Disparities in Early-Onset Colorectal Cancer
Abstract
1. Introduction
2. Early-Onset CRC Epidemiology and Disparities
3. Environmental/Lifestyle Risk Factors and Disparities
| Factor | Potential Impact on Disparities | References |
|---|---|---|
| Obesity | Increased prevalence of childhood obesity and extreme obesity in AAs and Hispanics | [50,55] |
| Type 2 diabetes | Increased prevalence in AAs and HispanicsIncreased prevalence of metabolic syndrome in Hispanics | [59,60] |
| Western diet | Poorer quality diet in AAs | [70] |
| Sedentary lifestyle | Increased rates of television viewing and decreased physical activity among minority children | [55,74] |
4. Genetic Risk Factors and Disparities
5. Tumor Characteristics and Disparities
6. CRC Outcomes and Disparities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; Murphy, C.C. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology 2020, 158, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.K.; Ward, E.; Kohler, B.A.; Eheman, C.; Zauber, A.G.; Anderson, R.N.; Jemal, A.; Schymura, M.J.; Lansdorp-Vogelaar, I.; Seeff, L.C.; et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010, 116, 544–573. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.E.; Hu, C.Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef]
- Murphy, C.C.; Singal, A.G.; Baron, J.A.; Sandler, R.S. Decrease in Incidence of Young-Onset Colorectal Cancer Before Recent Increase. Gastroenterology 2018, 155, 1716–1719.e4. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, R.; Frankel, W.L.; Swanson, B.; Zhao, W.; Yilmaz, A.; Miller, K.; Bacher, J.; Bigley, C.; Nelsen, L.; Goodfellow, P.J.; et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients with Early-Onset Colorectal Cancer. JAMA Oncol. 2017, 3, 464–471. [Google Scholar] [CrossRef]
- Stoffel, E.M.; Koeppe, E.; Everett, J.; Ulintz, P.; Kiel, M.; Osborne, J.; Williams, L.; Hanson, K.; Gruber, S.B.; Rozek, L.S. Germline Genetic Features of Young Individuals With Colorectal Cancer. Gastroenterology 2018, 154, 897–905.e1. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 153, 307–323. [Google Scholar] [CrossRef]
- Gupta, S.; Bharti, B.; Ahnen, D.J.; Buchanan, D.D.; Cheng, I.C.; Cotterchio, M.; Figueiredo, J.C.; Gallinger, S.J.; Haile, R.W.; Jenkins, M.A.; et al. Potential impact of family history-based screening guidelines on the detection of early-onset colorectal cancer. Cancer 2020, 126, 3013–3020. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. Healthy People 2020: Determinants of Health. Available online: https://www.healthypeople.gov/2020/about/foundation-health-measures/Determinants-of-Health (accessed on 26 February 2021).
- O’Connell, J.B.; Maggard, M.A.; Liu, J.H.; Etzioni, D.A.; Livingston, E.H.; Ko, C.Y. Rates of colon and rectal cancers are increasing in young adults. Am. Surg 2003, 69, 866–872. [Google Scholar]
- Murphy, C.C.; Wallace, K.; Sandler, R.S.; Baron, J.A. Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival. Gastroenterology 2019, 156, 958–965. [Google Scholar] [CrossRef]
- Siegel, R.L.; Jemal, A.; Ward, E.M. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 1695–1698. [Google Scholar] [CrossRef]
- You, Y.N.; Xing, Y.; Feig, B.W.; Chang, G.J.; Cormier, J.N. Young-onset colorectal cancer: Is it time to pay attention? Arch. Intern. Med. 2012, 172, 287–289. [Google Scholar] [CrossRef]
- Araghi, M.; Soerjomataram, I.; Bardot, A.; Ferlay, J.; Cabasag, C.J.; Morrison, D.S.; De, P.; Tervonen, H.; Walsh, P.M.; Bucher, O.; et al. Changes in colorectal cancer incidence in seven high-income countries: A population-based study. Lancet Gastroenterol. Hepatol. 2019, 4, 511–518. [Google Scholar] [CrossRef]
- Young, J.P.; Win, A.K.; Rosty, C.; Flight, I.; Roder, D.; Young, G.P.; Frank, O.; Suthers, G.K.; Hewett, P.J.; Ruszkiewicz, A.; et al. Rising incidence of early-onset colorectal cancer in Australia over two decades: Report and review. J. Gastroenterol. Hepatol. 2015, 30, 6–13. [Google Scholar] [CrossRef]
- Feletto, E.; Yu, X.Q.; Lew, J.B.; St John, D.J.B.; Jenkins, M.A.; Macrae, F.A.; Mahady, S.E.; Canfell, K. Trends in Colon and Rectal Cancer Incidence in Australia from 1982 to 2014: Analysis of Data on Over 375,000 Cases. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.; Davidson, C.; Hall, C.; Pearson, J.; Eglinton, T.; Wakeman, C.; Frizelle, F. Population-based study demonstrating an increase in colorectal cancer in young patients. Br. J. Surg. 2017, 104, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Larsen, I.K.; Bray, F. Trends in colorectal cancer incidence in Norway 1962-2006: An interpretation of the temporal patterns by anatomic subsite. Int. J. Cancer 2010, 126, 721–732. [Google Scholar] [CrossRef]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; De, P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15–49-year-olds in Canada, 1969–2010. Cancer Epidemiol. 2016, 42, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; Ruan, Y.; Shaw, E.; De, P.; Heitman, S.J.; Hilsden, R.J. Increasing colorectal cancer incidence trends among younger adults in Canada. Prev. Med. 2017, 105, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Bhurgri, Y.; Khan, T.; Kayani, N.; Ahmad, R.; Usman, A.; Bhurgri, A.; Bashir, I.; Hasan, S.H.; Zaidi, S. Incidence and current trends of colorectal malignancies in an unscreened, low risk Pakistan population. Asian Pac. J. Cancer Prev. 2011, 12, 703–708. [Google Scholar] [PubMed]
- Hessami Arani, S.; Kerachian, M.A. Rising rates of colorectal cancer among younger Iranians: Is diet to blame? Curr. Oncol. 2017, 24, e131–e137. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.J.Y.; Chiu, H.M.; Jung, K.W.; Jun, J.K.; Sekiguchi, M.; Matsuda, T.; Kyaw, M.H. Increasing Trend in Young-Onset Colorectal Cancer in Asia: More Cancers in Men and More Rectal Cancers. Am. J. Gastroenterol. 2019, 114, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Baby, B.; Wang, K.; Sirohi, B.; Lei, F.; Chen, Q.; Huang, B. Colorectal cancer incidence in younger adults in India. Gut 2020, 69, 1899–1900. [Google Scholar] [CrossRef]
- Kupfer, S.S.; Carr, R.M.; Carethers, J.M. Reducing colorectal cancer risk among African Americans. Gastroenterology 2015, 149, 1302–1304. [Google Scholar] [CrossRef]
- Wallace, K.; Hill, E.G.; Lewin, D.N.; Williamson, G.; Oppenheimer, S.; Ford, M.E.; Wargovich, M.J.; Berger, F.G.; Bolick, S.W.; Thomas, M.B.; et al. Racial disparities in advanced-stage colorectal cancer survival. Cancer Causes Control. 2013, 24, 463–471. [Google Scholar] [CrossRef]
- Paquette, I.M.; Ying, J.; Shah, S.A.; Abbott, D.E.; Ho, S.M. African Americans should be screened at an earlier age for colorectal cancer. Gastrointest. Endosc. 2015, 82, 878–883. [Google Scholar] [CrossRef]
- DeSantis, C.; Naishadham, D.; Jemal, A. Cancer statistics for African Americans, 2013. CA Cancer J. Clin. 2013, 63, 151–166. [Google Scholar] [CrossRef]
- Rahman, R.; Schmaltz, C.; Jackson, C.S.; Simoes, E.J.; Jackson-Thompson, J.; Ibdah, J.A. Increased risk for colorectal cancer under age 50 in racial and ethnic minorities living in the United States. Cancer Med. 2015, 4, 1863–1870. [Google Scholar] [CrossRef]
- Murphy, C.C.; Sandler, R.S.; Sanoff, H.K.; Yang, Y.C.; Lund, J.L.; Baron, J.A. Decrease in Incidence of Colorectal Cancer Among Individuals 50 Years or Older After Recommendations for Population-based Screening. Clin. Gastroenterol. Hepatol. 2017, 15, 903–909.e6. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Fidler, M.M.; Arnold, M.; Jemal, A.; Bray, F.; Soerjomataram, I. The Future Burden of Colorectal Cancer Among US Blacks and Whites. J. Natl. Cancer Inst. 2018, 110, 791–793. [Google Scholar] [CrossRef]
- Klabunde, C.N.; Cronin, K.A.; Breen, N.; Waldron, W.R.; Ambs, A.H.; Nadel, M.R. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 1611–1621. [Google Scholar] [CrossRef]
- CDC. Vital signs: Colorectal cancer screening test use—United States, 2012. MMWR Morb. Mortal Wkly. Rep. 2013, 62, 881–888. [Google Scholar]
- Ellis, L.; Abrahão, R.; McKinley, M.; Yang, J.; Somsouk, M.; Marchand, L.L.; Cheng, I.; Gomez, S.L.; Shariff-Marco, S. Colorectal Cancer Incidence Trends by Age, Stage, and Racial/Ethnic Group in California, 1990–2014. Cancer Epidemiol. Biomarkers Prev. 2018, 27, 1011–1018. [Google Scholar] [CrossRef]
- Levin, T.R.; Jensen, C.D.; Chawla, N.M.; Sakoda, L.C.; Lee, J.K.; Zhao, W.K.; Landau, M.A.; Herm, A.; Eby, E.; Quesenberry, C.P.; et al. Early Screening of African Americans (45–50 Years Old) in a Fecal Immunochemical Test-Based Colorectal Cancer Screening Program. Gastroenterology 2020, 159, 1695–1704.e1. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Suveillance, Epidemiology, and End Results Program. Available online: https://seer.cancer.gov/explorer/ (accessed on 9 April 2021).
- Stefanidis, D.; Pollock, B.H.; Miranda, J.; Wong, A.; Sharkey, F.E.; Rousseau, D.L.; Thomas, C.R.; Kahlenberg, M.S. Colorectal cancer in Hispanics: A population at risk for earlier onset, advanced disease, and decreased survival. Am. J. Clin. Oncol. 2006, 29, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Thrift, A.P.; Zarrin-Khameh, N.; Wichmann, A.; Armstrong, G.N.; Thompson, P.A.; Bondy, M.L.; Musher, B.L. Rising Incidence of Colorectal Cancer among Young Hispanics in Texas. J. Clin. Gastroenterol. 2017, 51, 34–42. [Google Scholar] [CrossRef]
- Garcia, S.; Pruitt, S.L.; Singal, A.G.; Murphy, C.C. Colorectal cancer incidence among Hispanics and non-Hispanic Whites in the United States. Cancer Causes Control. 2018, 29, 1039–1046. [Google Scholar] [CrossRef]
- Siegel, R.L.; Medhanie, G.A.; Fedewa, S.A.; Jemal, A. State Variation in Early-Onset Colorectal Cancer in the United States, 1995–2015. J. Natl. Cancer Inst. 2019, 111, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Obesity and colon and rectal cancer risk: A meta-analysis of prospective studies. Am. J. Clin. Nutr. 2007, 86, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.D.; Miller, A.B.; Rohan, T.E. Obesity and colorectal cancer risk in women. Gut 2002, 51, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.K.; Pirie, K.; Beral, V.; Green, J.; Spencer, E.; Bull, D.; Collaboration, M.W.S. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ 2007, 335, 1134. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.Y.; Kim, B.C.; Han, K.S.; Ryu, K.H.; Park, B.J.; Kim, H.B.; Nam, B.H. Abdominal visceral adipose tissue predicts risk of colorectal adenoma in both sexes. Clin. Gastroenterol. Hepatol. 2010, 8, 443.e2–450.e2. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.H.; Kim, R.; Greenwood, D.C.; Giovannucci, E.L. Visceral adiposity and colorectal adenomas: Dose-response meta-analysis of observational studies. Ann. Oncol. 2015, 26, 1101–1109. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; McDowell, M.A.; Tabak, C.J.; Flegal, K.M. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006, 295, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Liu, P.H.; Wu, K.; Ng, K.; Zauber, A.G.; Nguyen, L.H.; Song, M.; He, X.; Fuchs, C.S.; Ogino, S.; Willett, W.C.; et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol. 2019, 5, 37–44. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Torre, L.A.; Pearson-Stuttard, J.; Islami, F.; Fedewa, S.A.; Goding Sauer, A.; Shuval, K.; Gapstur, S.M.; Jacobs, E.J.; et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J. Clin. 2019, 69, 88–112. [Google Scholar] [CrossRef]
- Rosato, V.; Bosetti, C.; Levi, F.; Polesel, J.; Zucchetto, A.; Negri, E.; La Vecchia, C. Risk factors for young-onset colorectal cancer. Cancer Causes Control. 2013, 24, 335–341. [Google Scholar] [CrossRef]
- Low, E.E.; Demb, J.; Liu, L.; Earles, A.; Bustamante, R.; Williams, C.D.; Provenzale, D.; Kaltenbach, T.; Gawron, A.J.; Martinez, M.E.; et al. Risk Factors for Early-Onset Colorectal Cancer. Gastroenterology 2020, 159, 492–501.e7. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Lawman, H.G.; Fryar, C.D.; Kruszon-Moran, D.; Kit, B.K.; Flegal, K.M. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 through 2013–2014. JAMA 2016, 315, 2292–2299. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, Y.S.; Park, J.H.; Kim, H.J.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; Choi, K.Y.; Park, D.I. Different risk factors for advanced colorectal neoplasm in young adults. World J. Gastroenterol. 2016, 22, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Célind, J.; Ohlsson, C.; Bygdell, M.; Nethander, M.; Kindblom, J.M. Childhood Body Mass Index Is Associated with Risk of Adult Colon Cancer in Men: An Association Modulated by Pubertal Change in Body Mass Index. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 974–979. [Google Scholar] [CrossRef]
- Jensen, B.W.; Gamborg, M.; Gögenur, I.; Renehan, A.G.; Sørensen, T.I.A.; Baker, J.L. Childhood body mass index and height in relation to site-specific risks of colorectal cancers in adult life. Eur. J. Epidemiol. 2017, 32, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Ward, H.A.; Jenab, M.; Rothwell, J.A.; Boutron-Ruault, M.C.; Carbonnel, F.; Kvaskoff, M.; Kaaks, R.; Kühn, T.; Boeing, H.; et al. Heterogeneity of Colorectal Cancer Risk Factors by Anatomical Subsite in 10 European Countries: A Multinational Cohort Study. Clin. Gastroenterol. Hepatol. 2019, 17, 1323–1331.e6. [Google Scholar] [CrossRef]
- Iannotti, R.J.; Wang, J. Trends in physical activity, sedentary behavior, diet, and BMI among US adolescents, 2001–2009. Pediatrics 2013, 132, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Hennessy, S.; Lewis, J.D. Type 2 diabetes mellitus and the risk of colorectal cancer. Clin. Gastroenterol. Hepatol. 2005, 3, 587–594. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N.; Wolk, A. Diabetes mellitus and risk of colorectal cancer: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 1679–1687. [Google Scholar] [CrossRef]
- Deng, L.; Gui, Z.; Zhao, L.; Wang, J.; Shen, L. Diabetes mellitus and the incidence of colorectal cancer: An updated systematic review and meta-analysis. Dig. Dis. Sci. 2012, 57, 1576–1585. [Google Scholar] [CrossRef]
- Koopman, R.J.; Mainous, A.G.; Diaz, V.A.; Geesey, M.E. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann. Fam. Med. 2005, 3, 60–63. [Google Scholar] [CrossRef]
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R.J. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015, 313, 1973–1974. [Google Scholar] [CrossRef] [PubMed]
- Haile, R.W.; John, E.M.; Levine, A.J.; Cortessis, V.K.; Unger, J.B.; Gonzales, M.; Ziv, E.; Thompson, P.; Spruijt-Metz, D.; Tucker, K.L.; et al. A review of cancer in U.S. Hispanic populations. Cancer Prev. Res. 2012, 5, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, B.; Peleteiro, B.; Lunet, N. Dietary patterns and colorectal cancer: Systematic review and meta-analysis. Eur. J. Cancer Prev. 2012, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; Corpet, D. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Chan, A.T.; Giovannucci, E.L. Primary prevention of colorectal cancer. Gastroenterology 2010, 138, 2029–2043.e10. [Google Scholar] [CrossRef]
- Feng, Y.L.; Shu, L.; Zheng, P.F.; Zhang, X.Y.; Si, C.J.; Yu, X.L.; Gao, W.; Zhang, L. Dietary patterns and colorectal cancer risk: A meta-analysis. Eur. J. Cancer Prev. 2017, 26, 201–211. [Google Scholar] [CrossRef]
- O’Keefe, S.J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260.e16. [Google Scholar] [CrossRef]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Colorectal Cancer Risk-A Meta-Analysis. Nutrients 2017, 9, 1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Leung, C.W.; Li, Y.; Ding, E.L.; Chiuve, S.E.; Hu, F.B.; Willett, W.C. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern. Med. 2014, 174, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Sijtsma, F.P.; Meyer, K.A.; Steffen, L.M.; Shikany, J.M.; Van Horn, L.; Harnack, L.; Kromhout, D.; Jacobs, D.R. Longitudinal trends in diet and effects of sex, race, and education on dietary quality score change: The Coronary Artery Risk Development in Young Adults study. Am. J. Clin. Nutr. 2012, 95, 580–586. [Google Scholar] [CrossRef]
- Zheng, X.; Hur, J.; Nguyen, L.H.; Liu, J.; Song, M.; Wu, K.; Smith-Warner, S.A.; Ogino, S.; Willett, W.C.; Chan, A.T.; et al. Comprehensive Assessment of Diet Quality and Risk of Precursors of Early-Onset Colorectal Cancer. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef]
- Schmid, D.; Leitzmann, M.F. Television viewing and time spent sedentary in relation to cancer risk: A meta-analysis. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol 2017, 18, e457–e471. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Liu, P.H.; Zheng, X.; Keum, N.; Zong, X.; Li, X.; Wu, K.; Fuchs, C.S.; Ogino, S.; Ng, K.; et al. Sedentary Behaviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectr. 2018, 2, pky073. [Google Scholar] [CrossRef]
- Rogers, C.R.; Moore, J.X.; Qeadan, F.; Gu, L.Y.; Huntington, M.S.; Holowatyj, A.N. Examining factors underlying geographic disparities in early-onset colorectal cancer survival among men in the United States. Am. J. Cancer Res. 2020, 10, 1592–1607. [Google Scholar]
- Murphy, C.C.; Sanoff, H.K.; Stitzenberg, K.B.; Baron, J.A.; Lund, J.L.; Sandler, R.S. Patterns of Sociodemographic and Clinicopathologic Characteristics of Stages II and III Colorectal Cancer Patients by Age: Examining Potential Mechanisms of Young-Onset Disease. J. Cancer Epidemiol. 2017, 2017, 4024580. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, K.; Mehta, R.; Drew, D.A.; Song, M.; Lochhead, P.; Nguyen, L.H.; Izard, J.; Fuchs, C.S.; Garrett, W.S.; et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut 2018, 67, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Dik, V.K.; van Oijen, M.G.; Smeets, H.M.; Siersema, P.D. Frequent Use of Antibiotics Is Associated with Colorectal Cancer Risk: Results of a Nested Case-Control Study. Dig. Dis. Sci. 2016, 61, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Haynes, K.; Mamtani, R.; Yang, Y.X. Impact of antibiotic exposure on the risk of colorectal cancer. Pharmacoepidemiol. Drug Saf. 2015, 24, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Babic, A.; Tworoger, S.S.; Zhang, L.; Wu, K.; Smith-Warner, S.A.; Ogino, S.; Chan, A.T.; Meyerhardt, J.; Giovannucci, E.; et al. Periodontal disease, tooth loss and colorectal cancer risk: Results from the Nurses’ Health Study. Int. J. Cancer 2017, 140, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Carson, T.L.; Wang, F.; Cui, X.; Jackson, B.E.; Van Der Pol, W.J.; Lefkowitz, E.J.; Morrow, C.; Baskin, M.L. Associations Between Race, Perceived Psychological Stress, and the Gut Microbiota in a Sample of Generally Healthy Black and White Women: A Pilot Study on the Role of Race and Perceived Psychological Stress. Psychosom. Med. 2018, 80, 640–648. [Google Scholar] [CrossRef]
- Wallace, K.; Lewin, D.N.; Sun, S.; Spiceland, C.M.; Rockey, D.C.; Alekseyenko, A.V.; Wu, J.D.; Baron, J.A.; Alberg, A.J.; Hill, E.G. Tumor-Infiltrating Lymphocytes and Colorectal Cancer Survival in African American and Caucasian Patients. Cancer Epidemiol. Biomarkers Prev. 2018, 27, 755–761. [Google Scholar] [CrossRef]
- Fedirko, V.; Tramacere, I.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Negri, E.; Straif, K.; Romieu, I.; La Vecchia, C.; et al. Alcohol drinking and colorectal cancer risk: An overall and dose-response meta-analysis of published studies. Ann. Oncol. 2011, 22, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Iodice, S.; Bagnardi, V.; Raimondi, S.; Lowenfels, A.B.; Maisonneuve, P. Smoking and colorectal cancer: A meta-analysis. JAMA 2008, 300, 2765–2778. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Wilson, M.; Elwin, C.E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef]
- Yurgelun, M.B.; Kulke, M.H.; Fuchs, C.S.; Allen, B.A.; Uno, H.; Hornick, J.L.; Ukaegbu, C.I.; Brais, L.K.; McNamara, P.G.; Mayer, R.J.; et al. Cancer Susceptibility Gene Mutations in Individuals With Colorectal Cancer. J. Clin. Oncol. 2017, 35, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Mork, M.E.; You, Y.N.; Ying, J.; Bannon, S.A.; Lynch, P.M.; Rodriguez-Bigas, M.A.; Vilar, E. High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults With Colorectal Cancer. J. Clin. Oncol. 2015, 33, 3544–3549. [Google Scholar] [CrossRef] [PubMed]
- (NCCN), NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Colorectal Version 1. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf (accessed on 26 February 2021).
- Guindalini, R.S.; Win, A.K.; Gulden, C.; Lindor, N.M.; Newcomb, P.A.; Haile, R.W.; Raymond, V.; Stoffel, E.; Hall, M.; Llor, X.; et al. Mutation spectrum and risk of colorectal cancer in African American families with Lynch syndrome. Gastroenterology 2015, 149, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Lee, S.M.; Barge, W.; Siddique, S.M.; Berera, S.; Wideroff, G.; Tondon, R.; Chang, J.; Peterson, M.; Stoll, J.; et al. Low Referral Rate for Genetic Testing in Racially and Ethnically Diverse Patients Despite Universal Colorectal Cancer Screening. Clin. Gastroenterol. Hepatol. 2018, 16, 1911–1918.e2. [Google Scholar] [CrossRef] [PubMed]
- Dharwadkar, P.; Greenan, G.; Stoffel, E.M.; Burstein, E.; Pirzadeh-Miller, S.; Lahiri, S.; Mauer, C.; Singal, A.G.; Murphy, C.C. Racial and Ethnic Disparities in Germline Genetic Testing of Patients With Young-Onset Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef]
- Kupfer, S.S.; McCaffrey, S.; Kim, K.E. Racial and gender disparities in hereditary colorectal cancer risk assessment: The role of family history. J. Cancer Educ. 2006, 21, S32–S36. [Google Scholar] [CrossRef]
- Perencevich, M.; Ojha, R.P.; Steyerberg, E.W.; Syngal, S. Racial and ethnic variations in the effects of family history of colorectal cancer on screening compliance. Gastroenterology 2013, 145, 775–781.e2. [Google Scholar] [CrossRef]
- Scheuner, M.T.; McNeel, T.S.; Freedman, A.N. Population prevalence of familial cancer and common hereditary cancer syndromes. The 2005 California Health Interview Survey. Genet. Med. 2010, 12, 726–735. [Google Scholar] [CrossRef]
- Griffith, K.A.; McGuire, D.B.; Royak-Schaler, R.; Plowden, K.O.; Steinberger, E.K. Influence of family history and preventive health behaviors on colorectal cancer screening in African Americans. Cancer 2008, 113, 276–285. [Google Scholar] [CrossRef]
- Hofseth, L.J.; Hebert, J.R.; Chanda, A.; Chen, H.; Love, B.L.; Pena, M.M.; Murphy, E.A.; Sajish, M.; Sheth, A.; Buckhaults, P.J.; et al. Early-onset colorectal cancer: Initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 352–364. [Google Scholar] [CrossRef]
- Gausman, V.; Dornblaser, D.; Anand, S.; Hayes, R.B.; O’Connell, K.; Du, M.; Liang, P.S. Risk Factors Associated With Early-Onset Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 2752–2759.e2. [Google Scholar] [CrossRef]
- Chang, D.T.; Pai, R.K.; Rybicki, L.A.; Dimaio, M.A.; Limaye, M.; Jayachandran, P.; Koong, A.C.; Kunz, P.A.; Fisher, G.A.; Ford, J.M.; et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: An adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod. Pathol. 2012, 25, 1128–1139. [Google Scholar] [CrossRef]
- Yantiss, R.K.; Goodarzi, M.; Zhou, X.K.; Rennert, H.; Pirog, E.C.; Banner, B.F.; Chen, Y.T. Clinical, pathologic, and molecular features of early-onset colorectal carcinoma. Am. J. Surg. Pathol. 2009, 33, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Willauer, A.N.; Liu, Y.; Pereira, A.A.L.; Lam, M.; Morris, J.S.; Raghav, K.P.S.; Morris, V.K.; Menter, D.; Broaddus, R.; Meric-Bernstam, F.; et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer 2019, 125, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Xicola, R.M.; Manojlovic, Z.; Augustus, G.J.; Kupfer, S.S.; Emmadi, R.; Alagiozian-Angelova, V.; Triche, T.; Salhia, B.; Carpten, J.; Llor, X.; et al. Lack of APC somatic mutation is associated with early-onset colorectal cancer in African Americans. Carcinogenesis 2018, 39, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Aitchison, A.; Hakkaart, C.; Day, R.C.; Morrin, H.R.; Frizelle, F.A.; Keenan, J.I. Mutations Are Not Confined to Hotspot Regions in Early-Onset Colorectal Cancer. Cancers 2020, 12, 3829. [Google Scholar] [CrossRef] [PubMed]
- Antelo, M.; Balaguer, F.; Shia, J.; Shen, Y.; Hur, K.; Moreira, L.; Cuatrecasas, M.; Bujanda, L.; Giraldez, M.D.; Takahashi, M.; et al. A high degree of LINE-1 hypomethylation is a unique feature of early-onset colorectal cancer. PLoS ONE 2012, 7, e45357. [Google Scholar] [CrossRef]
- Holowatyj, A.N.; Gigic, B.; Herpel, E.; Scalbert, A.; Schneider, M.; Ulrich, C.M.; Consortium, M.; Study, C. Distinct Molecular Phenotype of Sporadic Colorectal Cancers Among Young Patients Based on Multiomics Analysis. Gastroenterology 2020, 158, 1155–1158.e2. [Google Scholar] [CrossRef]
- Carethers, J.M. Clinical and Genetic Factors to Inform Reducing Colorectal Cancer Disparitites in African Americans. Front. Oncol. 2018, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Xicola, R.M.; Gagnon, M.; Clark, J.R.; Carroll, T.; Gao, W.; Fernandez, C.; Mijic, D.; Rawson, J.B.; Janoski, A.; Pusatcioglu, C.K.; et al. Excess of proximal microsatellite-stable colorectal cancer in African Americans from a multiethnic study. Clin. Cancer Res. 2014, 20, 4962–4970. [Google Scholar] [CrossRef]
- Guda, K.; Veigl, M.L.; Varadan, V.; Nosrati, A.; Ravi, L.; Lutterbaugh, J.; Beard, L.; Willson, J.K.; Sedwick, W.D.; Wang, Z.J.; et al. Novel recurrently mutated genes in African American colon cancers. Proc. Natl. Acad. Sci. USA 2015, 112, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Ahuja, S.; Kannan, L.; Llor, X.; Ellis, N.A.; Xicola, R.M.; Laiyemo, A.O.; Carethers, J.M.; Brim, H.; Nouraie, M. A meta-analysis of MSI frequency and race in colorectal cancer. Oncotarget 2016, 7, 34546–34557. [Google Scholar] [CrossRef]
- Basa, R.C.; Davies, V.; Li, X.; Murali, B.; Shah, J.; Yang, B.; Li, S.; Khan, M.W.; Tian, M.; Tejada, R.; et al. Decreased Anti-Tumor Cytotoxic Immunity among Microsatellite-Stable Colon Cancers from African Americans. PLoS ONE 2016, 11, e0156660. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, B.; Lee, A.; Cabrera, B.L.; Miyai, K.; Luo, L.; Ramamoorthy, S.; Keku, T.; Sandler, R.S.; McGuire, K.L.; Carethers, J.M. Relationship of EMAST and microsatellite instability among patients with rectal cancer. J. Gastrointest. Surg. 2010, 14, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Devall, M.; Sun, X.; Yuan, F.; Cooper, G.S.; Willis, J.; Weisenberger, D.J.; Casey, G.; Li, L. Racial Disparities in Epigenetic Aging of the Right vs Left Colon. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef]
- Boardman, L.A.; Lanier, A.P.; French, A.J.; Schowalter, K.V.; Burgart, L.J.; Koller, K.R.; McDonnell, S.K.; Schaid, D.J.; Thibodeau, S.N. Frequency of defective DNA mismatch repair in colorectal cancer among the Alaska Native people. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2344–2350. [Google Scholar] [CrossRef]
- Dos Santos, W.; Sobanski, T.; de Carvalho, A.C.; Evangelista, A.F.; Matsushita, M.; Berardinelli, G.N.; de Oliveira, M.A.; Reis, R.M.; Guimarães, D.P. Mutation profiling of cancer drivers in Brazilian colorectal cancer. Sci. Rep. 2019, 9, 13687. [Google Scholar] [CrossRef]
- Madiba, T.; Moodley, Y.; Sartorius, B.; Sartorius, K.; Aldous, C.; Naidoo, M.; Govindasamy, V.; Bhadree, S.; Stopforth, L.; Ning, Y.; et al. Clinicopathological spectrum of colorectal cancer among the population of the KwaZulu-Natal Province in South Africa. Pan Afr. Med. J. 2020, 37, 74. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.D.; Eide, T.J.; Jass, J.R. Trends in colorectal cancer incidence and histologic findings in Maori and Polynesian residents of New Zealand. Cancer 1993, 71, 3839–3845. [Google Scholar] [CrossRef]
- Holowatyj, A.N.; Maude, A.S.; Musa, H.S.; Adamu, A.; Ibrahim, S.; Abdullahi, A.; Manko, M.; Aminu, S.M.; Mohammed, A.; Idoko, J.; et al. Patterns of Early-Onset Colorectal Cancer Among Nigerians and African Americans. JCO Glob. Oncol. 2020, 6, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Holowatyj, A.N.; Ruterbusch, J.J.; Rozek, L.S.; Cote, M.L.; Stoffel, E.M. Racial/Ethnic Disparities in Survival Among Patients With Young-Onset Colorectal Cancer. J. Clin. Oncol. 2016, 34, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Sineshaw, H.M.; Ng, K.; Flanders, W.D.; Brawley, O.W.; Jemal, A. Factors That Contribute to Differences in Survival of Black vs White Patients With Colorectal Cancer. Gastroenterology 2018, 154, 906–915.e7. [Google Scholar] [CrossRef] [PubMed]
- Holowatyj, A.N.; Langston, M.E.; Han, Y.; Viskochil, R.; Perea, J.; Cao, Y.; Rogers, C.R.; Lieu, C.H.; Moore, J.X. Community Health Behaviors and Geographic Variation in Early-Onset Colorectal Cancer Survival Among Women. Clin. Transl. Gastroenterol. 2020, 11, e00266. [Google Scholar] [CrossRef] [PubMed]
- Alese, O.B.; Jiang, R.; Zakka, K.M.; Wu, C.; Shaib, W.; Akce, M.; Behera, M.; El-Rayes, B.F. Analysis of racial disparities in the treatment and outcomes of colorectal cancer in young adults. Cancer Epidemiol. 2019, 63, 101618. [Google Scholar] [CrossRef] [PubMed]
- King, M.L., Jr. Untitled presentation. In Proceedings of the Second National Convention of the Medical Committee for Human Rights, Chicago, IL, USA, 25 March 1966. [Google Scholar]
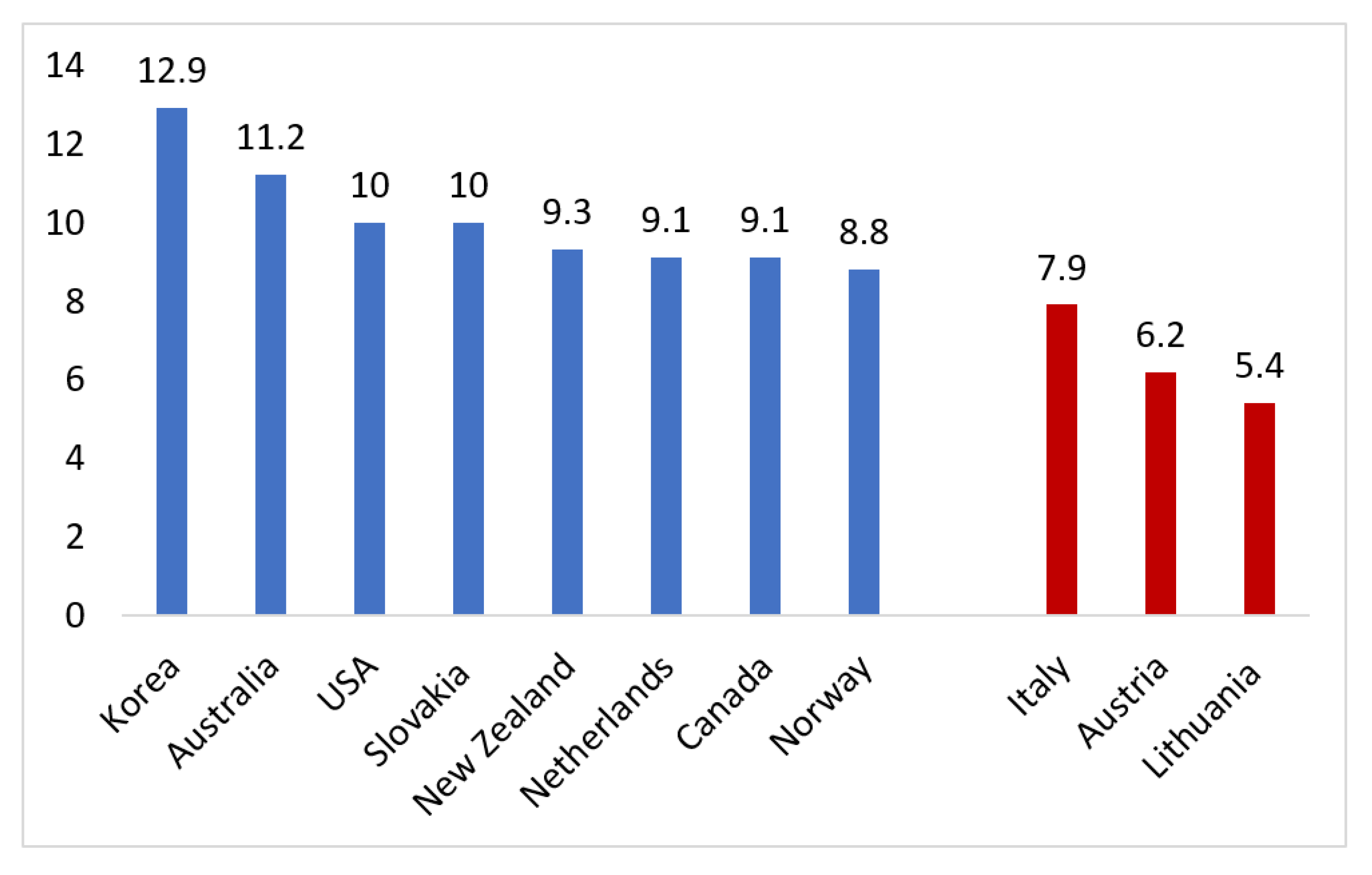
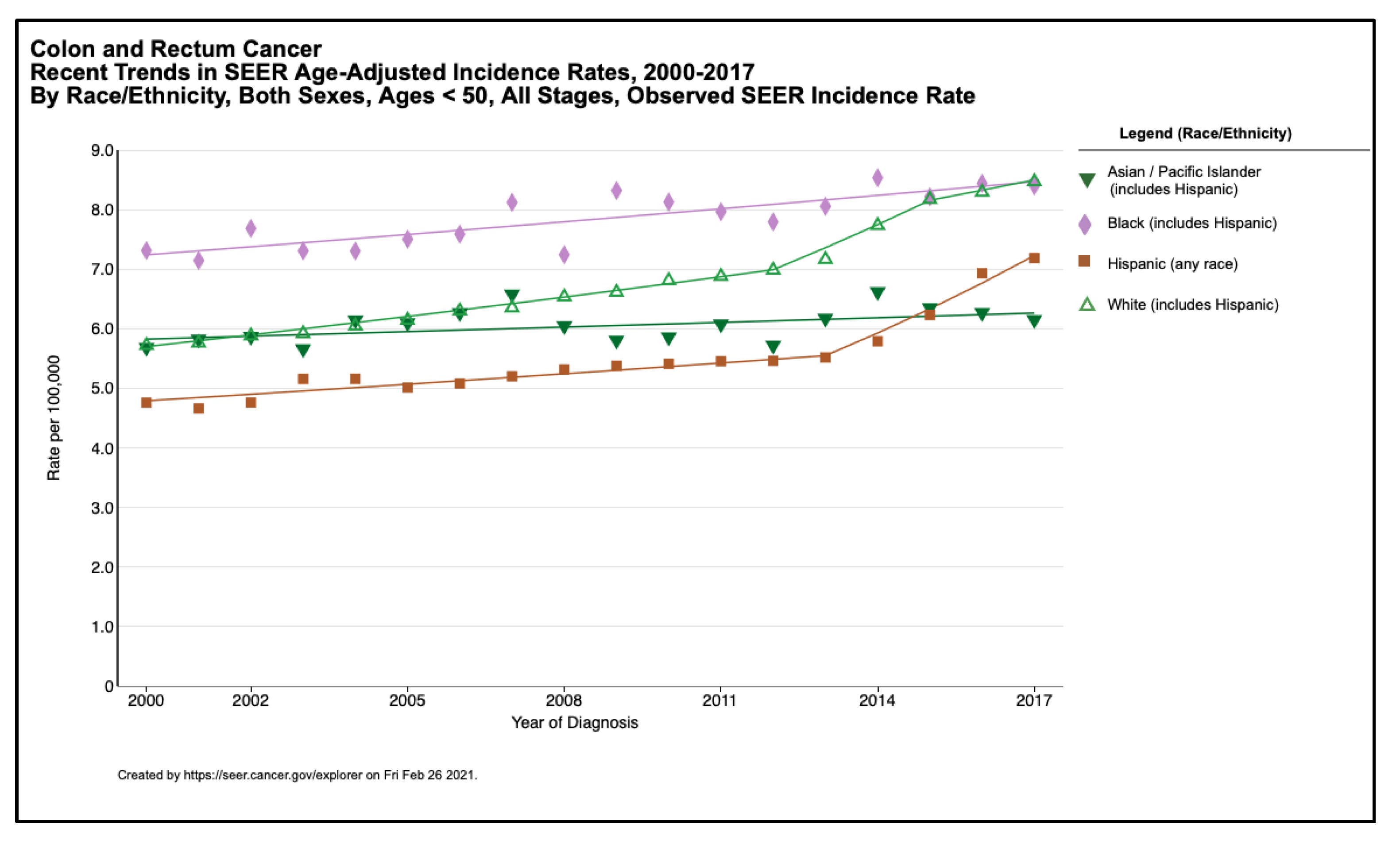

| Domain | Examples |
|---|---|
| Biology/Genetics | Age; sex; inherited condition; family history |
| Individual behavior | Diet; physical activity; habits (e.g., tobacco, alcohol) |
| Health services | Lack of or limited access to healthcare; lack of insurance |
| Social factors | Job; discrimination; exposure to crime; social support; socioeconomic status; neighborhood deprivation; quality schools; transportation; public safety; residential segregation; exposure to environmental toxins |
| Policies | Local, state, and federal policies affecting health |
| Characteristic | Details for African-American patients | References |
|---|---|---|
| Anatomic location | Overall more proximal vs. distal tumorsYounger AAs have higher prevalence of distal tumors vs. older AAs | [107] |
| Somatic mutations | Unique mutations in EPHA6, FLCN and CDH5APC-negative tumors more frequently in younger AA | [102,103,108] |
| Microsatellite instability | 20% lower rate of microsatellite instabilityHigher rate of EMAST in rectal tumors | [109,110,111] |
| Epigenetics | Unique pattern of epigenetic signature in proximal colon | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muller, C.; Ihionkhan, E.; Stoffel, E.M.; Kupfer, S.S. Disparities in Early-Onset Colorectal Cancer. Cells 2021, 10, 1018. https://doi.org/10.3390/cells10051018
Muller C, Ihionkhan E, Stoffel EM, Kupfer SS. Disparities in Early-Onset Colorectal Cancer. Cells. 2021; 10(5):1018. https://doi.org/10.3390/cells10051018
Chicago/Turabian StyleMuller, Charles, Ehizokha Ihionkhan, Elena M. Stoffel, and Sonia S. Kupfer. 2021. "Disparities in Early-Onset Colorectal Cancer" Cells 10, no. 5: 1018. https://doi.org/10.3390/cells10051018
APA StyleMuller, C., Ihionkhan, E., Stoffel, E. M., & Kupfer, S. S. (2021). Disparities in Early-Onset Colorectal Cancer. Cells, 10(5), 1018. https://doi.org/10.3390/cells10051018





