, C.; Fisicaro, P.; Valdatta, C.; Amadei, B.; Di Vincenzo, P.; Giuberti, T.; Laccabue, D.; Zerbini, A.; Cavalli, A.; Missale, G.; et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 2007, 81, 4215–4225. [Google Scholar] [CrossRef]
- Wang, H.; Luo, H.; Wan, X.; Fu, X.; Mao, Q.; Xiang, X.; Zhou, Y.; He, W.; Zhang, J.; Guo, Y.; et al. TNF-α/IFN-γ profile of HBV-specific CD4 T cells is associated with liver damage and viral clearance in chronic HBV infection. J. Hepatol. 2020, 72, 45–56. [Google Scholar] [CrossRef]
- Milich, D.R.; Schödel, F.; Hughes, J.L.; Jones, J.E.; Peterson, D.L. The hepatitis B virus core and e antigens elicit different Th cell subsets: Antigen structure can affect Th cell phenotype. J. Virol. 1997, 71, 2192–2201. [Google Scholar] [CrossRef]
- Milich, D.R.; Chen, M.K.; Hughes, J.L.; Jones, J.E. The secreted hepatitis B precore antigen can modulate the immune response to the nucleocapsid: A mechanism for persistence. J. Immunol. 1998, 160, 2013–2021. [Google Scholar] [PubMed]
- Livingston, B.D.; Alexander, J.; Crimi, C.; Oseroff, C.; Celis, E.; Daly, K.; Guidotti, L.G.; Chisari, F.V.; Fikes, J.; Chesnut, R.W.; et al. Altered helper T lymphocyte function associated with chronic hepatitis B virus infection and its role in response to therapeutic vaccination in humans. J. Immunol. 1999, 162, 3088–3095. [Google Scholar]
- Zhang, J.Y.; Zhang, Z.; Lin, F.; Zou, Z.S.; Xu, R.N.; Jin, L.; Fu, J.L.; Shi, F.; Shi, M.; Wang, H.F.; et al. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology 2010, 51, 81–91. [Google Scholar] [CrossRef]
- Ye, Y.; Xie, X.; Yu, J.; Zhou, L.; Xie, H.; Jiang, G.; Yu, X.; Zhang, W.; Wu, J.; Zheng, S. Involvement of Th17 and Th1 effector responses in patients with Hepatitis B. J. Clin. Immunol. 2010, 30, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Stoop, J.N.; van der Molen, R.G.; Baan, C.C.; van der Laan, L.J.; Kuipers, E.J.; Kusters, J.G.; Janssen, H.L. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 2005, 41, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Stoop, J.N.; Woltman, A.M.; Biesta, P.J.; Kusters, J.G.; Kuipers, E.J.; Janssen, H.L.; van der Molen, R.G. Tumor necrosis factor alpha inhibits the suppressive effect of regulatory T cells on the hepatitis B virus-specific immune response. Hepatology 2007, 46, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Manigold, T.; Racanelli, V. T-cell regulation by CD4 regulatory T cells during hepatitis B and C virus infections: Facts and controversies. Lancet Infect. Dis. 2007, 7, 804–813. [Google Scholar] [CrossRef]
- Wang, R.; Xie, R.; Song, Z. Circulating regulatory Tfh cells are enriched in patients with chronic hepatitis B infection and induce the differentiation of regulatory B cells. Exp. Cell Res. 2018, 365, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, Q.; Li, Q.; Li, Y.; Zhao, D.; Sun, J.; Fu, J.; Meng, F.; Lin, H.; Luan, J.; et al. Dysregulated Response of Follicular Helper T Cells to Hepatitis B Surface Antigen Promotes HBV Persistence in Mice and Associates With Outcomes of Patients. Gastroenterology 2018, 154, 2222–2236. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, S.; Tang, L.; Li, Y.; Wang, W.; Huang, X.; Lai, Q.; Zhang, M.; Sun, J.; Li, C.K.; et al. Circulating chemokine (C-X-C Motif) receptor 5(+) CD4(+) T cells benefit hepatitis B e antigen seroconversion through IL-21 in patients with chronic hepatitis B virus infection. Hepatology 2013, 58, 1277–1286. [Google Scholar] [CrossRef]
- Huang, Y.X.; Zhao, Q.Y.; Wu, L.L.; Xie, D.Y.; Gao, Z.L.; Deng, H. Increased CCR7(lo)PD-1(hi)CXCR5(+)CD4(+) T Cells in Peripheral Blood Mononuclear Cells Are Correlated with Immune Activation in Patients with Chronic HBV Infection. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1020925. [Google Scholar] [CrossRef]
- Lang, J.; Neumann-Haefelin, C.; Thimme, R. Immunological cure of HBV infection. Hepatol. Int. 2019, 13, 113–124. [Google Scholar] [CrossRef]
- Bertoletti, A.; Le Bert, N. Immunotherapy for Chronic Hepatitis B Virus Infection. Gut Liver 2018, 12, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Ishioka, G.; Grey, H.M.; Rose, R.; Farness, P.; LaFond, R.; Yuan, L.; Chisari, F.V.; Furze, J.; Bartholomeuz, R.; et al. Development of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. I. Induction of a primary cytotoxic T lymphocyte response in humans. J. Clin. Investig. 1995, 95, 341–349. [Google Scholar] [CrossRef]
- Ren, F.; Hino, K.; Yamaguchi, Y.; Funatsuki, K.; Hayashi, A.; Ishiko, H.; Furutani, M.; Yamasaki, T.; Korenaga, K.; Yamashita, S.; et al. Cytokine-dependent anti-viral role of CD4-positive T cells in therapeutic vaccination against chronic hepatitis B viral infection. J. Med. Virol. 2003, 71, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Couillin, I.; Pol, S.; Mancini, M.; Driss, F.; Bréchot, C.; Tiollais, P.; Michel, M.L. Specific vaccine therapy in chronic hepatitis B: Induction of T cell proliferative responses specific for envelope antigens. J. Infect. Dis. 1999, 180, 15–26. [Google Scholar] [CrossRef]
- Mancini-Bourgine, M.; Fontaine, H.; Bréchot, C.; Pol, S.; Michel, M.L. Immunogenicity of a hepatitis B DNA vaccine administered to chronic HBV carriers. Vaccine 2006, 24, 4482–4489. [Google Scholar] [CrossRef] [PubMed]
- Mancini-Bourgine, M.; Fontaine, H.; Scott-Algara, D.; Pol, S.; Bréchot, C.; Michel, M.L. Induction or expansion of T-cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology 2004, 40, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Lee, C.G.; Park, S.H.; Im, S.J.; Kim, Y.M.; Son, J.M.; Wang, J.S.; Yoon, S.K.; Song, M.K.; Ambrozaitis, A.; et al. Correlation of antiviral T-cell responses with suppression of viral rebound in chronic hepatitis B carriers: A proof-of-concept study. Gene Ther. 2006, 13, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.; Pan, C.Q.; Han, S.H.; Trinh, H.N.; Fessel, W.J.; Rodell, T.; Massetto, B.; Lin, L.; Gaggar, A.; Subramanian, G.M.; et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J. Hepatol. 2016, 65, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Boni, C.; Janssen, H.L.A.; Rossi, M.; Yoon, S.K.; Vecchi, A.; Barili, V.; Yoshida, E.M.; Trinh, H.; Rodell, T.C.; Laccabue, D.; et al. Combined GS-4774 and Tenofovir Therapy Can Improve HBV-Specific T-Cell Responses in Patients with Chronic Hepatitis. Gastroenterology 2019, 157, 227–241 e227. [Google Scholar] [CrossRef] [PubMed]
- Ahrends, T.; Spanjaard, A.; Pilzecker, B.; Bąbała, N.; Bovens, A.; Xiao, Y.; Jacobs, H.; Borst, J. CD4(+) T Cell Help Confers a Cytotoxic T Cell Effector Program Including Coinhibitory Receptor Downregulation and Increased Tissue Invasiveness. Immunity 2017, 47, 848–861 e845. [Google Scholar] [CrossRef]
- Isogawa, M.; Chung, J.; Murata, Y.; Kakimi, K.; Chisari, F.V. CD40 activation rescues antiviral CD8⁺ T cells from PD-1-mediated exhaustion. PLoS Pathog. 2013, 9, e1003490. [Google Scholar] [CrossRef]
- Salimzadeh, L.; Le Bert, N.; Dutertre, C.A.; Gill, U.S.; Newell, E.W.; Frey, C.; Hung, M.; Novikov, N.; Fletcher, S.; Kennedy, P.T.; et al. PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection. J. Clin. Investig. 2018, 128, 4573–4587. [Google Scholar] [CrossRef] [PubMed]
- de Niet, A.; Stelma, F.; Jansen, L.; Sinnige, M.J.; Remmerswaal, E.B.; Takkenberg, R.B.; Kootstra, N.A.; Reesink, H.W.; van Lier, R.A.; van Leeuwen, E.M. Restoration of T cell function in chronic hepatitis B patients upon treatment with interferon based combination therapy. J. Hepatol. 2016, 64, 539–546. [Google Scholar] [CrossRef]
- Szkaradkiewicz, A.; Jopek, A.; Wysocki, J. Effects of IL-12 and IL-18 on HBcAg-specific cytokine production by CD4 T lymphocytes of children with chronic hepatitis B infection. Antivir. Res. 2005, 66, 23–27. [Google Scholar] [CrossRef]
- Ozato, K.; Tsujimura, H.; Tamura, T. Toll-like receptor signaling and regulation of cytokine gene expression in the immune system. Biotechniques 2002, 33, S66–S75. [Google Scholar] [CrossRef]
- Jacobi, F.J.; Wild, K.; Smits, M.; Zoldan, K.; Csernalabics, B.; Flecken, T.; Lang, J.; Ehrenmann, P.; Emmerich, F.; Hofmann, M.; et al. OX40 stimulation and PD-L1 blockade synergistically augment HBV-specific CD4 T cells in patients with HBeAg-negative infection. J. Hepatol. 2019, 70, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Elsaesser, H.; Sauer, K.; Brooks, D.G. IL-21 is required to control chronic viral infection. Science 2009, 324, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, M.U.; Thimme, R.; Klenerman, P.; Semmo, N. Methodologies for the Analysis of HCV-Specific CD4(+) T Cells. Front. Immunol. 2015, 6, 57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boeijen, L.L.; Hoogeveen, R.C.; Boonstra, A.; Lauer, G.M. Hepatitis B virus infection and the immune response: The big questions. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Raziorrouh, B.; Heeg, M.; Kurktschiev, P.; Schraut, W.; Zachoval, R.; Wendtner, C.; Wächtler, M.; Spannagl, M.; Denk, G.; Ulsenheimer, A.; et al. Inhibitory phenotype of HBV-specific CD4+ T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS ONE 2014, 9, e105703. [Google Scholar] [CrossRef]
- Schuch, A.; Salimi Alizei, E.; Heim, K.; Wieland, D.; Kiraithe, M.M.; Kemming, J.; Llewellyn-Lacey, S.; Sogukpinar, Ö.; Ni, Y.; Urban, S.; et al. Phenotypic and functional differences of HBV core-specific versus HBV polymerase-specific CD8+ T cells in chronically HBV-infected patients with low viral load. Gut 2019, 68, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, P.; Barili, V.; Montanini, B.; Acerbi, G.; Ferracin, M.; Guerrieri, F.; Salerno, D.; Boni, C.; Massari, M.; Cavallo, M.C.; et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat. Med. 2017, 23, 327–336. [Google Scholar] [CrossRef]
- Acerbi, G.; Montali, I.; Ferrigno, G.D.; Barili, V.; Schivazappa, S.; Alfieri, A.; Laccabue, D.; Loglio, A.; Borghi, M.; Massari, M.; et al. Functional reconstitution of HBV-specific CD8 T cells by in vitro polyphenol treatment in chronic hepatitis B. J. Hepatol. 2020, 74, 783–793. [Google Scholar] [CrossRef]
- Barili, V.; Boni, C.; Rossi, M.; Vecchi, A.; Zecca, A.; Penna, A.; Missale, G.; Ferrari, C.; Fisicaro, P. Metabolic regulation of the HBV-specific T cell function. Antivir. Res. 2021, 185, 104989. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buschow, S.I.; Jansen, D.T.S.L. CD4+ T Cells in Chronic Hepatitis B and T Cell-Directed Immunotherapy. Cells 2021, 10, 1114. https://doi.org/10.3390/cells10051114
Buschow SI, Jansen DTSL. CD4+ T Cells in Chronic Hepatitis B and T Cell-Directed Immunotherapy. Cells. 2021; 10(5):1114. https://doi.org/10.3390/cells10051114
Chicago/Turabian StyleBuschow, Sonja I., and Diahann T. S. L. Jansen. 2021. "CD4+ T Cells in Chronic Hepatitis B and T Cell-Directed Immunotherapy" Cells 10, no. 5: 1114. https://doi.org/10.3390/cells10051114
APA StyleBuschow, S. I., & Jansen, D. T. S. L. (2021). CD4+ T Cells in Chronic Hepatitis B and T Cell-Directed Immunotherapy. Cells, 10(5), 1114. https://doi.org/10.3390/cells10051114






