Molecular Functions of WWOX Potentially Involved in Cancer Development
Abstract
1. Introduction
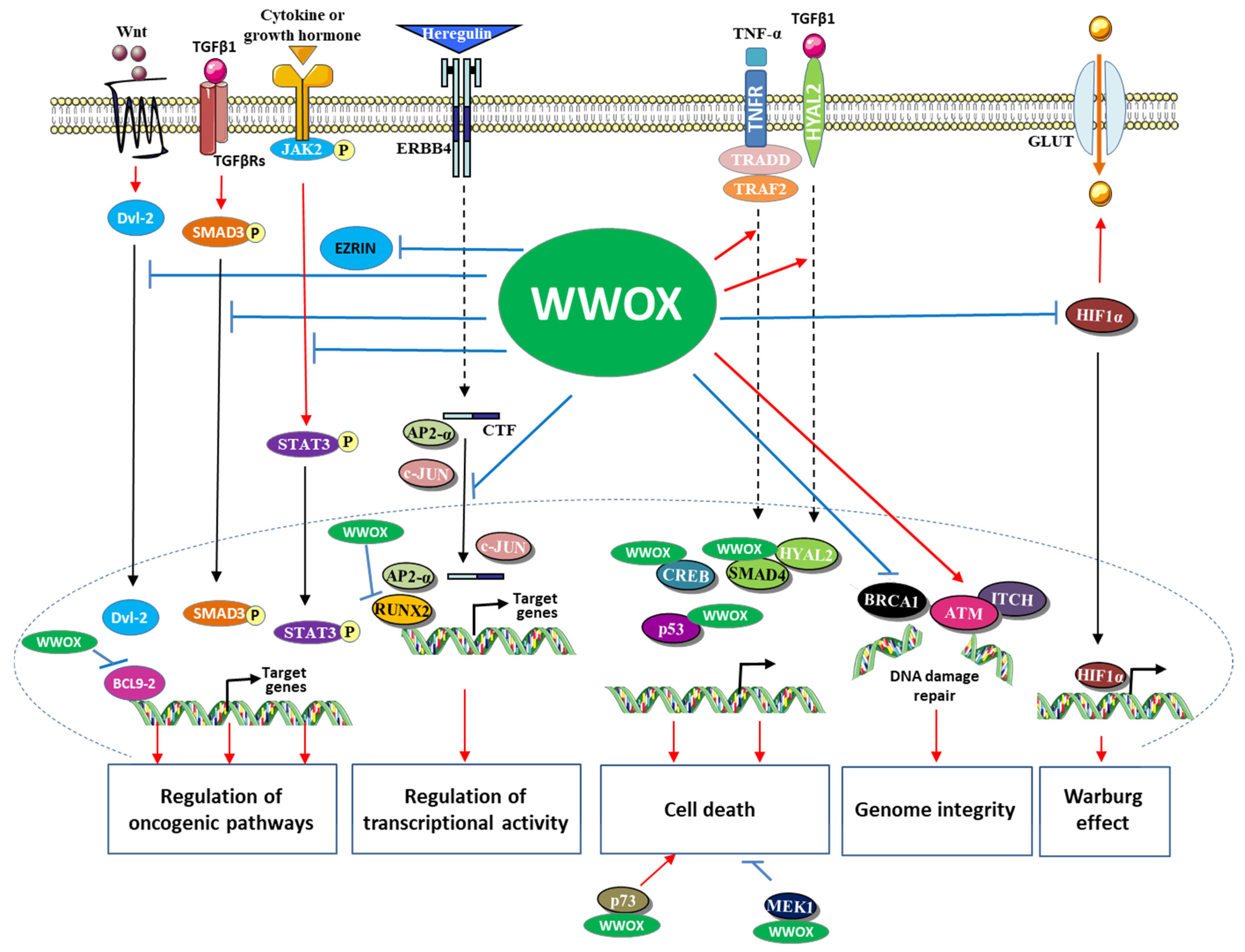
2. WWOX a Positive Regulator of Cell Death
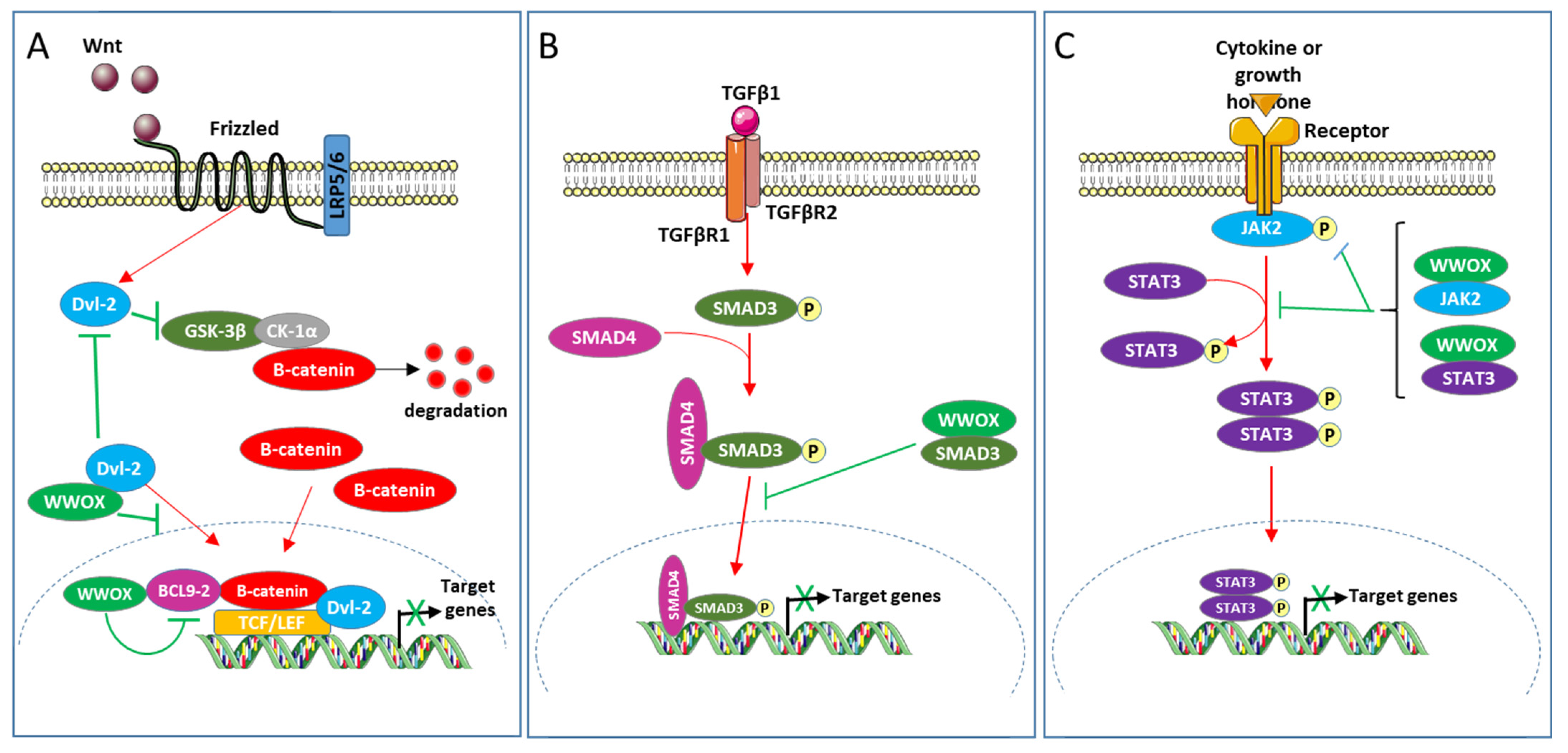
3. WWOX Associates with Cancer-Linked Transcription Factors and Inhibits Their Transcriptional Activity
4. WWOX Interacts and Affects the Function of Components of Oncogenic Pathways
5. WWOX, the Hypoxia-Inducible Transcription Factor 1α (HIF1α) and the Warburg Effect
6. WWOX, a Genome Stability Gatekeeper
7. WWOX in the Regulation of Protein Trafficking to the Endosomes, Golgi, and Lysosomes
8. The Function of WWOX in Cancer Development Is Regulated by Its Phosphorylation State
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bednarek, A.K.; Laflin, K.J.; Daniel, R.L.; Liao, Q.; Hawkins, K.A.; Aldaz, C.M. WWOX, a Novel WW Domain-Containing Protein Mapping to Human Chromosome 16q23.3-24.1, a Region Frequently Affected in Breast Cancer. Cancer Res. 2000, 60, 2140–2145. [Google Scholar]
- Bièche, I.; Lidereau, R. Genetic Alterations in Breast Cancer. Genes Chromosomes Cancer 1995, 14, 227–251. [Google Scholar] [CrossRef]
- Chang, N.S.; Pratt, N.; Heath, J.; Schultz, L.; Sleve, D.; Carey, G.B.; Zevotek, N. Hyaluronidase Induction of a WW Domain-Containing Oxidoreductase That Enhances Tumor Necrosis Factor Cytotoxicity. J. Biol. Chem. 2001, 276, 3361–3370. [Google Scholar] [CrossRef]
- Chen, T.; Sahin, A.; Aldaz, C.M. Deletion Map of Chromosome 16q in Ductal Carcinoma in Situ of the Breast: Refining a Putative Tumor Suppressor Gene Region. Cancer Res. 1996, 56, 5605–5609. [Google Scholar] [CrossRef]
- Driouch, K.; Dorion-Bonnet, F.; Briffod, M.; Champéme, M.H.; Longy, M.; Lidereau, R. Loss of Heterozygosity on Chromosome Arm 16q in Breast Cancer Metastases. Genes Chromosomes Cancer 1997, 19, 185–191. [Google Scholar] [CrossRef]
- Ried, K.; Finnis, M.; Hobson, L.; Mangelsdorf, M.; Dayan, S.; Nancarrow, J.K.; Woollatt, E.; Kremmidiotis, G.; Gardner, A.; Venter, D.; et al. Common Chromosomal Fragile Site FRA16D Sequence: Identification of the FOR Gene Spanning FRA16D and Homozygous Deletions and Translocation Breakpoints in Cancer Cells. Hum. Mol. Genet. 2000, 9, 1651–1663. [Google Scholar] [CrossRef]
- Paige, A.J.; Taylor, K.J.; Taylor, C.; Hillier, S.G.; Farrington, S.; Scott, D.; Porteous, D.J.; Smyth, J.F.; Gabra, H.; Watson, J.E. WWOX: A Candidate Tumor Suppressor Gene Involved in Multiple Tumor Types. Proc. Natl. Acad. Sci. USA 2001, 98, 11417–11422. [Google Scholar] [CrossRef] [PubMed]
- Abu-Odeh, M.; Bar-Mag, T.; Huang, H.; Kim, T.; Salah, Z.; Abdeen, S.K.; Sudol, M.; Reichmann, D.; Sidhu, S.; Kim, P.M.; et al. Characterizing WW Domain Interactions of Tumor Suppressor WWOX Reveals Its Association with Multiprotein Networks. J. Biol. Chem. 2014, 289, 8865–8880. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Hagan, J.P.; de Bruin, A.; Rawahneh, M.; Salah, Z.; Gaudio, E.; Siddiqui, H.; Volinia, S.; Alder, H.; Lian, J.B.; et al. Targeted Ablation of the WW Domain-Containing Oxidoreductase Tumor Suppressor Leads to Impaired Steroidogenesis. Endocrinology 2009, 150, 1530–1535. [Google Scholar] [CrossRef]
- Sałuda-Gorgul, A.; Seta, K.; Nowakowska, M.; Bednarek, A.K. WWOX Oxidoreductase--Substrate and Enzymatic Characterization. Z. Naturforsch. C J. Biosci. 2011, 66, 73–82. [Google Scholar] [CrossRef]
- Hussain, T.; Lee, J.; Abba, M.C.; Chen, J.; Aldaz, C.M. Delineating WWOX Protein Interactome by Tandem Affinity Purification-Mass Spectrometry: Identification of Top Interactors and Key Metabolic Pathways Involved. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef]
- Baryła, I.; Styczeń-Binkowska, E.; Bednarek, A.K. Alteration of WWOX in Human Cancer: A Clinical View. Exp. Biol. Med. 2015, 240, 305–314. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Donati, V.; Gaudio, E.; Nicoloso, M.S.; Sundvall, M.; Korhonen, A.; Lundin, J.; Isola, J.; Sudol, M.; Joensuu, H.; et al. Association of Wwox with ErbB4 in Breast Cancer. Cancer Res. 2007, 67, 9330–9336. [Google Scholar] [CrossRef]
- Bonin, F.; Taouis, K.; Azorin, P.; Petitalot, A.; Tariq, Z.; Nola, S.; Bouteille, N.; Tury, S.; Vacher, S.; Bièche, I.; et al. VOPP1 Promotes Breast Tumorigenesis by Interacting with the Tumor Suppressor WWOX. BMC Biol. 2018, 16, 109. [Google Scholar] [CrossRef]
- Chang, R.; Song, L.; Xu, Y.; Wu, Y.; Dai, C.; Wang, X.; Sun, X.; Hou, Y.; Li, W.; Zhan, X.; et al. Loss of Wwox Drives Metastasis in Triple-Negative Breast Cancer by JAK2/STAT3 Axis. Nat. Commun. 2018, 9, 3486. [Google Scholar] [CrossRef]
- Guler, G.; Uner, A.; Guler, N.; Han, S.-Y.; Iliopoulos, D.; Hauck, W.W.; McCue, P.; Huebner, K. The Fragile Genes FHIT and WWOX Are Inactivated Coordinately in Invasive Breast Carcinoma. Cancer 2004, 100, 1605–1614. [Google Scholar] [CrossRef]
- Khawaled, S.; Suh, S.S.; Abdeen, S.K.; Monin, J.; Distefano, R.; Nigita, G.; Croce, C.M.; Aqeilan, R.I. WWOX Inhibits Metastasis of Triple-Negative Breast Cancer Cells via Modulation of MiRNAs. Cancer Res. 2019, 79, 1784–1798. [Google Scholar] [CrossRef]
- Nunez, M.I.; Ludes-Meyers, J.; Abba, M.C.; Kil, H.; Abbey, N.W.; Page, R.E.; Sahin, A.; Klein-Szanto, A.J.P.; Aldaz, C.M. Frequent Loss of WWOX Expression in Breast Cancer: Correlation with Estrogen Receptor Status. Breast Cancer Res. Treat. 2005, 89, 99–105. [Google Scholar] [CrossRef]
- Płuciennik, E.; Kusińska, R.; Potemski, P.; Kubiak, R.; Kordek, R.; Bednarek, A.K. WWOX--the FRA16D Cancer Gene: Expression Correlation with Breast Cancer Progression and Prognosis. Eur. J. Surg. Oncol. 2006, 32, 153–157. [Google Scholar] [CrossRef]
- Nunez, M.I.; Rosen, D.G.; Ludes-Meyers, J.H.; Abba, M.C.; Kil, H.; Page, R.; Klein-Szanto, A.J.P.; Godwin, A.K.; Liu, J.; Mills, G.B.; et al. WWOX Protein Expression Varies among Ovarian Carcinoma Histotypes and Correlates with Less Favorable Outcome. BMC Cancer 2005, 5, 64. [Google Scholar] [CrossRef]
- Ramos, D.; Abba, M.; López-Guerrero, J.A.; Rubio, J.; Solsona, E.; Almenar, S.; Llombart-Bosch, A.; Aldaz, C.M. Low Levels of WWOX Protein Immunoexpression Correlate with Tumour Grade and a Less Favourable Outcome in Patients with Urinary Bladder Tumours. Histopathology 2008, 52, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Abu-Remaileh, M.; Khalaileh, A.; Pikarsky, E.; Aqeilan, R.I. WWOX Controls Hepatic HIF1α to Suppress Hepatocyte Proliferation and Neoplasia. Cell Death Dis. 2018, 9, 511. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, B.; Huang, A.; Wang, X. The relationship between FHIT and WWOX expression and clinicopathological features in hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi 2010, 18, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-T.; Tzai, T.-S.; Liao, C.-Y.; Wang, J.-S.; Wu, T.T.; Wang, H.-Y.; Wu, C.-H.; Yu, C.-C.; Lu, P.-J. WWOX Protein Expression Varies among RCC Histotypes and Downregulation of WWOX Protein Correlates with Less-Favorable Prognosis in Clear RCC. Ann. Surg. Oncol. 2013, 20, 193–199. [Google Scholar] [CrossRef]
- Aldaz, C.M.; Ferguson, B.W.; Abba, M.C. WWOX at the Crossroads of Cancer, Metabolic Syndrome Related Traits and CNS Pathologies. Biochim. Biophys. Acta 2014, 1846, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Del Mare, S.; Salah, Z.; Aqeilan, R.I. WWOX: Its Genomics, Partners, and Functions. J. Cell. Biochem. 2009, 108, 737–745. [Google Scholar] [CrossRef]
- Mahajan, N.P.; Whang, Y.E.; Mohler, J.L.; Earp, H.S. Activated Tyrosine Kinase Ack1 Promotes Prostate Tumorigenesis: Role of Ack1 in Polyubiquitination of Tumor Suppressor Wwox. Cancer Res. 2005, 65, 10514–10523. [Google Scholar] [CrossRef]
- Schrock, M.S.; Huebner, K. WWOX: A Fragile Tumor Suppressor. Exp. Biol. Med. 2015, 240, 296–304. [Google Scholar] [CrossRef]
- Aldaz, C.M.; Hussain, T. WWOX Loss of Function in Neurodevelopmental and Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 8922. [Google Scholar] [CrossRef]
- Piard, J.; Hawkes, L.; Milh, M.; Villard, L.; Borgatti, R.; Romaniello, R.; Fradin, M.; Capri, Y.; Héron, D.; Nougues, M.-C.; et al. The Phenotypic Spectrum of WWOX-Related Disorders: 20 Additional Cases of WOREE Syndrome and Review of the Literature. Genet. Med. 2019, 21, 1308–1318. [Google Scholar] [CrossRef]
- Bednarek, A.K.; Keck-Waggoner, C.L.; Daniel, R.L.; Laflin, K.J.; Bergsagel, P.L.; Kiguchi, K.; Brenner, A.J.; Aldaz, C.M. WWOX, the FRA16D Gene, Behaves as a Suppressor of Tumor Growth. Cancer Res. 2001, 61, 8068–8073. [Google Scholar]
- Aderca, I.; Moser, C.D.; Veerasamy, M.; Bani-Hani, A.H.; Bonilla-Guerrero, R.; Ahmed, K.; Shire, A.; Cazanave, S.C.; Montoya, D.P.; Mettler, T.A.; et al. The JNK Inhibitor SP600129 Enhances Apoptosis of HCC Cells Induced by the Tumor Suppressor WWOX. J. Hepatol. 2008, 49, 373–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gourley, C.; Paige, A.J.W.; Taylor, K.J.; Ward, C.; Kuske, B.; Zhang, J.; Sun, M.; Janczar, S.; Harrison, D.J.; Muir, M.; et al. WWOX Gene Expression Abolishes Ovarian Cancer Tumorigenicity In Vivo and Decreases Attachment to Fibronectin via Integrin A3. Cancer Res. 2009, 69, 4835–4842. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.W.; Gao, X.; Zelazowski, M.J.; Lee, J.; Jeter, C.R.; Abba, M.C.; Aldaz, C.M. The Cancer Gene WWOX Behaves as an Inhibitor of SMAD3 Transcriptional Activity via Direct Binding. BMC Cancer 2013, 13, 593. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Huang, Y.-F.; Li, M.-L.; Cheng, J.-H.; Hu, P.; Lu, C.-H.; Zhang, Y.; Liu, N.; Tzeng, C.-M.; et al. WW Domain-Binding Protein 2: An Adaptor Protein Closely Linked to the Development of Breast Cancer. Mol. Cancer 2017, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.-S.; Doherty, J.; Ensign, A. JNK1 Physically Interacts with WW Domain-Containing Oxidoreductase (WOX1) and Inhibits WOX1-Mediated Apoptosis. J. Biol. Chem. 2003, 278, 9195–9202. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.-S.; Doherty, J.; Ensign, A.; Schultz, L.; Hsu, L.-J.; Hong, Q. WOX1 Is Essential for Tumor Necrosis Factor-, UV Light-, Staurosporine-, and P53-Mediated Cell Death, and Its Tyrosine 33-Phosphorylated Form Binds and Stabilizes Serine 46-Phosphorylated P53. J. Biol. Chem. 2005, 280, 43100–43108. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Pekarsky, Y.; Herrero, J.J.; Palamarchuk, A.; Letofsky, J.; Druck, T.; Trapasso, F.; Han, S.-Y.; Melino, G.; Huebner, K.; et al. Functional Association between Wwox Tumor Suppressor Protein and P73, a P53 Homolog. Proc. Natl. Acad. Sci. USA 2004, 101, 4401–4406. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhu, K.; Liu, C.; Xie, F.; Xu, P.; Tang, Z. Expression of WW domain containing oxidoreductase gene in cholangiocarcinoma and its effect on the biological behavior of cancer cell line RBE. Zhonghua Wai Ke Za Zhi 2011, 49, 324–329. [Google Scholar]
- Chou, P.-Y.; Lin, S.-R.; Lee, M.-H.; Schultz, L.; Sze, C.-I.; Chang, N.-S. A P53/TIAF1/WWOX Triad Exerts Cancer Suppression but May Cause Brain Protein Aggregation Due to P53/WWOX Functional Antagonism. Cell Commun. Signal. 2019, 17, 76. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Trapasso, F.; Hussain, S.; Costinean, S.; Marshall, D.; Pekarsky, Y.; Hagan, J.P.; Zanesi, N.; Kaou, M.; Stein, G.S.; et al. Targeted Deletion of Wwox Reveals a Tumor Suppressor Function. Proc. Natl. Acad. Sci. USA 2007, 104, 3949–3954. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, S.K.; Salah, Z.; Maly, B.; Smith, Y.; Tufail, R.; Abu-Odeh, M.; Zanesi, N.; Croce, C.M.; Nawaz, Z.; Aqeilan, R.I. Wwox Inactivation Enhances Mammary Tumorigenesis. Oncogene 2011, 30, 3900–3906. [Google Scholar] [CrossRef] [PubMed]
- Ludes-Meyers, J.H.; Kil, H.; Nuñez, M.I.; Conti, C.J.; Parker-Thornburg, J.; Bedford, M.T.; Aldaz, C.M. WWOX Hypomorphic Mice Display a Higher Incidence of B-Cell Lymphomas and Develop Testicular Atrophy. Genes Chromosomes Cancer 2007, 46, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-Y.; Chou, Y.-T.; Lai, F.-J.; Jan, M.-S.; Chang, T.-H.; Jou, I.-M.; Chen, P.-S.; Lo, J.-Y.; Huang, S.-S.; Chang, N.-S.; et al. Wwox Deficiency Leads to Neurodevelopmental and Degenerative Neuropathies and Glycogen Synthase Kinase 3β-Mediated Epileptic Seizure Activity in Mice. Acta Neuropathol. Commun. 2020, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Ludes-Meyers, J.H.; Kil, H.; Parker-Thornburg, J.; Kusewitt, D.F.; Bedford, M.T.; Aldaz, C.M. Generation and Characterization of Mice Carrying a Conditional Allele of the Wwox Tumor Suppressor Gene. PLoS ONE 2009, 4, e7775. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-T.; Lai, F.-J.; Chang, N.-S.; Hsu, L.-J. Wwox Deficiency Causes Downregulation of Prosurvival ERK Signaling and Abnormal Homeostatic Responses in Mouse Skin. Front. Cell Dev. Biol. 2020, 8, 558432. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Katayama, K.; Takenaka, M.; Amakasu, K.; Saito, K.; Suzuki, K. A Spontaneous Mutation of the Wwox Gene and Audiogenic Seizures in Rats with Lethal Dwarfism and Epilepsy. Genes Brain Behav. 2009, 8, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, S.K.; Ben-David, U.; Shweiki, A.; Maly, B.; Aqeilan, R.I. Somatic Loss of WWOX Is Associated with TP53 Perturbation in Basal-like Breast Cancer. Cell Death Dis. 2018, 9, 832. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.W.; Gao, X.; Kil, H.; Lee, J.; Benavides, F.; Abba, M.C.; Aldaz, C.M. Conditional Wwox Deletion in Mouse Mammary Gland by Means of Two Cre Recombinase Approaches. PLoS ONE 2012, 7, e36618. [Google Scholar] [CrossRef]
- Abdeen, S.K.; Salah, Z.; Khawaled, S.; Aqeilan, R.I. Characterization of WWOX Inactivation in Murine Mammary Gland Development. J. Cell. Physiol. 2013, 228, 1391–1396. [Google Scholar] [CrossRef]
- Del Mare, S.; Husanie, H.; Iancu, O.; Abu-Odeh, M.; Evangelou, K.; Lovat, F.; Volinia, S.; Gordon, J.; Amir, G.; Stein, J.; et al. WWOX and P53 Dysregulation Synergize to Drive the Development of Osteosarcoma. Cancer Res. 2016, 76, 6107–6117. [Google Scholar] [CrossRef]
- Su, W.-P.; Wang, W.-J.; Chang, J.-Y.; Ho, P.-C.; Liu, T.-Y.; Wen, K.-Y.; Kuo, H.-L.; Chen, Y.-J.; Huang, S.-S.; Subhan, D.; et al. Therapeutic Zfra4-10 or WWOX7-21 Peptide Induces Complex Formation of WWOX with Selective Protein Targets in Organs That Leads to Cancer Suppression and Spleen Cytotoxic Memory Z Cell Activation In Vivo. Cancers 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Ho, P.-C.; Nagarajan, G.; Chen, Y.-A.; Kuo, H.-L.; Subhan, D.; Su, W.-P.; Chang, J.-Y.; Lu, C.-Y.; Chang, K.T.; et al. WWOX Possesses N-Terminal Cell Surface-Exposed Epitopes WWOX7-21 and WWOX7-11 for Signaling Cancer Growth Suppression and Prevention In Vivo. Cancers 2019, 11, 1818. [Google Scholar] [CrossRef]
- Chou, P.-Y.; Lai, F.-J.; Chen, Y.-A.; Sie, Y.-D.; Kuo, H.-L.; Su, W.-P.; Wu, C.-Y.; Liu, T.-Y.; Wen, K.-Y.; Hsu, L.-J.; et al. Strategies by Which WWOX-Deficient Metastatic Cancer Cells Utilize to Survive via Dodging, Compromising, and Causing Damage to WWOX-Positive Normal Microenvironment. Cell Death Discov. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.-S.; Doherty, J.; Ensign, A.; Lewis, J.; Heath, J.; Schultz, L.; Chen, S.-T.; Oppermann, U. Molecular Mechanisms Underlying WOX1 Activation during Apoptotic and Stress Responses. Biochem. Pharmacol. 2003, 66, 1347–1354. [Google Scholar] [CrossRef]
- Li, M.-Y.; Lai, F.-J.; Hsu, L.-J.; Lo, C.-P.; Cheng, C.-L.; Lin, S.-R.; Lee, M.-H.; Chang, J.-Y.; Subhan, D.; Tsai, M.-S.; et al. Dramatic Co-Activation of WWOX/WOX1 with CREB and NF-KappaB in Delayed Loss of Small Dorsal Root Ganglion Neurons upon Sciatic Nerve Transection in Rats. PLoS ONE 2009, 4, e7820. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.-J.; Hong, Q.; Chen, S.-T.; Kuo, H.-L.; Schultz, L.; Heath, J.; Lin, S.-R.; Lee, M.-H.; Li, D.-Z.; Li, Z.-L.; et al. Hyaluronan Activates Hyal-2/WWOX/Smad4 Signaling and Causes Bubbling Cell Death When the Signaling Complex Is Overexpressed. Oncotarget 2017, 8, 19137–19155. [Google Scholar] [CrossRef]
- Hsu, L.-J.; Schultz, L.; Hong, Q.; Van Moer, K.; Heath, J.; Li, M.-Y.; Lai, F.-J.; Lin, S.-R.; Lee, M.-H.; Lo, C.-P.; et al. Transforming Growth Factor Beta1 Signaling via Interaction with Cell Surface Hyal-2 and Recruitment of WWOX/WOX1. J. Biol. Chem. 2009, 284, 16049–16059. [Google Scholar] [CrossRef]
- Lin, H.-P.; Chang, J.-Y.; Lin, S.-R.; Lee, M.-H.; Huang, S.-S.; Hsu, L.-J.; Chang, N.-S. Identification of an In Vivo MEK/WOX1 Complex as a Master Switch for Apoptosis in T Cell Leukemia. Genes Cancer 2011, 2, 550–562. [Google Scholar] [CrossRef][Green Version]
- Bouteille, N.; Driouch, K.; Hage, P.E.; Sin, S.; Formstecher, E.; Camonis, J.; Lidereau, R.; Lallemand, F. Inhibition of the Wnt/Beta-Catenin Pathway by the WWOX Tumor Suppressor Protein. Oncogene 2009, 28, 2569–2580. [Google Scholar] [CrossRef]
- El-Hage, P.; Petitalot, A.; Monsoro-Burq, A.-H.; Maczkowiak, F.; Driouch, K.; Formstecher, E.; Camonis, J.; Sabbah, M.; Bièche, I.; Lidereau, R.; et al. The Tumor-Suppressor WWOX and HDAC3 Inhibit the Transcriptional Activity of the β-Catenin Coactivator BCL9-2 in Breast Cancer Cells. Mol. Cancer Res. 2015, 13, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Palamarchuk, A.; Weigel, R.J.; Herrero, J.J.; Pekarsky, Y.; Croce, C.M. Physical and Functional Interactions between the Wwox Tumor Suppressor Protein and the AP-2gamma Transcription Factor. Cancer Res. 2004, 64, 8256–8261. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Donati, V.; Palamarchuk, A.; Trapasso, F.; Kaou, M.; Pekarsky, Y.; Sudol, M.; Croce, C.M. WW Domain-Containing Proteins, WWOX and YAP, Compete for Interaction with ErbB-4 and Modulate Its Transcriptional Function. Cancer Res. 2005, 65, 6764–6772. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, E.; Palamarchuk, A.; Palumbo, T.; Trapasso, F.; Pekarsky, Y.; Croce, C.M.; Aqeilan, R.I. Physical Association with WWOX Suppresses C-Jun Transcriptional Activity. Cancer Res. 2006, 66, 11585–11589. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Hassan, M.Q.; de Bruin, A.; Hagan, J.P.; Volinia, S.; Palumbo, T.; Hussain, S.; Lee, S.-H.; Gaur, T.; Stein, G.S.; et al. The WWOX Tumor Suppressor Is Essential for Postnatal Survival and Normal Bone Metabolism. J. Biol. Chem. 2008, 283, 21629–21639. [Google Scholar] [CrossRef]
- Abu-Odeh, M.; Salah, Z.; Herbel, C.; Hofmann, T.G.; Aqeilan, R.I. WWOX, the Common Fragile Site FRA16D Gene Product, Regulates ATM Activation and the DNA Damage Response. Proc. Natl. Acad. Sci. USA 2014, 111, E4716–E4725. [Google Scholar] [CrossRef]
- Schrock, M.S.; Batar, B.; Lee, J.; Druck, T.; Ferguson, B.; Cho, J.H.; Akakpo, K.; Hagrass, H.; Heerema, N.A.; Xia, F.; et al. Wwox-Brca1 Interaction: Role in DNA Repair Pathway Choice. Oncogene 2017, 36, 2215–2227. [Google Scholar] [CrossRef]
- Jin, C.; Ge, L.; Ding, X.; Chen, Y.; Zhu, H.; Ward, T.; Wu, F.; Cao, X.; Wang, Q.; Yao, X. PKA-Mediated Protein Phosphorylation Regulates Ezrin–WWOX Interaction. Biochem. Biophys. Res. Commun. 2006, 341, 784–791. [Google Scholar] [CrossRef]
- Takeuchi, T.; Adachi, Y.; Nagayama, T. A WWOX-Binding Molecule, Transmembrane Protein 207, Is Related to the Invasiveness of Gastric Signet-Ring Cell Carcinoma. Carcinogenesis 2012, 33, 548–554. [Google Scholar] [CrossRef]
- Ludes-Meyers, J.H.; Kil, H.; Bednarek, A.K.; Drake, J.; Bedford, M.T.; Aldaz, C.M. WWOX Binds the Specific Proline-Rich Ligand PPXY: Identification of Candidate Interacting Proteins. Oncogene 2004, 23, 5049–5055. [Google Scholar] [CrossRef]
- Kołat, D.; Kałuzińska, Ż.; Bednarek, A.K.; Płuciennik, E. The Biological Characteristics of Transcription Factors AP-2α and AP-2γ and Their Importance in Various Types of Cancers. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Gee, J.M.W.; Eloranta, J.J.; Ibbitt, J.C.; Robertson, J.F.R.; Ellis, I.O.; Williams, T.; Nicholson, R.I.; Hurst, H.C. Overexpression of TFAP2C in Invasive Breast Cancer Correlates with a Poorer Response to Anti-Hormone Therapy and Reduced Patient Survival. J. Pathol. 2009, 217, 32–41. [Google Scholar] [CrossRef]
- Guler, G.; Himmetoglu, C.; Jimenez, R.E.; Geyer, S.M.; Wang, W.P.; Costinean, S.; Pilarski, R.T.; Morrison, C.; Suren, D.; Liu, J.; et al. Aberrant Expression of DNA Damage Response Proteins Is Associated with Breast Cancer Subtype and Clinical Features. Breast Cancer Res. Treat. 2011, 129, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Guler, G.; Iliopoulos, D.; Guler, N.; Himmetoglu, C.; Hayran, M.; Huebner, K. Wwox and Ap2gamma Expression Levels Predict Tamoxifen Response. Clin. Cancer Res. 2007, 13, 6115–6121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sundvall, M.; Iljin, K.; Kilpinen, S.; Sara, H.; Kallioniemi, O.-P.; Elenius, K. Role of ErbB4 in Breast Cancer. J. Mammary Gland Biol. Neoplasia 2008, 13, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.; Leech, J.T.; Kad, N.M.; Mason, J.M. Selective Antagonism of CJun for Cancer Therapy. J. Exp. Clin. Cancer Res. 2020, 39, 184. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-S.; Zhang, L.; Yang, J.; Zhou, X. Role of Runt-Related Transcription Factor 2 in Signal Network of Tumors as an Inter-Mediator. Cancer Lett. 2015, 361, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Runx2, an Inducer of Osteoblast and Chondrocyte Differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef]
- Barnes, G.L.; Hebert, K.E.; Kamal, M.; Javed, A.; Einhorn, T.A.; Lian, J.B.; Stein, G.S.; Gerstenfeld, L.C. Fidelity of Runx2 Activity in Breast Cancer Cells Is Required for the Generation of Metastases-Associated Osteolytic Disease. Cancer Res. 2004, 64, 4506–4513. [Google Scholar] [CrossRef]
- Javed, A.; Barnes, G.L.; Pratap, J.; Antkowiak, T.; Gerstenfeld, L.C.; van Wijnen, A.J.; Stein, J.L.; Lian, J.B.; Stein, G.S. Impaired Intranuclear Trafficking of Runx2 (AML3/CBFA1) Transcription Factors in Breast Cancer Cells Inhibits Osteolysis in Vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 1454–1459. [Google Scholar] [CrossRef]
- Del Mare, S.; Aqeilan, R.I. Tumor Suppressor WWOX Inhibits Osteosarcoma Metastasis by Modulating RUNX2 Function. Sci. Rep. 2015, 5, 12959. [Google Scholar] [CrossRef]
- Zheng, Q.-W.; Zhou, Y.-L.; You, Q.-J.; Shou, F.; Pang, Q.-F.; Chen, J.-L. WWOX Inhibits the Invasion of Lung Cancer Cells by Downregulating RUNX2. Cancer Gene Ther. 2016, 23, 433–438. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/Beta-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- Dey, N.; Barwick, B.G.; Moreno, C.S.; Ordanic-Kodani, M.; Chen, Z.; Oprea-Ilies, G.; Tang, W.; Catzavelos, C.; Kerstann, K.F.; Sledge, G.W.; et al. Wnt Signaling in Triple Negative Breast Cancer Is Associated with Metastasis. BMC Cancer 2013, 13, 537. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Kikuchi, A.; Kishida, S.; Yamamoto, H. Regulation of Wnt Signaling by Protein-Protein Interaction and Post-Translational Modifications. Exp. Mol. Med. 2006, 38, 1–10. [Google Scholar] [CrossRef]
- Brembeck, F.H.; Schwarz-Romond, T.; Bakkers, J.; Wilhelm, S.; Hammerschmidt, M.; Birchmeier, W. Essential Role of BCL9-2 in the Switch between Beta-Catenin’s Adhesive and Transcriptional Functions. Genes Dev. 2004, 18, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Brott, B.K.; Bae, G.-U.; Ratcliffe, M.J.; Sokol, S.Y. Nuclear Localization Is Required for Dishevelled Function in Wnt/Beta-Catenin Signaling. J. Biol. 2005, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Wang, J.; Xi, Y.; Wu, Z.; Li, Y.; Li, L. Nuclear Dvl, c-Jun, Beta-Catenin, and TCF Form a Complex Leading to Stabilization of Beta-Catenin-TCF Interaction. J. Cell Biol. 2008, 180, 1087–1100. [Google Scholar] [CrossRef]
- Khawaled, S.; Nigita, G.; Distefano, R.; Oster, S.; Suh, S.-S.; Smith, Y.; Khalaileh, A.; Peng, Y.; Croce, C.M.; Geiger, T.; et al. Pleiotropic Tumor Suppressor Functions of WWOX Antagonize Metastasis. Signal Transduct. Target. Ther. 2020, 5, 43. [Google Scholar] [CrossRef]
- Seoane, J.; Gomis, R.R. TGF-β Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef]
- Alunno, A.; Padjen, I.; Fanouriakis, A.; Boumpas, D.T. Pathogenic and Therapeutic Relevance of JAK/STAT Signaling in Systemic Lupus Erythematosus: Integration of Distinct Inflammatory Pathways and the Prospect of Their Inhibition with an Oral Agent. Cells 2019, 8, 898. [Google Scholar] [CrossRef]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The Role of JAK/STAT Signalling in the Pathogenesis, Prognosis and Treatment of Solid Tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef]
- Song, Y.; Ma, X.; Zhang, M.; Wang, M.; Wang, G.; Ye, Y.; Xia, W. Ezrin Mediates Invasion and Metastasis in Tumorigenesis: A Review. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Soni, S.; Padwad, Y.S. HIF-1 in Cancer Therapy: Two Decade Long Story of a Transcription Factor. Acta Oncol. 2017, 56, 503–515. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Aqeilan, R.I. Tumor Suppressor WWOX Regulates Glucose Metabolism via HIF1α Modulation. Cell Death Differ. 2014, 21, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Van Gent, D.C.; Hoeijmakers, J.H.; Kanaar, R. Chromosomal Stability and the DNA Double-Stranded Break Connection. Nat. Rev. Genet. 2001, 2, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H.J. DNA Damage, Aging, and Cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-Damage Response in Human Biology and Disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.R.; Taylor, M.R.G.; Boulton, S.J. Playing the End Game: DNA Double-Strand Break Repair Pathway Choice. Mol. Cell 2012, 47, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Her, J.; Bunting, S.F. How Cells Ensure Correct Repair of DNA Double-Strand Breaks. J. Biol. Chem. 2018, 293, 10502–10511. [Google Scholar] [CrossRef]
- Abu-Odeh, M.; Hereema, N.A.; Aqeilan, R.I. WWOX Modulates the ATR-Mediated DNA Damage Checkpoint Response. Oncotarget 2016, 7, 4344–4355. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Venugopalan, A.; Lynberg, M.; Cultraro, C.M.; Nguyen, K.D.P.; Zhang, X.; Waris, M.; Dayal, N.; Abebe, A.; Maity, T.K.; Guha, U. SCAMP3 Is a Mutant EGFR Phosphorylation Target and a Tumor Suppressor in Lung Adenocarcinoma. Oncogene 2021. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, J.; Yu, X.; Jiang, X.; Shi, Y.; Weng, Y.; Kuai, Y.; Lei, L.; Ren, G.; Feng, X.; et al. LITAF Is a Potential Tumor Suppressor in Pancreatic Cancer. Oncotarget 2017, 9, 3131–3142. [Google Scholar] [CrossRef]
- Bunai, K.; Okubo, H.; Hano, K.; Inoue, K.; Kito, Y.; Saigo, C.; Shibata, T.; Takeuchi, T. TMEM207 Hinders the Tumour Suppressor Function of WWOX in Oral Squamous Cell Carcinoma. J. Cell. Mol. Med. 2018, 22, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Saigo, C.; Kito, Y.; Takeuchi, T. Cancerous Protein Network That Inhibits the Tumor Suppressor Function of WW Domain-Containing Oxidoreductase (WWOX) by Aberrantly Expressed Molecules. Front. Oncol. 2018, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-S.; Chang, N.-S. Phosphorylation/de-Phosphorylation in Specific Sites of Tumor Suppressor WWOX and Control of Distinct Biological Events. Exp. Biol. Med. 2018, 243, 137–147. [Google Scholar] [CrossRef]
- Lee, M.-H.; Shih, Y.-H.; Lin, S.-R.; Chang, J.-Y.; Lin, Y.-H.; Sze, C.-I.; Kuo, Y.-M.; Chang, N.-S. Zfra Restores Memory Deficits in Alzheimer’s Disease Triple-Transgenic Mice by Blocking Aggregation of TRAPPC6AΔ, SH3GLB2, Tau, and Amyloid β, and Inflammatory NF-ΚB Activation. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2017, 3, 189–204. [Google Scholar] [CrossRef]

| Molecular Partner | WWOX Domain Involved | Partner Domain Involved | Complex Localization | Involved in | Reference |
|---|---|---|---|---|---|
| p53 | nd | nd | nucleus | apoptosis | [37] |
| p73 | nd | PPPY487 | cytoplasm | apoptosis | [38] |
| TRADD | nd | nd | nd | TNF signaling | [55] |
| TRAF2 | nd | nd | nucleus | TNF signaling | [55] |
| CREB | WW | nd | nucleus | apoptosis | [56] |
| Hyal-2 | WW1 | nd | Membrane, nucleus, cytoplasm | cell death | [57,58] |
| SMAD4 | WW1 | nd | nucleus | cell death | [57,58] |
| MEK1 | WW and SDR | nd | lysosome | apoptosis | [59] |
| SMAD3 | WW1 | PPGY184 | cytoplasm | TGFβ1 signaling | [34] |
| Dvl-2 | WW1 | PPPY568 | cytoplasm | Wnt signaling | [60] |
| BCL9-2 | WW1 | PPPY561 | nucleus | Wnt signaling | [61] |
| JAK2 | WW1 | nd | cytoplasm | JAK2/STAT3 signaling | [15] |
| STAT3 | WW1 | nd | cytoplasm | JAK2/STAT3 signaling | [15] |
| AP-2γ | WW1 | PPPY64 | cytoplasm | transcription | [62] |
| ERBB4 | WW1 | PPIY1037, PPPY1285 | cytoplasm | transcription | [63] |
| C-JUN | WW1 | PPPY64 | cytoplasm | transcription | [64] |
| RUNX2 | WW1 | nd | nucleus | transcription | [65] |
| ATM | WW1 | nd | nd | DNA repair response | [66] |
| BRCA1 | WW1 | nd | nd | DNA repair response | [67] |
| ITCH | WW1 | LPXY623 and LPXY839 | nucleus | DNA repair response | [8,66] |
| EZRIN | WW1 | PPPPPPPVY477 | Apical membrane | Signal transduction | [68] |
| VOPP1 | WW1 | PPPY165 | cytoplasm | Protein trafficking/sorting | [14] |
| TMEM207 | nd | PPPY139 | cytoplasm | Protein trafficking/sorting | [69] |
| SEC23IP | WW1 | PPSY167 | nd | Protein trafficking/sorting | [11] |
| SCAMP3 | WW1 | PPAY53 or LPSF141 | nd | Protein trafficking/sorting | [11] |
| LITAF | WW1 | PPSY23 | Golgi apparatus | Protein trafficking/sorting | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taouis, K.; Driouch, K.; Lidereau, R.; Lallemand, F. Molecular Functions of WWOX Potentially Involved in Cancer Development. Cells 2021, 10, 1051. https://doi.org/10.3390/cells10051051
Taouis K, Driouch K, Lidereau R, Lallemand F. Molecular Functions of WWOX Potentially Involved in Cancer Development. Cells. 2021; 10(5):1051. https://doi.org/10.3390/cells10051051
Chicago/Turabian StyleTaouis, Karim, Keltouma Driouch, Rosette Lidereau, and François Lallemand. 2021. "Molecular Functions of WWOX Potentially Involved in Cancer Development" Cells 10, no. 5: 1051. https://doi.org/10.3390/cells10051051
APA StyleTaouis, K., Driouch, K., Lidereau, R., & Lallemand, F. (2021). Molecular Functions of WWOX Potentially Involved in Cancer Development. Cells, 10(5), 1051. https://doi.org/10.3390/cells10051051





