Peripheral Blood from Rheumatoid Arthritis Patients Shows Decreased Treg CD25 Expression and Reduced Frequency of Effector Treg Subpopulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. PBMC Isolation
2.3. Flow Cytometric Analysis
2.4. Statistical Analysis
3. Results
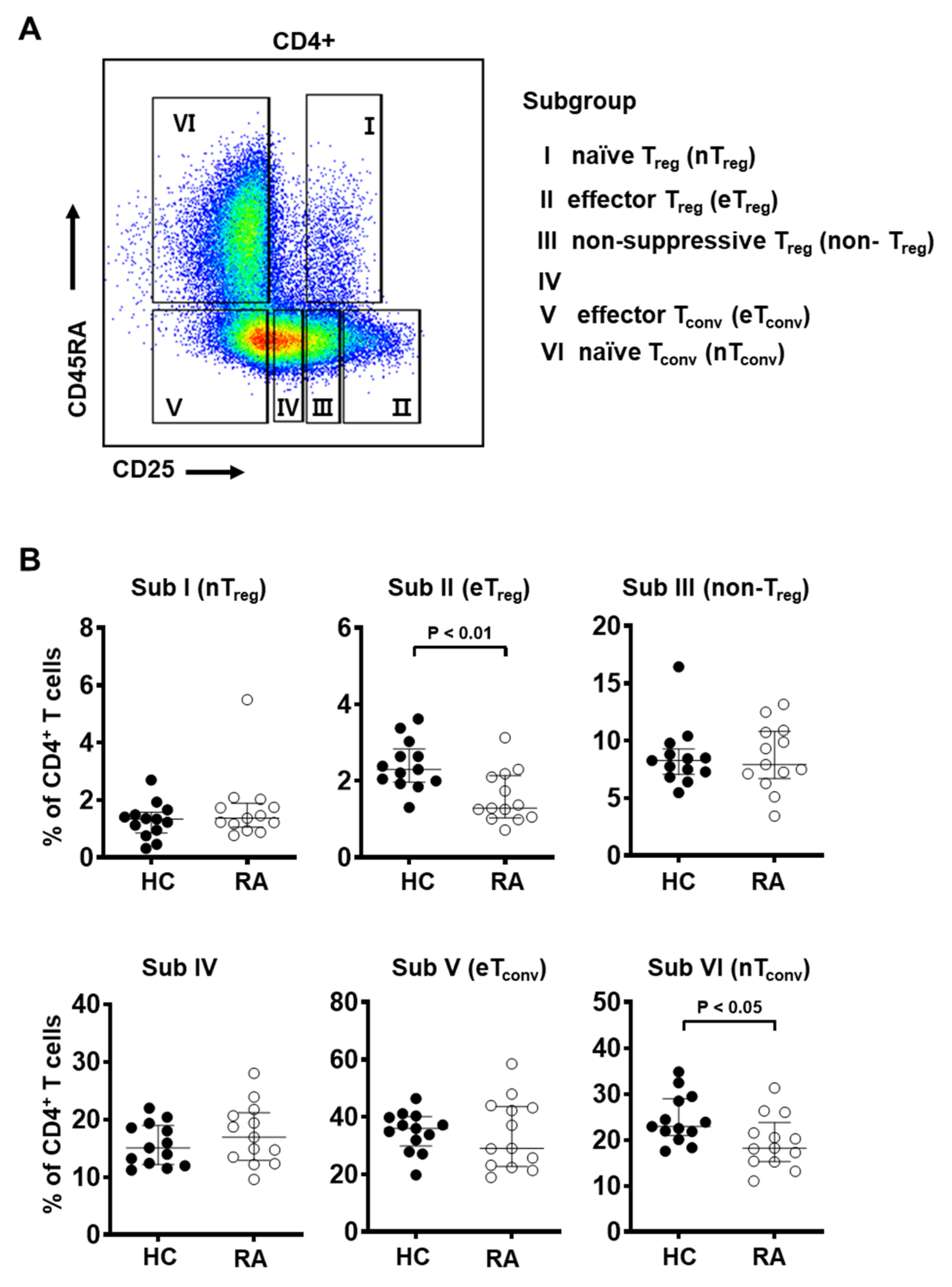
3.1. Total Frequency of Treg Cells in Peripheral Blood Did Not Show Significant Difference between RA and Control Subjects
3.2. Frequency of Effector Treg Cells Is Decreased in the Peripheral Blood from RA Patients
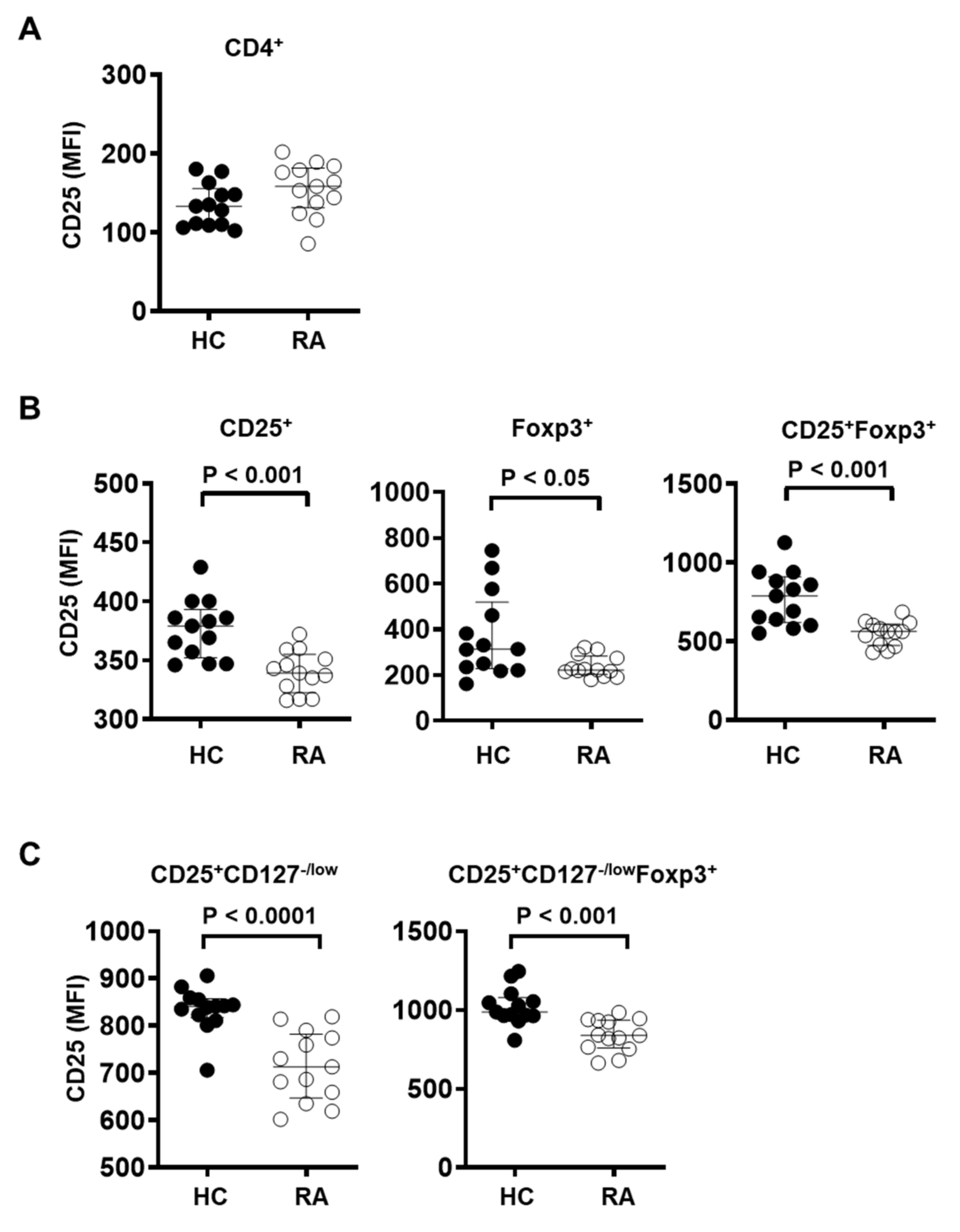
3.3. CD25 Expression Is Significantly Reduced in Treg Cells in the Peripheral Blood of RA Patients
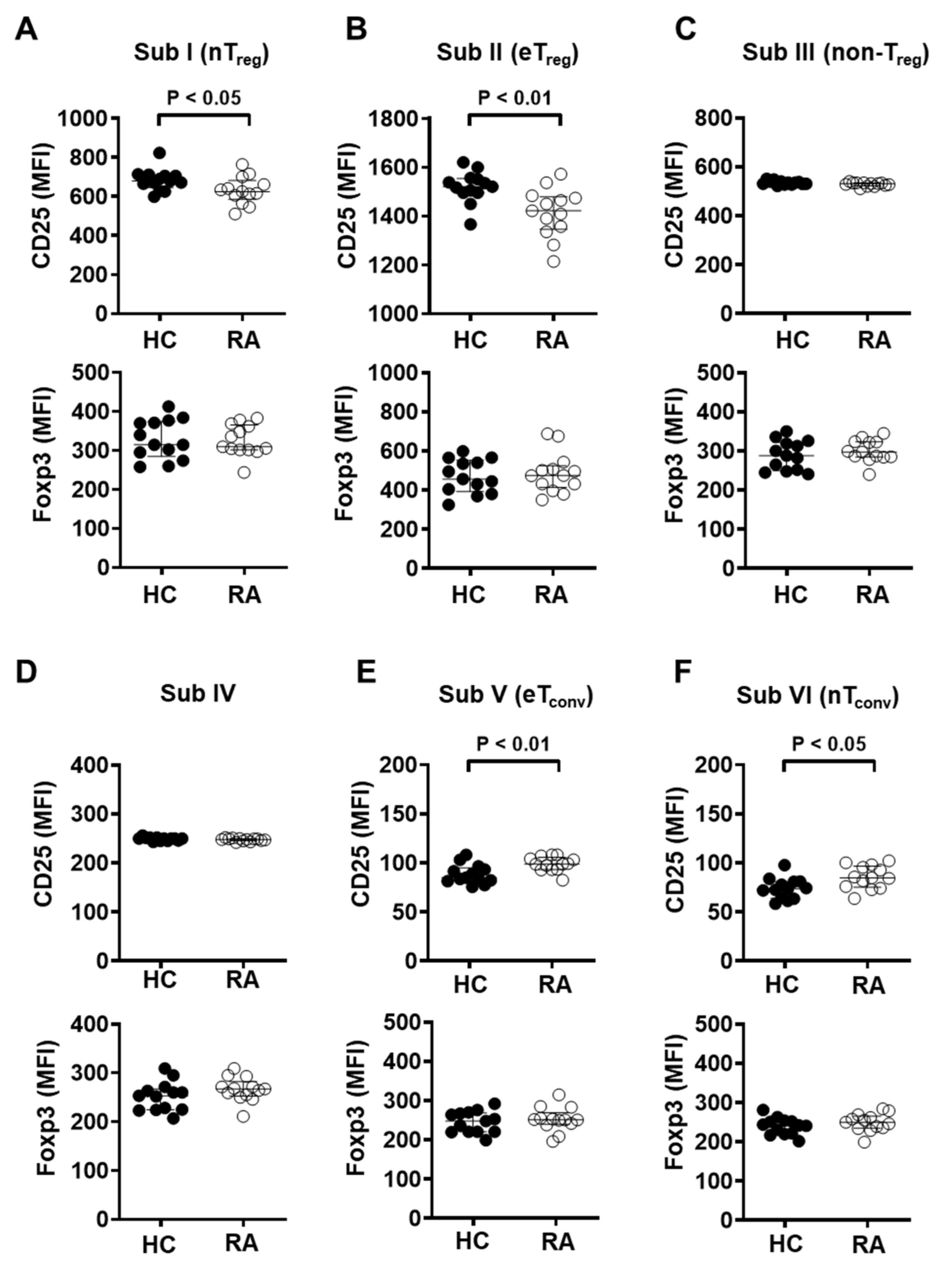
3.4. CD25 Expression in Naïve and Effector Treg Cells Is Significantly Reduced in the Peripheral Blood of RA Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feldmann, M.; Brennan, F.M.; Maini, R.N. Rheumatoid arthritis. Cell 1996, 85, 307–310. [Google Scholar] [CrossRef]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 2018, 4, 18001. [Google Scholar] [CrossRef]
- Shevach, E.M. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 2000, 18, 423–449. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [PubMed]
- Miyara, M.; Gorochov, G.; Ehrenstein, M.; Musset, L.; Sakaguchi, S.; Amoura, Z. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun. Rev. 2011, 10, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Shima, Y.; Wing, J.B.; Sakaguchi, S.; Ogata, A.; Kumanogoh, A. The Proportion of Regulatory T Cells in Patients with Rheumatoid Arthritis: A Meta-Analysis. PLoS ONE 2016, 11, e0162306. [Google Scholar] [CrossRef]
- Ranganathan, P. Pharmacogenomics of tumor necrosis factor antagonists in rheumatoid arthritis. Pharmacogenomics 2005, 6, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Valencia, X.; Stephens, G.; Goldbach-Mansky, R.; Wilson, M.; Shevach, E.M.; Lipsky, P.E. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood 2006, 108, 253–261. [Google Scholar] [CrossRef]
- Grant, C.R.; Liberal, R.; Mieli-Vergani, G.; Vergani, D.; Longhi, M.S. Regulatory T-cells in autoimmune diseases: Challenges, controversies and—Yet—Unanswered questions. Autoimmun. Rev. 2015, 14, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Khattri, R.; Cox, T.; Yasayko, S.A.; Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003, 4, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Colamatteo, A.; Carbone, F.; Bruzzaniti, S.; Galgani, M.; Fusco, C. Molecular Mechanisms Controlling Foxp3 Expression in Health and Autoimmunity: From Epigenetic to Post-translational Regulation. Front. Immunol. 2019, 10, 3136. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Mazor, O.; Wilson, R.I. Thermosensory processing in the Drosophila brain. Nature 2015, 519, 353–357. [Google Scholar] [CrossRef]
- O’Gorman, W.E.; Dooms, H.; Thorne, S.H.; Kuswanto, W.F.; Simonds, E.F. The initial phase of an immune response functions to activate regulatory T cells. J. Immunol. 2009, 183, 332–339. [Google Scholar] [CrossRef]
- Liu, W.; Putnam, A.L.; Xu-Yu, Z.; Szot, G.L.; Lee, M.R. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef]
- Seddiki, N.; Santner-Nanan, B.; Martinson, J.; Zaunders, J.; Sasson, S. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 2006, 203, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Aerts, N.E.; Dombrecht, E.J.; Ebo, D.G.; Bridts, C.H.; Stevens, W.J.; de Clerck, L.S. Activated T cells complicate the identification of regulatory T cells in rheumatoid arthritis. Cell Immunol. 2008, 251, 109–115. [Google Scholar] [CrossRef]
- Kmieciak, M.; Gowda, M.; Graham, L.; Godder, K.; Bear, H.D. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. J. Transl. Med. 2009, 7, 89. [Google Scholar] [CrossRef]
- Letourneau, S.; Krieg, C.; Pantaleo, G.; Boyman, O. IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. J. Allergy Clin. Immunol. 2009, 123, 758–762. [Google Scholar] [CrossRef]
- Malek, T.R.; Castro, I. Interleukin-2 receptor signaling: At the interface between tolerance and immunity. Immunity 2010, 33, 153–165. [Google Scholar] [CrossRef]
- Permanyer, M.; Bosnjak, B.; Glage, S.; Friedrichsen, M.; Floess, S. Efficient IL-2R signaling differentially affects the stability, function, and composition of the regulatory T-cell pool. Cell Mol. Immunol. 2021, 18, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Toomer, K.H.; Lui, J.B.; Altman, N.H.; Ban, Y.; Chen, X.; Malek, T.R. Essential and non-overlapping IL-2Ralpha-dependent processes for thymic development and peripheral homeostasis of regulatory T cells. Nat. Commun. 2019, 10, 1037. [Google Scholar] [CrossRef]
- Toomer, K.H.; Malek, T.R. Cytokine Signaling in the Development and Homeostasis of Regulatory T cells. Cold Spring Harb. Perspect. Biol. 2018, 10, a028597. [Google Scholar] [CrossRef] [PubMed]
- Marquez, A.; Kerick, M.; Zhernakova, A.; Gutierrez-Achury, J.; Chen, W.M. Meta-analysis of Immunochip data of four autoimmune diseases reveals novel single-disease and cross-phenotype associations. Genome Med. 2018, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Eyre, S.; Suzuki, A.; Kochi, Y.; Yamamoto, K. Genetics of rheumatoid arthritis: 2018 status. Ann. Rheum. Dis. 2019, 78, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Knevel, R.; de Rooy, D.P.; Zhernakova, A.; Grondal, G.; Krabben, A. Association of variants in IL2RA with progression of joint destruction in rheumatoid arthritis. Arthritis Rheum. 2013, 65, 1684–1693. [Google Scholar] [CrossRef]
- Van Steenbergen, H.W.; van Nies, J.A.; Ruyssen-Witrand, A.; Huizinga, T.W.; Cantagrel, A. IL2RA is associated with persistence of rheumatoid arthritis. Arthritis Res. Ther. 2015, 17, 244. [Google Scholar] [CrossRef] [PubMed]
- Hulme, M.A.; Wasserfall, C.H.; Atkinson, M.A.; Brusko, T.M. Central role for interleukin-2 in type 1 diabetes. Diabetes 2012, 61, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Golding, A.; Hasni, S.; Illei, G.; Shevach, E.M. The percentage of FoxP3+Helios+ Treg cells correlates positively with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2013, 65, 2898–2906. [Google Scholar] [CrossRef]
- Abbas, A.K.; Trotta, E.; Simeonov, D.R.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3, eaat1482. [Google Scholar] [CrossRef]
- Klatzmann, D.; Abbas, A.K. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol. 2015, 15, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Rosenzwajg, M.; Lorenzon, R.; Cacoub, P.; Pham, H.P.; Pitoiset, F. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis. 2019, 78, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Von Spee-Mayer, C.; Siegert, E.; Abdirama, D.; Rose, A.; Klaus, A. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2016, 75, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, G.; Gray, E.; Mastoridis, S.; Merritt, E.; Kodela, E. IL-2 therapy restores regulatory T-cell dysfunction induced by calcineurin inhibitors. Proc. Natl. Acad. Sci. USA 2017, 114, 7083–7088. [Google Scholar] [CrossRef]
- Miao, J.; Zhu, P. Functional Defects of Treg Cells: New Targets in Rheumatic Diseases, Including Ankylosing Spondylitis. Curr. Rheumatol. Rep. 2018, 20, 30. [Google Scholar] [CrossRef]
- Miyara, M.; Ito, Y.; Sakaguchi, S. TREG-cell therapies for autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 2014, 10, 543–551. [Google Scholar] [CrossRef]
- Sun, H.; Gao, W.; Pan, W.; Zhang, Q.; Wang, G. Tim3(+) Foxp3 (+) Treg Cells Are Potent Inhibitors of Effector T Cells and Are Suppressed in Rheumatoid Arthritis. Inflammation 2017, 40, 1342–1350. [Google Scholar] [CrossRef]
- Malemud, C.J. Defective T-Cell Apoptosis and T-Regulatory Cell Dysfunction in Rheumatoid Arthritis. Cells 2018, 7, 223. [Google Scholar] [CrossRef]
- Cribbs, A.P.; Kennedy, A.; Penn, H.; Amjadi, P.; Green, P. Methotrexate Restores Regulatory T Cell Function Through Demethylation of the FoxP3 Upstream Enhancer in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2015, 67, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Ahmed, S.; Jain, A.; Misra, R. Emerging role of metabolomics in rheumatology. Int. J. Rheum. Dis. 2018, 21, 1468–1477. [Google Scholar] [CrossRef]
- Nguyen, D.X.; Cotton, A.; Attipoe, L.; Ciurtin, C.; Dore, C.J.; Ehrenstein, M.R. Regulatory T cells as a biomarker for response to adalimumab in rheumatoid arthritis. J. Allergy Clin. Immunol. 2018, 142, 978–980.e9. [Google Scholar] [CrossRef]
- Tada, Y.; Ono, N.; Suematsu, R.; Tashiro, S.; Sadanaga, Y. The balance between Foxp3 and Ror-gammat expression in peripheral blood is altered by tocilizumab and abatacept in patients with rheumatoid arthritis. BMC Musculoskelet. Disord. 2016, 17, 290. [Google Scholar] [CrossRef] [PubMed]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Prevoo, M.L.; van ’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perea, A.L.; Arcia, E.D.; Rueda, C.M.; Velilla, P.A. Phenotypical characterization of regulatory T cells in humans and rodents. Clin. Exp. Immunol. 2016, 185, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Santegoets, S.J.; Dijkgraaf, E.M.; Battaglia, A.; Beckhove, P.; Britten, C.M. Monitoring regulatory T cells in clinical samples: Consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol. Immunother. 2015, 64, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, E.; van den Biggelaar, M.; de Kivit, S.; Chen, Y.Y.; Slot, M. Proteomic Analyses of Human Regulatory T Cells Reveal Adaptations in Signaling Pathways that Protect Cellular Identity. Immunity 2018, 48, 1046–1059.e6. [Google Scholar] [CrossRef]
- Wing, J.B.; Tanaka, A.; Sakaguchi, S. Human FOXP3(+) Regulatory T Cell Heterogeneity and Function in Autoimmunity and Cancer. Immunity 2019, 50, 302–316. [Google Scholar] [CrossRef]
- Combe, B.; Pope, R.M.; Fischbach, M.; Darnell, B.; Baron, S.; Talal, N. Interleukin-2 in rheumatoid arthritis: Production of and response to interleukin-2 in rheumatoid synovial fluid, synovial tissue and peripheral blood. Clin. Exp. Immunol. 1985, 59, 520–528. [Google Scholar] [PubMed]
- Kitas, G.D.; Salmon, M.; Farr, M.; Gaston, J.S.; Bacon, P.A. Deficient interleukin 2 production in rheumatoid arthritis: Association with active disease and systemic complications. Clin. Exp. Immunol. 1988, 73, 242–249. [Google Scholar] [PubMed]
- Kitas, G.D.; Salmon, M.; Farr, M.; Young, S.P.; Bacon, P.A. T-cell functional defects in rheumatoid arthritis: Intrinsic or extrinsic? J. Autoimmun. 1988, 1, 339–351. [Google Scholar] [CrossRef]
- Skapenko, A.; Wendler, J.; Lipsky, P.E.; Kalden, J.R.; Schulze-Koops, H. Altered memory T cell differentiation in patients with early rheumatoid arthritis. J. Immunol. 1999, 163, 491–499. [Google Scholar] [PubMed]
- Cao, S.; Xiang, Z.; Ma, X. Global gene expression profiling in interleukin-12-induced activation of CD8(+) cytotoxic T lymphocytes against mouse mammary Carcinoma. Cell Mol. Immunol. 2004, 1, 357–366. [Google Scholar] [PubMed]
- Jiao, Z.; Wang, W.; Jia, R.; Li, J.; You, H. Accumulation of FoxP3-expressing CD4+CD25+ T cells with distinct chemokine receptors in synovial fluid of patients with active rheumatoid arthritis. Scand. J. Rheumatol. 2007, 36, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Sempere-Ortells, J.M.; Perez-Garcia, V.; Marin-Alberca, G.; Peris-Pertusa, A.; Benito, J.M. Quantification and phenotype of regulatory T cells in rheumatoid arthritis according to disease activity score-28. Autoimmunity 2009, 42, 636–645. [Google Scholar] [CrossRef]
- Ehrenstein, M.R.; Evans, J.G.; Singh, A.; Moore, S.; Warnes, G. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J. Exp. Med. 2004, 200, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Han, G.M.; O’Neil-Andersen, N.J.; Zurier, R.B.; Lawrence, D.A. CD4+CD25high T cell numbers are enriched in the peripheral blood of patients with rheumatoid arthritis. Cell Immunol. 2008, 253, 92–101. [Google Scholar] [CrossRef]
- Van Amelsfort, J.M.; Jacobs, K.M.; Bijlsma, J.W.; Lafeber, F.P.; Taams, L.S. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: Differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004, 50, 2775–2785. [Google Scholar] [CrossRef]
- Fonseka, C.Y.; Rao, D.A.; Teslovich, N.C.; Korsunsky, I.; Hannes, S.K. Mixed-effects association of single cells identifies an expanded effector CD4(+) T cell subset in rheumatoid arthritis. Sci. Transl. Med. 2018, 10, eaaq0305. [Google Scholar] [CrossRef]
- Liao, W.; Lin, J.X.; Leonard, W.J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013, 38, 13–25. [Google Scholar] [CrossRef]
- Haque, R.; Lei, F.; Xiong, X.; Wu, Y.; Song, J. FoxP3 and Bcl-xL cooperatively promote regulatory T cell persistence and prevention of arthritis development. Arthritis Res. Ther. 2010, 12, R66. [Google Scholar] [CrossRef] [PubMed]
- Issa, F.; Milward, K.; Goto, R.; Betts, G.; Wood, K.J.; Hester, J. Transiently Activated Human Regulatory T Cells Upregulate BCL-XL Expression and Acquire a Functional Advantage in vivo. Front. Immunol. 2019, 10, 889. [Google Scholar] [CrossRef]
- Murase, K.; Kawano, Y.; Ryan, J.; Matsuoka, K.; Gregory, B. Low-Dose IL-2 Induces Bcl2 Expression and Resistance To Apoptosis In CD4 Regulatory T Cells. Blood 2013, 122, 3475. [Google Scholar] [CrossRef]
- Sharabi, A.; Lapter, S.; Mozes, E. Bcl-xL is required for the development of functional regulatory CD4 cells in lupus-afflicted mice following treatment with a tolerogenic peptide. J. Autoimmun. 2010, 34, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Tischner, D.; Gaggl, I.; Peschel, I.; Kaufmann, M.; Tuzlak, S. Defective cell death signalling along the Bcl-2 regulated apoptosis pathway compromises Treg cell development and limits their functionality in mice. J. Autoimmun. 2012, 38, 59–69. [Google Scholar] [CrossRef] [PubMed][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Go, E.; Yoo, S.-J.; Choi, S.; Sun, P.; Jung, M.K.; Kwon, S.; Heo, B.Y.; Kim, Y.; Kang, J.-G.; Kim, J.; et al. Peripheral Blood from Rheumatoid Arthritis Patients Shows Decreased Treg CD25 Expression and Reduced Frequency of Effector Treg Subpopulation. Cells 2021, 10, 801. https://doi.org/10.3390/cells10040801
Go E, Yoo S-J, Choi S, Sun P, Jung MK, Kwon S, Heo BY, Kim Y, Kang J-G, Kim J, et al. Peripheral Blood from Rheumatoid Arthritis Patients Shows Decreased Treg CD25 Expression and Reduced Frequency of Effector Treg Subpopulation. Cells. 2021; 10(4):801. https://doi.org/10.3390/cells10040801
Chicago/Turabian StyleGo, Eunbyeol, Su-Jin Yoo, Suyoung Choi, Pureum Sun, Min Kyung Jung, Somin Kwon, Bu Yeon Heo, Yeeun Kim, Ju-Gyeong Kang, Jinhyun Kim, and et al. 2021. "Peripheral Blood from Rheumatoid Arthritis Patients Shows Decreased Treg CD25 Expression and Reduced Frequency of Effector Treg Subpopulation" Cells 10, no. 4: 801. https://doi.org/10.3390/cells10040801
APA StyleGo, E., Yoo, S.-J., Choi, S., Sun, P., Jung, M. K., Kwon, S., Heo, B. Y., Kim, Y., Kang, J.-G., Kim, J., Shin, E.-C., Kang, S. W., & Kwon, J. (2021). Peripheral Blood from Rheumatoid Arthritis Patients Shows Decreased Treg CD25 Expression and Reduced Frequency of Effector Treg Subpopulation. Cells, 10(4), 801. https://doi.org/10.3390/cells10040801






