Therapeutic Potential of Niche-Specific Mesenchymal Stromal Cells for Spinal Cord Injury Repair
Abstract
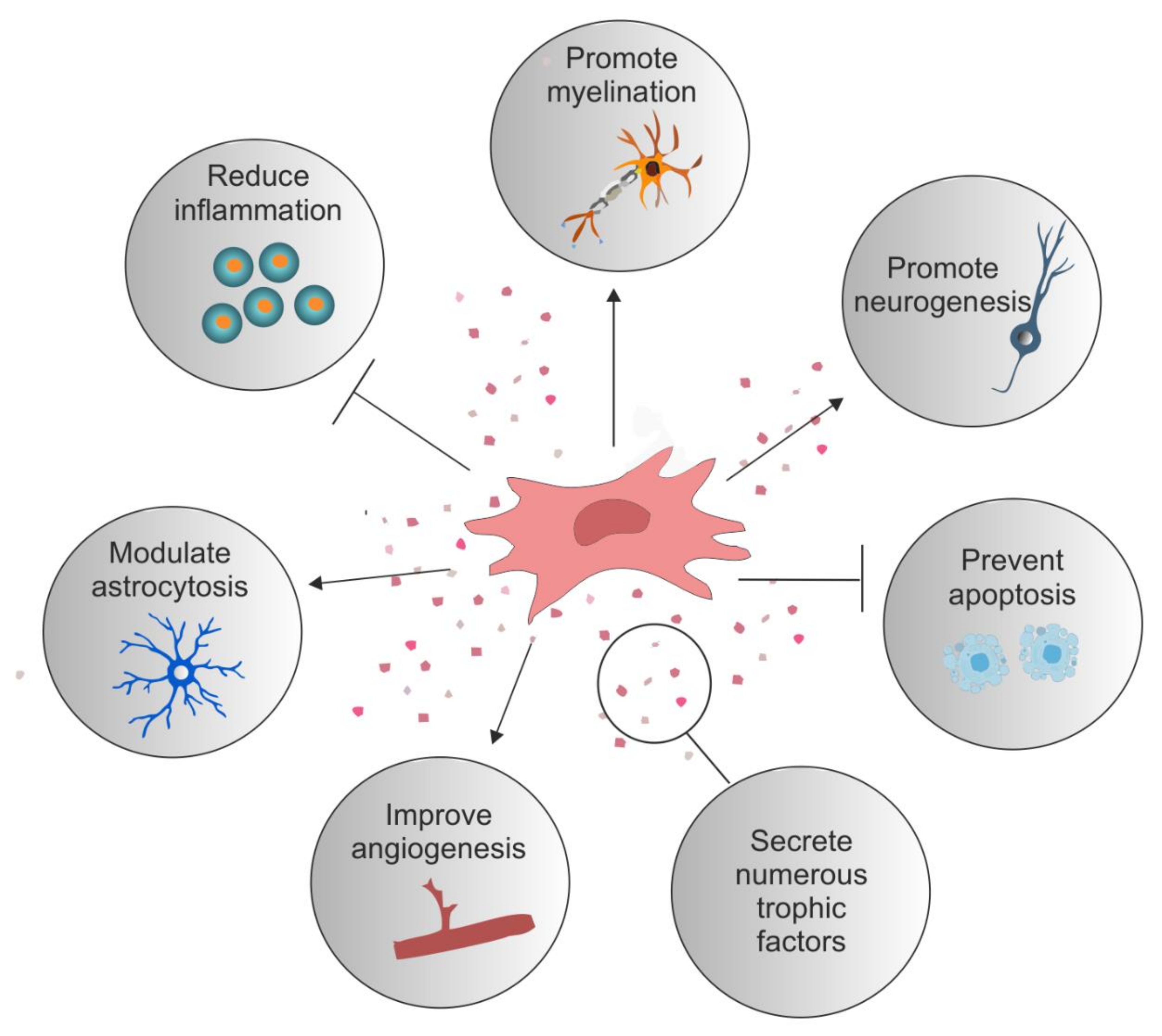
1. Introduction
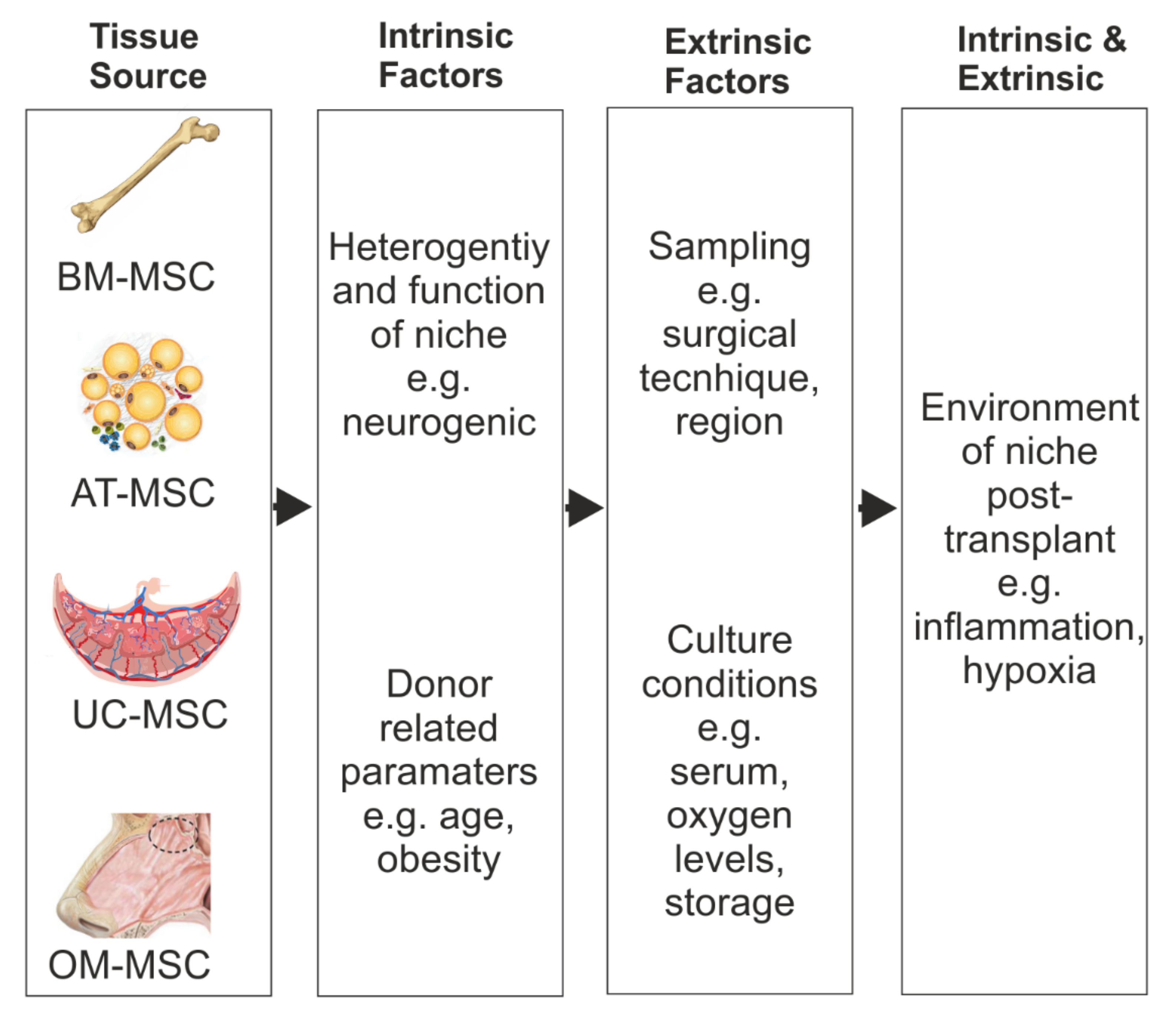
2. Bone Marrow Mesenchymal Stromal Cells (BM-MSCs)
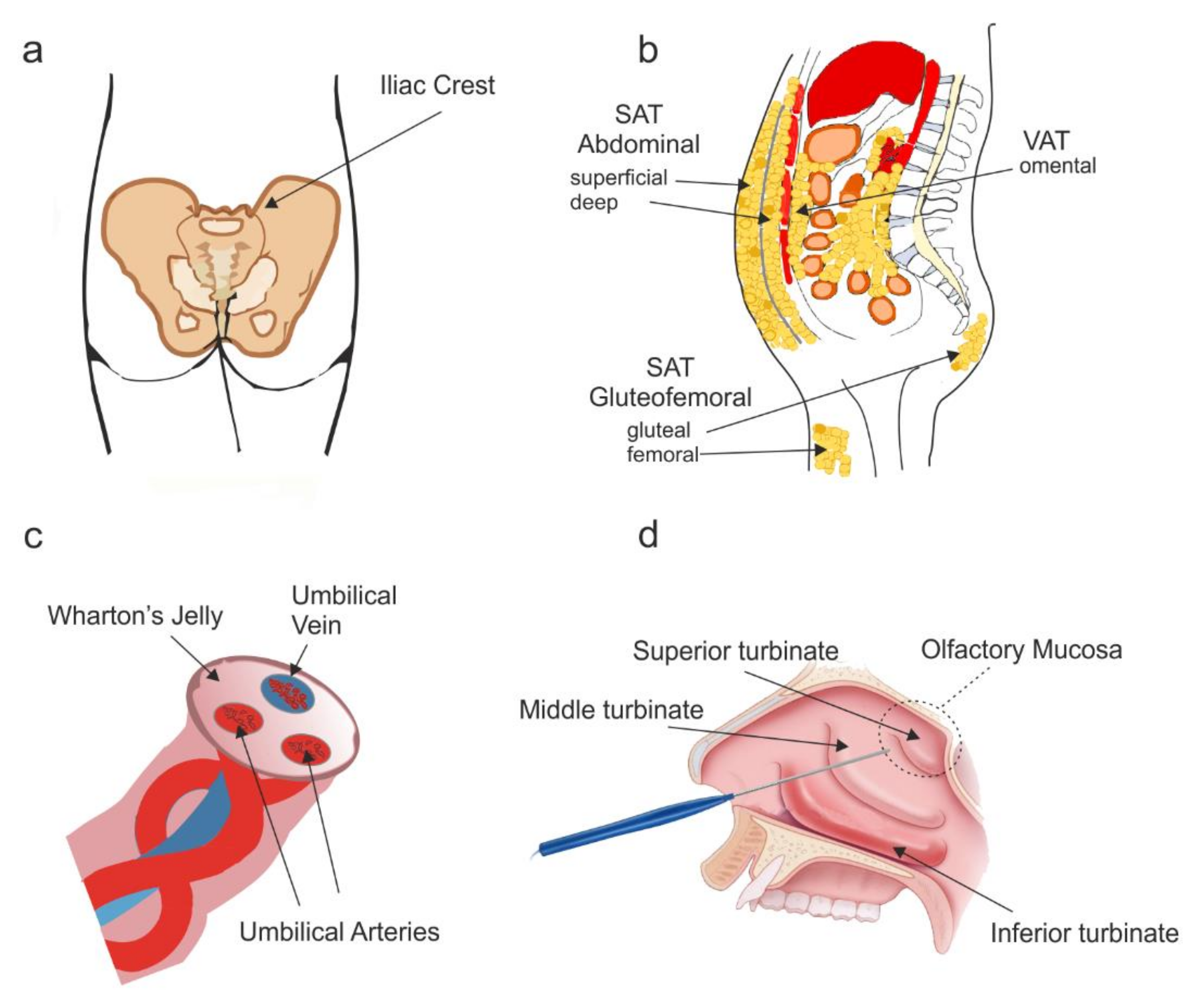
2.1. Anatomical Origin and Culture
2.2. Parameters Related to Donor of MSCs
3. Adipose-Derived Mesenchymal Stromal Cells
3.1. Anatomical Location and Culture
3.2. Parameters Related to Donor of MSCs
4. Umbilical-Cord-Derived Mesenchymal Stromal Cells (UC-MSCs)
4.1. Anatomical Location and Cell Culture
4.2. Parameters Related to Donor of MSCs
5. Olfactory-Mucosa-Derived Mesenchymal Stromal Cells (OM-MSCs)
5.1. Anatomical Location and Cell Culture
5.2. Parameters Related to Donor of MSC
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Norenberg, M.D.; Smith, J.; Marcillo, A. The Pathology of Human Spinal Cord Injury: Defining the Problems. J. Neurotrauma 2004, 21, 429–440. [Google Scholar] [CrossRef]
- Brennan, F.H.; Popovich, P.G. Emerging targets for reprograming the immune response to promote repair and recovery of function after spinal cord injury. Curr. Opin. Neurol. 2018, 31, 334–344. [Google Scholar] [CrossRef]
- Fawcett, J.W.; Asher, R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999, 49, 377–391. [Google Scholar] [CrossRef]
- Tetzlaff, W.; Okon, E.B.; Karimi-Abdolrezaee, S.; Hill, C.E.; Sparling, J.S.; Plemel, J.R.; Plunet, W.T.; Tsai, E.C.; Baptiste, D.; Smithson, L.J.; et al. A Systematic Review of Cellular Transplantation Therapies for Spinal Cord Injury. J. Neurotrauma 2011, 28, 1611–1682. [Google Scholar] [CrossRef] [PubMed]
- Zavvarian, M.-M.; Toossi, A.; Khazaei, M.; Hong, J.; Fehlings, M. Novel innovations in cell and gene therapies for spinal cord injury. F1000Research 2020, 9, 279. [Google Scholar] [CrossRef]
- Biernaskie, J.; Sparling, J.S.; Liu, J.; Shannon, C.P.; Plemel, J.R.; Xie, Y.; Miller, F.D.; Tetzlaff, W. Skin-Derived Precursors Generate Myelinating Schwann Cells That Promote Remyelination and Functional Recovery after Contusion Spinal Cord Injury. J. Neurosci. 2007, 27, 9545–9559. [Google Scholar] [CrossRef]
- Sparling, J.S.; Bretzner, F.; Biernaskie, J.; Assinck, P.; Jiang, Y.; Arisato, H.; Plunet, W.T.; Borisoff, J.; Liu, J.; Miller, F.D.; et al. Schwann Cells Generated from Neonatal Skin-Derived Precursors or Neonatal Peripheral Nerve Improve Functional Recovery after Acute Transplantation into the Partially Injured Cervical Spinal Cord of the Rat. J. Neurosci. 2015, 35, 6714–6730. [Google Scholar] [CrossRef]
- Toft, A.; Scott, D.T.; Barnett, S.C.; Riddell, J.S. Electrophysiological evidence that olfactory cell transplants improve function after spinal cord injury. Brain J. Neurol. 2006, 130, 970–984. [Google Scholar] [CrossRef]
- Barnett, S.C.; Riddell, J.S. Olfactory ensheathing cell transplantation as a strategy for spinal cord repair—What can it achieve? Nature clinical practice. Neurology 2007, 3, 152–161. [Google Scholar] [PubMed]
- Rosenzweig, E.S.; Brock, J.H.; Lu, P.; Kumamaru, H.; A Salegio, E.; Kadoya, K.; Weber, J.L.; Liang, J.J.; Moseanko, R.; Hawbecker, S.; et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018, 24, 484–490. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef]
- Hunt, M.; Lu, P.; Tuszynski, M.H. Myelination of axons emerging from neural progenitor grafts after spinal cord injury. Exp. Neurol. 2017, 296, 69–73. [Google Scholar] [CrossRef]
- Faulkner, J.; Keirstead, H.S. Human embryonic stem cell-derived oligodendrocyte progenitors for the treatment of spinal cord injury. Transpl. Immunol. 2005, 15, 131–142. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Petrakova, K.V.; I Kurolesova, A.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Deriglasova, U.F.; Kulagina, N.N.; Panasuk, A.F.; Rudakowa, S.F.; Luriá, E.A.; Ruadkow, I.A. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 1974, 2, 83–92. [Google Scholar] [PubMed]
- Lindsay, S.L.; Barnett, S.C. Are nestin-positive mesenchymal stromal cells a better source of cells for CNS repair? Neurochem. Int. 2017, 106, 101–107. [Google Scholar] [CrossRef]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119 Pt 11, 2204–2213. [Google Scholar] [CrossRef]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med J. 2018, 18, e264–e277. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Pickard, M.; Johnson, W. The Comparative Effects of Mesenchymal Stem Cell Transplantation Therapy for Spinal Cord Injury in Humans and Animal Models: A Systematic Review and Meta-Analysis. Biology 2021, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Fu, C.; Xiong, F.; He, C.; Wei, Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transplant. 2021, 30. [Google Scholar] [CrossRef]
- Mendonça, M.V.P.; LaRocca, T.F.; de Freitas Souza, B.S.; Villarreal, C.F.; Silva, L.F.M.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.P.; de Oliveira Melo Martinez, A.C.; Kaneto, C.M.; et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014, 5, 126. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.W.; Cho, T.-H.; Park, D.-H.; Lee, J.-B.; Park, J.-Y.; Chung, Y.-G. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J. Spinal Cord Med. 2015, 39, 655–664. [Google Scholar] [CrossRef]
- Willison, A.G.; Smith, S.; Davies, B.M.; Kotter, M.R.N.; Barnett, S.C. A scoping review of trials for cell-based therapies in human spinal cord injury. Spinal Cord 2020, 58, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Liau, L.L.; Looi, Q.H.; Chia, W.C.; Subramaniam, T.; Ng, M.H.; Law, J.X. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Scadden, D.T. The stem-cell niche as an entity of action. Nature 2006, 441, 1075–1079. [Google Scholar] [CrossRef]
- Dazzi, F.; Ramasamy, R.; Glennie, S.; Jones, S.P.; Roberts, I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006, 20, 161–171. [Google Scholar] [CrossRef]
- Ciciarello, M.; Corradi, G.; Loscocco, F.; Visani, G.; Monaco, F.; Cavo, M.; Curti, A.; Isidori, A. The Yin and Yang of the Bone Marrow Microenvironment: Pros and Cons of Mesenchymal Stromal Cells in Acute Myeloid Leukemia. Front. Oncol. 2019, 9, 1135. [Google Scholar] [CrossRef]
- Lo Celso, C.; Fleming, H.E.; Wu, J.W.; Zhao, C.X.; Miake-Lye, S.; Fujisaki, J.; Côté, D.; Rowe, D.W.; Lin, C.P.; Scadden, D.T. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nat. Cell Biol. 2008, 457, 92–96. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’Ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nat. Cell Biol. 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Lucas, D.; Battista, M.; Frenette, P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nat. Cell Biol. 2008, 452, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Liao, Y.; Zhu, S.-H.; Chen, Q.-C.; Xie, D.-M.; Liao, J.-J.; Feng, X.; Jiang, M.H.; He, W. Bone-derived Nestin-positive mesenchymal stem cells improve cardiac function via recruiting cardiac endothelial cells after myocardial infarction. Stem Cell Res. Ther. 2019, 10, 127. [Google Scholar] [CrossRef]
- Machado, C.D.V.; Telles, P.D.D.S.; Nascimento, I.L.O. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. Hemoter. 2013, 35, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Uchida, K.; Guerrero, A.R.; Watanabe, S.; Sugita, D.; Takeura, N.; Yoshida, A.; Long, G.; Wright, K.T.; Johnson, W.E.; et al. Transplantation of Mesenchymal Stem Cells Promotes an Alternative Pathway of Macrophage Activation and Functional Recovery after Spinal Cord Injury. J. Neurotrauma 2012, 29, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wu, C.; Xiong, Q.; Zhou, L.; Tian, Y. Anti-inflammatory Mechanism of Bone Marrow Mesenchymal Stem Cell Transplantation in Rat Model of Spinal Cord Injury. Cell Biochem. Biophys. 2015, 71, 1341–1347. [Google Scholar] [CrossRef]
- Reagan, M.R.; Rosen, C.J. Navigating the bone marrow niche: Translational insights and cancer-driven dysfunction. Nat. Rev. Rheumatol. 2016, 12, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Guan, J.; Zhang, C. Mesenchymal stem cells: Mechanisms and role in bone regeneration. Postgrad. Med J. 2014, 90, 643–647. [Google Scholar] [CrossRef]
- Sivasubramaniyan, K.; Ilas, D.C.; Harichandan, A.; Bos, P.K.; Santos, D.L.; De Zwart, P.; Koevoet, W.J.; Owston, H.; Bühring, H.-J.; Jones, E.; et al. Bone Marrow–Harvesting Technique Influences Functional Heterogeneity of Mesenchymal Stem/Stromal Cells and Cartilage Regeneration. Am. J. Sports Med. 2018, 46, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadis, A.V.; Galanis, N. Human bone marrow-derived mesenchymal stem cells from different bone sources: A panorama. Stem Cell Investig. 2020, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- McLain, R.F.; Fleming, J.E.; Boehm, C.A.; Muschler, G.F. Aspiration of osteoprogenitor cells for augmenting spinal fusion: Comparison of progenitor cell concentrations from the vertebral body and iliac crest. J. Bone Joint Surg. Am. 2005, 87, 2655–2661. [Google Scholar] [PubMed]
- Hyer, C.F.; Berlet, G.C.; Bussewitz, B.W.; Hankins, T.; Ziegler, H.L.; Philbin, T.M. Quantitative Assessment of the Yield of Osteoblastic Connective Tissue Progenitors in Bone Marrow Aspirate from the Iliac Crest, Tibia, and Calcaneus. J. Bone Jt. Surgery Am. Vol. 2013, 95, 1312–1316. [Google Scholar] [CrossRef]
- Li, H.; Ghazanfari, R.; Zacharaki, D.; Lim, H.C.; Scheding, S. Isolation and characterization of primary bone marrow mes-enchymal stromal cells. Ann. N. Y. Acad. Sci. 2016, 1370, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.T.; El Masri, W.; Osman, A.; Chowdhury, J.; Johnson, W.E.B. Concise Review: Bone Marrow for the Treatment of Spinal Cord Injury: Mechanisms and Clinical Applications. STEM Cells 2011, 29, 169–178. [Google Scholar] [CrossRef]
- Boxall, S.A.; Jones, E. Markers for Characterization of Bone Marrow Multipotential Stromal Cells. Stem Cells Int. 2012, 2012, 975871. [Google Scholar] [CrossRef]
- Codinach, M.; Blanco, M.; Ortega, I.; Lloret, M.; Reales, L.; Coca, M.I.; Torrents, S.; Doral, M.; Oliver-Vila, I.; Requena-Montero, M.; et al. Design and validation of a consistent and reproducible manufacture process for the production of clinical-grade bone marrow–derived multipotent mesenchymal stromal cells. Cytotherapy 2016, 18, 1197–1208. [Google Scholar] [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Klein-Nulend, J.; Bacabac, R.; Mullender, M. Mechanobiology of bone tissue. Pathol. Biol. Paris 2005, 53, 576–580. [Google Scholar] [CrossRef]
- Minaire, P.; Edouard, C.; Arlot, M.; Meunier, P.J. Marrow changes in paraplegic patients. Calcif. Tissue Int. 1984, 36, 338–340. [Google Scholar] [CrossRef]
- Carpenter, R.S.; Marbourg, J.M.; Brennan, F.H.; Mifflin, K.A.; Hall, J.C.E.; Jiang, R.R.; Mo, X.M.; Karunasiri, M.; Burke, M.H.; Dorrance, A.M.; et al. Spinal cord injury causes chronic bone marrow failure. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Bourgeois, C.; Gorwood, J.; Barrail-Tran, A.; Lagathu, C.; Capeau, J.; Desjardins, D.; Le Grand, R.; Damouche, A.; Béréziat, V.; Lambotte, O. Specific Biological Features of Adipose Tissue, and Their Impact on HIV Persistence. Front. Microbiol. 2019, 10, 2837. [Google Scholar] [CrossRef]
- Panina, Y.A.; Yakimov, A.S.; Komleva, Y.K.; Morgun, A.V.; Lopatina, O.L.; Malinovskaya, N.A.; Shuvaev, A.N.; Salmin, V.V.; Taranushenko, T.E.; Salmina, A.B. Plasticity of Adipose Tissue-Derived Stem Cells and Regulation of Angiogenesis. Front. Physiol. 2018, 9, 1656. [Google Scholar] [CrossRef] [PubMed]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or Not the Same? Comparison of Adipose Tissue-Derived Versus Bone Marrow-Derived Mesenchymal Stem and Stromal Cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Suga, H.; Eto, H. Adipose-derived stem/progenitor cells: Roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen. Med. 2009, 4, 265–273. [Google Scholar] [CrossRef]
- Estève, D.; Boulet, N.; Belles, C.; Zakaroff-Girard, A.; Decaunes, P.; Briot, A.; Veeranagouda, Y.; Didier, M.; Remaury, A.; Guillemot, J.C.; et al. Lobular architecture of human adipose tissue defines the niche and fate of progenitor cells. Nat. Commun. 2019, 10, 2549. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Carter, G.; El Bassit, G.; Patel, A.A.; Cooper, D.R.; Murr, M.; Patel, N.A. Adipose-derived stem cells from lean and obese humans show depot specific differences in their stem cell markers, exosome contents and senescence: Role of protein kinase C delta (PKCδ) in adipose stem cell niche. Stem Cell Investig. 2016, 3, 2. [Google Scholar]
- Gesta, S.; Tseng, Y.-H.; Kahn, C.R. Developmental Origin of Fat: Tracking Obesity to Its Source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef]
- Grandl, G.; Müller, S.; Moest, H.; Moser, C.; Wollscheid, B.; Wolfrum, C. Depot specific differences in the adipogenic potential of precursors are mediated by collagenous extracellular matrix and Flotillin 2 dependent signaling. Mol. Metab. 2016, 5, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Schoettl, T.; Fischer, I.P.; Ussar, S. Heterogeneity of adipose tissue in development and metabolic function. J. Exp. Biol. 2018, 221 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
- Mariman, E.C.M.; Wang, P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell. Mol. Life Sci. 2010, 67, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Kiuchi, S.; Ouchi, A.; Hase, T.; Murase, T. Characteristic Expression of Extracellular Matrix in Subcutaneous Adipose Tissue Development and Adipogenesis; Comparison with Visceral Adipose Tissue. Int. J. Biol. Sci. 2014, 10, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qin, G. Adipocyte dysfunction and hypertension. Am. J. Cardiovasc. Dis. 2012, 2, 143–149. [Google Scholar] [PubMed]
- Ishii, M.; Iadecola, C. Adipocyte-derived factors in age-related dementia and their contribution to vascular and Alzheimer pathology. Biochim. Biophys. Acta 2016, 1862, 966–974. [Google Scholar] [CrossRef]
- Di Taranto, G.; Cicione, C.; Visconti, G.; Isgrò, M.A.; Barba, M.; Di Stasio, E.; Stigliano, E.; Bernardini, C.; Michetti, F.; Salgarello, M.; et al. Qualitative and quantitative differences of adipose-derived stromal cells from superficial and deep subcutaneous lipoaspirates: A matter of fat. Cytotherapy 2015, 17, 1076–1089. [Google Scholar] [CrossRef]
- Bajek, A.; Gurtowska, N.; Olkowska, J.; Kazmierski, L.; Maj, M.; Drewa, T. Adipose-Derived Stem Cells as a Tool in Cell-Based Therapies. Arch. Immunol. Ther. Exp. Warsz. 2016, 64, 443–454. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Zannettino, A.; Paton, S.; Arthur, A.; Khor, F.; Itescu, S.; Gimble, J.; Gronthos, S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J. Cell. Physiol. 2007, 214, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Nakajima, H.; Uchida, K.; Takeura, N.; Honjoh, K.; Watanabe, S.; Kitade, M.; Kokubo, Y.; Johnson, W.E.B.; Matsumine, A. Comparison of Mesenchymal Stromal Cells Isolated from Murine Adipose Tissue and Bone Marrow in the Treatment of Spinal Cord Injury. Cell Transplant. 2018, 27, 1126–1139. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Y.; Zhang, H.; Min, S.; Yu, B.; He, B.; Jin, A. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy 2013, 15, 434–448. [Google Scholar] [CrossRef]
- Ménard, C.; Dulong, J.; Roulois, D.; Hébraud, B.; Verdière, L.; Pangault, C.; Sibut, V.; Bezier, I.; Bescher, N.; Monvoisin, C.; et al. Integrated transcriptomic, phenotypic, and functional study reveals tissue-specific immune properties of mesenchymal stromal cells. Stem Cells 2020, 38, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S.; Pontecorvi, P.; Anastasiadou, E.; Napoli, C.; Marchese, C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front. Cell Dev. Biol. 2020, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef]
- Pérez, L.M.; de Lucas, B.; Gálvez, B.G. Unhealthy Stem Cells: When Health Conditions Upset Stem Cell Properties. Cell Physiol. Biochem. 2018, 46, 1999–2016. [Google Scholar] [CrossRef]
- Girard, A.-C.; Atlan, M.; Bencharif, K.; Gunasekaran, M.K.; Delarue, P.; Hulard, O.; Lefebvre-D’Hellencourt, C.; Roche, R.; Hoareau, L.; Festy, F. New Insights into Lidocaine and Adrenaline Effects on Human Adipose Stem Cells. Aesthetic Plast. Surg. 2012, 37, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Keck, M.; Zeyda, M.; Gollinger, K.; Burjak, S.; Kamolz, L.-P.; Frey, M.; Stulnig, T.M. Local Anesthetics Have a Major Impact on Viability of Preadipocytes and Their Differentiation into Adipocytes. Plast. Reconstr. Surg. 2010, 126, 1500–1505. [Google Scholar] [CrossRef]
- Breu, A.; Eckl, S.; Zink, W.; Kujat, R.; Angele, P. Cytotoxicity of Local Anesthetics on Human Mesenchymal Stem Cells In Vitro. Arthroscopy 2013, 29, 1676–1684. [Google Scholar] [CrossRef]
- Kubrova, E.; Su, M.; Galeano-Garces, C.; Galvan, M.L.; Jerez, S.; Dietz, A.B.; Smith, J.; Qu, W.; Van Wijnen, A.J. Differences in Cytotoxicity of Lidocaine, Ropivacaine, and Bupivacaine on the Viability and Metabolic Activity of Human Adipose-Derived Mesenchymal Stem Cells. Am. J. Phys. Med. Rehabil. 2021, 100, 82–91. [Google Scholar] [CrossRef]
- Caggiati, A.; Germani, A.; Di Carlo, A.; Borsellino, G.; Capogrossi, M.C.; Picozza, M. Naturally Adipose Stromal Cell-Enriched Fat Graft: Comparative Polychromatic Flow Cytometry Study of Fat Harvested by Barbed or Blunt Multihole Cannula. Aesthetic Surg. J. 2017, 37, 591–602. [Google Scholar] [CrossRef][Green Version]
- Leto Barone, A.A.; Khalifian, S.; Lee, W.P.A.; Brandacher, G. Immunomodulatory Effects of Adipose-Derived Stem Cells: Fact or Fiction? BioMed Res. Int. 2013, 2013, 383685. [Google Scholar] [CrossRef] [PubMed]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of Multi-Lineage Cells from Human Adipose Tissue and Bone Marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef]
- Izadpanah, R.; Trygg, C.; Patel, B.; Kriedt, C.; Dufour, J.; Gimble, J.M.; Bunnell, B.A. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J. Cell. Biochem. 2006, 99, 1285–1297. [Google Scholar] [CrossRef]
- Neri, S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci. 2019, 20, 2406. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. STEM Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Bravo, S.B.; Pérez-Sotelo, D.; Alonso, J.; Castro, A.I.; Baamonde, I.; Baltar, J.; Casanueva, F.F.; Pardo, M. CILAIR-Based Secretome Analysis of Obese Visceral and Subcutaneous Adipose Tissues Reveals Distinctive ECM Remodeling and Inflammation Mediators. Sci. Rep. 2015, 5, 12214. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, M.H.; Alavinia, S.M.; Craven, B.C. Management of obesity after spinal cord injury: A systematic review. J. Spinal Cord Med. 2017, 40, 783–794. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.E.; Weiss, M.L.; Mitchell, B.M.; Martin, P.; Davis, D.; Morales, L.; Helwig, B.; Beerenstrauch, M.; Abou-Easa, K.; Hildreth, T.; et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells 2003, 21, 50–60. [Google Scholar] [CrossRef] [PubMed]
- McElreavey, K.D.; Irvine, A.I.; Ennis, K.T.; McLean, W.H. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem. Soc. Trans. 1991, 19, 29S. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T.; He, H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells 2014, 6, 195–202. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, R.; Jiang, J.; Peng, J.; Yang, C.; Zhang, W.; Wang, S.; Song, J. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res. Ther. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Troyer, D.L.; Weiss, M.L. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells 2008, 26, 591–599. [Google Scholar] [CrossRef]
- Carlin, R.; Davis, D.; Weiss, M.; Schultz, B.; Troyer, D. Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod. Biol. Endocrinol. 2006, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.-Y.; Chak, L.-L.; Biswas, A.; Tan, J.-H.; Gauthaman, K.; Chan, W.-K.; Bongso, A. Human Wharton’s Jelly Stem Cells Have Unique Transcriptome Profiles Compared to Human Embryonic Stem Cells and Other Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2011, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.; Hatlapatka, T.; Marten, D.; Lavrentieva, A.; Majore, I.; Hass, R.; Kasper, C. Mesenchymal stromal cells derived from human umbilical cord tissues: Primitive cells with potential for clinical and tissue engineering applications. Adv. Biochem. Eng. Biotechnol. 2010, 123, 29–54. [Google Scholar] [PubMed]
- Dulugiac, M.; Moldovan, L.; Zărnescu, O. Comparative studies of mesenchymal stem cells derived from different cord tissue compartments – The influence of cryopreservation and growth media. Placenta 2015, 36, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.-Y.; Subramanian, A.; Biswas, A.; Gauthaman, K.; Srikanth, P.; Hande, M.P.; Bongso, A. Derivation efficiency, cell proliferation, freeze–thaw survival, stem-cell properties and differentiation of human Wharton’s jelly stem cells. Reprod. Biomed. Online 2010, 21, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. STEM Cells Transl. Med. 2019, 8, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.; Cooper, K.; Viswanathan, C. Comparison of proliferative and multilineage differentiation potentials of cord matrix, cord blood, and bone marrow mesenchymal stem cells. Asian J. Transfus. Sci. 2010, 4, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.S.; Bhonde, R.R. Islet neogenesis from the constitutively nestin expressing human umbilical cord matrix derived mesenchymal stem cells. Islets 2010, 2, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, F.; Hu, X.; Tang, Z.; Fu, Z.; Liang, X.; Zeng, G.; Zeng, W.; Liao, Y.; Ren, Y.; et al. Recovery and maintenance of NESTIN expression in umbilical cord-MSC using a novel culture medium. AMB Express 2020, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.L.; Medicetty, S.; Bledsoe, A.R.; Rachakatla, R.S.; Choi, M.; Merchav, S.; Luo, Y.; Rao, M.S.; Velagaleti, G.; Troyer, D. Human umbilical cord matrix stem cells: Preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 2006, 24, 781–792. [Google Scholar] [CrossRef]
- Skiles, M.L.; Marzan, A.J.; Brown, K.S.; Shamonki, J.M. Comparison of umbilical cord tissue-derived mesenchymal stromal cells isolated from cryopreserved material and extracted by explantation and digestion methods utilizing a split manufacturing model. Cytotherapy 2020, 22, 581–591. [Google Scholar] [CrossRef]
- Hua, J.; Gong, J.; Meng, H.; Xu, B.; Yao, L.; Qian, M.; He, Z.; Zou, S.; Zhou, B.; Song, Z. Comparison of different methods for the isolation of mesenchymal stem cells from umbilical cord matrix: Proliferation and multilineage differentiation as compared to mesenchymal stem cells from umbilical cord blood and bone marrow. Cell Biol. Int. 2013, 38, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.L.; Troyer, D.L. Stem cells in the umbilical cord. Stem Cell Rev. 2006, 2, 155–162. [Google Scholar] [CrossRef]
- Tabakow, P.; Jarmundowicz, W.; Czapiga, B.; Fortuna, W.; Miȩdzybrodzki, R.; Czyż, M.; Huber, J.; Szarek, D.; Okurowski, S.; Szewczyk, P.; et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 2013, 22, 1591–1612. [Google Scholar] [CrossRef]
- Tabakow, P.; Raisman, G.; Fortuna, W.; Czyz, M.; Huber, J.; Li, D.; Szewczyk, P.; Okurowski, S.; Miedzybrodzki, R.; Czapiga, B.; et al. Functional Regeneration of Supraspinal Connections in a Patient with Transected Spinal Cord following Transplantation of Bulbar Olfactory Ensheathing Cells with Peripheral Nerve Bridging. Cell Transplant. 2014, 23, 1631–1655. [Google Scholar] [CrossRef]
- Lima, C.; Escada, P.; Pratas-Vital, J.; Branco, C.; Arcangeli, C.A.; Lazzeri, G.; Maia, C.A.S.; Capucho, C.; Hasse-Ferreira, A.; Peduzzi, J.D. Olfactory Mucosal Autografts and Rehabilitation for Chronic Traumatic Spinal Cord Injury. Neurorehabilit. Neural Repair 2009, 24, 10–22. [Google Scholar] [CrossRef]
- Lima, C.; Pratas-Vital, J.; Escada, P.; Hasse-Ferreira, A.; Capucho, C.; Peduzzi, J.D. Olfactory Mucosa Autografts in Human Spinal Cord Injury: A Pilot Clinical Study. J. Spinal Cord Med. 2006, 29, 191–203; discussion 204–206. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, H.S.; Lima, C.; Sachdeva, S.; Mittal, A.; Nigam, V.; Chaturvedi, D.; Arora, M.; Aggarwal, A.; Kapur, R.; Khan, T.A. Autologous olfactory [corrected] mucosal transplant in chronic spinal cord injury: An Indian Pilot Study. Spinal Cord 2009, 47, 887–895. [Google Scholar] [CrossRef]
- Lindsay, S.L.; McCanney, G.A.; Willison, A.G.; Barnett, S.C. Multi-target approaches to CNS repair: Olfactory mucosa-derived cells and heparan sulfates. Nat. Rev. Neurol. 2020, 16, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Tomé, M.; Lindsay, S.L.; Riddell, J.S.; Barnett, S.C. Identification of Nonepithelial Multipotent Cells in the Embryonic Olfactory Mucosa. STEM Cells 2009, 27, 2196–2208. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, S.L.; Johnstone, S.A.; Mountford, J.C.; Sheikh, S.; Allan, D.B.; Clark, L.; Barnett, S.C. Human mesenchymal stem cells isolated from olfactory biopsies but not bone enhance CNS myelination in vitro. Glia 2013, 61, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, S.L.; Johnstone, S.A.; McGrath, M.A.; Mallinson, D.; Barnett, S.C. Comparative miRNA-Based Fingerprinting Reveals Biological Differences in Human Olfactory Mucosa- and Bone-Marrow-Derived Mesenchymal Stromal Cells. Stem Cell Rep. 2016, 6, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Delorme, B.; Nivet, E.; Gaillard, J.; Häupl, T.; Ringe, J.; Devèze, A.; Magnan, J.; Sohier, J.; Khrestchatisky, M.; Roman, F.S.; et al. The Human Nose Harbors a Niche of Olfactory Ectomesenchymal Stem Cells Displaying Neurogenic and Osteogenic Properties. Stem Cells Dev. 2010, 19, 853–866. [Google Scholar] [CrossRef]
- Lindsay, S.L.; Riddell, J.S.; Barnett, S.C. Olfactory mucosa for transplant-mediated repair: A complex tissue for a complex injury? Glia 2010, 58, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Graziadei, G.A.M.; Graziadei, P.P.C. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J. Neurocytol. 1979, 8, 197–213. [Google Scholar] [CrossRef]
- Perry, C.; Mackay-Sim, A.; Féron, F.; McGrath, J. Olfactory Neural Cells: An Untapped Diagnostic and Therapeutic Resource. The 2000 Ogura Lecture. Laryngoscope 2002, 112, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Escada, P.A.; Lima, C.; Da Silva, J.M. The human olfactory mucosa. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 1675–1680. [Google Scholar] [CrossRef]
- LaMantia, A.-S.; Bhasin, N.; Rhodes, K.; Heemskerk, J. Mesenchymal/epithelial induction mediates olfactory pathway formation. Neuron 2000, 28, 411–425. [Google Scholar] [CrossRef]
- Girard, S.D.; Jacquet, M.; Baranger, K.; Migliorati, M.; Escoffier, G.; Bernard, A.; Khrestchatisky, M.; Feron, F.; Rivera, S.; Roman, F.S.; et al. Onset of hippocampus-dependent memory impairments in 5XFAD transgenic mouse model of Alzheimer’s disease. Hippocampus 2014, 24, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Di Trapani, M.; Bassi, G.; Ricciardi, M.; Fontana, E.; Bifari, F.; Pacelli, L.; Giacomello, L.; Pozzobon, M.; Féron, F.; De Coppi, P.; et al. Comparative Study of Immune Regulatory Properties of Stem Cells Derived from Different Tissues. Stem Cells Dev. 2013, 22, 2990–3002. [Google Scholar] [CrossRef]
- Antonevich, N.; Hancharou, A.; Buschik, O.; Rydna, A.; Chekan, V.; Strinkevich, E.; DuBuske, L. Human Olfactory Mucosa-Derived Mesenchymal Stem Cells Suppress Cytotoxic Functions of CD8+ T-Lymphocytes and Natural Killer Cells. J. Allergy Clin. Immunol. 2018, 141. [Google Scholar] [CrossRef]
- Lindsay, S.L.; Toft, A.; Griffin, J.; Emraja, A.M.M.; Barnett, S.C.; Riddell, J.S. Human olfactory mesenchymal stromal cell transplants promote remyelination and earlier improvement in gait co-ordination after spinal cord injury. Glia 2017, 65, 639–656. [Google Scholar] [CrossRef]
- Johnstone, S.A.; Liley, M.; Dalby, M.J.; Barnett, S.C. Comparison of human olfactory and skeletal MSCs using osteogenic nanotopography to demonstrate bone-specific bioactivity of the surfaces. Acta Biomater. 2015, 13, 266–276. [Google Scholar] [CrossRef]
- Suzuki, J.; Yoshizaki, K.; Kobayashi, T.; Osumi, N. Neural crest-derived horizontal basal cells as tissue stem cells in the adult olfactory epithelium. Neurosci. Res. 2013, 75, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Osumi, N. Neural Crest and Placode Contributions to Olfactory Development. Current Topics in Developmental Biology 2015, 111, 351–374. [Google Scholar] [CrossRef] [PubMed]
- Barraud, P.; Seferiadis, A.A.; Tyson, L.D.; Zwart, M.F.; Szabo-Rogers, H.L.; Ruhrberg, C.; Liu, K.J.; Baker, C.V.H. Neural crest origin of olfactory ensheathing glia. Proc. Natl. Acad. Sci. USA 2010, 107, 21040–21045. [Google Scholar] [CrossRef]
- Goldstein, B.J.; Hare, J.M.; Lieberman, S.; Casiano, R. Adult human nasal mesenchymal stem cells have an unexpected broad anatomic distribution. Int. Forum Allergy Rhinol. 2013, 3, 550–555. [Google Scholar] [CrossRef]
- Féron, F.; Perry, C.; McGrath, J.J.; Mackay-Sim, A. New Techniques for Biopsy and Culture of Human Olfactory Epithelial Neurons. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Lanza, D.C.; Deems, D.A.; Doty, R.L.; Moran, D.; Crawford, D.; Rowley, J.C., 3rd; Sajjadian, A.; Kennedy, D.W. The effect of human olfactory biopsy on olfaction: A preliminary report. Laryngoscope 1994, 104, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.; Poirrier, A.; Lund, V.; Choi, D. Safety of human olfactory mucosal biopsy for the purpose of olfactory ensheathing cell harvest and nerve repair: A prospective controlled study in patients undergoing endoscopic sinus surgery. Rhinol. J. 2016, 54, 183–191. [Google Scholar] [CrossRef]
- Gross, E.A.; Swenberg, J.A.; Fields, S.; Popp, J.A. Comparative morphometry of the nasal cavity in rats and mice. J. Anat. 1982, 135, 83–88. [Google Scholar] [PubMed]
- Sveiven, S.N.; Nordgren, T.M. Lung-resident mesenchymal stromal cells are tissue-specific regulators of lung homeostasis. Am. J. Physiol. Cell. Mol. Physiol. 2020, 319, L197–L210. [Google Scholar] [CrossRef]
- Dlouhy, B.J.; Awe, O.; Rao, R.C.; Kirby, P.A.; Hitchon, P.W. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: Case report. J. Neurosurg. Spine 2014, 21, 618–622. [Google Scholar] [CrossRef]
- Woodworth, C.F.; Jenkins, G.; Barron, J.; Hache, N. Intramedullary cervical spinal mass after stem cell transplantation using an olfactory mucosal cell autograft. Can. Med Assoc. J. 2019, 191, E761–E764. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, S.; Barnett, S. Culture of Rat Olfactory Ensheathing Cells Using EasySep® Magnetic Nanoparticle Separation. Bio-Protocol 2013, 3. [Google Scholar] [CrossRef]
- Yao, R.; Murtaza, M.; Velasquez, J.T.; Todorovic, M.; Rayfield, A.; Ekberg, J.; Barton, M.; St John, J. Olfactory Ensheathing Cells for Spinal Cord Injury: Sniffing Out the Issues. Cell Transplant. 2018, 27, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Thirlwell, K.L.; Colligan, D.; Mountford, J.C.; Samuel, K.; Bailey, L.; Cuesta-Gomez, N.; Hewit, K.D.; Kelly, C.J.; West, C.C.; McGowan, N.W.; et al. Pancreas-derived mesenchymal stromal cells share immune response-modulating and angiogenic potential with bone marrow mesenchymal stromal cells and can be grown to therapeutic scale under Good Manufacturing Practice conditions. Cytotherapy 2020, 22, 762–771. [Google Scholar] [CrossRef]
- Moran, D.T.; Rowley, J.C., 3rd; Jafek, B.W.; Lovell, M.A. The fine structure of the olfactory mucosa in man. J. Neurocytol. 1982, 11, 721–746. [Google Scholar] [CrossRef] [PubMed]
- Moran, D.T.; Jafek, B.W.; Eller, P.M.; Rowley, J.C., 3rd. Ultrastructural histopathology of human olfactory dysfunction. Microsc. Res. Tech. 1992, 23, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.T.; Guo, Z.; Lu, C.; Klueber, K.M.; Khalyfa, A.; Cooper, N.G.; Roisen, F.J. Human adult olfactory neuroepithelial derived progenitors retain telomerase activity and lack apoptotic activity. Brain Res. 2005, 1045, 45–56. [Google Scholar] [CrossRef] [PubMed]
| BM-MSC | AD-MSC | UC-MSC | OM-MSC | |
|---|---|---|---|---|
| Niche | Haematopoietic | Angiogenic | Haematopoietic | Neurogenic |
| Tissue Availability | ++ | +++ | +++ | +++ |
| Use in SCI clinical trials | +++ | ++ | +++ | + |
| Procedure | Invasive | Minimally Invasive | Not Invasive | Minimally Invasive |
| Proliferative Capacity | + | ++ | +++ | +++ |
| Cell Yield | + | +++ | ++ | +++ |
| Autologous use | +++ | +++ | + | +++ |
| Allogenic use | +++ | +++ | +++ | + |
| Nestin expression | ++ | + | ++ | +++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindsay, S.L.; Barnett, S.C. Therapeutic Potential of Niche-Specific Mesenchymal Stromal Cells for Spinal Cord Injury Repair. Cells 2021, 10, 901. https://doi.org/10.3390/cells10040901
Lindsay SL, Barnett SC. Therapeutic Potential of Niche-Specific Mesenchymal Stromal Cells for Spinal Cord Injury Repair. Cells. 2021; 10(4):901. https://doi.org/10.3390/cells10040901
Chicago/Turabian StyleLindsay, Susan L., and Susan C. Barnett. 2021. "Therapeutic Potential of Niche-Specific Mesenchymal Stromal Cells for Spinal Cord Injury Repair" Cells 10, no. 4: 901. https://doi.org/10.3390/cells10040901
APA StyleLindsay, S. L., & Barnett, S. C. (2021). Therapeutic Potential of Niche-Specific Mesenchymal Stromal Cells for Spinal Cord Injury Repair. Cells, 10(4), 901. https://doi.org/10.3390/cells10040901







