A Molecular Networking Strategy: High-Throughput Screening and Chemical Analysis of Brazilian Cerrado Plant Extracts against Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Extracts
2.2. Antiproliferative Bioassays
2.3. HRESIMS Data Acquisition
2.4. Data Processing and Molecular Networking Construction
3. Results
3.1. High-Throughput Screening
3.2. Molecular Networking
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Lahsen, M.; Mercedes, M.C.; Bustamante, M.M.C.; Dalla-Nora, E.L. Undervaluing and Overexploiting the Brazilian Cerrado at Our Peril. Environ. Sci. Policy Sustain. Dev. 2016, 58, 4–15. [Google Scholar] [CrossRef]
- Wang, M.X.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechn. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Pilon, A.C.; Selegato, D.M.; Fernandes, R.P.; Bueno, P.C.P.; Pinho, D.R.; Neto, F.C.; Freire, R.T.; Castro-Gamboa, I.; Bolzani, V.S.; Lopes, N.P. Metabolômica de Plantas: Métodos e Desafios. Quim. Nova 2020, 43, 329–354. [Google Scholar] [CrossRef]
- Demarque, D.P.; Dusi, R.G.; de Sousa, F.D.M.; Grossi, S.M.; Silverio, M.R.S.; Lopes, N.P.; Espindola, L.S. Mass Spectrometry-Based Metabolomics Approach in the Isolation of Bioactive Natural Products. Sci. Rep. 2020, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Tosun, F.; Mihoglugil, F.; Beutler, J.A.A.; Eroglu Ozkan, E.; Miski, M. Neopapillarine, an Unusual Coumarino-Alkaloid from the Root Extract of Neocryptodiscus papillaris with Cytotoxic Activity on Renal Cancer Cells. Molecules 2020, 25, 3040. [Google Scholar] [CrossRef] [PubMed]
- Devkota, K.P.; Covell, D.; Ransom, T.; McMahon, J.B.; Beutler, J.A. Growth Inhibition of Human Colon Carcinoma Cells by Sesquiterpenoids and Tetralones of Zygogynum Calothyrsum. J. Nat. Prod. 2013, 76, 710–714. [Google Scholar] [CrossRef]
- Long, S.A.; Huang, S.; Kambala, A.; Ren, L.; Wilson, J.; Goetz, M.; Hao, X.; Yang, X.; Goncharova, E.I.; Jia, L.; et al. Identification of Potential Modulators Of Osteosarcoma Metastasis by High-Throughput Cellular Screening of Natural Products. Chem. Biol. Drug Des. 2021, 97, 77–86. [Google Scholar] [CrossRef]
- Henrich, C.J.; Budhu, A.; Yu, Z.; Evans, J.R.; Goncharova, E.I.; Ransom, T.T.; Wang, X.W.; McMahon, J.B. High-Throughput Screening for Identification of Inhibitors of EpCAM-Dependent Growth of Hepatocellular Carcinoma Cells. Chem. Biol. Drug Des. 2013, 82, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.H. The NCI60 Human Tumour Cell Line Anticancer Drug Screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- Su, G.; Burant, C.F.; Beecher, C.W.; Athey, B.D.; Meng, F. Integrated Metabolome and Transcriptome Analysis of the NCI60 Dataset. BMC Bioinform. 2011, 12, 36. [Google Scholar] [CrossRef]
- Holbeck, S.L.; Collins, J.M.; Doroshow, J.H. Analysis of Food and Drug Administration-Approved Anticancer Agents in the NCI60 Panel of Human Tumor Cell Lines. Mol. Cancer Ther 2010, 9, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- EMBRAPA. Empresa Brasileira de Pesquisa Agropecuária. Mapa de Solos do Distrito Federal (1:100.000); EMBRAPA-PI. Serviço de Produção de Informação: Brasília, Brazil, 1978. [Google Scholar]
- United States. Science, & Education Administration. Soil taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; US Department of Agriculture: Beltsville, MD, USA, 1975.
- Tang, H.F.; Yun, J.; Lin, H.W.; Chen, X.L.; Wang, X.J.; Cheng, G. Two New Triterpenoid Saponins Cytotoxic to Human Glioblastoma U251MG Cells from Ardisia Pusilla. Chem. Biodivers. 2009, 6, 1443–1452. [Google Scholar] [CrossRef]
- Dong, W.; Liu, X.; Li, X.; Yang, D.; Ding, L. A New Triterpene Saponin. Andr. Integr. Fitoter. 2011, 82, 782–785. [Google Scholar] [CrossRef]
- Katajamaa, M.; Miettinen, J.; Oresic, M. MZmine: Toolbox for Processing and Visualization of Mass Spectrometry Based Molecular Profile Data. Bioinformatics 2006, 22, 634–636. [Google Scholar] [CrossRef]
- Olivon, F.; Grelier, G.; Roussi, F.; Litaudon, M.; Touboul, D. MZmine 2 Data-Preprocessing to Enhance Molecular Networking Reliability. Anal. Chem. 2017, 89, 7836–7840. [Google Scholar] [CrossRef] [PubMed]
- Myers, O.D.; Sumner, S.J.; Li, S.; Barnes, S.; Du, X. One Step Forward for Reducing False Positive and False Negative Compound Identifications from Mass Spectrometry Metabolomics Data: New Algorithms for Constructing Extracted Ion Chromatograms and Detecting Chromatographic Peaks. Anal. Chem. 2017, 89, 8696–8703. [Google Scholar] [CrossRef]
- Nothias, L.F.; Petras, D.; Schmid, R.; Duhrkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Dictionary of Natural Products 28.1. Available online: http://dnp.chemnetbase.com/ (accessed on 9 January 2020).
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS Online: A Web-Based Platform to Process Untargeted Metabolomic Data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Domingo-Almenara, X.; Palermo, A.; Warth, B.; Hermann, G.; Koellensperger, G.; Huan, T.; Uritboonthai, W.; Aisporna, A.E.; et al. METLIN: A Technology Platform for Identifying Knowns and Unknowns. Anal. Chem. 2018, 90, 3156–3164. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.C.; Santana, D.B.; Araujo, R.M.; de Paula, J.E.; do Nascimento, P.C.; Lopes, N.P.; Braz-Filho, R.; Espindola, L.S. Discovery of the Rapanone and Suberonone Mixture as a Motif for Leishmanicidal and Antifungal Applications. Bioorg. Med. Chem. 2014, 22, 135–140. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhao, X.E.; Zhu, S.; Dang, J.; Qiao, X.; Qiu, Z.; Tao, Y. Simultaneous Determination of Oleanolic Acid and Ursolic Acid by in Vivo Microdialysis via UHPLC-MS/MS Using Magnetic Dispersive Solid Phase Extraction Coupling with Microwave-Assisted Derivatization and Its Application to a Pharmacokinetic Study of Arctiumlappa, L. Root Extract in Rats. J. Agric. Food Chem. 2018, 66, 3975–3982. [Google Scholar] [CrossRef]
- Amara, S.; Zheng, M.; Tiriveedhi, V. Oleanolic Acid Inhibits High Salt-Induced Exaggeration of Warburg-like Metabolism in Breast Cancer Cells. Cell Biochem. Biophys 2016, 74, 427–434. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Novotny, L.; Abdel-Hamid, M.E.; Hamza, H.; Masterova, I.; Grancai, D. Development of LC-MS Method for Determination of Ursolic Acid: Application to the Analysis of Ursolic Acid in Staphylea holocarpa Hemsl. J. Pharm. Biomed. Anal. 2003, 31, 961–968. [Google Scholar] [CrossRef]
- Kahnt, M.; Heller, L.; Grabandt, P.; Al-Harrasi, A.; Csuk, R. Platanic acid: A New Scaffold for the Synthesis of Cytotoxic Agents. Eur. J. Med. Chem. 2018, 143, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, I.; Peixoto, J.L.B.; Silva, C.C.D.; Shuquel, I.T.A.; Basso, E.A.; Vidotti, G.J. Triterpenic Acids from Eugenia Moraviana. J. Braz. Chem. Soc. 2001, 12, 180–183. [Google Scholar] [CrossRef]
- Fujioka, T.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, L.M.; Ballas, L.M.; Jiang, J.B.; Janzen, W.P.; Chen, I.S.; Lee, K.H. Anti-AIDS Agents, 11. Betulinic Acid and Platanic Acid as Anti-HIV Principles from Syzigium Claviflorum, and the Anti-HIV Activity of Structurally Related Triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef]
- Dhar Dubey, K.K.; Sharma, G.; Kumar, A. Conjugated Linolenic Acids: Implication in Cancer. J. Agric. Food Chem. 2019, 67, 6091–6101. [Google Scholar] [CrossRef]
- Giordano, C.; Plastina, P.; Barone, I.; Catalano, S.; Bonofiglio, D. N-3 Polyunsaturated Fatty Acid Amides: New Avenues in the Prevention and Treatment of Breast Cancer. Int. J. Mol. Sci. 2020, 21, 2279. [Google Scholar] [CrossRef] [PubMed]
- Okubo, R.; Noguchi, H.; Hamazaki, K.; Sekiguchi, M.; Kinoshita, T.; Katsumata, N.; Narisawa, T.; Uezono, Y.; Xiao, J.; Matsuoka, Y.J. Fear of Cancer Recurrence Among Breast Cancer Survivors Could be Controlled by Prudent Dietary Modification with Polyunsaturated Fatty Acids. J. Affect. Disord 2019, 245, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Espindola, L.S.; Dusi, R.G.; Demarque, D.P.; Braz, R.; Yan, P.C.; Bokesch, H.R.; Gustafson, K.R.; Beutler, J.A. Cytotoxic Triterpenes from Salacia Crassifolia and Metabolite Profiling of Celastraceae Species. Molecules 2018, 23, 1494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, Y.; Zhong, J.; Bi, Y.; Liu, Y.; Ren, Z.; Li, X.; Jia, J.; Yu, M.; Yu, X. Pristimerin Induces Apoptosis and Autophagy via Activation of ROS/ASK1/JNK Pathway in Human Breast Cancer in Vitro and in Vivo. Cell Death Discov. 2019, 5, 125. [Google Scholar] [CrossRef]
- Kucera, O.; Mezera, V.; Moravcova, A.; Endlicher, R.; Lotkova, H.; Drahota, Z.; Cervinkova, Z. In Vitro Toxicity of Epigallocatechin Gallate in Rat Liver Mitochondria and Hepatocytes. Oxid Med. Cell Longev. 2015, 2015, 476180. [Google Scholar] [CrossRef] [PubMed]
- Kurbitz, C.; Heise, D.; Redmer, T.; Goumas, F.; Arlt, A.; Lemke, J.; Rimbach, G.; Kalthoff, H.; Trauzold, A. Epicatechin Gallate and Catechin Gallate are Superior to Epigallocatechin Gallate in Growth Suppression and Anti-Inflammatory Activities in Pancreatic Tumor Cells. Cancer Sci. 2011, 102, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.; Braca, A.; De Tommasi, N.; Morelli, I. Further Constituents from Caralluma Negevensis. Phytochemistry 2003, 62, 1277–1281. [Google Scholar] [CrossRef]
- Wishart, D.S. Is Cancer a Genetic Disease or a Metabolic Disease? EBioMedicine 2015, 2, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Albernaz, L.C.; de Paula, J.E.; Romero, G.A.; Silva Mdo, R.; Grellier, P.; Mambu, L.; Espindola, L.S. Investigation of Plant Extracts in Traditional Medicine of the Brazilian Cerrado Against Protozoans and Yeasts. J. Ethnopharmacol 2010, 131, 116–121. [Google Scholar] [CrossRef]
- De Mesquita, M.L.; Leao, W.F.; Ferreira, M.R.; de Paula, J.E.; Espindola, L.S.; Soares, L.A. Reversed-Phase-Liquid Chromatography Method for Separation and Quantification of Gallic Acid from Hydroalcoholic Extracts of Qualea Grandiflora and Qualea Parviflora. Pharmacogn. Mag. 2015, 11, 316–321. [Google Scholar] [CrossRef]
- De Mesquita, M.L.; de Paula, J.E.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V.; Grougnet, R.; Michel, S.; Tillequin, F.; Espindola, L.S. Cytotoxic Activity of Brazilian Cerrado Plants Used in Traditional Medicine Against Cancer Cell Lines. J. Ethnopharmaco. 2009, 123, 439–445. [Google Scholar] [CrossRef] [PubMed]
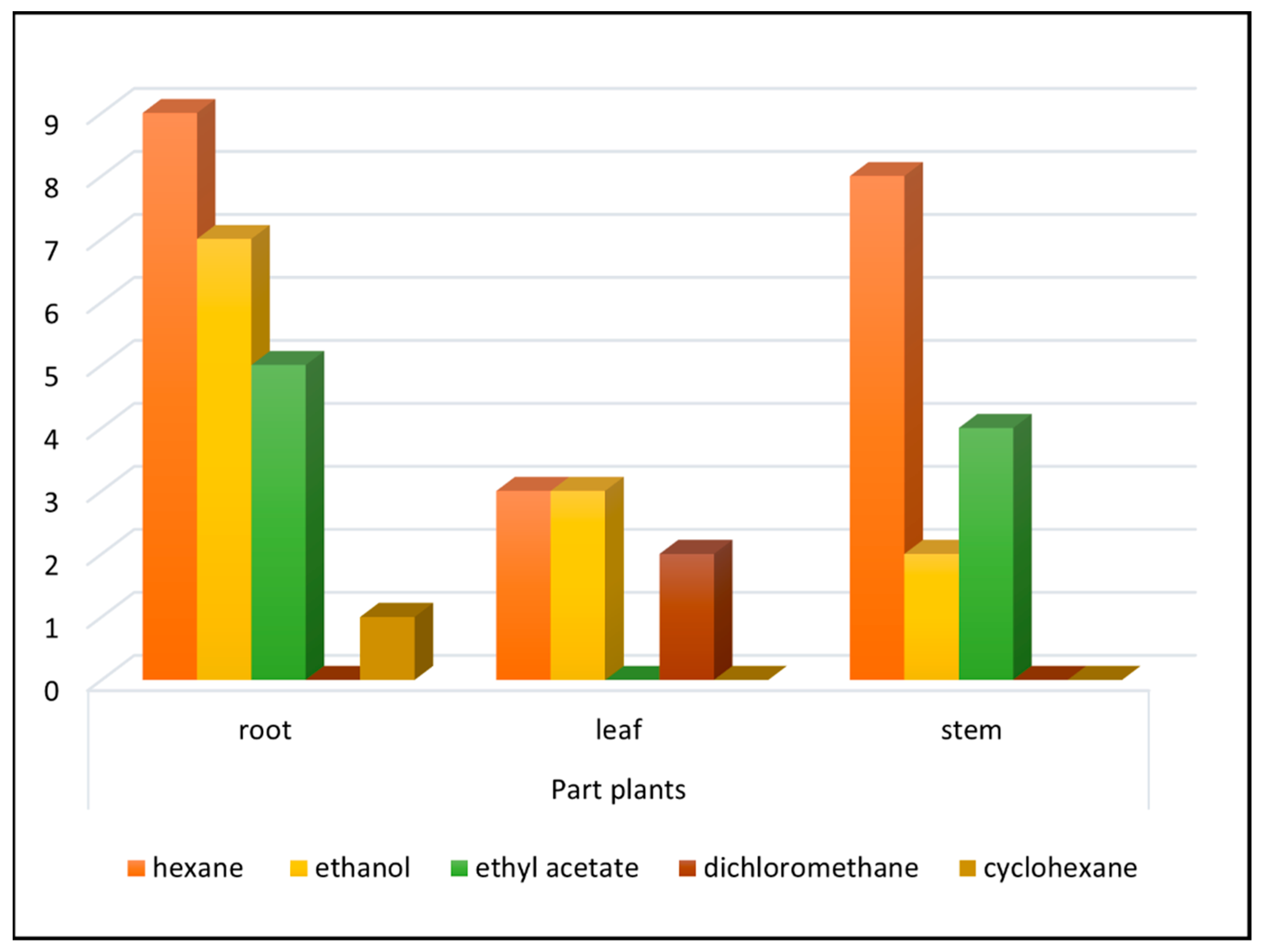
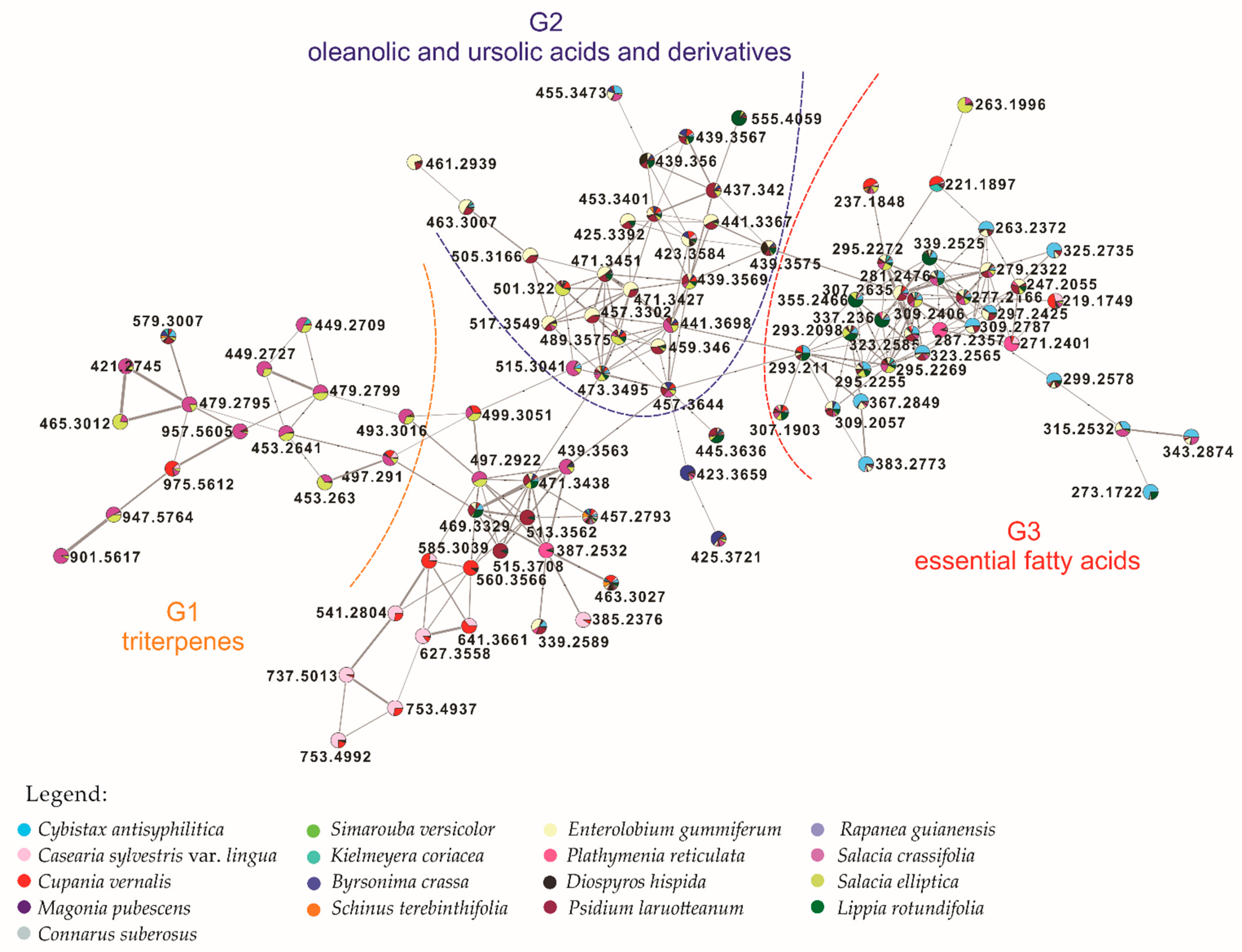
| Plant Species/Plant Organ (Solvent)* | Codes BR/NCI60 | IC50 (μg/mL) | NCI-60 Sensitivity Potency (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Colon | Renal | Osteosarcoma | ||||||||
| Colo205 | KM12 | A498 | U031 | MG63 | MG 63.3 | Mean GI50/Most Sensitive Cell Line | Mean TGI | Mean LC50 | ||
| Peschiera affinis SB (h) | BR 075 | 17.8 | 19.1 | >20 | >20 | >20 | >20 | - | - | - |
| Cybistax antisyphilitica SB (h) | BR 125 N192795 | - | - | - | - | - | - | 85 | >100 | >100 |
| Tabebuia caraiba L (h) | BR 139 | >20 | 18.9 | >20 | >20 | >20 | >20 | - | - | - |
| Casearia sylvestris var. lingua SW (h) | BR 177 N192825 | 4.6 | 4.5 | 3.6 | 2.7 | 3.9 | 5.7 | 11/2.5 NCI-H522 | 28 | 76 |
| Cupania vernalis L (h) | BR 193 N192827 | 12.3 | 10 | 18.7 | 7.6 | 13.8 | 15 | 43 | 140 | 490 |
| Magonia pubescens R (e) | BR 197 | 4.2 | 7.2 | 3.6 | 3.9 | 14.5 | 14.5 | - | - | - |
| Magonia pubescens RW (e) | BR 204 N192797 | 3.5 | 5.5 | 2.9 | 3.8 | 15.4 | 15.6 | 15/0.3SR | 34 | 81 |
| Simarouba versicolor RB (e) | BR 254 N192829 | 6.8 | >20 | 3.7 | 5 | <1.3 | <1.3 | 4.9/0.7 NCI-H522 | 29 | 620 |
| Simaba suffruticosa L (a) | BR 261 | 16.8 | 1.6 | >20 | 17.6 | 2.2 | 4.9 | - | - | - |
| Kielmeyera coriacea SW (h) | BR 331 N192831 | 11.9 | 4.9 | 15.9 | 14.9 | >20 | >20 | 56 | 210 | 600 |
| Byrsonima crassa RB (h) | BR 411 N192833 | 15 | 15 | >20 | >20 | >20 | >20 | 220 | 530 | 910 |
| Schinus terebinthifolia L (d) | BR 436 N192835 | 12.4 | 6.9 | 16.2 | 7.8 | 3.4 | 14.8 | 100/0.9 NCI-H522 | 87 | 360 |
| Enterolobium gummiferum RW (e) | BR 467 | 10.5 | 10.9 | <1.3 | 4.6 | 12.6 | 14.9 | - | - | - |
| Enterolobium gummiferum SB (h) | BR 469 N192837 | 2.2 | 4.9 | >20 | >20 | >20 | >20 | 180 | 710 | 980 |
| Plathymenia reticulata RW (h) | BR 489 N192839 | 8.9 | 6.7 | 12.4 | 4.9 | >20 | >20 | 60 | 330 | 680 |
| Diospyros hispida R (a) | BR 501 N192799 | - | - | - | - | - | - | 7/0.7 NCI-H522 | 30 | 81 |
| Maprounea guianensis RB (a) | BR 536 | 8.1 | 9.9 | 6.7 | >20 | 9.6 | 8.5 | - | - | - |
| Psidium laruotteanum SB (h) | BR 549 N192841 | - | - | - | - | - | - | 87 | 340 | 830 |
| Andira humilis SB (e) | BR 587 | >20 | 18.6 | >20 | >20 | >20 | >20 | - | - | - |
| Rapanea guianensis SW (h) | BR 624 | 5.3 | 3.2 | 4.3 | 4.4 | 17.8 | 14.9 | - | - | - |
| Rapanea guianensis RW (e) | BR 627 N192801 | 3.5 | 4.0 | 2.7 | 3.7 | 14.6 | 14 | 16/2.4 SR | 361 | 81 |
| Salacia crassifolia RW (h) | BR 640 N192803 | >20 | 1.7 | 1.6 | >20 | 3.7 | 5.7 | 0.3/0.1 HCT-15 | 1.0 | 5.0 |
| Salacia elliptica RW (a) | BR 652 N192805 | 3.6 | 3.4 | 3.5 | 2.0 | 5.1 | 7.1 | 2.0/0.7 MOLT-4 | 5.1 | 20 |
| Lippia rotundifolia SW (a) | BR 660 N192843 | 12.3 | 9.9 | >20 | >20 | >20 | >20 | - | - | - |
| Connarus suberosus RW (a) | BR 693 N192845 | 5.7 | 4.5 | 4.7 | 7.7 | 17.6 | 19 | 44 | 200 | 830 |
| Compound Name | Molecular Formula | m/z | Rt (min) |
|---|---|---|---|
| oleanolic acid* | C30H48O3 | 439.3567 | 9.7 |
| ursolic acid* | C30H48O3 | 439.3567 | 9.7 |
| 3α-cyclopenta[α]chrysene-3α-carboxylic acid | C29H46O4 | 441.3367 | 7.5 |
| platanic acid | C29H46O4 | 459.346 | 7.6 |
| linoleic acid | C18H32O2 | 281.2476 | 8.4 |
| linoleic acid ethyl ester | C20H36O3 | 309.2787 | 10.0 |
| linolenic acid ethyl ester | C20H34O2 | 307.2635 | 9.6 |
| 13-keto-9Z,11E-octadecadienoic acid | C18H30O3 | 295.2272 | 8.4 |
| 9-oxo-10E,12Z-octadecadienoic acid | C18H30O3 | 295.2269 | 8.5 |
| stearidonic acid | C18H28O2 | 277.2166 | 8.3 |
| 9(10)-epoxy-12Z-octadecenoic acid | C18H32O3 | 279.2322 | 8.1 |
| 9S,13R-12-oxophytodienoic acid | C18H28O3 | 293.2098 | 5.3 |
| pristimerin | C30H40O4 | 465.3012 | 10.3 |
| tingenone | C28H36O3 | 421.2745 | 8.6 |
| 20-oxo-20,21-seco-tingen-21-oic acid | C28H36O5 | 453.263 | 5.9 |
| (-)-catechin gallate | C22H18O10 | 443.0972 | 3.7 |
| epigallocatechin gallate | C22H17O11 | 459.0944 | 3.2 |
| luteolin 3′,4′-di-O-beta-D-glucopyranoside | C27H29O16 | 628.1959 | 10.7 |
| 13-docosenamide | C22H43NO3 | 338.342 | 11.7 |
| 9-octadecenamide | C18H35NO | 282.2793 | 10.1 |
| N-phenyl-1-naphthylamine | C16H13N | 220.1124 | 8.2 |
| (2R,3S,4S,5R,6S)-2-[[(2S,3R,4R)-3,4-dihydroxy-4-(hydroxymetil)oxolan-2-yl]oxymetil]-6-(3,4,5-trimethoxyphenoxy)oxane-3,4,5-triol | C20H30O13 | 496.1988 | 3.0 |
| (3R,5R,6R,7S,9S,10R,13R,17R)-17-((R)-5-ethoxy-5-oxypentan-2-yl)-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthreno-3,6,7-triyl triacetate | C32H50O | 585.3039 | 9.0 |
| 1-linoleoilglycerol | C16H36O3 | 355.282 | 7.9 |
| hesperidine | C28H34O15 | 611.498 | 4.1 |
| palmitamide | C20H41NO | 256.2639 | 10.0 |
| 4-(2,6,6-trimethyl-4-oxo-2-ciclohexen-1-yl)-2-butanyl beta-D-glucopyranoside | C19H32O7 | 373.2192 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortelo, P.C.; Demarque, D.P.; Dusi, R.G.; Albernaz, L.C.; Braz-Filho, R.; Goncharova, E.I.; Bokesch, H.R.; Gustafson, K.R.; Beutler, J.A.; Espindola, L.S. A Molecular Networking Strategy: High-Throughput Screening and Chemical Analysis of Brazilian Cerrado Plant Extracts against Cancer Cells. Cells 2021, 10, 691. https://doi.org/10.3390/cells10030691
Cortelo PC, Demarque DP, Dusi RG, Albernaz LC, Braz-Filho R, Goncharova EI, Bokesch HR, Gustafson KR, Beutler JA, Espindola LS. A Molecular Networking Strategy: High-Throughput Screening and Chemical Analysis of Brazilian Cerrado Plant Extracts against Cancer Cells. Cells. 2021; 10(3):691. https://doi.org/10.3390/cells10030691
Chicago/Turabian StyleCortelo, Patrícia C., Daniel P. Demarque, Renata G. Dusi, Lorena C. Albernaz, Raimundo Braz-Filho, Ekaterina I. Goncharova, Heidi R. Bokesch, Kirk R. Gustafson, John A. Beutler, and Laila S. Espindola. 2021. "A Molecular Networking Strategy: High-Throughput Screening and Chemical Analysis of Brazilian Cerrado Plant Extracts against Cancer Cells" Cells 10, no. 3: 691. https://doi.org/10.3390/cells10030691
APA StyleCortelo, P. C., Demarque, D. P., Dusi, R. G., Albernaz, L. C., Braz-Filho, R., Goncharova, E. I., Bokesch, H. R., Gustafson, K. R., Beutler, J. A., & Espindola, L. S. (2021). A Molecular Networking Strategy: High-Throughput Screening and Chemical Analysis of Brazilian Cerrado Plant Extracts against Cancer Cells. Cells, 10(3), 691. https://doi.org/10.3390/cells10030691











