Elevation of Pro-Inflammatory Cytokine Levels Following Intra-Articular Fractures—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
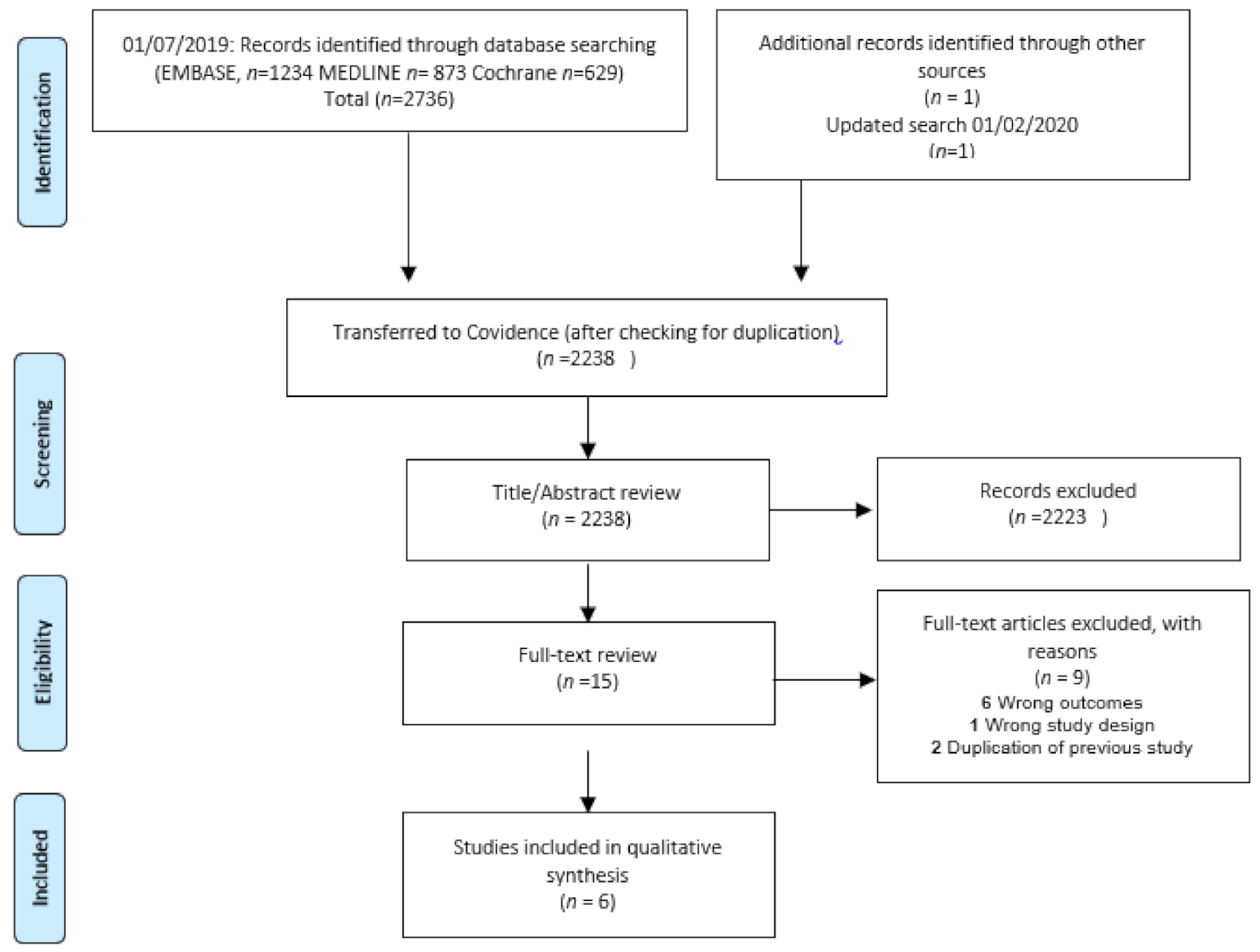
2.3. Study Selection and Data Collection Process
2.4. Risk of Bias in Individual Studies
2.5. Quality Assessment
3. Results
4. Discussion
Suggestions for Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emery, C.; Roos, E.; Verhagen, E.; Finch, C.; Bennell, K.; Story, B.; Spindler, K.; Kemp, J.; Lohmander, L. OARSI Clinical Trials Recommendations: Design and conduct of clinical trials for primary prevention of osteoarthritis by joint injury prevention in sport and recreation. Osteoarthr. Cartil. 2015, 23, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Schmal, H.; Salzmann, G.M.; Niemeyer, P.; Langenmair, E.; Guo, R.; Schneider, C.; Habel, M.; Riedemann, N. Early Intra-Articular Complement Activation in Ankle Fractures. BioMed Res. Int. 2014, 2014, 426893. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, C.L.; Salamon, M.L.; Blanchard, G.M.; Huff, T.; Hayes, A.; Buckwalter, J.A.; Amendola, A. Epidemiology of ankle arthritis: Report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop. J. 2005, 25, 44–46. [Google Scholar] [PubMed]
- Valderrabano, V.; Horisberger, M.; Russell, I.; Dougall, H.; Hintermann, B. Etiology of Ankle Osteoarthritis. Clin. Orthop. Relat. Res. 2009, 467, 1800–1806. [Google Scholar] [CrossRef]
- Horisberger, M.; Valderrabano, V.; Hintermann, B. Posttraumatic Ankle Osteoarthritis After Ankle-Related Fractures. J. Orthop. Trauma 2009, 23, 60–67. [Google Scholar] [CrossRef]
- Giannoudis, P.; Tzioupis, C.; Papathanassopoulos, A.; Obakponovwe, O.; Roberts, C. Articular step-off and risk of post-traumatic osteoarthritis. Evidence today. Injury 2010, 41, 986–995. [Google Scholar] [CrossRef]
- Marsh, J.L.; Buckwalter, J.; Gelberman, R.; Dirschl, D.; Olson, S.; Brown, T.; Llinias, A. Articular fractures: Does an anatomic reduction really change the result? J. Bone Jt. Surg. Am. 2002, 84, 1259–1271. [Google Scholar] [CrossRef]
- Donken, C.C.; Verhofstad, M.H.; Edwards, M.J.; van Laarhoven, C.J. Twenty-one-year follow-up of supination-external rotation type II-IV (OTA type B) ankle fractures: A retrospective cohort study. J. Orthop. Trauma 2012, 26, e108–e114. [Google Scholar] [CrossRef]
- Lübbeke, A.; Salvo, D.; Stern, R.; Hoffmeyer, P.; Holzer, N.; Assal, M. Risk factors for post-traumatic osteoarthritis of the ankle: An eighteen year follow-up study. Int. Orthop. 2012, 36, 1403–1410. [Google Scholar] [CrossRef]
- Dirschl, D.R.; Marsh, L.J.; Buckwalter, J.A.; Gelberman, R.; Olson, S.A.; Brown, T.D.; Llinias, A. Articular Fractures. J. Am. Acad. Orthop. Surg. 2004, 12, 416–423. [Google Scholar] [CrossRef]
- Adams, S.B.; Setton, L.A.; Bell, R.D.; Easley, M.E.; Huebner, J.L.; Stabler, T.; Kraus, V.B.; Leimer, E.M.; Olson, S.A.; Nettles, D.L. Inflammatory Cytokines and Matrix Metalloproteinases in the Synovial Fluid After Intra-articular Ankle Fracture. Foot Ankle Int. 2015, 36, 1264–1271. [Google Scholar] [CrossRef]
- Haller, J.M.; McFadden, M.; Kubiak, E.N.; Higgins, T.F. Inflammatory Cytokine Response Following Acute Tibial Plateau Fracture. J. Bone Jt. Surg. Am. Vol. 2015, 97, 478–483. [Google Scholar] [CrossRef]
- Lewis, J.; Hembree, W.; Furman, B.; Tippets, L.; Cattel, D.; Huebner, J.; Little, D.; DeFrate, L.; Kraus, V.; Guilak, F.; et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthr. Cartil. 2011, 19, 864–873. [Google Scholar] [CrossRef]
- Swärd, P.; Struglics, A.; Englund, M.; Roos, H.P.; Frobell, R.B. Soft Tissue Knee Injury With Concomitant Osteochondral Fracture Is Associated With Higher Degree of Acute Joint Inflammation. Am. J. Sports Med. 2014, 42, 1096–1102. [Google Scholar] [CrossRef]
- Kaplan, D.J.; Cuellar, V.G.; Jazrawi, L.M.; Strauss, E.J. Biomarker Changes in Anterior Cruciate Ligament-Deficient Knees Compared With Healthy Controls. Arthroscopy 2017, 33, 1053–1061. [Google Scholar] [CrossRef]
- Irie, K.; Uchiyama, E.; Iwaso, H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee 2003, 10, 93–96. [Google Scholar] [CrossRef]
- Haslauer, C.M.; Proffen, B.L.; Johnson, V.M.; Murray, M.M. Expression of modulators of extracellular matrix structure after anterior cruciate ligament injury. Wound Repair Regen. 2014, 22, 103–110. [Google Scholar] [CrossRef]
- Siqueira, M.B.; Frangiamore, S.; Klika, A.K.; Gajewski, N.; Barsoum, W.K.; Higuera, C.A. Comparison of synovial fluid cytokine levels between traumatic knee injury and end-stage osteoarthritis. J. Knee Surg. 2017, 30, 128–133. [Google Scholar] [CrossRef]
- Anderson, D.D.; Chubinskaya, S.; Guilak, F.; Martin, J.A.; Oegema, T.R.; Olson, S.A.; Buckwalter, J.A. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. J. Orthop. Res. 2011, 29, 802–809. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Wahl, E.P.; Lampley, A.J.; Chen, A.; Adams, S.B.; Nettles, D.L.; Richard, M.J. Inflammatory cytokines and matrix metalloproteinases in the synovial fluid after intra-articular elbow fracture. J. Shoulder Elb. Surg. 2020, 29, 736–742. [Google Scholar] [CrossRef]
- Furman, B.D.; Kimmerling, K.A.; Zura, R.D.; Reilly, R.M.; Zlowodzki, M.P.; Huebner, J.L.; Kraus, V.B.; Guilak, F.; Olson, S.A. Articular ankle fracture results in increased synovitis, synovial macrophage infiltration, and synovial fluid concentrations of inflammatory cytokines and chemokines. Arthritis Rheumatol. 2015, 67, 1234–1239. [Google Scholar] [CrossRef]
- Godoy-Santos, A.L.; Ranzoni, L.; Teodoro, W.R.; Capelozzi, V.; Giglio, P.; Fernandes, T.D.; Rammelt, S. Increased cytokine levels and histological changes in cartilage, synovial cells and synovial fluid after malleolar fractures. Injury 2017, 48, S27–S33. [Google Scholar] [CrossRef]
- Chubinskaya, S.; Wimmer, M.A. Key Pathways to Prevent Posttraumatic Arthritis for Future Molecule-Based Therapy. Cartilage 2013, 4, 13S–21S. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 1–19. [Google Scholar] [CrossRef]
- Bigoni, M.; Sacerdote, P.; Turati, M.; Franchi, S.; Gandolla, M.; Gaddi, D.; Torsello, A. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J. Orthop. Res. 2013, 31, 315–321. [Google Scholar] [CrossRef]
- Adams, S.B.; Leimer, E.M.; Setton, L.A.; Bell, R.D.; Easley, M.E.; Huebner, J.L.; Stabler, T.V.; Kraus, V.B.; Olson, S.A.; Nettles, D.L. Inflammatory Microenvironment Persists After Bone Healing in Intra-articular Ankle Fractures. Foot Ankle Int. 2017, 38, 479–484. [Google Scholar] [CrossRef]
- Langenmair, E.R.; Kubosch, E.J.; Salzmann, G.M.; Beck, S.; Schmal, H. Clinical Trial andIn VitroStudy for the Role of Cartilage and Synovia in Acute Articular Infection. Mediat. Inflamm. 2015, 2015, 430324. [Google Scholar] [CrossRef]
- Schmal, H.; Marintschev, I.; Salzmann, G.M. Current status of anti-inflammatory therapy for posttraumatic osteoarthritis. Acta Orthop. Belg. 2016, 82, 427–439. [Google Scholar]
- Wang, C.-W.; Gao, L.-H.; Jin, X.-Y.; Chen, P.-B.; Zhang, G.-M. Clinical study of sodium hyaluronate in supplementary treatment of comminuted fracture of ankle. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2002, 16, 21–22. [Google Scholar]
- Kraus, V.B.; Birmingham, J.; Stabler, T.V.; Feng, S.; Taylor, D.C.; Moorman, C.T., III; Toth, A.P. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: A randomized controlled pilot trial (NCT00332254). Osteoarthr. Cartil. 2012, 20, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Appleton, B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2009, 61, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.M.; Bliddal, H.; Blanco, F.J.; Schnitzer, T.J.; Peterfy, C.; Chen, S.; Levesque, M.C. A Phase II Trial of Lutikizumab, an Anti-Interleukin-1α/β Dual Variable Domain Immunoglobulin, in Knee Osteoarthritis Patients With Synovitis. Arthritis Rheumatol. 2019, 71, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
| Year/Author/Country | Study Design | Fracture Joint | Control Group | Time of Synovial Fluid Collection (Days after Injury) | Non-Significant Outcomes (Fracture vs. Control) | Significant Outcomes (Fracture vs. Control) | Newcastle-Ottawa Scale (NOS) |
|---|---|---|---|---|---|---|---|
| 2015/Adams/USA (11) | Cross-sectional | Ankle (n = 21) | Contralateral ankle (n = 21) | 8–40 days | IL-1α, IL-2, CTX-II | IFN-γ, IL-1β, IL-6, IL-8, IL-10, IL-12p70, MMP-1, MMP-3, MMP-9, TNF-α | Good study (8 stars) |
| 2015/Furman/USA (22) | Cross-sectional | Ankle (n = 6) | Knee OA (n = 6) | 5–21 days | None | IFN-γ, IL-1β, IL-6, IL-8, IL-10, IL-12p70, TNF-α | Unsatisfactory study (6 stars) |
| 2014/Schmal/DE (2) | Cross-sectional | Ankle (n = 8) | OCD grade 2 (n = 8) | 0–4 days | ACG, IL-1β | bFGF | Unsatisfactory study (4 stars) |
| 2015/Haller/USA (12) | Cross-sectional | Knee (n = 45) | Contralateral knee (n = 45) | 0–1 day | IL-1α, IL-4, IL-12p70, IL-13, TNF-α | IFN-y, IL-1β, IL-2, IL-6, IL-8, IL-10, IL-1RA | Very good study (9 stars) |
| 2017/Godoy-Santos/BRA | Cross-sectional | Ankle (n = 16) | Cadavers (n = 5) | 2–5 days | IFN-y, TGF-β1 | IL-2, IL-6, and IL-10 | Unsatisfactory study (4 stars) |
| 2019/Wahl/ USA (21) | Cross-sectional/Case-control | Elbow (n = 11) | Contralateral elbow (n = 11) | 0–17 days | CTX-II | IFN-y, IL-1 β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, MMP-1, MMP-3, MMP-9, TNF-α | Satisfactory study (7 stars) |
| Study | Pro-Inflammatory Cytokines | Anti-Inflammatory Cytokines | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year/Author/Country | Joint Involved | IL-1α (pg/mL) | IL-1β (pg/mL) | IL-2 (pg/mL) | IL-6 (ng/mL) | IL-8 (ng/mL) | IL-12p70 (pg/mL) | TNF-α (pg/mL) | IFN-Y (pg/mL) | MMP-1 (ng/mL) | MMP-3 (ng/mL) | MMP-9 (ng/mL) | IL-4 (pg/mL) | IL-10 (pg/mL) | IL-1RA (pg/mL) |
| 2015/Adams/USA [11] | Fractured ankle | 1.81 ± 2.97 (ns) | 2.12 ± 2.90 | 1.11 ± 2.26 (ns) | 1.83 ± 1.78 | 1.13 ± 1.07 | 1.06 ± 3.65 | 3.78 ± 3.56 | 0.44 ± 0.34 | 830.3 ± 247.2 | 1776.7 ± 629.1 | 38.8 ± 50.6 | 10.12 ± 12.95 | ||
| Contralateral ankle | 2.44 ± 4.24 | 0.29 ± 0.51 | 0.31 ± 0.67 | 0.12 ± 0.52 | 0.009 ± 0.016 | 0.18 ± 0.00 | 0.55 ± 1.04 | 0.37 ± 0.00 | 13.5 ± 37.5 | 59.7 ± 124.0 | 9.4 ± 20.9 | 0.33 ± 0.52 | |||
| 2015/Furman/USA [22] | Fractured ankle vs. knee OA | Significant increase | Significant increase | Significant increase | Significant increase | Significant increase | Significant increase | Significant increase | |||||||
| 2014/Schmal/DE [2] | Fractured ankle | 18.7 ± 24.8 (ns) | |||||||||||||
| OCD grade 2 ankle | 10.9 ± 3.70 | ||||||||||||||
| 2015/Haller/USA [12] | Fractured knee | Below LLOD | 1.9 (1.2–2.8) | 3.5 (2.1–5.3) | 3.1 (1.4–6.7) | 0.22 (0.14–0.36) | 5.4 (3.8–7.4) (ns) | 9.6 (7.5–12.4) (ns) | 3.3 (2.2–4.9) | Below LLOD | 88.6 (63.5–123.5) | 113.6 (68.7–187.5) | |||
| Contralateral knee | 0.8 (0.4–1.3) | 1.6 (0.8–2.6) | 0.006 (0.002–0.014) | 0.004 (0.002–0.006) | 7.8 (5.6–10.7) | 9.5 (7.3–12.1) | 1.7 (1–2.6) | 2.4 (1.5–3.8) | 12.6 (7.3–21.4) | ||||||
| 2017/Godoy-Santos/BRA [23] | Fractured ankle vs. cadavers | Significant increase | Significant increase | Non- significant increase | Significant increase | ||||||||||
| 2019/Wahl/USA [21] | Fractured elbow | 4.47 ± 3.81 | 1.36 ± 0.93 | 1.76 ± 0.37 | 1.38 ± 0.80 | 5.62 ± 2.91 | 2.58 ± 0.60 | 8.37 ± 5.32 | 240 ± 430 | 1300 ± 910 | 280 ± 250 | 45.6 ± 93.7 | 2.89 ± 1.84 | ||
| Contralateral elbow | 0.17 ± 0.15 | 0.42 ± 0.7 | 0.004 ± 0.007 | 0.012 ± 0.009 | 0.07 ± 0.08 | 0.80 ± 0.82 | 0.33 ± 0.39 | 2.0 ± 2.0 | 100.0 ± 70.0 | 30.0 ± 40.0 | 0.10 ± 0.23 | 0.15 ± 0.29 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, T.M.; Erichsen, J.L.; Kowal, J.M.; Overgaard, S.; Schmal, H. Elevation of Pro-Inflammatory Cytokine Levels Following Intra-Articular Fractures—A Systematic Review. Cells 2021, 10, 902. https://doi.org/10.3390/cells10040902
Pham TM, Erichsen JL, Kowal JM, Overgaard S, Schmal H. Elevation of Pro-Inflammatory Cytokine Levels Following Intra-Articular Fractures—A Systematic Review. Cells. 2021; 10(4):902. https://doi.org/10.3390/cells10040902
Chicago/Turabian StylePham, That Minh, Julie Ladeby Erichsen, Justyna Magdalena Kowal, Søren Overgaard, and Hagen Schmal. 2021. "Elevation of Pro-Inflammatory Cytokine Levels Following Intra-Articular Fractures—A Systematic Review" Cells 10, no. 4: 902. https://doi.org/10.3390/cells10040902
APA StylePham, T. M., Erichsen, J. L., Kowal, J. M., Overgaard, S., & Schmal, H. (2021). Elevation of Pro-Inflammatory Cytokine Levels Following Intra-Articular Fractures—A Systematic Review. Cells, 10(4), 902. https://doi.org/10.3390/cells10040902






