Sirtuin 1 and Skin: Implications in Intrinsic and Extrinsic Aging—A Systematic Review
Abstract
1. Introduction
2. Objective
3. Materials and Methods
4. Results
5. Discussion
5.1. Sirtuin 1 Relevance in Intrinsic Aging
5.1.1. Cutaneous Sirtuin 1 Expression Decreases with Age
5.1.2. Premature Aging Related to Genetic Background
5.1.3. Progressive Tissue Fibrosis as a Hallmark of Aging
5.2. Sirtuin 1 Relevance in Extrinsic Aging
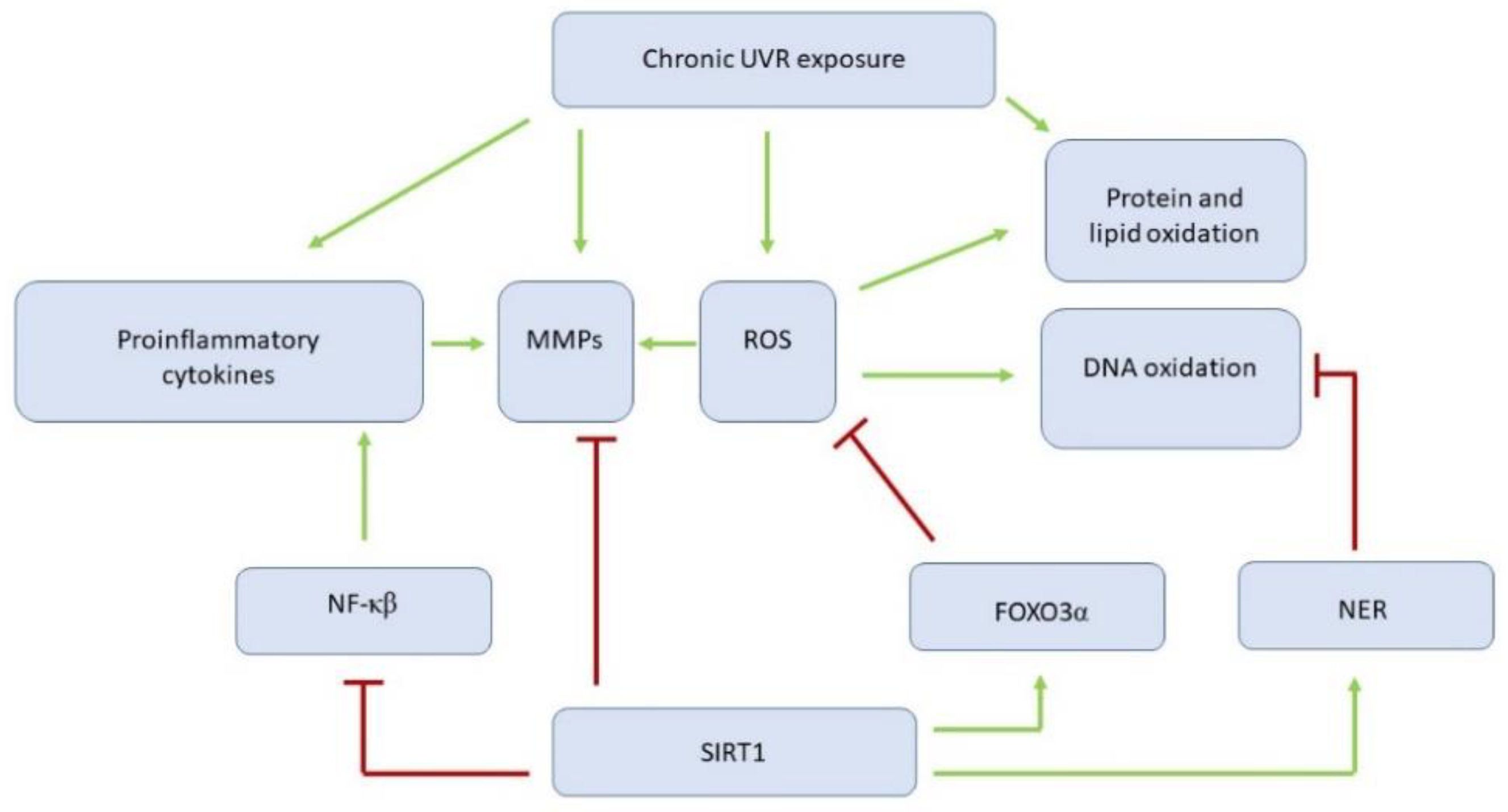
5.2.1. Sirtuin 1 Affects Ultraviolet Irradiation-Related Skin Changes by Numerous Targets
5.2.2. Sirtuin 1 as a Promising ‘Weapon’ against Pollutant-Related Premature Aging
5.3. Potential Interventions in Aging Process by Sirtuin 1 Activation
5.3.1. Sirtuin 1 Mediates the Benefits of Caloric Restriction on Healthspan
5.3.2. Sirtuin 1 Activators for the Topical Treatment of Premature Aging Symptoms
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Rodero, S.; Fernandez-Morera, J.L.; Menéndez-Torre, E.; Calvanese, V.; Fernández, A.F.; Fraga, M.F. Aging Genetics and Aging. Aging Dis. 2011, 2, 186–195. [Google Scholar] [PubMed]
- Market Data Forecast. Available online: https://www.marketdataforecast.com/market-reports/anti-aging-market (accessed on 6 January 2021).
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018. Available online: dietandcancerreport.org (accessed on 15 February 2021).
- Bazyluk, A.; Malyszko, J.; Hryszko, T.; Zbroch, E. State of the art–sirtuin 1 in kidney pathology–clinical relevance. Adv. Med. Sci. 2019, 64, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Bruno, J.; Easlon, E.; Lin, S.-J.; Cheng, H.-L.; Alt, F.W.; Guarente, L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008, 22, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S.-I. Sirt1 Extends Life Span and Delays Aging in Mice through the Regulation of Nk2 Homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef]
- Lin, S.-J.; Ford, E.; Haigis, M.; Liszt, G.; Guarente, L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004, 18, 12–16. [Google Scholar] [CrossRef]
- Ota, H.; Akishita, M.; Eto, M.; Iijima, K.; Kaneki, M.; Ouchi, Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J. Mol. Cell. Cardiol. 2007, 43, 571–579. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Lee, K.; Liu, B.; Deng, Y.; Chen, Y.; Zhang, X.; He, J.C.; Zhong, Y. Puerarin attenuates diabetic kidney injury through the suppression of NOX4 expression in podocytes. Sci. Rep. 2017, 7, 14603. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wang, X.-C.; Xu, Y.-M.; Luo, N.-C.; Luo, S.; Hao, X.-Y.; Cheng, S.-Y.; Fang, J.-S.; Wang, Q.; Zhang, S.-J.; et al. Berberine Improves Diabetic Encephalopathy through the SIRT1/ER Stress Pathway indb/dbMice. Rejuvenation Res. 2018, 21, 200–209. [Google Scholar] [CrossRef]
- Rahman, S.; Islam, R. Mammalian Sirt1: Insights on its biological functions. Cell Commun. Signal. 2011, 9, 11. [Google Scholar] [CrossRef]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, J.; Wu, S.; Li, B.; Liu, S.; Cheng, J. SIRT1 promotes proliferation and inhibits apoptosis of human malignant glioma cell lines. Neurosci. Lett. 2012, 525, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-H.; Sengupta, K.; Li, C.; Kim, H.-S.; Cao, L.; Xiao, C.; Kim, S.; Xu, X.; Zheng, Y.; Chilton, B.; et al. Impaired DNA Damage Response, Genome Instability, and Tumorigenesis in SIRT1 Mutant Mice. Cancer Cell 2008, 14, 312–323. [Google Scholar] [CrossRef]
- Sommer, M.; Poliak, N.; Upadhyay, S.; Ratovitski, E.A.; Nelkin, B.D.; Donehower, L.A.; Sidransky, D. ΔNp63&alpha Overexpression Induces Downregulation of Sirt1 and an Accelerated Aging Phenotype in the Mouse. Cell Cycle 2006, 5, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.-C.; Song, T.-Y.; Chen, M.-Y.; Hu, M.-L. Effects of 2-deoxyglucose and dehydroepiandrosterone on intracellular NAD+ level, SIRT1 activity and replicative lifespan of human Hs68 cells. Biogerontology 2011, 12, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Kalfalah, F.; Sobek, S.; Bornholz, B.; Götz-Rösch, C.; Tigges, J.; Fritsche, E.; Krutmann, J.; Köhrer, K.; Deenen, R.; Ohse, S.; et al. Inadequate mito-biogenesis in primary dermal fibroblasts from old humans is associated with impairment of PGC1A-independent stimulation. Exp. Gerontol. 2014, 56, 59–68. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, H.-K.; Lee, J.-W.; Kim, Y.I.; Shin, M.K. Investigate correlation between mechanical property and aging biomarker in passaged human dermal fibroblasts. Microsc. Res. Tech. 2015, 78, 277–282. [Google Scholar] [CrossRef]
- Lee, J.-H.; Moon, J.-H.; Nazim, U.M.; Lee, Y.-J.; Seol, J.-W.; Eo, S.-K.; Lee, J.-H.; Park, S.-Y. Melatonin protects skin keratinocyte from hydrogen peroxide-mediated cell death via the SIRT1 pathway. Oncotarget 2016, 7, 12075–12088. [Google Scholar] [CrossRef]
- Golubtsova, N.N.; Filippov, F.N.; Gunin, A.G. [Age-related changes in the content of sirtuin 1 in fibroblasts of human dermis]. Adv. Gerontol. Uspekhi Gerontol. 2017, 30, 375–380. [Google Scholar]
- Sutter, C.H.; Olesen, K.M.; Bhuju, J.; Guo, Z.; Sutter, T.R. AHR Regulates Metabolic Reprogramming to Promote SIRT1-Dependent Keratinocyte Differentiation. J. Investig. Dermatol. 2019, 139, 818–826. [Google Scholar] [CrossRef]
- Wei, J.; Ghosh, A.K.; Chu, H.; Fang, F.; Hinchcliff, M.E.; Wang, J.; Marangoni, R.G.; Varga, J. The Histone Deacetylase Sirtuin 1 Is Reduced in Systemic Sclerosis and Abrogates Fibrotic Responses by Targeting Transforming Growth Factor β Signaling. Arthritis Rheumatol. 2015, 67, 1323–1334. [Google Scholar] [CrossRef]
- Zerr, P.; Palumbo-Zerr, K.; Huang, J.; Tomcik, M.; Sumova, B.; Distler, O.; Schett, G.; Distler, J.H.W. Sirt1 regulates canonical TGF-β signalling to control fibroblast activation and tissue fibrosis. Ann. Rheum. Dis. 2016, 75, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chu, H.; Jiang, S.; Liu, Q.; Liu, L.; Xue, Y.; Zheng, S.; Wan, W.; Qiu, J.; Wang, J.; et al. Sirt1 ameliorates systemic sclerosis by targeting the mTOR pathway. J. Dermatol. Sci. 2017, 87, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kahyo, T.; Mostoslavsky, R.; Goto, M.; Setou, M. Sirtuin-mediated deacetylation pathway stabilizes Werner syndrome protein. FEBS Lett. 2008, 582, 2479–2483. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Luo, J. SIRT1 Regulates UV-Induced DNA Repair through Deacetylating XPA. Mol. Cell 2010, 39, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Scheibye-Knudsen, M.; Brace, L.E.; Kassahun, H.; SenGupta, T.; Nilsen, H.; Mitchell, J.R.; Croteau, D.L.; Bohr, V.A. Defective Mitophagy in XPA via PARP-1 Hyperactivation and NAD+/SIRT1 Reduction. Cell 2014, 157, 882–896. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.G.; Carter, K.M.; Bautista, R.-M.; He, D.; Wang, C.; D’Orazio, J.A. Sirtuin 1-mediated deacetylation of XPA DNA repair protein enhances its interaction with ATR protein and promotes cAMP-induced DNA repair of UV damage. J. Biol. Chem. 2018, 293, 19025–19037. [Google Scholar] [CrossRef]
- Ming, M.; Shea, C.R.; Guo, X.; Li, X.; Soltani, K.; Han, W.; He, Y.-Y. Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C. Proc. Natl. Acad. Sci. USA 2010, 107, 22623–22628. [Google Scholar] [CrossRef]
- Velez-Cruz, R.; Zadorin, A.S.; Coin, F.; Egly, J.-M. Sirt1 suppresses RNA synthesis after UV irradiation in combined xeroderma pigmentosum group D/Cockayne syndrome (XP-D/CS) cells. Proc. Natl. Acad. Sci. USA 2012, 110, E212–E220. [Google Scholar] [CrossRef]
- Scheibye-Knudsen, M.; Mitchell, S.J.; Fang, E.F.; Iyama, T.; Ward, T.; Wang, J.; Dunn, C.A.; Singh, N.; Veith, S.; Hasan-Olive, M.; et al. A High-Fat Diet and NAD + Activate Sirt1 to Rescue Premature Aging in Cockayne Syndrome. Cell Metab. 2014, 20, 840–855. [Google Scholar] [CrossRef]
- Fang, E.F.; Kassahun, H.; Croteau, D.L.; Scheibye-Knudsen, M.; Marosi, K.; Lu, H.; Shamanna, R.A.; Kalyanasundaram, S.; Bollineni, R.C.; Wilson, M.A.; et al. NAD + Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016, 24, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ghosh, S.; Yang, X.; Zheng, H.; Liu, X.; Wang, Z.; Jin, G.; Zheng, B.; Kennedy, B.K.; Suh, Y.; et al. Resveratrol Rescues SIRT1-Dependent Adult Stem Cell Decline and Alleviates Progeroid Features in Laminopathy-Based Progeria. Cell Metab. 2012, 16, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Ohguchi, K.; Itoh, T.; Akao, Y.; Inoue, H.; Nozawa, Y.; Ito, M. SIRT1 modulates expression of matrix metalloproteinases in human dermal fibroblasts. Br. J. Dermatol. 2010, 163, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Arai, N.; Kohno, K.; Ushio, S.; Fukuda, S. Anti-oxidative and anti-aging activities of 2-O-α-glucopyranosyl-L-ascorbic acid on human dermal fibroblasts. Eur. J. Pharmacol. 2012, 674, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Jeong, D.; Park, S.H.; Shin, K.K.; Hong, Y.H.; Kim, E.; Yu, Y.-G.; Kim, T.-R.; Kim, H.; Lee, J.; et al. Antiwrinkle and Antimelanogenesis Effects of Tyndallized Lactobacillus acidophilus KCCM12625P. Int. J. Mol. Sci. 2020, 21, 1620. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Lu, S.; Kivlin, R.; Wallin, B.; Card, E.; Bagdasarian, A.; Tamakloe, T.; Wang, W.-J.; Song, X.; Chu, W.-M.; et al. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J. Cell. Mol. Med. 2008, 13, 3632–3643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wen, C.; Lin, J.; Shen, G. Protective effect of pyrroloquinoline quinine on ultraviolet A irradiation-induced human dermal fibroblast senescence in vitro proceeds via the anti-apoptotic sirtuin 1/nuclear factor-derived erythroid 2-related factor 2/heme oxygenase 1 pathway. Mol. Med. Rep. 2015, 12, 4382–4388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chung, K.W.; Choi, Y.J.; Park, M.H.; Jang, E.J.; Kim, D.H.; Park, B.H.; Yu, B.P.; Chung, H.Y. Molecular Insights into SIRT1 Protection Against UVB-Induced Skin Fibroblast Senescence by Suppression of Oxidative Stress and p53 Acetylation. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014, 70, 959–968. [Google Scholar] [CrossRef]
- Calapre, L.; Gray, E.S.; Kurdykowski, S.; David, A.; Descargues, P.; Ziman, M. SIRT1 activation mediates heat-induced survival of UVB damaged Keratinocytes. BMC Dermatol. 2017, 17, 8. [Google Scholar] [CrossRef]
- Lei, D.; Huang, Y.; Xie, H.; Yi, Y.; Long, J.; Lin, S.; Huang, C.; Jian, D.; Li, J. Fluorofenidone inhibits UV-A induced senescence in human dermal fibroblasts via the mammalian target of rapamycin-dependent SIRT1 pathway. J. Dermatol. 2018, 45, 791–798. [Google Scholar] [CrossRef]
- Ding, S.; Chen, J.; Zeng, Q.; Lu, J.; Tan, L.; Guo, A.; Kang, J.; Yang, S.; Xiang, Y.; Zuo, C.; et al. Chronic sun exposure is associated with distinct histone acetylation changes in human skin. Br. J. Dermatol. 2018, 179, 110–117. [Google Scholar] [CrossRef]
- Li, Q.; Bai, D.; Qin, L.; Shao, M.; Liu, X.; Zhang, S.; Yan, C.; Yu, G.; Hao, J. Protective Effect of l-Hexaguluroic Acid Hexasodium Salt on UVA-Induced Photo-Aging in HaCaT Cells. Int. J. Mol. Sci. 2020, 21, 1201. [Google Scholar] [CrossRef]
- Hida, Y.; Kubo, Y.; Murao, K.; Arase, S. Strong expression of a longevity-related protein, SIRT1, in Bowen’s disease. Arch. Dermatol. Res. 2007, 299, 103–106. [Google Scholar] [CrossRef]
- Ming, M.; Soltani, K.; Shea, C.R.; Li, X.; He, Y.-Y. Dual role of SIRT1 in UVB-induced skin tumorigenesis. Oncogene 2014, 34, 357–363. [Google Scholar] [CrossRef]
- Brandl, L.; Hartmann, D.; Kirchner, T.; Menssen, A. Expression of n-MYC, NAMPT and SIRT1 in Basal Cell Carcinomas and their Cells of Origin. Acta Derm. Venereol. 2018, 1, 63–71. [Google Scholar] [CrossRef]
- Moreau, M.; Neveu, M.; Stéphan, S.; Noblesse, E.; Nizard, C.; Sadick, N.S.; Schnebert, S.; Bonté, F.; Dumas, M.; Andre, P.; et al. Enhancing cell longevity for cosmetic application: A complementary approach. J. Drugs Dermatol. 2007, 6, s14–s19. [Google Scholar]
- Lee, J.-S.; Park, K.-Y.; Min, H.-G.; Lee, S.J.; Kim, J.-J.; Choi, J.-S.; Kim, W.-S.; Cha, H.-J. Negative regulation of stress-induced matrix metalloproteinase-9 by Sirt1 in skin tissue. Exp. Dermatol. 2010, 19, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-H.; Kim, M.-M. Spermidine inhibits MMP-2 via modulation of histone acetyltransferase and histone deacetylase in HDFs. Int. J. Biol. Macromol. 2012, 51, 1003–1007. [Google Scholar] [CrossRef]
- Han, D.-W.; Lee, M.H.; Kim, B.; Lee, J.J.; Hyon, S.-H.; Park, J.-C. Preventive Effects of Epigallocatechin-3-O-Gallate against Replicative Senescence Associated with p53 Acetylation in Human Dermal Fibroblasts. Oxidative Med. Cell. Longev. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ruszová, E.; Cheel, J.; Pávek, S.; Moravcová, M.; Hermannová, M.; Matějková, I.; Spilková, J.; Velebný, V.; Kubala, L. Epilobium angustifolium extract demonstrates multiple effects on dermal fibroblasts in vitro and skin photo-protection in vivo. Gen. Physiol. Biophys. 2013, 32, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Joven, J.; Aragonès, G.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Borrás-Linares, I.; Camps, J.; Corominas-Faja, B.; Cufí, S.; Fernández-Arroyo, S.; et al. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil. Cell Cycle 2013, 12, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Motoo, Y.; Tomosugi, N. Effect of Saikokeishito, a Kampo medicine, on hydrogen peroxide-induced premature senescence of normal human dermal fibroblasts. J. Integr. Med. 2014, 12, 495–503. [Google Scholar] [CrossRef]
- Watanabe, K.; Shibuya, S.; Ozawa, Y.; Izuo, N.; Shimizu, T. Resveratrol Derivative-Rich Melinjo Seed Extract Attenuates Skin Atrophy inSod1-Deficient Mice. Oxidative Med. Cell. Longev. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- De Cabo, R.; Liu, L.; Ali, A.; Price, N.; Zhang, J.; Wang, M.; Lakatta, E.; Irusta, P.M. Serum from calorie-restricted animals delays senescence and extends the lifespan of normal human fibroblasts in vitro. Aging 2015, 7, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K. Protective Effect of Garlic on Cellular Senescence in UVB-Exposed HaCaT Human Keratinocytes. Nutrition 2016, 8, 464. [Google Scholar] [CrossRef] [PubMed]
- Wahedi, H.M.; Lee, T.H.; Moon, E.-Y.; Kim, S.Y.; Information, P.E.K.F.C. Juglone up-regulates sirt1 in skin cells under normal and UVB irradiated conditions. J. Dermatol. Sci. 2016, 81, 210–212. [Google Scholar] [CrossRef]
- Senthil, K.K.; Gokila, V.M.; Mau, J.-L.; Lin, C.-C.; Chu, F.-H.; Wei, C.-C.; Liao, V.H.-C.; Wang, S.-Y. A steroid like phytochemical Antcin M is an anti-aging reagent that eliminates hyperglycemia-accelerated premature senescence in dermal fibroblasts by direct activation of Nrf2 and SIRT-1. Oncotarget 2016, 7, 62836–62861. [Google Scholar] [CrossRef]
- Shen, T.; Duan, C.; Chen, B.; Li, M.; Ruan, Y.; Xu, D.; Shi, D.; Yu, D.; Li, J.; Wang, C. Tremella fuciformis polysaccharide suppresses hydrogen peroxide-triggered injury of human skin fibroblasts via upregulation of SIRT1. Mol. Med. Rep. 2017, 16, 1340–1346. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Shi, T.; Wang, Y.; Guan, C. Possible treatment for cutaneous lichen planus: An in vitro anti-inflammatory role of Angelica polysaccharide in human keratinocytes HaCaT. Int. J. Immunopathol. Pharmacol. 2019, 33. [Google Scholar] [CrossRef]
- Chen, D.; Steele, A.D.; Lindquist, S.; Guarente, L. Increase in Activity during Calorie Restriction Requires Sirt1. Science 2005, 310, 1641. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Koya, D. The protective role of Sirt1 in vascular tissue: Its relationship to vascular aging and atherosclerosis. Aging 2016, 8, 2290–2307. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Wakino, S.; Simic, P.; Sakamaki, Y.; Minakuchi, H.; Fujimura, K.; Hosoya, K.; Komatsu, M.; Kaneko, Y.; Kanda, T.; et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat. Med. 2013, 19, 1496–1504. [Google Scholar] [CrossRef]
- Luo, G.; Jian, Z.; Zhu, Y.; Chen, B.; Ma, R.; Tang, F.; Xiao, Y. Sirt1 promotes autophagy and inhibits apoptosis to protect cardiomyocytes from hypoxic stress. Int. J. Mol. Med. 2019, 43, 2033–2043. [Google Scholar] [CrossRef]
- Ryu, D.R.; Yu, M.R.; Kong, K.H.; Kim, H.; Kwon, S.H.; Jeon, J.S.; Han, D.C.; Noh, H. Sirt1-hypoxia-inducible factor-1α interaction is a key mediator of tubulointerstitial damage in the aged kidney. Aging Cell 2019, 18, e12904. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, Z.; Ke, L.; Li, Z.; Li, W.; Zhang, Z.; Zhou, Y.; Feng, X.; Zhu, W. Resveratrol improves glucose uptake in insulin-resistant adipocytes via Sirt1. J. Nutr. Biochem. 2018, 55, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Bonta, M.; Daina, L.; Muţiu, G. The process of ageing reflected by histological changes in the skin. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2013, 54, 797–804. [Google Scholar]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; Di Fagagna, F.D. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Gladyshev, V.N. The Free Radical Theory of Aging Is Dead. Long Live the Damage Theory! Antioxid. Redox Signal. 2014, 20, 727–731. [Google Scholar] [CrossRef]
- Janson, D.; Rietveld, M.; Willemze, R.; El Ghalbzouri, A. Effects of serially passaged fibroblasts on dermal and epidermal morphogenesis in human skin equivalents. Biogerontology 2013, 14, 131–140. [Google Scholar] [CrossRef]
- Farra, C.D.; Domloge, N. SIRT1, the human homologue to SIR2, is expressed in human skin and in cultured keratinocytes fibroblasts and HaCaT cells; and its levels is closely related to stress and aging. J. Cosmet. Sci. 2006, 57, 187–188. [Google Scholar]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Tezuka, T. The content of free amino acids in the stratum corneum is increased in senile xerosis. Arch. Dermatol. Res. 2004, 295, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, C.L.; Cadiñanos, J.; Varela, I.; Freije, J.M.P.; López-Otín, C. Human progeroid syndromes, aging and cancer: New genetic and epigenetic insights into old questions. Cell. Mol. Life Sci. 2007, 64, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.B.; Massaro, J.; D’Agostino, R.B.; Campbell, S.E.; Brazier, J.; Brown, W.T.; Kleinman, M.E.; Kieran, M.W. Impact of Farnesylation Inhibitors on Survival in Hutchinson-Gilford Progeria Syndrome. Circulation 2014, 130, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.B.; Brown, W.T.; Collins, F.S. Hutchinson-Gilford Progeria Syndrome. 2003 Dec 12 [Updated 2019 Jan 17]. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Eds.; University of Washington: Seattle, WA, USA, 1993–2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1121/# (accessed on 6 April 2021).
- Scaffidi, P. Lamin A-Dependent Nuclear Defects in Human Aging. Science 2006, 312, 1059–1063. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-hutchinson-gilford-progeria-syndrome-and-some-progeroid-laminopathies (accessed on 8 February 2021).
- Oshima, J.; Martin, G.M.; Hisama, F.M. Werner Syndrome. 2002 Dec 2 [Updated 2016 Sep 29]. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Eds.; University of Washington: Seattle, WA, USA, 1993–2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1514/ (accessed on 6 April 2021).
- Gorbunova, V.; Seluanov, A.; Mao, Z.; Hine, C. Changes in DNA repair during aging. Nucleic Acids Res. 2007, 35, 7466–7474. [Google Scholar] [CrossRef]
- Karikkineth, A.C.; Scheibye-Knudsen, M.; Fivenson, E.; Croteau, D.L.; Bohr, V.A. Cockayne syndrome: Clinical features, model systems and pathways. Ageing Res. Rev. 2017, 33, 3–17. [Google Scholar] [CrossRef]
- Spivak, G. Nucleotide excision repair in humans. DNA Repair 2015, 36, 13–18. [Google Scholar] [CrossRef]
- Shah, P.; He, Y.-Y. Molecular Regulation of UV-Induced DNA Repair. Photochem. Photobiol. 2015, 91, 254–264. [Google Scholar] [CrossRef]
- Wu, X.; Shell, S.M.; Yang, Z.; Zou, Y. Phosphorylation of Nucleotide Excision Repair Factor Xeroderma Pigmentosum Group A by Ataxia Telangiectasia Mutated and Rad3-Related–Dependent Checkpoint Pathway Promotes Cell Survival in Response to UV Irradiation. Cancer Res. 2006, 66, 2997–3005. [Google Scholar] [CrossRef]
- Luckhardt, T.R.; Thannickal, V.J. Systemic sclerosis-associated fibrosis. Curr. Opin. Rheumatol. 2015, 27, 571–576. [Google Scholar] [CrossRef]
- Huang, X.-Z.; Wen, D.; Zhang, M.; Xie, Q.; Ma, L.; Guan, Y.; Ren, Y.; Chen, J.; Hao, C.-M. Sirt1 Activation Ameliorates Renal Fibrosis by Inhibiting the TGF-β/Smad3 Pathway. J. Cell. Biochem. 2014, 115, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Zhang, Y.; Wang, X.; Fan, X.-F.; Zhang, Y.; Li, X.; Gong, Y.-S.; Han, L.-P. SIRT1 activation attenuates cardiac fibrosis by endothelial-to-mesenchymal transition. Biomed. Pharmacother. 2019, 118, 109227. [Google Scholar] [CrossRef]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Flament, F.; Bazin, R.; Rubert, V.; Simonpietri, E.; Piot, B.; Laquieze, S. Effect of the sun on visible clinical signs of aging in Caucasian skin. Clin. Cosmet. Investig. Dermatol. 2013, 6, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M.; Fujiwara, H. Cell-Extracellular Matrix Interactions in Normal and Diseased Skin. Cold Spring Harb. Perspect. Biol. 2011, 3, a005124. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased Collagen Production in Chronologically Aged Skin: Roles of Age-Dependent Alteration in Fibroblast Function and Defective Mechanical Stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Quan, T.; Wang, F.; Shao, Y.; Rittié, L.; Xia, W.; Orringer, J.S.; Voorhees, J.J.; Fisher, G.J. Enhancing Structural Support of the Dermal Microenvironment Activates Fibroblasts, Endothelial Cells, and Keratinocytes in Aged Human Skin In Vivo. J. Investig. Dermatol. 2013, 133, 658–667. [Google Scholar] [CrossRef]
- Madzharova, E.; Kastl, P.; Sabino, F.; Keller, U.A.D. Post-Translational Modification-Dependent Activity of Matrix Metalloproteinases. Int. J. Mol. Sci. 2019, 20, 3077. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-Degrading Metalloproteinases in Photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Fonseca-Kelly, Z.; Callinan, C.; Zuo, L.; Sachdeva, M.M.; Shindler, K.S. SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front. Cell. Neurosci. 2012, 6, 63. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-W.; Chen, K.-C.; Wang, Y.-S.; Wang, J.-Y.; Liang, C.-L.; Juo, S.-H.H. The role of SIRT1/AKT/ERK pathway in ultraviolet B induced damage on human retinal pigment epithelial cells. Toxicol. Vitr. 2013, 27, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- De Pedro, I.; Alonso-Lecue, P.; Sanz-Gómez, N.; Freije, A.; Gandarillas, A. Sublethal UV irradiation induces squamous differentiation via a p53-independent, DNA damage-mitosis checkpoint. Cell Death Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Raynes, R.; Brunquell, J.; Westerheide, S.D. Stress Inducibility of SIRT1 and Its Role in Cytoprotection and Cancer. Genes Cancer 2013, 4, 172–182. [Google Scholar] [CrossRef] [PubMed]
- van der Leun, J.C.; Piacentini, R.D.; de Gruijl, F.R. Climate change and human skin cancer. Photochem. Photobiol. Sci. 2008, 7, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.M.; Kitahara, C.M.; Linet, M.S.; Alexander, B.H.; Neta, G.; Little, M.P.; Cahoon, E.K. Ambient temperature and risk of first primary basal cell carcinoma: A nationwide United States cohort study. J. Photochem. Photobiol. B Biol. 2015, 148, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Westerheide, S.D.; Anckar, J.; Stevens, S.M.; Sistonen, L.; Morimoto, R.I. Stress-Inducible Regulation of Heat Shock Factor 1 by the Deacetylase SIRT1. Science 2009, 323, 1063–1066. [Google Scholar] [CrossRef]
- WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide, Global Update 2005, Summary of Risk Assessment. 2006. Available online: http://www.who.int/phe/health_topics/outdoorair/outdoorair_aqg/en/ (accessed on 15 February 2021).
- Kathuria, S.; Puri, P.; Nandar, S.; Ramesh, V. Effects of air pollution on the skin: A review. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 415–423. [Google Scholar] [CrossRef]
- Harris, C.C. Tobacco smoking, E-cigarettes, and nicotine harm. Proc. Natl. Acad. Sci. USA 2018, 115, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- Freiman, A.; Bird, G.; Metelitsa, A.I.; Barankin, B.; Lauzon, G.J. Cutaneous Effects of Smoking. J. Cutan. Med. Surg. 2004, 8, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Knuutinen, A.; Kokkonen, N.; Risteli, J.; Vahakangas, K.; Kallioinen, M.; Salo, T.; Sorsa, T.; Oikarinen, A. Smoking affects collagen synthesis and extracellular matrix turnover in human skin. Br. J. Dermatol. 2002, 146, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Torii, K.; Maeda, A.; Yamaguchi, Y. Molecular Basis of Tobacco Smoke-Induced Premature Skin Aging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 53–55. [Google Scholar] [CrossRef]
- Lai, T.; Wen, X.; Wu, D.; Su, G.; Gao, Y.; Chen, C.; Wu, W.; Lv, Y.; Chen, Z.; Lv, Q.; et al. SIRT1 protects against urban particulate matter-induced airway inflammation. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1741–1752. [Google Scholar] [CrossRef]
- Yang, L.; Duan, Z.; Liu, X.; Yuan, Y. N-acetyl-l-cysteine ameliorates the PM2.5-induced oxidative stress by regulating SIRT-1 in rats. Environ. Toxicol. Pharmacol. 2018, 57, 70–75. [Google Scholar] [CrossRef]
- Tien, C.-P.; Chen, C.-H.; Lin, W.-Y.; Liu, C.-S.; Liu, K.-J.; Hsiao, M.; Chang, Y.-C.; Hung, S.-C. Ambient particulate matter attenuates Sirtuin1 and augments SREBP1-PIR axis to induce human pulmonary fibroblast inflammation: Molecular mechanism of microenvironment associated with COPD. Aging 2019, 11, 4654–4671. [Google Scholar] [CrossRef]
- Yao, H.; Hwang, J.-W.; Sundar, I.K.; Friedman, A.E.; McBurney, M.W.; Guarente, L.; Gu, W.; Kinnula, V.L.; Rahman, I. SIRT1 redresses the imbalance of tissue inhibitor of matrix metalloproteinase-1 and matrix metalloproteinase-9 in the development of mouse emphysema and human COPD. Am. J. Physiol. Cell. Mol. Physiol. 2013, 305, L615–L624. [Google Scholar] [CrossRef]
- Raitio, A.; Tuomas, H.; Kokkonen, N.; Salo, T.; Sorsa, T.; Hanemaaijer, R.; Oikarinen, A. Levels of matrix metalloproteinase-2, −9 and −8 in the skin, serum and saliva of smokers and non-smokers. Arch. Dermatol. Res. 2005, 297, 242–248. [Google Scholar] [CrossRef]
- Yin, L.; Morita, A.; Tsuji, T. Alterations of extracellular matrix induced by tobacco smoke extract. Arch. Dermatol. Res. 2000, 292, 188–194. [Google Scholar] [CrossRef]
- Anderson, R.M.; Bitterman, K.J.; Wood, J.G.; Medvedik, O.; Sinclair, D.A. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nat. Cell Biol. 2003, 423, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Masoro, E.J. Role of Hormesis in Life Extension by Caloric Restriction. Dose-Response 2007, 5, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular Stress Responses, The Hormesis Paradigm, and Vitagenes: Novel Targets for Therapeutic Intervention in Neurodegenerative Disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Bell-Farrow, A.D.; Wang, Z.Q.; Sonntag, W.E.; Fu, M.-X.; Baynes, J.W.; Thorpe, S.R. Caloric Restriction Decreases Age-Dependent Accumulation of the Glycoxidation Products, Nisin-(Carboxymethyl)lysine and Pentosidine, in Rat Skin Collagen. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 1995, 50, B337–B341. [Google Scholar] [CrossRef]
- Smith, J.D.L.; Mattison, J.A.; Desmond, R.A.; Gardner, J.P.; Kimura, M.; Roth, G.S.; Ingram, D.K.; Allison, D.B.; Aviv, A. Telomere Dynamics in Rhesus Monkeys: No Apparent Effect of Caloric Restriction. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2011, 66, 1163–1168. [Google Scholar] [CrossRef]
- Lekli, I.; Ray, D.; Das, D.K. Longevity nutrients resveratrol, wines and grapes. Genes Nutr. 2009, 5, 55–60. [Google Scholar] [CrossRef]
- Öztürk, E.; Arslan, A.K.K.; Yerer, M.B.; Bishayee, A. Resveratrol and diabetes: A critical review of clinical studies. Biomed. Pharmacother. 2017, 95, 230–234. [Google Scholar] [CrossRef]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxidative Med. Cell. Longev. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Blander, G.; Bhimavarapu, A.; Mammone, T.; Maes, D.H.; Elliston, K.; Reich, C.; Matsui, M.S.; Guarente, L.; Loureiro, J.J. SIRT1 Promotes Differentiation of Normal Human Keratinocytes. J. Investig. Dermatol. 2009, 129, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Holian, O.; Walter, R.J. Resveratrol inhibits the proliferation of normal human keratinocytes in vitro. J. Cell. Biochem. Suppl. 2001, 81, 55–62. [Google Scholar] [CrossRef]
- Ido, Y.; Duranton, A.; Lan, F.; Weikel, K.A.; Breton, L.; Ruderman, N.B. Resveratrol Prevents Oxidative Stress-Induced Senescence and Proliferative Dysfunction by Activating the AMPK-FOXO3 Cascade in Cultured Primary Human Keratinocytes. PLoS ONE 2015, 10, e0115341. [Google Scholar] [CrossRef]
- Potapovich, A.I.; Lulli, D.; Fidanza, P.; Kostyuk, V.A.; De Luca, C.; Pastore, S.; Korkina, L.G. Plant polyphenols differentially modulate inflammatory responses of human keratinocytes by interfering with activation of transcription factors NFκB and AhR and EGFR–ERK pathway. Toxicol. Appl. Pharmacol. 2011, 255, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Sticozzi, C.; Belmonte, G.; Cervellati, F.; Muresan, X.; Pessina, F.; Lim, Y.; Forman, H.J.; Valacchi, G. Resveratrol protects SR-B1 levels in keratinocytes exposed to cigarette smoke. Free. Radic. Biol. Med. 2014, 69, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Afaq, F.; Ahmad, N. Prevention of ultraviolet B radiation-damage by resveratrol in mouse skin is mediated via modulation in Survivin. Photochem. Photobiol. 2004, 81, 25–31. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Feddi, F.; Mena, S.; Rodriguez, M.L.; Sirera, P.; Aupí, M.; Pérez, S.; Asensi, M.; Ortega, A.; Estrela, J.M. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free. Radic. Biol. Med. 2015, 85, 1–11. [Google Scholar] [CrossRef]
- Afaq, F.; Adhami, V.M.; Ahmad, N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice☆☆Part of this work was conducted at the Department of Dermatology, Case Western Reserve University and the Research Institute of University Hospitals of Cleveland, 11100 Euclid Avenue, Cleveland, Ohio 44106. Toxicol. Appl. Pharmacol. 2003, 186, 28–37. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Szaefer, H.; Stefański, T.; Sobiak, S.; Cichocki, M.; Baer-Dubowska, W. The effect of resveratrol and its methylthio-derivatives on the Nrf2-ARE pathway in mouse epidermis and HaCaT keratinocytes. Cell. Mol. Biol. Lett. 2014, 19, 500–516. [Google Scholar] [CrossRef]
- Alonso, C.; Martí, M.; Barba, C.; Carrer, V.; Rubio, L.; Coderch, L. Skin permeation and antioxidant efficacy of topically applied resveratrol. Arch. Dermatol. Res. 2017, 309, 423–431. [Google Scholar] [CrossRef]
- Detoni, C.B.; Souto, G.D.; Da Silva, A.L.M.; Pohlmann, A.R.; Guterres, S.S. Photostability and Skin Penetration of Different E-Resveratrol-Loaded Supramolecular Structures. Photochem. Photobiol. 2012, 88, 913–921. [Google Scholar] [CrossRef]
- Lepak, A.; Gutmann, A.; Kulmer, S.T.; Nidetzky, B. Creating a Water-Soluble Resveratrol-Based Antioxidant by Site-Selective Enzymatic Glucosylation. ChemBioChem 2015, 16, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Andrus, M.B. Human skin gene expression: Natural (trans) resveratrol versus five resveratrol analogs for dermal applications. Exp. Biol. Med. 2017, 242, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Correia-Da-Silva, M.; Rocha, V.; Marques, C.; Deus, C.M.; Marques-Carvalho, A.; Oliveira, P.J.; Palmeira, A.; Pinto, M.; Sousa, E.; Lobo, J.M.S.; et al. SULFATION PATHWAYS: Potential benefits of a sulfated resveratrol derivative for topical application. J. Mol. Endocrinol. 2018, 61, M27–M39. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, D.; Nobile, V.; Cestone, E.; Santin, G.; Bottone, M.G.; Marzatico, F.; Lazzeretti, A.; Tocabens, P. Resveratrol-procyanidin blend: Nutraceutical and antiaging efficacy evaluated in a placebo-controlled, double-blind study. Clin. Cosmet. Investig. Dermatol. 2012, 5, 159–165. [Google Scholar] [CrossRef]
- Bertuccelli, G.; Zerbinati, N.; Marcellino, M.; Kumar, N.S.N.; He, F.; Tsepakolenko, V.; Cervi, J.; Lorenzetti, A.; Marotta, F. Effect of a quality-controlled fermented nutraceutical on skin aging markers: An antioxidant-control, double-blind study. Exp. Ther. Med. 2016, 11, 909–916. [Google Scholar] [CrossRef]
- Farris, P.; Zeichner, J.; Berson, D. Efficacy and Tolerability of a Skin Brightening/Anti-Aging Cosmeceutical Containing Retinol 0.5%, Niacinamide, Hexylresorcinol, and Resveratrol. J. Drugs Dermatol. 2016, 15, 863–868. [Google Scholar] [PubMed]
- Wen, S.; Zhang, J.; Yang, B.; Elias, P.M.; Man, M.-Q. Role of Resveratrol in Regulating Cutaneous Functions. Evid.-Based Complement. Altern. Med. 2020, 2020, 1–20. [Google Scholar] [CrossRef]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Pazyar, N.; Feily, A. Garlic in dermatology. Dermatol. Rep. 2011, 3, e4. [Google Scholar] [CrossRef]
- de Boer, V.C.; de Goffau, M.C.; Arts, I.C.; Hollman, P.C.; Keijer, J. SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech. Ageing Dev. 2006, 127, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.; Calabrese, E. Best in small doses. New Sci. 2008, 199, 36–39. [Google Scholar] [CrossRef]
- Brunetti, G.; Di Rosa, G.; Scuto, M.; Leri, M.; Stefani, M.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan Maintenance and Prevention of Parkinson’s-like Phenotypes with Hydroxytyrosol and Oleuropein Aglycone in C. elegans. Int. J. Mol. Sci. 2020, 21, 2588. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Scuto, M.; Fusco, R.; Trovato, A.; Ontario, M.L.; Crea, R.; Di Paola, R.; Cuzzocrea, S.; Calabrese, V. Anti-inflammatory and Anti-oxidant Activity of Hidrox® in Rotenone-Induced Parkinson’s Disease in Mice. Antioxidants 2020, 9, 824. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Population | Key Observation |
|---|---|---|---|
| Intrinsic Aging–Basic Information | |||
| Sommer et al. [16] | 2006 | ∆Np63α transgenic mice Normal lung epithelial cells transfected with a vector containing∆Np63α | transgenic mice exhibited an accelerated aging phenotype in the skin accompanied by a decrease in longevity correlated with levels of SIRT1. In cell culture beta-galactosidase accumulation and typical senescent morphology was rescued by SIRT1. |
| Yang et al. [17] | 2011 | HDFs, Hs68 cell culture exposed to 2-DG and DHEA | 2-DG, but not DHEA, at non-cytotoxic concentrations extends lifespan in parallel with increased intracellular NAD+ levels and SIRT1 activities |
| Kalfalah et al. [18] | 2014 | Skin biopsies of females aged 20–67 | Age-dependent decrease in SIRT1 |
| Kim et al. [19] | 2015 | Passaged HDFs culture | SIRT 1 is down regulated by increasing passage of HDFS. |
| Lee et al. [20] | 2016 | HaCaT keratinocytes | Melatonin-induced autophagy play a protective role through SIRT1 pathway against skin cell damage as a result of hydrogen peroxide-induced cell death. |
| Golubtsova et al. [21] | 2017 | Skin biopsies retrieved from deceased donors: fetuses at pregnant age 20–40 week, people from birth to 85 years old | Age-related decrease in sirtuin 1 content in HDFs is correlated with age-dependent decrease in fibroblasts proliferation. The highest level of SIRT1 is found between 20- to 40-week of pregnancy. |
| Sutter et al. [22] | 2019 | NHEKs and N/TERT-1 | Decreased glucose metabolism increases keratinocytes differentiation by SIRT1 activation. |
| Systemic Sclerosis | |||
| Wei et al. [23] | 2015 | Skin biopsy samples of healthy adults and patients with SSc. Sirt1−/− and wild-type mouse embryonic fibroblasts | Reduced SIRT1 expression and protein level in SSc skin biopsy samples compared to healthy. Activation of Sirt1 attenuated fibrosis, while inhibition had profibrotic effects. |
| Zerr et al. [24] | 2016 | Skin biopsies of patients with SSc and healthy volunteers Sirt1−/− and wild-type mice | SIRT1 is decreased in TGF- β-dependent manner in patients with SSc and in experimental fibrosis. SIRT1 activation enhances the profibrotic effects of TGF-β with increased Smad reporter activity, elevated transcription of TGF-β target genes and raised release of collagen. Sirt1 KO inhibited TGF-β/SMAD signaling and reduced release of collagen in fibroblasts. Sirt1−/− mice were less susceptible to fibrosis |
| Zhu et al. [25] | 2017 | Skin biopsy specimens of SSc patients and healthy controls Mice treated with BLM | SIRT1, activated by RSV, ameliorated cutaneous inflammation and fibrosis in BLM- induced scleroderma. The enhancement of mTOR expression in the skin of the mice was significantly inhibited by Sirt1 activation. |
| Author | Year | Mutation | Population | Key Observations |
|---|---|---|---|---|
| Kayho et al. [26] | 2008 | WRN | Sirt1-heterozygous mice, homozygous mutant offspring Sirt1−/− HEK293T cells | CR of rats led to a simultaneous increase in the level of WRN and SIRT1 protein. WRN was decreased in Sirt1-deficient mice and HEK293T cells, treated with sirtuin inhibitors. |
| Fan et al. [27] | 2010 | XPA | HeLa, H1299, HEK293T cells and XPA-deficient fibroblasts | UVR augments the XPA and SIRT1 interaction, which leads to cell survival. Phosphorylation works in acute response to UV-induced damage, while acetylation is involved in DNA repair complex Downregulation of SIRT1 delays the removal of CPD, but no 6-4PPs lesion. SIRT1 functions as a tumor suppressor. |
| Fang et al. [28] | 2014 | XPA | In silico on-line database www.mitodb.com, accessed on 6 April 2021 (Scheibye-Knudsen et al., 2013) | In XPA, CS, and AT, SIRT1 attenuation leads to decreased mitophagy through the depression of PGC-1α and UCP2. The mitochondrial abnormalities appear to be caused by decreased activation of the NAD+-SIRT1-PGC-1α axis triggered by hyperactivation of the DNA damage sensor PARP1. |
| Jarrett et al. [29] | 2018 | XPA | A375 melanoma cells UV-irradiated | SIRT1-dependent deacetylation of XPA augments cAMP-enhanced NER. |
| Ming et al. [30] | 2010 | XPC | Human skin tumor samples UV irradiated | Inhibition of SIRT1 impairs global genome NER through suppressing the transcription of XPC in a SIRT1 dependent manner. SIRT1 levels are significantly reduced inhuman skin tumors from Caucasian patients. SIRT1 acts as a tumor suppressor. |
| Velez-Cruz et al. [31] | 2013 | XPD subunit of TFIIH | Human primary fibroblast | Transcriptional arrest upon UVR in XP-D/CS cells results from gene repression mediated by SIRT1 and may be restored with a Sirt1-specific inhibitor or downregulation by siRNA. |
| Scheibye-Knudsen et al. [32] | 2014 | CSB | Four-month-old mice SV40-transformed CS1AN cells Bristol N2 (WT) and csb-1 worms | Premature aging results from aberrant PARP activation due to deficient DNA repair leading to decreased SIRT1 activity and mitochondrial dysfunction. B-hydroxybutyrate levels are increased by the high-fat diet, and b-hydroxybutyrate, PARP inhibition, or NAD+ supplementation can activate SIRT1 and rescue CS-associated phenotypes. |
| Fang et al. [33] | 2016 | ATM | Primary neurons, nematodes, Atm−/− mice | A-T laboratory animal models exhibit NAD+ depletion and impaired SIRT1 activity. NAD+ replenishment improves lifespan and healthspan in worms and mice and ameliorates A-T phenotypes through upregulation of mitophagy and DNA repair. |
| Liu et al. [34] | 2012 | LMNA | HEK293 cells, mouse embryonic fibroblasts, HDFs derived from HGPS patients and healthy individuals, cells harboring LMNA mutations, Zmpste24/mice, bone marrow stromal cell and hematopoietic stem | Lamin A activates SIRT1 deacetylase. Resveratrol enhances SIRT1 activity by increasing its interaction with lamin A. Prelamin A or progerin has significantly reduced association with SIRT1 in cells. SIRT1 deacetylase activity is compromised in progeroid cells. Resveratrol alleviates progeroid features and extends life span in progeria mice. |
| Author | Year | Population | Key Observation |
|---|---|---|---|
| Extrinsic Aging | |||
| Ohguchi et al. [35] | 2010 | HDFs treated with SIRT1 inhibitor, activator and IL-1β | SIRT1 negatively regulates transcription of MMP-1 and MMP-3 and controls both basal and IL-1β-induced MMP expression |
| Taniguchi et al. [36] | 2012 | HDFs exposed to H2O2, AA, AA-2G | H2O2 reduced SIRT1 in HDFs. Pretreatment with AA-2G significantly inhibits reduction, whereas AA had no effect. |
| Lim et al. [37] | 2020 | HaCaT, HDFs and B16F10 cells exposed to UVB irradiation | AL has an antiwrinkle activity in damaged skin and can inhibit melanogenesis, regulates baseline MMP expression and induces collagen production in HDFs. AL inhibits elastase and MMP-1 and induces type 1 procollagen. AL increased the expression of SIRT1 |
| Extrinsic Aging–UV Irradiation | |||
| Cao et al. [38] | 2009 | HaCaT, p53 wild-type mouse, MEFs and p53 knockout MEFs | UVR and H2O2 downregulate SIRT1. RSV protects against cell death, whereas SIRT inhibitors enhance it |
| Zhang et al. [39] | 2015 | HDFs exposed to UVA irradiation | PQQ reduces the expression of senescence markers MMP1, MMP3 and beta-galactosidase by up-regulation of SIRT1 |
| Chung et al. [40] | 2015 | HS27 culture cell and HR1 hairless mouse exposed to UVB irradiation | SIRT1 overexpression protects fibroblasts from UVB-induced cell cycle arrest by p53 deacetylation. SIRT1 thorough FOXO3α increases resistance to the oxidative stress. |
| Calapre et al. [41] | 2017 | ex vivo skin models, taken from non-sun exposed skin of healthy donors and NHEKs exposed to UVB plus heat | SIRT1 mediates UVB plus heat induced survival of DNA damaged keratinocytes by: decrease in p-53 acetylation and downregulation of its downstream pathways, including: BAX, ERCC1, XPC Increase in Ki67. |
| Lei et al. [42] | 2018 | Primary HDFs obtained from foreskins of healthy human donors aged 5–20 years exposed to UVA irradiation | Fluorofenidon alleviates HDFs senescence by inhibiting the mTOR and increasing SIRT1 |
| Ding et al. [43] | 2018 | Epidermis isolated from skin biopsies obtained from the outer forearm and the buttock of healthy females | Lower expression of HDAC1 and SIRT1 in sun-exposed skin compared with matched non-exposed skin |
| Li et al. [44] | 2020 | HaCaT exposed to UVA irradiation | α-l-Hexaguluroic acid hexasodium salt (G6) increases mitochondrial metabolism, alleviates oxidative stress, reverses the downregulation of SIRT1 and pGC-1a expression levels |
| UV-Related Carcinogenesis | |||
| Hida et al. [45] | 2007 | Immunohistochemical staining for SIRT1 expression in 87 cases of skin tumors and 20 normal skin samples. | Sun-exposed and sun-protected skin regions did not differ in SIRT1 expression. SIRT1 was overexpressed in all samples of AK, BD, SCC and BCC. SIRT1 overexpression may have some relevance to the early stage of skin carcinogenesis. |
| Ming et al. [46] | 2015 | Sirt1 cKO and cHet, WT mice, and NHEKs exposed to UVB irradiation. Human skin samples of SCC. | Sirt1 cHET promotes UVB-induced skin tumorigenesis, whereas cKO Sirt1 suppresses skin tumor development but sensitizes the mice to chronic solar injury. In mouse skin, Sirt1 is haploinsufficient for UVB-induced DNA damage repair. SIRT1 is downregulated in parallel with XPC in human SCC. cKO deletion of Sirt1 augments p53 acetylation and sensitizes the epidermis to UVB-induced apoptosis in vivo, while heterozygous has no such effect. UVB induced tumor formation in Sirt1 WT and Sirt1 cHet mice but not in Sirt1 cKO mice. |
| Brandl et al. [47] | 2019 | human BCC human and murine normal skin | The c-MYC-NAMPT-DBC1-SIRT1 positive feedback loop may play a role in the development of BCCs. |
| Dietary and Supplementary Interventions | ||||
|---|---|---|---|---|
| Author | Year | Population | Substance | Key Observations |
| Moreau et al. [48] | 2007 | HDFs skin samples of females aged 37 to 64. | emulsion enriched with 1% of the yeast Kluyveromyces biopeptides | SIRT1 is expressed in epidermis and dermis. SIRT1 expression is greater in proliferating compared to differentiated keratinocytes. Kluyveromyces biopeptides stimulate SIRT1 expression in keratinocytes and HDFs, decrease beta-galactosidase and enhance DNA integrity. Objective improvement in multiple skin aging symptoms after topical use of the formulation. |
| Lee et al. [49] | 2010 | HDFs and keratinocytes isolated from human skin sample. Mouse skin model. | Resveratrol Metformin | SIRT1 activation by RSV and metformin inhibits MMP-9 expression under UVR exposure |
| Park et al. [50] | 2012 | HDFs | Spermidine | SIRT1 gene expression was increased by spermidine Spermidine inhibits activity and expression of MMP-2 |
| Han et al. [51] | 2012 | HDFs | EGCG | EGCG at high concentration prevents serial passage and H2O2-induced senescence in HDFs via suppression of the p53 without affecting the SIRT1 activity |
| Ruszova et al. [52] | 2013 | HDFs Healthy volunteers aged 40–50 | Epilobium angustifolium (EA) extract | EA extract downregulated MMP-1,-3 and TIMP-1,-2 by repeatedly UV irradiated HDFs. EA extract diminished SIRT1 downregulation dampened by UV-irradiation and decreased UV-induced erythema formation in vivo. |
| Menendez et al. [53] | 2013 | HDFs | EVOO | EVOO prevents age-related changes in the cell size, morphological heterogeneity, arrayed cell arrangement and senescence-associated β-galactosidase staining of HDFs by the activation of ER stress and the unfolded protein response, spermidine and polyamine metabolism, SIRT1 and NRF2 signaling. |
| Takata et al. [54] | 2014 | HDFs | Saikokeishito | Saikokeishito protects HDFs from premature senescence by hydrogen peroxide, but had no effect on SIRT1 expression. |
| Watanabe et al. [55] | 2015 | Sod1−/− mice primary dermal fibroblasts from skin tissue of Sod1−/− neonates | MSE | Orally MSE and RSV treatment reversed the skin thinning associated with increased oxidative damage in the Sod1−/− mice. MSE and RSV normalized gene expression of Col1a1 and p53 and upregulated gene expression of Sirt1 in skin tissues. |
| De Cabo et al. [56] | 2015 | HDFs | Serum from rats fed on caloric restriction (40%) versus ad libitum diets | CR serum delays the passage-induced senescent phenotype, reduces SA-β-Gal and MMP-2 activity. CR serum prevents SIRT1 downregulation. Overexpression of SIRT1 in late passage human fibroblasts resulted in delayed senescent growth arrest. KO of SIRT1 in early passage cells enhanced MMP-2 activation. |
| Kim [57] | 2016 | UVB-exposed human keratinocytes, HaCaT cells | Garlic | Garlic pretreatment attenuated in a dose-dependent manner the production of ROS, proinflammatory cytokines and MMP-1 protein expressions. SA-β-gal and SIRT1 activity were ameliorated by garlic treatment. |
| Wahedi et al. [58] | 2016 | UVB-irradiated HaCaT and HDFs | Juglone | Juglone restored the expression of SIRT1 and Pin1 in almost dose dependent manner in irradiated cells. Juglone treatment upregulated SIRT1 in unirradiated skin cells. |
| Kumar et al. [59] | 2016 | HDFs | Antcins (Antrodia cinnamomea) | Antcin M protects HDFs from hyperglycemia-induced cell-cycle arrest and oxidative injury by upregulation of SIRT1 expression. |
| Shen et al. [60] | 2017 | HDFs | Tremella fuciformis polysaccharide | TFPS relieves H2O2-induced HDFs injury by attenuating oxidative stress and cell apoptosis by upregulation of the SIRT1 pathway. |
| Wang et al. [61] | 2019 | HaCaT | Angelica polysaccharide | AP alleviates LPS-induced injury through upregulating SIRT1 expression and then activating Nrf2/ HO-1 pathway but inactivating NF-κB pathway |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielach-Bazyluk, A.; Zbroch, E.; Mysliwiec, H.; Rydzewska-Rosolowska, A.; Kakareko, K.; Flisiak, I.; Hryszko, T. Sirtuin 1 and Skin: Implications in Intrinsic and Extrinsic Aging—A Systematic Review. Cells 2021, 10, 813. https://doi.org/10.3390/cells10040813
Bielach-Bazyluk A, Zbroch E, Mysliwiec H, Rydzewska-Rosolowska A, Kakareko K, Flisiak I, Hryszko T. Sirtuin 1 and Skin: Implications in Intrinsic and Extrinsic Aging—A Systematic Review. Cells. 2021; 10(4):813. https://doi.org/10.3390/cells10040813
Chicago/Turabian StyleBielach-Bazyluk, Angelika, Edyta Zbroch, Hanna Mysliwiec, Alicja Rydzewska-Rosolowska, Katarzyna Kakareko, Iwona Flisiak, and Tomasz Hryszko. 2021. "Sirtuin 1 and Skin: Implications in Intrinsic and Extrinsic Aging—A Systematic Review" Cells 10, no. 4: 813. https://doi.org/10.3390/cells10040813
APA StyleBielach-Bazyluk, A., Zbroch, E., Mysliwiec, H., Rydzewska-Rosolowska, A., Kakareko, K., Flisiak, I., & Hryszko, T. (2021). Sirtuin 1 and Skin: Implications in Intrinsic and Extrinsic Aging—A Systematic Review. Cells, 10(4), 813. https://doi.org/10.3390/cells10040813








