Misshapen Disruption Cooperates with RasV12 to Drive Tumorigenesis
Abstract
1. Introduction
2. Results
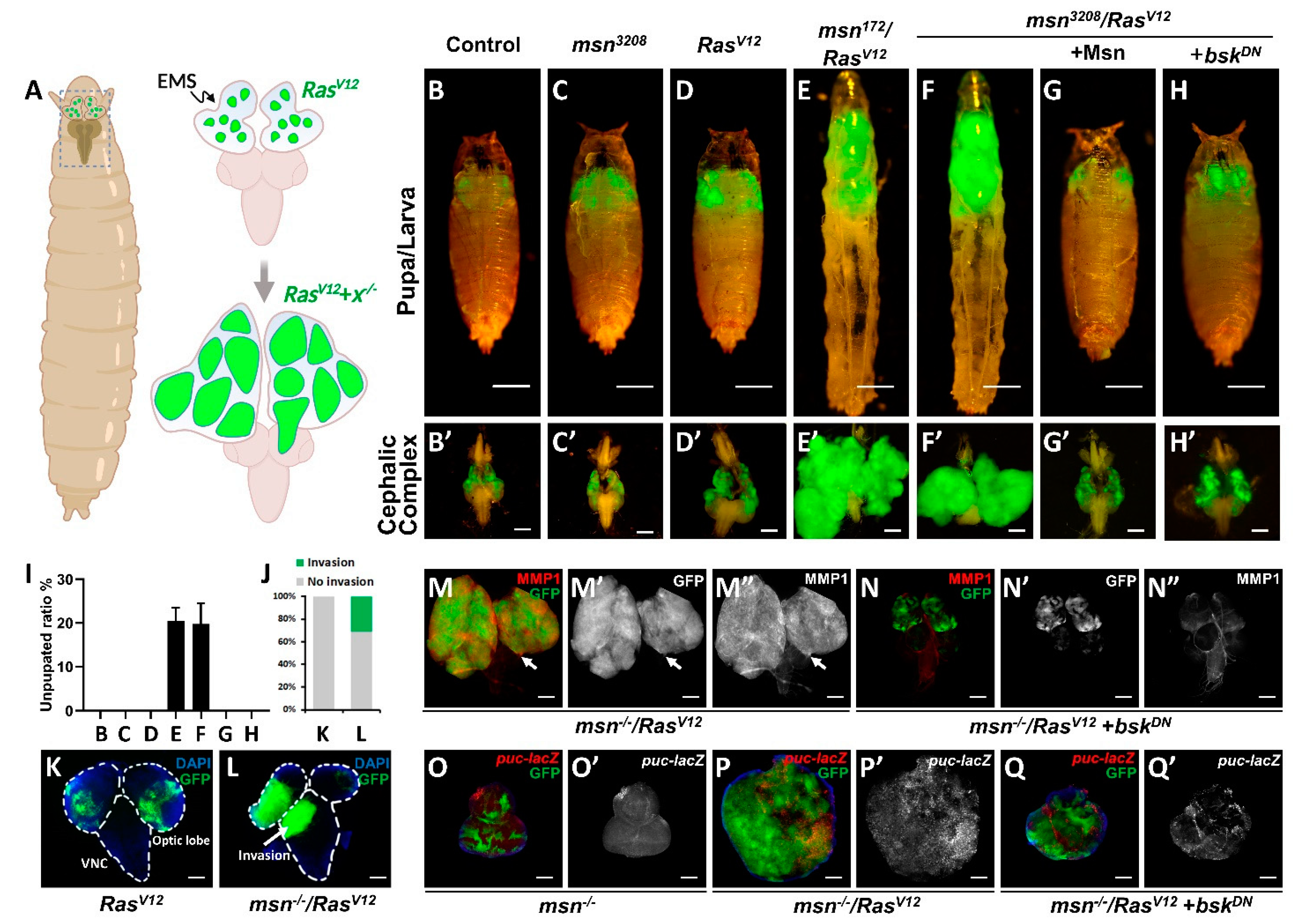
2.1. msn Mutant Synergizes with Oncogenic RasV12 to Drive Tumorigenesis and Invasion
2.2. msn−/− Collaborates with RasV12 to Activate JNK Signaling
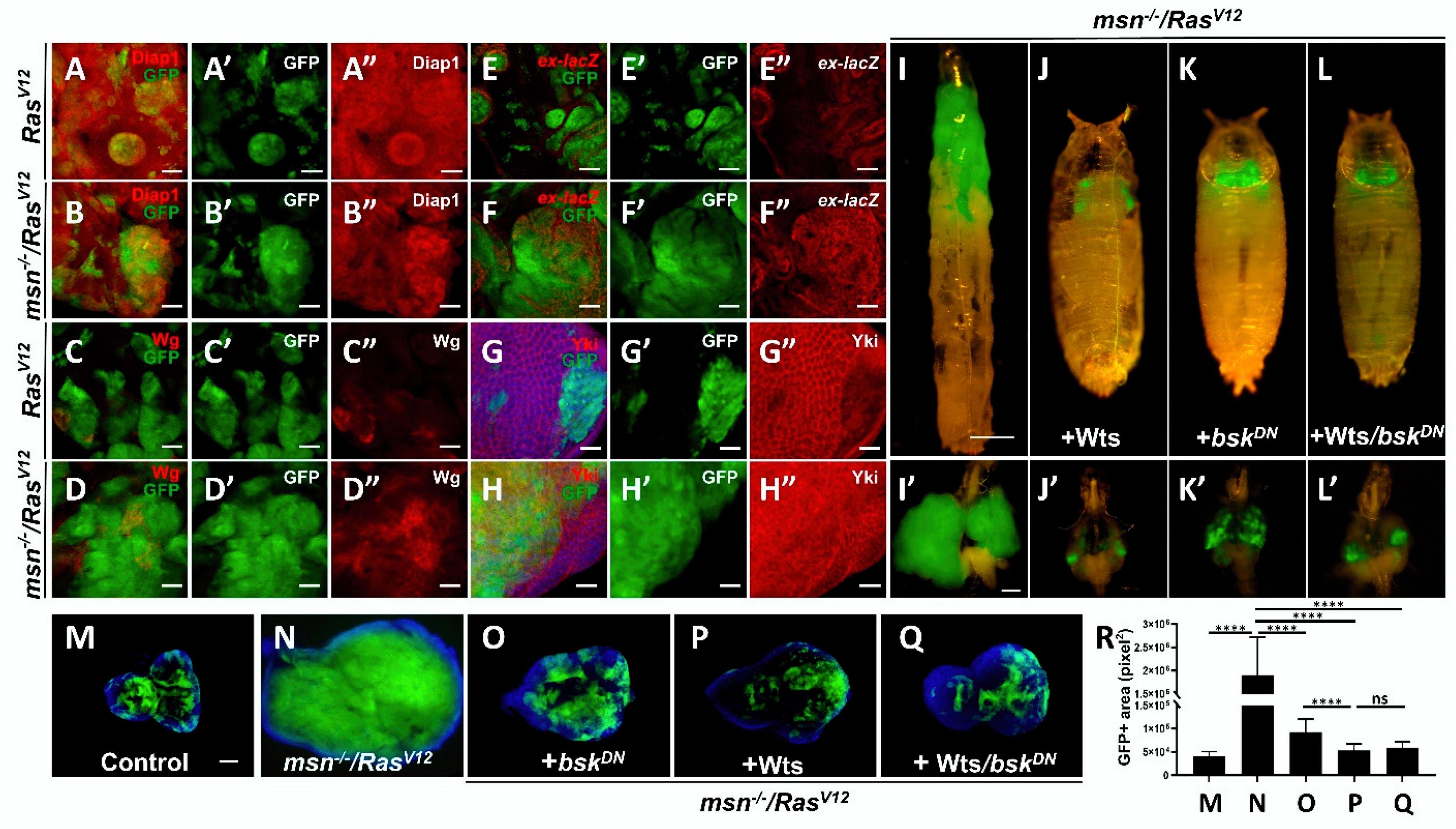
2.3. Loss of msn Synergizes with RasV12 to Inactivate Hippo Pathway
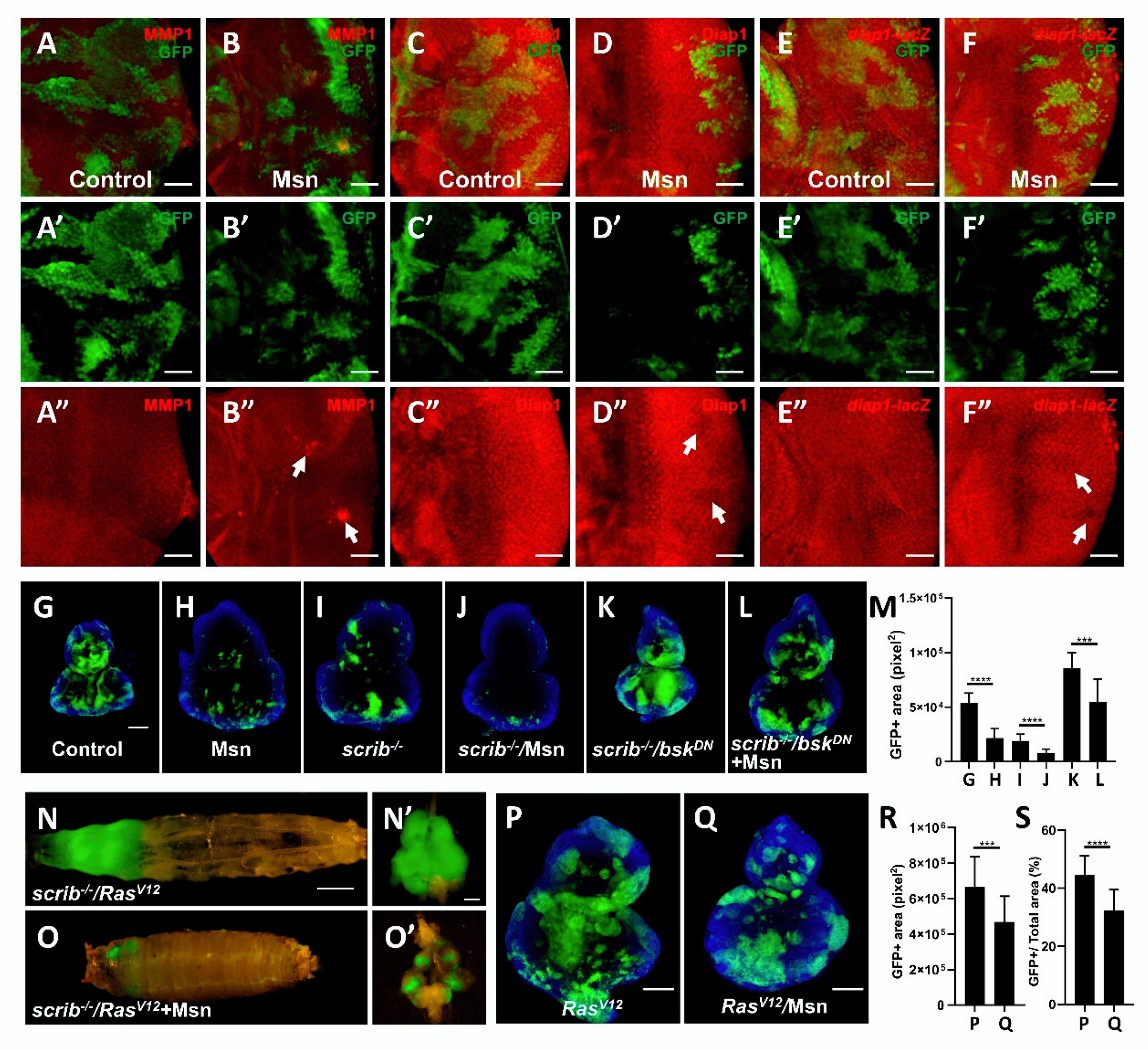
2.4. Msn Positively Regulates Hippo Signaling in Tumorigenesis
2.5. Msn Acts Downstream of Ft in Modulating Hippo Signaling
2.6. Msn Acts as a Hippo Target Gene in a Negative Feedback Manner
3. Discussion
4. Experimental Procedures
4.1. Drosophila Stocks and Genetics
4.2. EMS Mutagenesis and Genetic Screen
4.3. Immunostaining
4.4. Cell Culture
4.5. Immunoprecipitation and Western Blotting
4.6. Quantitative Real-Time PCR
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ryan, M.B.; Der, C.J.; Wang-Gillam, A.; Cox, A.D. Targeting RAS-mutant cancers: Is ERK the key? Trends Cancer 2015, 1, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.G.; Esposito, D.; Bagni, R.K.; McCormick, F. Dragging ras back in the ring. Cancer Cell 2014, 25, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.D.; Fesik, S.W.; Kimmelman, A.C.; Luo, J.; Der, C.J. Drugging the undruggable RAS: Mission possible? Nat. Rev. Drug Discov. 2014, 13, 828–851. [Google Scholar] [CrossRef] [PubMed]
- Samatar, A.A.; Poulikakos, P.I. Targeting RAS-ERK signalling in cancer: Promises and challenges. Nat. Rev. Drug Discov. 2014, 13, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, P.; Perrimon, N. Drosophila as a Model for Tumor-Induced Organ Wasting. Adv. Exp. Med. Biol. 2019, 1167, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, M.; Siow, C.; Igaki, T. Drosophila As a Cancer Model. Adv. Exp. Med. Biol. 2018, 1076, 173–194. [Google Scholar] [CrossRef]
- Mirzoyan, Z.; Sollazzo, M.; Allocca, M.; Valenza, A.M.; Grifoni, D.; Bellosta, P. Drosophila melanogaster: A Model Organism to Study Cancer. Front. Genet. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H.E.; Cordero, J.B.; Grifoni, D. Basic and Translational Models of Cooperative Oncogenesis. Int. J. Mol. Sci. 2020, 21, 5919. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Pareja, J.C.; Xu, T. Dissecting social cell biology and tumors using Drosophila genetics. Annu. Rev. Genet. 2013, 47, 51–74. [Google Scholar] [CrossRef]
- Gonzalez, C. Drosophila melanogaster: A model and a tool to investigate malignancy and identify new therapeutics. Nat. Rev. Cancer 2013, 13, 172–183. [Google Scholar] [CrossRef]
- Figueroa-Clarevega, A.; Bilder, D. Malignant Drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev. Cell 2015, 33, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Pagliarini, R.A.; Xu, T. A genetic screen in Drosophila for metastatic behavior. Science 2003, 302, 1227–1231. [Google Scholar] [CrossRef]
- Brumby, A.M.; Richardson, H.E. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003, 22, 5769–5779. [Google Scholar] [CrossRef] [PubMed]
- Igaki, T.; Pagliarini, R.A.; Xu, T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 2006, 16, 1139–1146. [Google Scholar] [CrossRef]
- Ma, X.; Li, W.; Yu, H.; Yang, Y.; Li, M.; Xue, L.; Xu, T. Bendless modulates JNK-mediated cell death and migration in Drosophila. Cell Death Differ. 2014, 21, 407–415. [Google Scholar] [CrossRef]
- Ma, X.; Lu, J.Y.; Dong, Y.; Li, D.; Malagon, J.N.; Xu, T. PP6 Disruption Synergizes with Oncogenic Ras to Promote JNK-Dependent Tumor Growth and Invasion. Cell Rep. 2017, 19, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, W.; Zhang, D.; Yang, Y.; Li, W.; Xue, L. Wallenda regulates JNK-mediated cell death in Drosophila. Cell Death Dis. 2015, 6, e1737. [Google Scholar] [CrossRef]
- Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 2010, 19, 491–505. [Google Scholar] [CrossRef]
- Staley, B.K.; Irvine, K.D. Hippo signaling in Drosophila: Recent advances and insights. Dev. Dyn. 2012, 241, 3–15. [Google Scholar] [CrossRef]
- Zeng, R.; Dong, J. The Hippo Signaling Pathway in Drug Resistance in Cancer. Cancers 2021, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Pan, D. The Hippo Signaling Pathway in Development and Disease. Dev. Cell 2019, 50, 264–282. [Google Scholar] [CrossRef]
- Ma, X.; Lu, J.Y.; Moraru, A.; Teleman, A.A.; Fang, J.; Qiu, Y.; Liu, P.; Xu, T. A novel regulator of ER Ca(2+) drives Hippo-mediated tumorigenesis. Oncogene 2020, 39, 1378–1387. [Google Scholar] [CrossRef]
- Su, Y.C.; Treisman, J.E.; Skolnik, E.Y. The Drosophila Ste20-related kinase misshapen is required for embryonic dorsal closure and acts through a JNK MAPK module on an evolutionarily conserved signaling pathway. Genes Dev. 1998, 12, 2371–2380. [Google Scholar] [CrossRef]
- Srivastava, A.; Pastor-Pareja, J.C.; Igaki, T.; Pagliarini, R.; Xu, T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc. Natl. Acad. Sci. USA 2007, 104, 2721–2726. [Google Scholar] [CrossRef] [PubMed]
- Uhlirova, M.; Bohmann, D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006, 25, 5294–5304. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Su, Y.C.; Becker, E.; Treisman, J.; Skolnik, E.Y. A Drosophila TNF-receptor-associated factor (TRAF) binds the ste20 kinase Misshapen and activates Jun kinase. Curr. Biol. 1999, 9, 101–104. [Google Scholar] [CrossRef]
- Paricio, N.; Feiguin, F.; Boutros, M.; Eaton, S.; Mlodzik, M. The Drosophila STE20-like kinase misshapen is required downstream of the Frizzled receptor in planar polarity signaling. EMBO J. 1999, 18, 4669–4678. [Google Scholar] [CrossRef]
- Wu, M.; Pastor-Pareja, J.C.; Xu, T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature 2010, 463, 545–548. [Google Scholar] [CrossRef]
- Li, Q.; Li, S.; Mana-Capelli, S.; Roth Flach, R.J.; Danai, L.V.; Amcheslavsky, A.; Nie, Y.; Kaneko, S.; Yao, X.; Chen, X.; et al. The conserved misshapen-warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev. Cell 2014, 31, 291–304. [Google Scholar] [CrossRef]
- Li, Q.; Nirala, N.K.; Chen, H.J.; Nie, Y.; Wang, W.; Zhang, B.; Czech, M.P.; Wang, Q.; Xu, L.; Mao, J.; et al. The Misshapen subfamily of Ste20 kinases regulate proliferation in the aging mammalian intestinal epithelium. J. Cell. Physiol. 2019, 234, 21925–21936. [Google Scholar] [CrossRef]
- Li, Q.; Nirala, N.K.; Nie, Y.; Chen, H.J.; Ostroff, G.; Mao, J.; Wang, Q.; Xu, L.; Ip, Y.T. Ingestion of Food Particles Regulates the Mechanosensing Misshapen-Yorkie Pathway in Drosophila Intestinal Growth. Dev. Cell 2018, 45, 433–449.e436. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cho, Y.S.; Yue, T.; Ip, Y.T.; Jiang, J. Overlapping functions of the MAP4K family kinases Hppy and Msn in Hippo signaling. Cell Discov. 2015, 1, 15038. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Moroishi, T.; Mottier-Pavie, V.; Plouffe, S.W.; Hansen, C.G.; Hong, A.W.; Park, H.W.; Mo, J.S.; Lu, W.; Lu, S.; et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat. Commun. 2015, 6, 8357. [Google Scholar] [CrossRef]
- Meng, Z.; Qiu, Y.; Lin, K.C.; Kumar, A.; Placone, J.K.; Fang, C.; Wang, K.C.; Lu, S.; Pan, M.; Hong, A.W.; et al. RAP2 mediates mechanoresponses of the Hippo pathway. Nature 2018, 560, 655–660. [Google Scholar] [CrossRef]
- Chen, C.L.; Schroeder, M.C.; Kango-Singh, M.; Tao, C.; Halder, G. Tumor suppression by cell competition through regulation of the Hippo pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 484–489. [Google Scholar] [CrossRef]
- Nagata, R.; Igaki, T. Cell competition: Emerging mechanisms to eliminate neighbors. Dev. Growth Differ. 2018, 60, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Doggett, K.; Grusche, F.A.; Richardson, H.E.; Brumby, A.M. Loss of the Drosophila cell polarity regulator Scribbled promotes epithelial tissue overgrowth and cooperation with oncogenic Ras-Raf through impaired Hippo pathway signaling. BMC Dev. Biol. 2011, 11, 57. [Google Scholar] [CrossRef]
- Enomoto, M.; Igaki, T. Deciphering tumor-suppressor signaling in flies: Genetic link between Scribble/Dlg/Lgl and the Hippo pathways. J. Genet. Genomics 2011, 38, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Fahey-Lozano, N.; La Marca, J.E.; Portela, M.; Richardson, H.E. Drosophila Models of Cell Polarity and Cell Competition in Tumourigenesis. Adv. Exp. Med. Biol. 2019, 1167, 37–64. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Strutt, D. The roles of the cadherins Fat and Dachsous in planar polarity specification in Drosophila. Dev. Dyn. 2012, 241, 27–39. [Google Scholar] [CrossRef]
- Montes, A.J.; Morata, G. Homeostatic response to blocking cell division in Drosophila imaginal discs: Role of the Fat/Dachsous (Ft/Ds) pathway. Dev. Biol. 2017, 424, 113–123. [Google Scholar] [CrossRef]
- Cho, E.; Feng, Y.; Rauskolb, C.; Maitra, S.; Fehon, R.; Irvine, K.D. Delineation of a Fat tumor suppressor pathway. Nat. Genet. 2006, 38, 1142–1150. [Google Scholar] [CrossRef]
- Willecke, M.; Hamaratoglu, F.; Kango-Singh, M.; Udan, R.; Chen, C.L.; Tao, C.; Zhang, X.; Halder, G. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr. Biol. 2006, 16, 2090–2100. [Google Scholar] [CrossRef]
- Verghese, S.; Waghmare, I.; Kwon, H.; Hanes, K.; Kango-Singh, M. Scribble acts in the Drosophila fat-hippo pathway to regulate warts activity. PLoS ONE 2012, 7, e47173. [Google Scholar] [CrossRef]
- Kwon, Y.; Vinayagam, A.; Sun, X.; Dephoure, N.; Gygi, S.P.; Hong, P.; Perrimon, N. The Hippo signaling pathway interactome. Science 2013, 342, 737–740. [Google Scholar] [CrossRef]
- Hao, J.M.; Chen, J.Z.; Sui, H.M.; Si-Ma, X.Q.; Li, G.Q.; Liu, C.; Li, J.L.; Ding, Y.Q.; Li, J.M. A five-gene signature as a potential predictor of metastasis and survival in colorectal cancer. J. Pathol. 2010, 220, 475–489. [Google Scholar] [CrossRef]
- Liang, J.J.; Wang, H.; Rashid, A.; Tan, T.H.; Hwang, R.F.; Hamilton, S.R.; Abbruzzese, J.L.; Evans, D.B.; Wang, H. Expression of MAP4K4 is associated with worse prognosis in patients with stage II pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2008, 14, 7043–7049. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.H.; Qian, Y.M.; Zhao, X.L.; Wang, S.M.; Feng, X.J.; Chen, X.F.; Zhang, S.H. Expression and prognostic significance of MAP4K4 in lung adenocarcinoma. Pathol. Res. Pract. 2012, 208, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Rizzardi, A.E.; Rosener, N.K.; Koopmeiners, J.S.; Isaksson Vogel, R.; Metzger, G.J.; Forster, C.L.; Marston, L.O.; Tiffany, J.R.; McCarthy, J.B.; Turley, E.A.; et al. Evaluation of protein biomarkers of prostate cancer aggressiveness. BMC Cancer 2014, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.W.; Cai, J.; Zhao, X.L.; Jiang, T.H.; He, T.F.; Fu, H.Q.; Zhu, M.H.; Zhang, S.H. ShRNA-targeted MAP4K4 inhibits hepatocellular carcinoma growth. Clin. Cancer Res. 2011, 17, 710–720. [Google Scholar] [CrossRef]
- Park, G.S.; Oh, H.; Kim, M.; Kim, T.; Johnson, R.L.; Irvine, K.D.; Lim, D.S. An evolutionarily conserved negative feedback mechanism in the Hippo pathway reflects functional difference between LATS1 and LATS2. Oncotarget 2016, 7, 24063–24075. [Google Scholar] [CrossRef]
- Lee, T.; Luo, L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001, 24, 251–254. [Google Scholar] [CrossRef]
- Lee, T.; Luo, L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 1999, 22, 451–461. [Google Scholar] [CrossRef]
- Kline, A.; Curry, T.; Lewellyn, L. The Misshapen kinase regulates the size and stability of the germline ring canals in the Drosophila egg chamber. Dev. Biol. 2018, 440, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Maurel-Zaffran, C.; Treisman, J.E.; Skolnik, E.Y. The Ste20 kinase misshapen regulates both photoreceptor axon targeting and dorsal closure, acting downstream of distinct signals. Mol. Cell Biol. 2000, 20, 4736–4744. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.; Liu, B.; Deng, H.; Uster, E.; Pan, D. Identification of Happyhour/MAP4K as Alternative Hpo/Mst-like Kinases in the Hippo Kinase Cascade. Dev. Cell 2015, 34, 642–655. [Google Scholar] [CrossRef]
- Jiang, J. Misshapen Connects Food, Mechanosensing, and Intestinal Growth. Dev. Cell 2018, 45, 417–418. [Google Scholar] [CrossRef]
- Dey, A.; Varelas, X.; Guan, K.L. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat. Rev. Drug Discov. 2020, 19, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Igaki, T.; Pastor-Pareja, J.C.; Aonuma, H.; Miura, M.; Xu, T. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev. Cell 2009, 16, 458–465. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Y.; Xu, W.; Wu, N.; Li, M.; Cao, Y.; Wu, S.; Li, Q.; Xue, L. Impaired Hippo signaling promotes Rho1-JNK-dependent growth. Proc. Natl. Acad. Sci. USA 2015, 112, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, L.; Yang, Y.; Li, M.; Li, W.; Xue, L. dUev1a modulates TNF-JNK mediated tumor progression and cell death in Drosophila. Dev. Biol. 2013, 380, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Matakatsu, H.; Fehon, R.; Blair, S.S. The novel SH3 domain protein Dlish/CG10933 mediates fat signaling in Drosophila by binding and regulating Dachs. Elife 2016, 5, e16624. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Lu, J.-Y.; Li, X.; Zhao, S.; Xu, W.; Fang, J.; Wang, X.; Ma, X. Misshapen Disruption Cooperates with RasV12 to Drive Tumorigenesis. Cells 2021, 10, 894. https://doi.org/10.3390/cells10040894
Kong D, Lu J-Y, Li X, Zhao S, Xu W, Fang J, Wang X, Ma X. Misshapen Disruption Cooperates with RasV12 to Drive Tumorigenesis. Cells. 2021; 10(4):894. https://doi.org/10.3390/cells10040894
Chicago/Turabian StyleKong, Du, Jin-Yu Lu, Xiaoqin Li, Sihua Zhao, Wenyan Xu, Jinan Fang, Xing Wang, and Xianjue Ma. 2021. "Misshapen Disruption Cooperates with RasV12 to Drive Tumorigenesis" Cells 10, no. 4: 894. https://doi.org/10.3390/cells10040894
APA StyleKong, D., Lu, J.-Y., Li, X., Zhao, S., Xu, W., Fang, J., Wang, X., & Ma, X. (2021). Misshapen Disruption Cooperates with RasV12 to Drive Tumorigenesis. Cells, 10(4), 894. https://doi.org/10.3390/cells10040894






