DiseaseLinc: Disease Enrichment Analysis of Sets of Differentially Expressed LincRNAs
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Web Server Implementation
2.3. A Case Study Using Breast Cancer LincRNAs
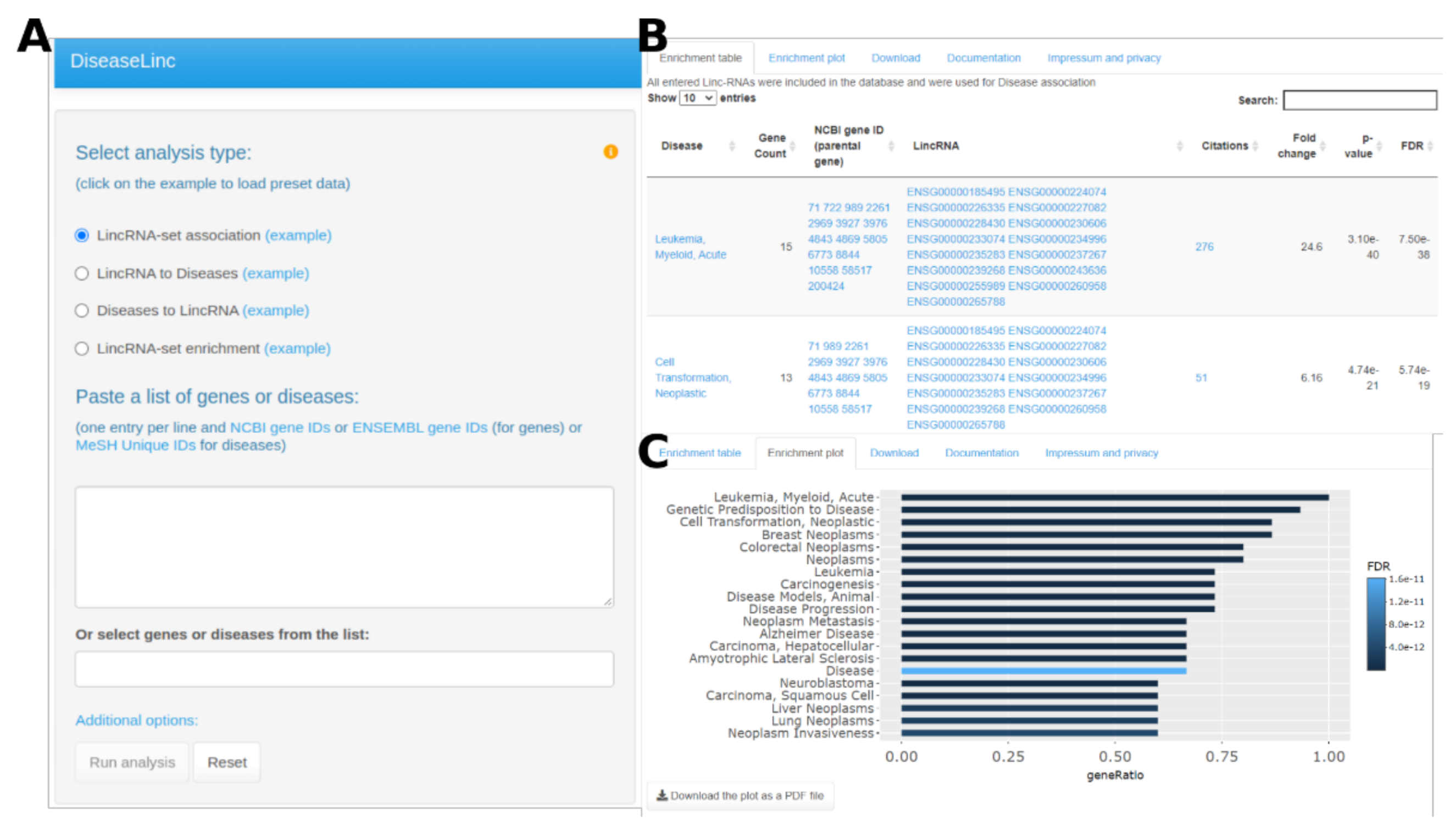
3. Results
3.1. Web Server Usage
3.2. LincRNA Expression Is Associated with Worse Prognosis in Breast Cancer
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, J.C.R.; Acuña, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Non-Coding Rna 2019, 5. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, J.; Gu, C.; Yang, Y. Alternative Splicing and Cancer: A Systematic Review. Signal Transduct. Target. 2021, 6, 78. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Lu, F.-H.; Huang, X.-R.; Zhang, L.; Mao, W.; Yu, X.-Q.; Liu, X.-S.; Lan, H.-Y. Non-Coding RNAs as Biomarkers and Therapeutic Targets for Diabetic Kidney Disease. Front. Pharm. 2020, 11, 583528. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Zhou, X.-Y.; Du, X. Circulating Long Non-Coding RNAs in Cancer: Current Status and Future Perspectives. Mol. Cancer 2016, 15, 39. [Google Scholar] [CrossRef]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szcześniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A Survey of Best Practices for RNA-Seq Data Analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Forne, I.; Imhof, A. Bioinformatic Analysis of Proteomics Data. BMC Syst. Biol. 2014, 8 (Suppl. S2), S3. [Google Scholar] [CrossRef] [PubMed]
- Muro, E.M.; Mah, N.; Andrade-Navarro, M.A. Functional Evidence of Post-Transcriptional Regulation by Pseudogenes. Biochimie 2011, 93, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- Muro, E.M.; Andrade-Navarro, M.A. Pseudogenes as an Alternative Source of Natural Antisense Transcripts. BMC Evol. Biol. 2010, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Poliseno, L.; Salmena, L.; Zhang, J.; Carver, B.; Haveman, W.J.; Pandolfi, P.P. A Coding-Independent Function of Gene and Pseudogene mRNAs Regulates Tumour Biology. Nature 2010, 465, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Ghosh, T.C. Pseudogenes and Their Composers: Delving in the “debris” of Human Genome. Brief Funct. Genom. 2013, 12, 536–547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Talyan, S.; Andrade-Navarro, M.A.; Muro, E.M. Identification of Transcribed Protein Coding Sequence Remnants within LincRNAs. Nucleic Acids Res. 2018, 46, 8720–8729. [Google Scholar] [CrossRef] [PubMed]
- Ebersberger, I.; Metzler, D.; Schwarz, C.; Pääbo, S. Genomewide Comparison of DNA Sequences between Humans and Chimpanzees. Am. J. Hum. Genet. 2002, 70, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, J.F.; Andrade-Navarro, M.A. Gene Set to Diseases (GS2D): Disease Enrichment Analysis on Human Gene Sets with Literature Data. Genom. Comput. Biol. 2016, 2, 33. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Li, J.; Han, L.; Roebuck, P.; Diao, L.; Liu, L.; Yuan, Y.; Weinstein, J.N.; Liang, H. TANRIC: An Interactive Open Platform to Explore the Function of LncRNAs in Cancer. Cancer Res. 2015, 75, 3728–3737. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-C.; Spitale, R.C.; Chang, H.Y. Long Intergenic Noncoding RNAs: New Links in Cancer Progression. Cancer Res. 2011, 71, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.-C.; Ni, J.-J.; Cui, W.-Y.; Wang, B.-Y.; Zhuo, W. Emerging Roles of LncRNA in Cancer and Therapeutic Opportunities. Am. J. Cancer Res. 2019, 9, 1354–1366. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
More, P.; Talyan, S.; Fontaine, J.-F.; Muro, E.M.; Andrade-Navarro, M.A. DiseaseLinc: Disease Enrichment Analysis of Sets of Differentially Expressed LincRNAs. Cells 2021, 10, 751. https://doi.org/10.3390/cells10040751
More P, Talyan S, Fontaine J-F, Muro EM, Andrade-Navarro MA. DiseaseLinc: Disease Enrichment Analysis of Sets of Differentially Expressed LincRNAs. Cells. 2021; 10(4):751. https://doi.org/10.3390/cells10040751
Chicago/Turabian StyleMore, Piyush, Sweta Talyan, Jean-Fred Fontaine, Enrique M. Muro, and Miguel A. Andrade-Navarro. 2021. "DiseaseLinc: Disease Enrichment Analysis of Sets of Differentially Expressed LincRNAs" Cells 10, no. 4: 751. https://doi.org/10.3390/cells10040751
APA StyleMore, P., Talyan, S., Fontaine, J.-F., Muro, E. M., & Andrade-Navarro, M. A. (2021). DiseaseLinc: Disease Enrichment Analysis of Sets of Differentially Expressed LincRNAs. Cells, 10(4), 751. https://doi.org/10.3390/cells10040751








