Cytokines Involved in the Pathogenesis of SSc and Problems in the Development of Anti-Cytokine Therapy
Abstract
1. Introduction
2. Clinical Stages of SSc
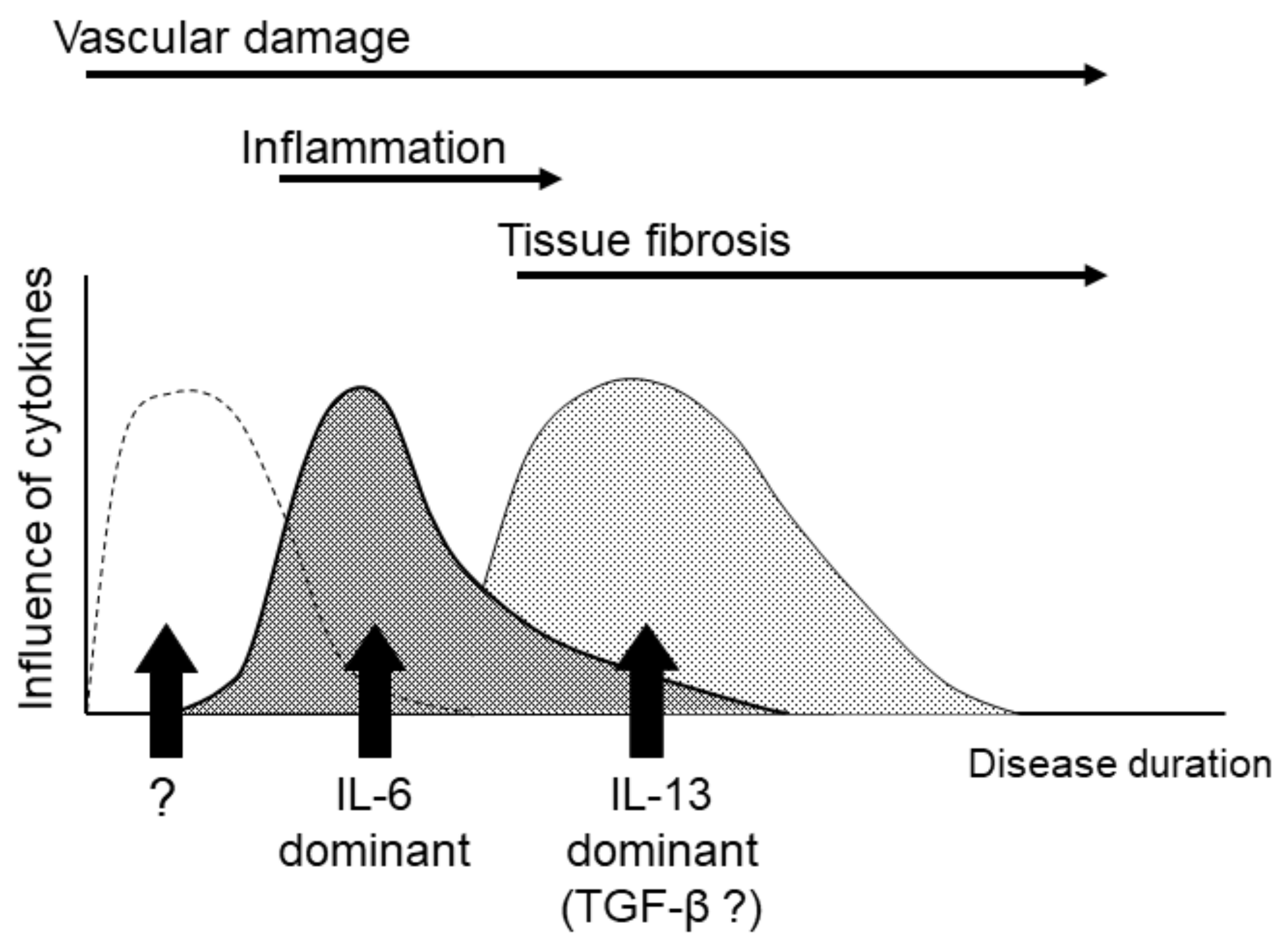
3. Progression of Pathological Stages
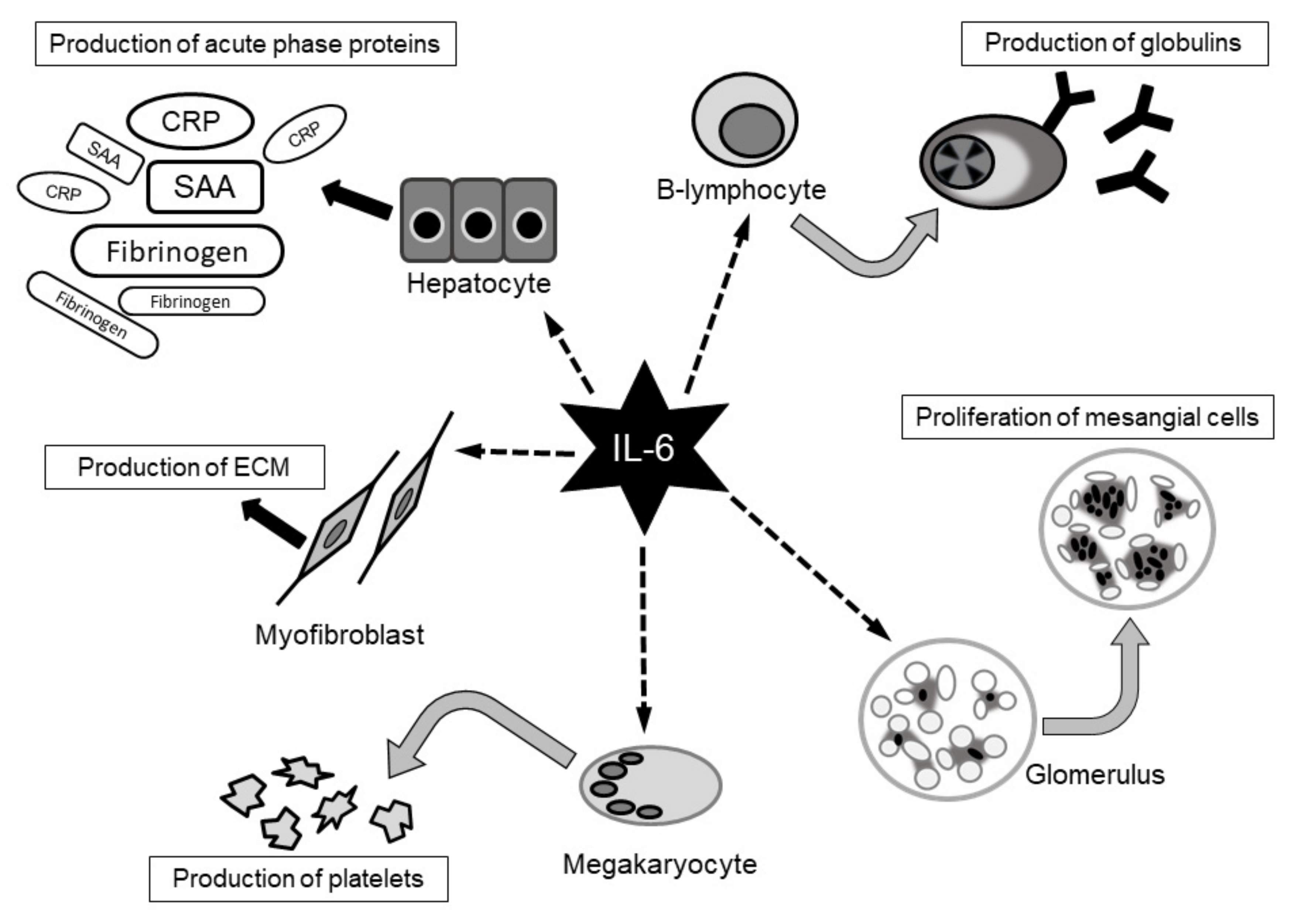
4. Cytokines Involved in the Pathology of SSc
5. Problems with Pathogenic Cytokines
6. Anti-Cytokine Therapy
7. Evaluation of Anti-Cytokine Therapy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar] [PubMed]
- Reveille, J.D.; Solomon, D.H. Evidence-based guidelines for the use of immunologic tests: Anticentromere, Scl-70, and nucleolar antibodies. Arthritis Rheum. 2003, 49, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.D. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005, 35, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Flavahan, N.A. A vascular mechanistic approach to understanding Raynaud phenomenon. Nat. Rev. Rheumatol. 2015, 11, 146–158. [Google Scholar] [CrossRef]
- Cutolo, M.; Herrick, A.L.; Distler, O.; Becker, M.O.; Beltran, E.; Carpentier, P.; Ferri, C.; Inanç, M.; Vlachoyiannopoulos, P.; Chadha-Boreham, H.; et al. Nailfold videocapillaroscopic features and other clinical risk factors for digital ulcers in systemic sclerosis: A multicenter, prospective cohort study. Arthritis Rheumatol. 2016, 68, 2527–2539. [Google Scholar] [CrossRef]
- Baron, M.; Lee, P.; Keystone, E.C. The articular manifestations of progressive systemic sclerosis (scleroderma). Ann. Rheum Dis. 1982, 41, 147–152. [Google Scholar] [CrossRef]
- Poole, J.L.; Gallegos, M.; O’Linc, S. Reliability and validity of the Arthritis Hand Function Test in adults with systemic sclerosis (scleroderma). Arthritis Care Res. 2000, 13, 69–73. [Google Scholar] [CrossRef]
- Steen, V.D.; Conte, C.; Owens, G.R.; Medsger, T.A., Jr. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994, 37, 1283–1289. [Google Scholar] [CrossRef]
- Steen, V.D.; Graham, G.; Conte, C.; Owens, G.; Medsger, T.A., Jr. Isolated diffusing capacity reduction in systemic sclerosis. Arthritis Rheum. 1992, 35, 765–770. [Google Scholar] [CrossRef]
- Monro, A.K. The anatomy, physiology and surgical consideration of the oesophagus. Postgrad. Med. J. 1944, 20, 273–277. [Google Scholar] [CrossRef][Green Version]
- Thoua, N.M.; Bunce, C.; Brough, G.; Forbes, A.; Emmanuel, A.V.; Denton, C.P. Assessment of gastrointestinal symptoms in patients with systemic sclerosis in a UK tertiary referral centre. Rheumatology (Oxford) 2010, 49, 1770–1775. [Google Scholar] [CrossRef]
- Ebert, E.C. Esophageal disease in scleroderma. J. Clin. Gastroenterol. 2006, 40, 769–775. [Google Scholar] [CrossRef]
- Weston, S.; Thumshirn, M.; Wiste, J.; Camilleri, M. Clinical and upper gastrointestinal motility features in systemic sclerosis and related disorders. Am. J. Gastroenterol. 1998, 93, 1085–1089. [Google Scholar] [CrossRef]
- Zamost, B.J.; Hirschberg, J.; Ippoliti, A.F.; Furst, D.E.; Clements, P.J.; Weinstein, W.M. Esophagitis in scleroderma. Prevalence and risk factors. Gastroenterology 1987, 92, 421–428. [Google Scholar] [CrossRef]
- Caporali, R.; Caccialanza, R.; Bonino, C.; Klersy, C.; Cereda, E.; Xoxi, B.; Crippa, A.; Rava, M.L.; Orlandi, M.; Bonardi, C.; et al. Disease-related malnutrition in outpatients with systemic sclerosis. Clin. Nutr. 2012, 31, 666–671. [Google Scholar] [CrossRef]
- Domsic, R.T.; Medsger, T.A., Jr. Disease subsets in clinical practice. In Scleroderma; Varga, J., Denton, C.P., Allanore, Y., Kuwana, M., Eds.; Springer: Boston, MA, USA, 2017; pp. 39–48. [Google Scholar]
- Matucci-Cerinic, M.; Kahaleh, B.; Wigley, F.M. Review: Evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. 2013, 65, 1953–1962. [Google Scholar] [CrossRef]
- Katsumoto, T.R.; Whitfield, M.L.; Connolly, M.K. The pathogenesis of systemic sclerosis. Annu. Rev. Pathol. Mech. Did. 2011, 6, 509–537. [Google Scholar] [CrossRef]
- Prescott, R.J.; Freemont, A.J.; Jones, C.J.; Hoyland, J.; Fielding, P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J. Pathol. 1992, 166, 255–263. [Google Scholar] [CrossRef]
- Montgomery, H.; O’Leary, P.A.; Ragsdale, W.E., Jr. Dermatohistopathology of various types of scleroderma. AMA Arch. Derm. 1957, 75, 78–87. [Google Scholar]
- De Larco, J.E.; Todaro, G.J. Growth factors from murine sarcoma virus-transformed cells. Proc. Natl. Acad. Sci. USA 1978, 75, 4001–4005. [Google Scholar] [CrossRef]
- Kulozik, M.; Hogg, A.; Lankat-Buttgereit, B.; Krieg, T. Co-localization of transforming growth factor beta 2 with alpha 1(I) procollagen mRNA in tissue sections of patients with systemic sclerosis. J. Clin. Investig. 1990, 86, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Kitani, A.; Hara, M.; Harigai, M.; Hirose, T.; Suzuki, K.; Kawakami, M.; Hidaka, T.; Ishizuka, T.; Kawagoe, M.; et al. Cytokine regulation of prolyl 4-hydroxylase production in skin fibroblast cultures from patients with systemic sclerosis: Contribution to collagen synthesis and fibrosis. J. Rheumatol. 1992, 19, 1195–1201. [Google Scholar] [PubMed]
- Yamakage, A.; Kikuchi, K.; Smith, E.A.; LeRoy, E.C.; Trojanowska, M. Selective upregulation of platelet-derived growth factor alpha receptors by transforming growth factor beta in scleroderma fibroblasts. J. Exp. Med. 1992, 175, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.A.; Ruscetti, F.W.; Gallo, R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 1976, 193, 1007–1008. [Google Scholar] [CrossRef]
- Umehara, H.; Kumagai, S.; Ishida, H.; Suginoshita, T.; Maeda, M.; Imura, H. Enhanced production of interleukin-2 in patients with progressive systemic sclerosis. Hyperactivity of CD4-positive T cells? Arthritis Rheum. 1988, 31, 401–407. [Google Scholar] [CrossRef]
- Degiannis, D.; Seibold, J.R.; Czarnecki, M.; Raskova, J.; Raska, K., Jr. Soluble interleukin-2 receptors in patients with systemic sclerosis. Clinical and laboratory correlations. Arthritis Rheum. 1990, 33, 375–380. [Google Scholar] [CrossRef]
- Hawrylko, E.; Spertus, A.; Mele, C.A.; Oster, N.; Frieri, M. Increased interleukin-2 production in response to human type I collagen stimulation in patients with systemic sclerosis. Arthritis Rheum. 1991, 34, 580–587. [Google Scholar] [CrossRef]
- Grabstein, K.; Eisenman, J.; Mochizuki, D.; Shanebeck, K.; Conlon, P.; Hopp, T.; March, C.; Gillis, S. Purification to homogeneity of B cell stimulating factor. A molecule that stimulates proliferation of multiple lymphokine-dependent cell lines. J. Exp. Med. 1986, 163, 1405–1414. [Google Scholar] [CrossRef]
- Furue, M.; Ohtsuki, M.; Ogata, F.; Ishibashi, Y. Responsiveness to interleukin 4 and interleukin 2 of peripheral blood mononuclear cells in atopic dermatitis. J. Investig. Dermatol. 1991, 96, 468–472. [Google Scholar] [CrossRef]
- Postlethwaite, A.E.; Holness, M.A.; Katai, H.; Raghow, R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J. Clin. Investig. 1992, 90, 1479–1485. [Google Scholar] [CrossRef]
- Famularo, G.; Procopio, A.; Giacomelli, R.; Danese, C.; Sacchetti, S.; Perego, M.A.; Santoni, A.; Tonietti, G. Soluble interleukin-2 receptor, interleukin-2 and interleukin-4 in sera and supernatants from patients with progressive systemic sclerosis. Clin. Exp. Immunol. 1990, 81, 368–372. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujimoto, M.; Kikuchi, K.; Takehara, K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J. Rheumatol. 1997, 24, 328–332. [Google Scholar]
- Makhluf, H.A.; Stepniakowska, J.; Hoffman, S.; Smith, E.; LeRoy, E.C.; Trojanowska, M. IL-4 upregulates tenascin synthesis in scleroderma and healthy skin fibroblasts. J. Investig. Dermatol. 1996, 107, 856–859. [Google Scholar] [CrossRef]
- Jinnin, M.; Ihn, H.; Asano, Y.; Yamane, K.; Trojanowska, M.; Tamaki, K. Upregulation of tenascin-C expression by IL-13 in human dermal fibroblasts via the phosphoinositide 3-kinase/Akt and the protein kinase C signaling pathways. J. Investig. Dermatol. 2006, 126, 551–560. [Google Scholar] [CrossRef]
- Chomarat, P.; Banchereau, J. Interleukin-4 and interleukin-13: Their similarities and discrepancies. Int. Rev. Immunol. 1998, 17, 1–52. [Google Scholar] [CrossRef]
- Wills-Karp, M.; Luyimbazi, J.; Xu, X.; Schofield, B.; Neben, T.Y.; Karp, C.L.; Donaldson, D.D. Interleukin-13: Central mediator of allergic asthma. Science 1998, 282, 2258–2261. [Google Scholar] [CrossRef]
- Fuschiotti, P.; Medsger, T.A., Jr.; Morel, P.A. Effector CD8+ T cells in systemic sclerosis patients produce abnormally high levels of interleukin-13 associated with increased skin fibrosis. Arthritis Rheum. 2009, 60, 1119–1128. [Google Scholar] [CrossRef]
- Chang, Y.J.; Kim, H.Y.; Albacker, L.A.; Baumgarth, N.; McKenzie, A.N.J.; Smith, D.E.; Dekruyff, R.H.; Umetsu, D.T. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 2011, 12, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt, T.; Usherenko, S.; Englbrecht, M.; Dees, C.; Weber, S.; Beyer, C.; Gelse, K.; Distler, O.; Schett, G.; Distler, J.H.W.; et al. Type 2 innate lymphoid cell counts are increased in patients with systemic sclerosis and correlate with the extent of fibrosis. Ann. Rheum Dis. 2016, 75, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Zlotnik, A.; Vieira, P.; Mosmann, T.R.; Howard, M.; Moore, K.W.; O’Garra, A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991, 146, 3444–3451. [Google Scholar] [PubMed]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional specialization of interleukin-17 family members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Kurasawa, K.; Hirose, K.; Sano, H.; Endo, H.; Shinkai, H.; Nawata, Y.; Takabayashi, K.; Iwamoto, I. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum. 2000, 43, 2455–2463. [Google Scholar] [CrossRef]
- Olewicz-Gawlik, A.; Danczak-Pazdrowska, A.; Kuznar-Kaminska, B.; Gornowicz-Porowska, J.; Katulska, K.; Trzybulska, D.; Batura-Gabryel, H.; Silny, W.; Poplawski, D.; Hrycaj, P. Interleukin-17 and interleukin-23: Importance in the pathogenesis of lung impairment in patients with systemic sclerosis. Int. J. Rheum. Dis. 2014, 17, 664–670. [Google Scholar] [CrossRef]
- Okada, M.; Sakaguchi, N.; Yoshimura, N.; Hara, H.; Shimizu, K.; Yoshida, N.; Yoshizaki, K.; Kishimoto, S.; Yamamura, Y.; Kishimoto, T. B cell growth factors and B cell differentiation factor from human T hybridomas. Two distinct kinds of B cell growth factor and their synergism in B cell proliferation. J. Exp. Med. 1983, 157, 583–590. [Google Scholar] [CrossRef]
- Hirano, T.; Yasukawa, K.; Harada, H.; Taga, T.; Watanabe, Y.; Matsuda, T.; Kashiwamura, S.I.; Nakajima, K.; Koyama, K.; Iwamatsu, A.; et al. Complementary DNA for a novel human interleukin (BSF-2) that induces Bc lymphocytes to produce immunoglobulin. Nature 1986, 324, 73–76. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Nishimoto, N.; Mihara, M.; Kishimoto, T. Therapy of rheumatoid arthritis by blocking IL-6 signal transduction with a humanized anti-IL-6 receptor antibody. Springer Semin Immunopathol. 1998, 20, 247–259. [Google Scholar] [CrossRef]
- Needleman, B.W.; Wigley, F.M.; Stair, R.W. Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumor necrosis factor alpha, and interferon-gamma levels in sera from patients with scleroderma. Arthritis Rheum. 1992, 35, 67–72. [Google Scholar] [CrossRef]
- Feghali, C.A.; Bost, K.L.; Boulware, D.W.; Levy, L.S. Mechanisms of pathogenesis in scleroderma. I. Overproduction of interleukin 6 by fibroblasts cultured from affected skin sites of patients with scleroderma. J. Rheumatol. 1992, 19, 1207–1211. [Google Scholar]
- Gurram, M.; Pahwa, S.; Frieri, M. Augmented interleukin-6 secretion in collagen-stimulated peripheral blood mononuclear cells from patients with systemic sclerosis. Ann. Allergy 1994, 73, 493–496. [Google Scholar]
- Sato, S.; Hasegawa, M.; Takehara, K. Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J. Dermatol. Sci. 2001, 27, 140–146. [Google Scholar] [CrossRef]
- Scala, E.; Pallotta, S.; Frezzolini, A.; Abeni, D.; Barbieri, C.; Sampogna, F.; De Pità, O.; Puddu, P.; Paganelli, R.; Russo, G. Cytokine and chemokine levels in systemic sclerosis: Relationship with cutaneous and internal organ involvement. Clin. Exp. Immunol. 2004, 138, 540–546. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sato, S.; Fujimoto, M.; Ihn, H.; Kikuchi, K.; Takehara, K. Serum levels of interleukin 6 (IL-6), oncostatin M, soluble IL-6 receptor, and soluble gp130 in patients with systemic sclerosis. J. Rheumatol. 1998, 25, 308–313. [Google Scholar]
- Matsushita, T.; Hasegawa, M.; Hamaguchi, Y.; Takehara, K.; Sato, S. Longitudinal analysis of serum cytokine concentrations in systemic sclerosis: Association of interleukin 12 elevation with spontaneous regression of skin sclerosis. J. Rheumatol. 2006, 33, 275–284. [Google Scholar]
- Shima, Y.; Kawaguchi, Y.; Kuwana, M. Add-on tocilizumab versus conventional treatment for systemic sclerosis, and cytokine analysis to identify an endotype to tocilizumab therapy. Mod. Rheumatol. 2019, 29, 134–139. [Google Scholar] [CrossRef]
- Di Luigi, L.; Sgrò, P.; Duranti, G.; Sabatini, S.; Caporossi, D.; Del Galdo, F.; Dimauro, I.; Antinozzi, C. Sildenafil Reduces Expression and Release of IL-6 and IL-8 Induced by Reactive Oxygen Species in Systemic Sclerosis Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3161. [Google Scholar] [CrossRef]
- Crestani, B.; Seta, N.; De Bandt, M.; Soler, P.; Rolland, C.; Dehoux, M.; Boutten, A.; Dombret, M.C.; Palazzo, E.; Kahn, M.F. Interleukin 6 secretion by monocytes and alveolar macrophages in systemic sclerosis with lung involvement. Am. J. Respir. Crit. Care Med. 1994, 149, 1260–1265. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujimoto, M.; Matsushita, T.; Hamaguchi, Y.; Takehara, K.; Sato, S. Serum chemokine and cytokine levels as indicators of disease activity in patients with systemic sclerosis. Clin. Rheumatol. 2011, 30, 231–237. [Google Scholar] [CrossRef]
- Denton, C.P.; Merkel, P.A.; Furst, D.E.; Khanna, D.; Emery, P.; Hsu, V.M.; Silliman, N.; Streisand, J.; Powell, J.; Akesson, A.; et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: A multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2007, 56, 323–333. [Google Scholar] [CrossRef]
- Yawata, H.; Yasukawa, K.; Natsuka, S.; Murakami, M.; Yamasaki, K.; Hibi, M.; Taga, T.; Kishimoto, T. Structure-function analysis of human IL-6 receptor: Dissociation of amino acid residues required for IL-6-binding and for IL-6 signal transduction through gp130. EMBO J. 1993, 12, 1705–1712. [Google Scholar] [CrossRef]
- Shima, Y.; Kuwahara, Y.; Murota, H.; Kitaba, S.; Kawai, M.; Hirano, T.; Arimitsu, J.; Narazaki, M.; Hagihara, K.; Ogata, A.; et al. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology (Oxford) 2010, 49, 2408–2412. [Google Scholar] [CrossRef]
- Khanna, D.; Denton, C.P.; Jahreis, A.; van Laar, J.M.; Frech, T.M.; Anderson, M.E.; Baron, M.; Chung, L.; Fierlbeck, G.; Lakshminarayanan, S.; et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): A phase 2, randomised, controlled trial. Lancet 2016, 387, 2630–2640. [Google Scholar] [CrossRef]
- Khanna, D.; Lin, C.J.; Furst, D.E.; Goldin, J.; Kim, G.; Kuwana, M.; Allanore, Y.; Matucci-Cerinic, M.; Distler, O.; Shima, Y.; et al. Tocilizumab in systemic sclerosis: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2020, 8, 963–974. [Google Scholar] [CrossRef]
- Allanore, Y.; Wung, P.; Soubrane, C.; Esperet, C.; Marrache, F.; Bejuit, R.; Lahmar, A.; Khanna, D.; Denton, C.P. A randomised, double-blind, placebo-controlled, 24-week, phase II, proof-of-concept study of romilkimab (SAR156597) in early diffuse cutaneous systemic sclerosis. Ann. Rheum. Dis. 2020, 79, 1600–1607. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Shima, Y.; Shirayama, D.; Kawai, M.; Hagihara, K.; Hirano, T.; Arimitsu, J.; Ogata, A.; Tanaka, T.; Kawase, I. Quantification of hardness, elasticity and viscosity of the skin of patients with systemic sclerosis using a novel sensing device (Vesmeter): A proposal for a new outcome measurement procedure. Rheumatology (Oxford) 2008, 47, 1018–1024. [Google Scholar] [CrossRef][Green Version]
- Kissin, E.Y.; Schiller, A.M.; Gelbard, R.B.; Anderson, J.J.; Falanga, V.; Simms, R.W.; Korn, J.H.; Merkel, P.A. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum. 2006, 55, 603–609. [Google Scholar] [CrossRef]
- Piérard, G.E.; Nikkels-Tassoudji, N.; Piérard-Franchimont, C. Influence of the test area on the mechanical properties of skin. Dermatology 1995, 191, 9–15. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shima, Y. Cytokines Involved in the Pathogenesis of SSc and Problems in the Development of Anti-Cytokine Therapy. Cells 2021, 10, 1104. https://doi.org/10.3390/cells10051104
Shima Y. Cytokines Involved in the Pathogenesis of SSc and Problems in the Development of Anti-Cytokine Therapy. Cells. 2021; 10(5):1104. https://doi.org/10.3390/cells10051104
Chicago/Turabian StyleShima, Yoshihito. 2021. "Cytokines Involved in the Pathogenesis of SSc and Problems in the Development of Anti-Cytokine Therapy" Cells 10, no. 5: 1104. https://doi.org/10.3390/cells10051104
APA StyleShima, Y. (2021). Cytokines Involved in the Pathogenesis of SSc and Problems in the Development of Anti-Cytokine Therapy. Cells, 10(5), 1104. https://doi.org/10.3390/cells10051104




