Key Signaling Pathways in Aging and Potential Interventions for Healthy Aging
Abstract
1. Introduction
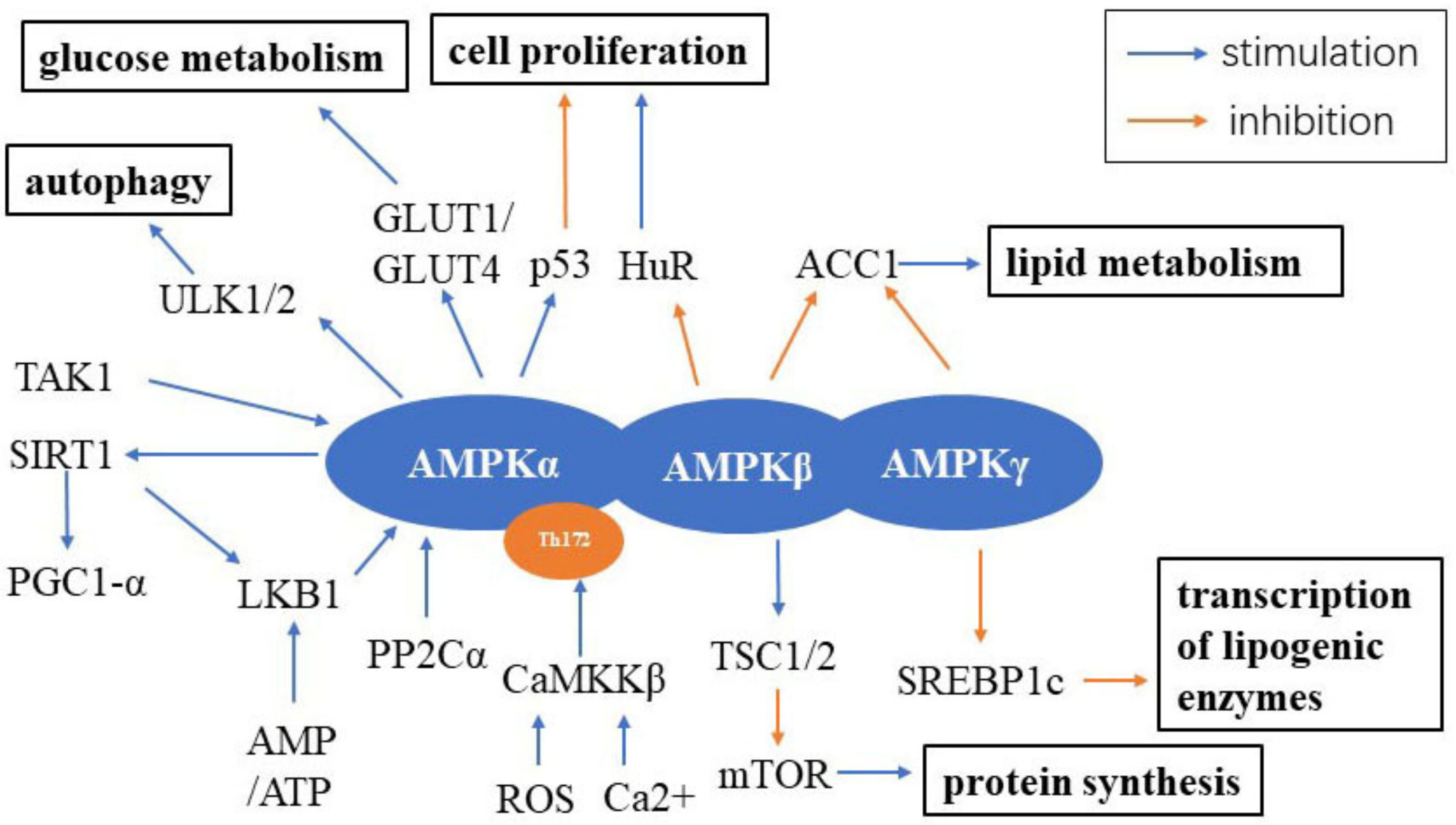
2. AMPK Signaling
2.1. AMPK Signaling and Aging
2.2. Metformin and AMPK Signaling
2.3. Resveratrol and AMPK Signaling
2.4. Physical Exercise, a Link between AMPK Signaling and Aging
2.5. Autophagy and AMPK Signaling in Aging
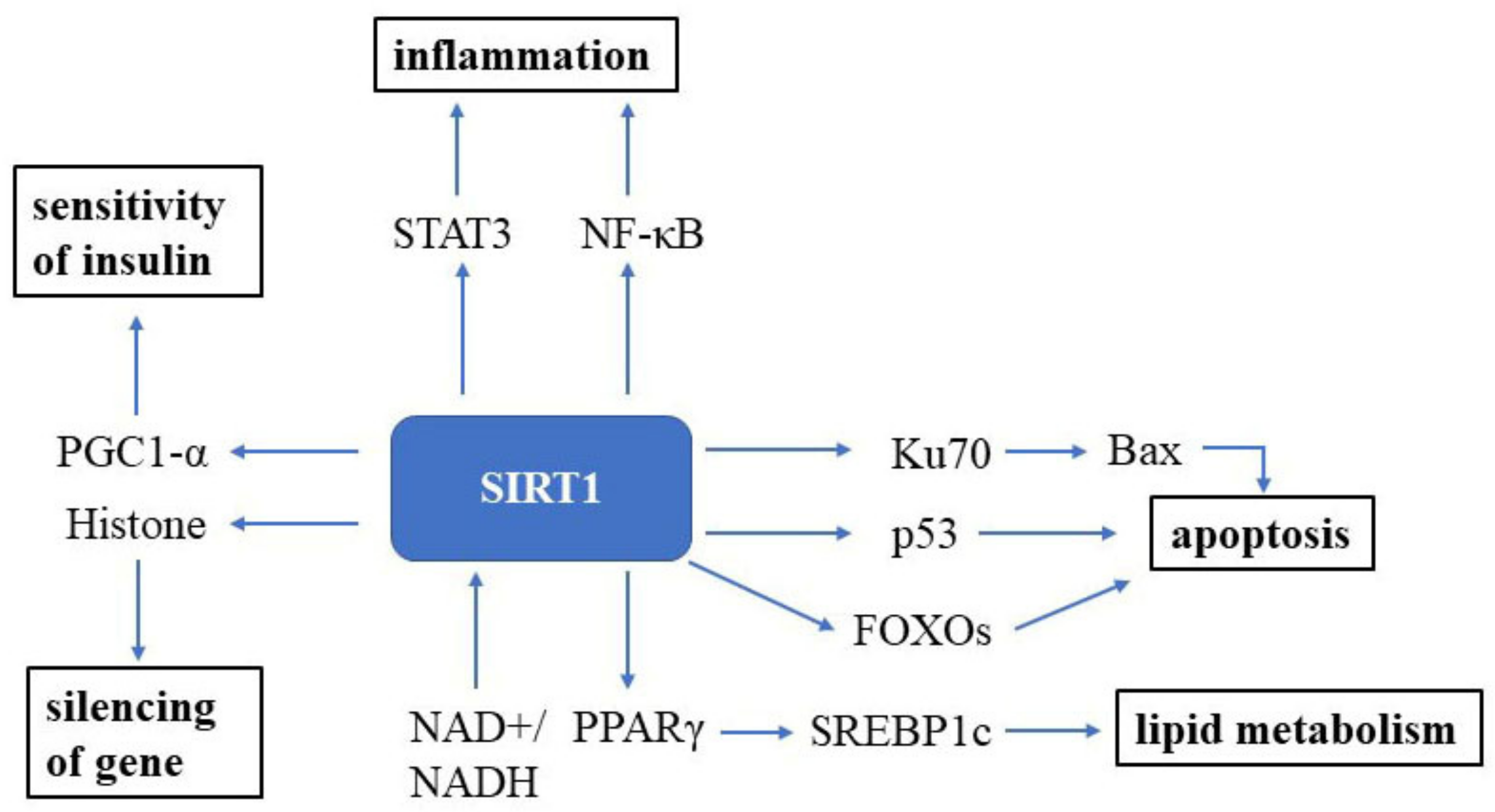
3. Sirtuin Signaling
3.1. SIRT1 Signaling and Aging
3.2. NAD+ and SIRT1 Signaling
3.3. Resveratrol and SIRT1 Signaling
3.4. Exercise and SIRT1 Signaling
3.5. Caloric Restriction and SIRT1 Signaling
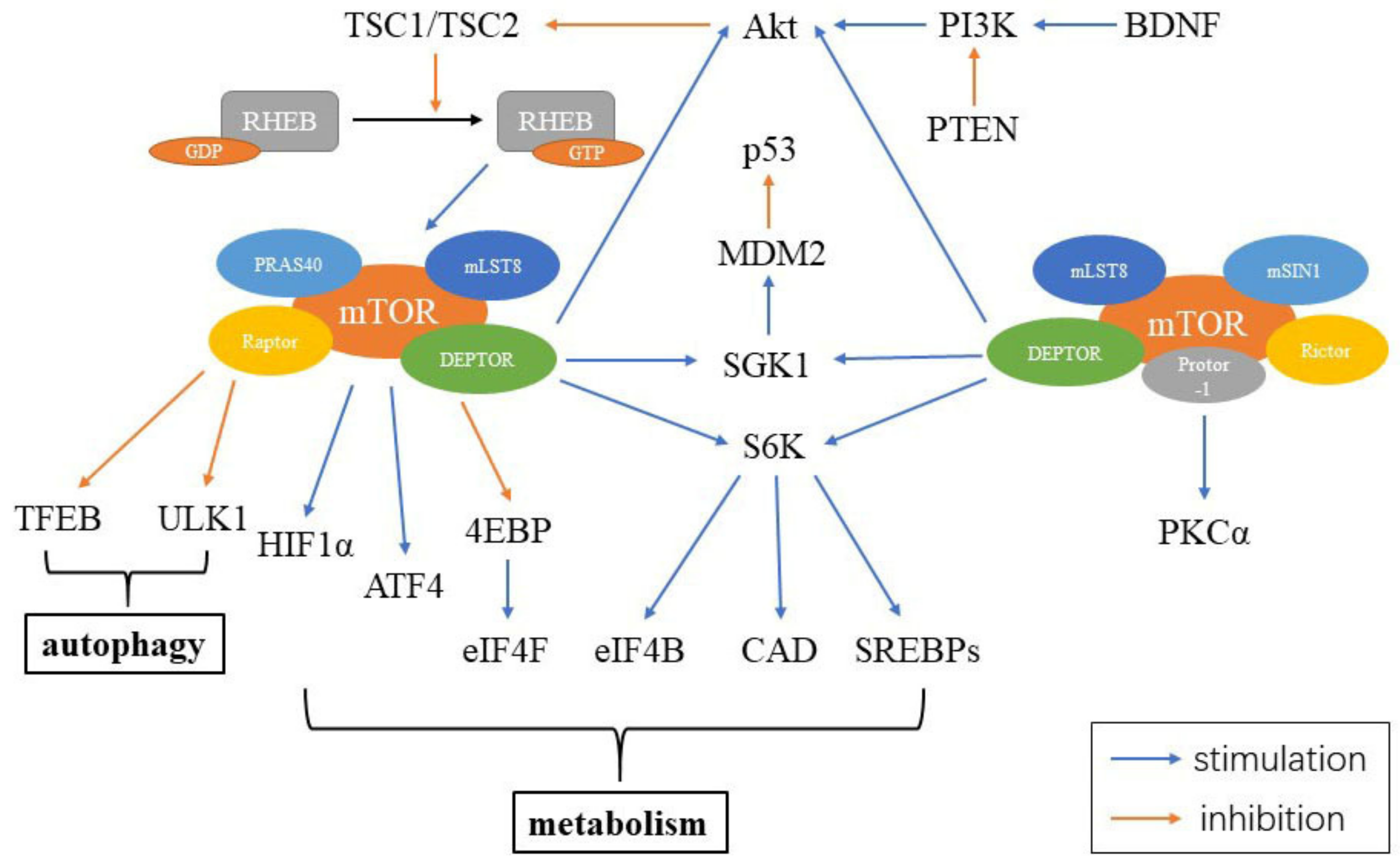
4. MTOR Signaling
4.1. MTOR Signaling and Aging
4.2. Rapamycin and mTOR Signaling
4.3. Resveratrol and mTOR Signaling
4.4. Exercise and mTOR Signaling
4.5. Caloric Restriction and mTOR Signaling
4.6. Autophagy and mTOR Signaling
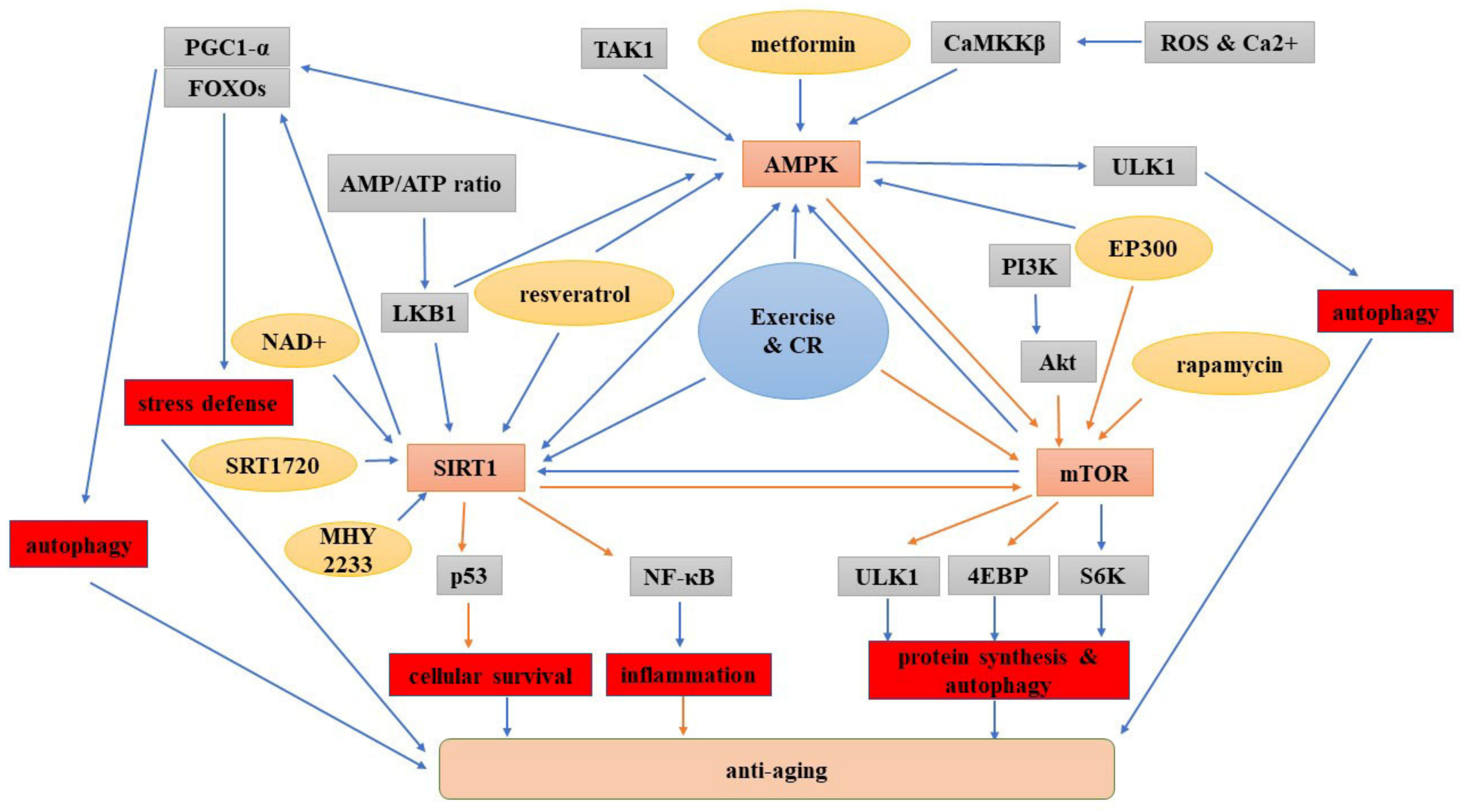
5. Interplay between the AMPK, SIRT1 and mTOR Signaling Pathways
5.1. Crosstalk between AMPK and SIRT1 Signaling
5.2. Interaction between AMPK and mTOR Signaling
5.3. Interplay between SIRT1 and mTOR Signaling
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, R.; Stambler, I. The Urgent Need for International Action for Anti-aging and Disease Prevention. Aging Dis. 2020, 11, 212–215. [Google Scholar] [CrossRef]
- Brayne, C.; Miller, B. Dementia and aging populations-A global priority for contextualized research and health policy. PLoS Med. 2017, 14, e1002275. [Google Scholar] [CrossRef]
- Harada, C.; Natelson Love, M.; Triebel, K. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Evitt, C.; Quigley, P. Fear of falling in older adults: A guide to its prevalence, risk factors, and consequences. Rehabil. Nurs. Off. J. Assoc. Rehabil. Nurses 2004, 29, 207–210. [Google Scholar]
- Novelle, M.; Ali, A.; Diéguez, C.; Bernier, M.; de Cabo, R. Metformin: A Hopeful Promise in Aging Research. Cold Spring Harb. Perspect. Med. 2016, 6, a025932. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, J.; Zhang, H.; Smith, C.; Jin, K. AMPK Signaling Regulates the Age-Related Decline of Hippocampal Neurogenesis. Aging Dis. 2019, 10, 1058–1074. [Google Scholar] [CrossRef] [PubMed]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef]
- Morgunova, G.V.; Klebanov, A.A. Age-related AMP-activated protein kinase alterations: From cellular energetics to longevity. Cell Biochem. Funct. 2019, 37, 169–176. [Google Scholar] [CrossRef]
- Tamás, P.; Hawley, S.; Clarke, R.; Mustard, K.; Green, K.; Hardie, D.; Cantrell, D. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J. Exp. Med. 2006, 203, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Choi, H. Quercetin-Induced AMP-Activated Protein Kinase Activation Attenuates Vasoconstriction Through LKB1-AMPK Signaling Pathway. J. Med. Food 2018, 21, 146–153. [Google Scholar] [CrossRef]
- Mitsuhashi, K.; Senmaru, T.; Fukuda, T.; Yamazaki, M.; Shinomiya, K.; Ueno, M.; Kinoshita, S.; Kitawaki, J.; Katsuyama, M.; Tsujikawa, M.; et al. Testosterone stimulates glucose uptake and GLUT4 translocation through LKB1/AMPK signaling in 3T3-L1 adipocytes. Endocrine 2016, 51, 174–184. [Google Scholar] [CrossRef]
- D’Eon, T.M.; Rogers, N.H.; Stancheva, Z.S.; Greenberg, A.S. Estradiol and the estradiol metabolite, 2-hydroxyestradiol, activate AMP-activated protein kinase in C2C12 myotubes. Obesity 2008, 16, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Lee, K.Y.; Kim, J.R.; Choi, H.C. Estrogenic compound attenuates angiotensin II-induced vascular smooth muscle cell proliferation through interaction between LKB1 and estrogen receptor α. J. Pharm. Sci. 2016, 132, 78–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.; Kandadi, M.; Ren, J. Double knockout of Akt2 and AMPK predisposes cardiac aging without affecting lifespan: Role of autophagy and mitophagy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1865–1875. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Cordero, M.D.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Santos-Buelga, C.; González-Paramás, A.M.; Astolfi, P.; Rubini, C.; et al. Strawberry consumption improves aging-associated impairments, mitochondrial biogenesis and functionality through the AMP-activated protein kinase signaling cascade. Food Chem. 2017, 234, 464–471. [Google Scholar] [CrossRef]
- Zawada, I.; Masternak, M.M.; List, E.O.; Stout, M.B.; Berryman, D.E.; Lewinski, A.; Kopchick, J.J.; Bartke, A.; Karbownik-Lewinska, M.; Gesing, A. Gene expression of key regulators of mitochondrial biogenesis is sex dependent in mice with growth hormone receptor deletion in liver. Aging 2015, 7, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. FGF21 activates AMPK signaling: Impact on metabolic regulation and the aging process. J. Mol. Med. 2017, 95, 123–131. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Li, H.; Shen, Q.; Shen, J.; An, X.; Wu, J.; Zhang, J.; Wu, Y.; Xiao, H.; et al. Exacerbated cardiac fibrosis induced by β-adrenergic activation in old mice due to decreased AMPK activity. Clin. Exp. Pharmacol. Physiol. 2016, 43, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 2012, 11, 230–241. [Google Scholar] [CrossRef]
- Ulgherait, M.; Rana, A.; Rera, M.; Graniel, J.; Walker, D. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014, 8, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Kaisar, M.A.; Villalba, H.; Prasad, S.; Liles, T.; Sifat, A.E.; Sajja, R.K.; Abbruscato, T.J.; Cucullo, L. Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: Is Metformin a viable countermeasure? Redox Biol. 2017, 13, 353–362. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, R.; Carmona-Gutierrez, D.; Bernier, M.; Hall, M.N.; Madeo, F. The search for antiaging interventions: From elixirs to fasting regimens. Cell 2014, 157, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Soukas, A.A.; Hao, H.; Wu, L. Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol. Metab. TEM 2019, 30, 745–755. [Google Scholar] [CrossRef]
- Onken, B.; Driscoll, M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS ONE 2010, 5, e8758. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.R.; Miller, B.F. Taming expectations of metformin as a treatment to extend healthspan. GeroScience 2019, 41, 101–108. [Google Scholar] [CrossRef]
- Barzilai, N.; Crandall, J.P.; Kritchevsky, S.B.; Espeland, M.A. Metformin as a Tool to Target Aging. Cell Metab. 2016, 23, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Castoldi, F.; Markaki, M.; Lachkar, S.; Chen, G.; Enot, D.P.; Durand, S.; Bossut, N.; Tong, M.; Malik, S.A.; et al. Aspirin Recapitulates Features of Caloric Restriction. Cell Rep. 2018, 22, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated protein kinase: Maintaining energy homeostasis at the cellular and whole-body levels. Annu. Rev. Nutr. 2014, 34, 31–55. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Finley, J. Cellular stress and AMPK activation as a common mechanism of action linking the effects of metformin and diverse compounds that alleviate accelerated aging defects in Hutchinson-Gilford progeria syndrome. Med. Hypotheses 2018, 118, 151–162. [Google Scholar] [CrossRef]
- Zhao, P.; Sui, B.-D.; Liu, N.; Lv, Y.-J.; Zheng, C.-X.; Lu, Y.-B.; Huang, W.-T.; Zhou, C.-H.; Chen, J.; Pang, D.-L.; et al. Anti-aging pharmacology in cutaneous wound healing: Effects of metformin, resveratrol, and rapamycin by local application. Aging Cell 2017, 16, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lee, S.; Sousa-Lima, I.; Kim, S.; Hwang, W.; Dagon, Y.; Yang, W.; Cho, S.; Kang, M.; Seo, J.; et al. Rho-kinase/AMPK axis regulates hepatic lipogenesis during overnutrition. J. Clin. Investig. 2018, 128, 5335–5350. [Google Scholar] [CrossRef]
- Luo, T.; Nocon, A.; Fry, J.; Sherban, A.; Rui, X.; Jiang, B.; Xu, X.; Han, J.; Yan, Y.; Yang, Q.; et al. AMPK Activation by Metformin Suppresses Abnormal Extracellular Matrix Remodeling in Adipose Tissue and Ameliorates Insulin Resistance in Obesity. Diabetes 2016, 65, 2295–2310. [Google Scholar] [CrossRef]
- Hunt, N.; Lockwood, G.; Kang, S.; Pulpitel, T.; Clark, X.; Mao, H.; McCourt, P.; Cooney, G.; Wali, J.; Le Couteur, F.; et al. The Effects of Metformin on Age-Related Changes in the Liver Sinusoidal Endothelial Cell. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 278–285. [Google Scholar] [CrossRef]
- Cai, H.; Han, B.; Hu, Y.; Zhao, X.; He, Z.; Chen, X.; Sun, H.; Yuan, J.; Li, Y.; Yang, X.; et al. Metformin attenuates the D-galactose-induced aging process via the UPR through the AMPK/ERK1/2 signaling pathways. Int. J. Mol. Med. 2020, 45, 715–730. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, Z.; Gaur, U.; Fang, J.; Peng, T.; Li, S.; Zheng, W. Metformin protects PC12 cells and hippocampal neurons from H2O2-induced oxidative damage through activation of AMPK pathway. J. Cell. Physiol. 2019, 234, 16619–16629. [Google Scholar] [CrossRef]
- Ryu, Y.; Go, J.; Park, H.; Choi, Y.; Seo, Y.; Choi, J.; Rhee, M.; Lee, T.; Lee, C.; Kim, K. Metformin regulates astrocyte reactivity in Parkinson’s disease and normal aging. Neuropharmacology 2020, 175, 108173. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.; Angelopoulou, E.; Piperi, C.; Shaikh, M.; Othman, I. Emerging neuroprotective effect of metformin in Parkinson’s disease: A molecular crosstalk. Pharmacol. Res. 2020, 152, 104593. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging 2019, 11, 2217–2240. [Google Scholar] [CrossRef]
- Campagna, J.; Spilman, P.; Jagodzinska, B.; Bai, D.; Hatami, A.; Zhu, C.; Bilousova, T.; Jun, M.; Elias, C.J.; Pham, J.; et al. A small molecule ApoE4-targeted therapeutic candidate that normalizes sirtuin 1 levels and improves cognition in an Alzheimer’s disease mouse model. Sci. Rep. 2018, 8, 17574. [Google Scholar] [CrossRef]
- Haramizu, S.; Asano, S.; Butler, D.C.; Stanton, D.A.; Hajira, A.; Mohamed, J.S.; Alway, S.E. Dietary resveratrol confers apoptotic resistance to oxidative stress in myoblasts. J. Nutr. Biochem. 2017, 50, 103–115. [Google Scholar] [CrossRef]
- Siman, R.; Cocca, R.; Dong, Y. The mTOR Inhibitor Rapamycin Mitigates Perforant Pathway Neurodegeneration and Synapse Loss in a Mouse Model of Early-Stage Alzheimer-Type Tauopathy. PLoS ONE 2015, 10, e0142340. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhang, D.; Liu, Y.; Li, L. Geniposide-mediated protection against amyloid deposition and behavioral impairment correlates with downregulation of mTOR signaling and enhanced autophagy in a mouse model of Alzheimer’s disease. Aging 2019, 11, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhong, Y.; Li, Q.; Cui, L.; Huang, G. Autophagy of macrophages is regulated by PI3k/Akt/mTOR signalling in the development of diabetic encephalopathy. Aging 2018, 10, 2772–2782. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Cheng, Y. Inhibition of miR-20 promotes proliferation and autophagy in articular chondrocytes by PI3K/AKT/mTOR signaling pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 97, 607–615. [Google Scholar] [CrossRef]
- Cunha-Santos, J.; Duarte-Neves, J.; Carmona, V.; Guarente, L.; Pereira de Almeida, L.; Cavadas, C. Caloric restriction blocks neuropathology and motor deficits in Machado-Joseph disease mouse models through SIRT1 pathway. Nat. Commun. 2016, 7, 11445. [Google Scholar] [CrossRef]
- Liu, P.; Feng, T.; Zuo, X.; Wang, X.; Luo, J.; Li, N.; Han, X.; Zhu, N.; Xu, S.; Xu, Y.; et al. A novel SIRT1 activator E6155 improves insulin sensitivity in type 2 diabetic KKA mice. Biochem. Biophys. Res. Commun. 2018, 498, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Li, F.H.; Yu, H.T.; Xiao, L.; Liu, Y.Y. Response of BAX, Bcl-2 Proteins, and SIRT1/PGC-1α mRNA Expression to 8-Week Treadmill Running in the Aging Rat Skeletal Muscle. Adv. Exp. Med. Biol. 2016, 923, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Houssaini, A.; Breau, M.; Kebe, K.; Abid, S.; Marcos, E.; Lipskaia, L.; Rideau, D.; Parpaleix, A.; Huang, J.; Amsellem, V.; et al. mTOR pathway activation drives lung cell senescence and emphysema. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Kueck, A.; Opipari, A.W., Jr.; Griffith, K.A.; Tan, L.; Choi, M.; Huang, J.; Wahl, H.; Liu, J.R. Resveratrol inhibits glucose metabolism in human ovarian cancer cells. Gynecol. Oncol. 2007, 107, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Lamming, D.; Cummings, N.; Rastelli, A.; Gao, F.; Cava, E.; Bertozzi, B.; Spelta, F.; Pili, R.; Fontana, L. Restriction of dietary protein decreases mTORC1 in tumors and somatic tissues of a tumor-bearing mouse xenograft model. Oncotarget 2015, 6, 31233–31240. [Google Scholar] [CrossRef]
- Ros, M.; Carrascosa, J.M. Current nutritional and pharmacological anti-aging interventions. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 165612. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Du, L.; Chen, E.; Wu, T.; Ruan, Y.; Wu, S. Resveratrol attenuates hydrogen peroxide-induced aging through upregulation of autophagy in human umbilical vein endothelial cells. Drug Des. Dev. Ther. 2019, 13, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yan, Y.; Zhao, C.; Xu, Y.; Wang, Q.; Xu, N. viaResveratrol improves osteogenic differentiation of senescent bone mesenchymal stem cells through inhibiting endogenous reactive oxygen species production AMPK activation. Redox Rep. Commun. Free Radic. Res. 2019, 24, 62–69. [Google Scholar] [CrossRef]
- Liu, C.; Sung, H.; Lin, S.; Wu, C.; Lee, C.; Lee, I.; Yang, Y.; Yu, I.; Lin, S.; Chiang, M.; et al. Resveratrol attenuates ICAM-1 expression and monocyte adhesiveness to TNF-α-treated endothelial cells: Evidence for an anti-inflammatory cascade mediated by the miR-221/222/AMPK/p38/NF-κB pathway. Sci. Rep. 2017, 7, 44689. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Duranton, A.; Lan, F.; Weikel, K.; Breton, L.; Ruderman, N. Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLoS ONE 2015, 10, e0115341. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef]
- Chan, A.; Dolinsky, V.; Soltys, C.; Viollet, B.; Baksh, S.; Light, P.; Dyck, J. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J. Biol. Chem. 2008, 283, 24194–24201. [Google Scholar] [CrossRef]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Moran, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef] [PubMed]
- Gronek, P.; Balko, S.; Gronek, J.; Zajac, A.; Maszczyk, A.; Celka, R.; Doberska, A.; Czarny, W.; Podstawski, R.; Clark, C.C.T.; et al. Physical Activity and Alzheimer’s Disease: A Narrative Review. Aging Dis. 2019, 10, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Gronek, P.; Haas, A.N.; Czarny, W.; Podstawski, R.; Delabary, M.D.S.; Clark, C.C.; Boraczynski, M.; Tarnas, M.; Wycichowska, P.; Pawlaczyk, M.; et al. The Mechanism of Physical Activity-induced Amelioration of Parkinson’s Disease: A Narrative Review. Aging Dis. 2021, 12, 192–202. [Google Scholar] [CrossRef]
- Li, T.; Mu, N.; Yin, Y.; Yu, L.; Ma, H. Targeting AMP-Activated Protein Kinase in Aging-Related Cardiovascular Diseases. Aging Dis. 2020, 11, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Tarnopolsky, M.A.; Beckman, K.; Felkey, K.; Hubbard, A. Resistance exercise reverses aging in human skeletal muscle. PLoS ONE 2007, 2, e465. [Google Scholar] [CrossRef]
- Brett, J.O.; Arjona, M.; Ikeda, M.; Quarta, M.; de Morree, A.; Egner, I.M.; Perandini, L.A.; Ishak, H.D.; Goshayeshi, A.; Benjamin, D.I.; et al. Exercise rejuvenates quiescent skeletal muscle stem cells in old mice through restoration of Cyclin D1. Nat. Metab. 2020, 2, 307–317. [Google Scholar] [CrossRef]
- Liu, W.; Xia, Y.; Kuang, H.; Wang, Z.; Liu, S.; Tang, C.; Yin, D. Proteomic Profile of Carbonylated Proteins Screen the Regulation of Calmodulin-Dependent Protein Kinases-AMPK-Beclin1 in Aerobic Exercise-Induced Autophagy in Middle-Aged Rat Hippocampus. Gerontology 2019, 65, 620–633. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Xia, Y.; Kuang, H.; Liu, S.; Li, L.; Tang, C.; Yin, D. The balance of apoptosis and autophagy via regulation of the AMPK signal pathway in aging rat striatum during regular aerobic exercise. Exp. Gerontol. 2019, 124, 110647. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sun, L.; Wu, D.; Gao, H.; Min, Z. Proteomics-based identification of different training adaptations of aged skeletal muscle following long-term high-intensity interval and moderate-intensity continuous training in aged rats. Aging 2019, 11, 4159–4182. [Google Scholar] [CrossRef]
- Yoon, K.; Zhang, D.; Kim, S.; Lee, M.; Moon, H. Exercise-induced AMPK activation is involved in delay of skeletal muscle senescence. Biochem. Biophys. Res. Commun. 2019, 512, 604–610. [Google Scholar] [CrossRef]
- Fan, J.; Yang, X.; Li, J.; Shu, Z.; Dai, J.; Liu, X.; Li, B.; Jia, S.; Kou, X.; Yang, Y.; et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from d-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget 2017, 8, 17475–17490. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, T.; Sato, K.; Iwanaka, N.; Honda, H.; Higashida, K.; Iemitsu, M.; Hayashi, T.; Hashimoto, T. Dehydroepiandrosterone activates AMP kinase and regulates GLUT4 and PGC-1α expression in C2C12 myotubes. Biochem. Biophys. Res. Commun. 2015, 463, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Diman, A.; Boros, J.; Poulain, F.; Rodriguez, J.; Purnelle, M.; Episkopou, H.; Bertrand, L.; Francaux, M.; Deldicque, L.; Decottignies, A. Nuclear respiratory factor 1 and endurance exercise promote human telomere transcription. Sci. Adv. 2016, 2, e1600031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, S.; Lu, K.; Wang, F.; Deng, J.; Xu, Z.; Wang, X.; Zhou, Q.; Le, W.; Zhao, Y. Verapamil Ameliorates Motor Neuron Degeneration and Improves Lifespan in the SOD1(G93A) Mouse Model of ALS by Enhancing Autophagic Flux. Aging Dis. 2019, 10, 1159–1173. [Google Scholar] [CrossRef]
- Han, X.; Tai, H.; Wang, X.; Wang, Z.; Zhou, J.; Wei, X.; Ding, Y.; Gong, H.; Mo, C.; Zhang, J.; et al. AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD(+) elevation. Aging Cell 2016, 15, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, L.; Xue, H.; Yang, Z.; Yin, Y.; Zhang, B.; Chen, M.; Ma, H. Nuclear AMPK regulated CARM1 stabilization impacts autophagy in aged heart. Biochem. Biophys. Res. Commun. 2017, 486, 398–405. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; Jiang, Y.; Silva, M.; Zhen, X.; Zheng, W. Protective Effect of Metformin against Hydrogen Peroxide-Induced Oxidative Damage in Human Retinal Pigment Epithelial (RPE) Cells by Enhancing Autophagy through Activation of AMPK Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 2524174. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tu, Y.; He, W.; Liu, Y.; Wu, W.; Fang, Q.; Tang, H.; Tang, R.; Wan, Z.; Sun, W.; et al. Hyperoside attenuates renal aging and injury induced by d-galactose via inhibiting AMPK-ULK1 signaling-mediated autophagy. Aging 2018, 10, 4197–4212. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.W.; Jeong, H.O.; Lee, B.; Chung, K.W.; Lee, Y.; Jung, H.J.; Hyun, M.K.; Lee, A.K.; Kim, B.M.; et al. Novel Role of Lck in Leptin-Induced Inflammation and Implications for Renal Aging. Aging Dis. 2019, 10, 1174–1186. [Google Scholar] [CrossRef]
- Li, Y.; Ouyang, S.; Tu, L.; Wang, X.; Yuan, W.; Wang, G.; Wu, Y.; Duan, W.; Yu, H.; Fang, Z.; et al. Caffeine Protects Skin from Oxidative Stress-Induced Senescence through the Activation of Autophagy. Theranostics 2018, 8, 5713–5730. [Google Scholar] [CrossRef]
- Kim, J.; Sim, H.; Jung, D.; Lim, E.; Kim, Y.; Kim, B.; Jung, M. Poria cocus Wolf Extract Ameliorates Hepatic Steatosis through Regulation of Lipid Metabolism, Inhibition of ER Stress, and Activation of Autophagy via AMPK Activation. Int. J. Mol. Sci. 2019, 20, 4801. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wu, G.; Dai, W.; Wang, G.; Su, H.; Shen, X.; Zhan, R.; Xie, J.; Wang, Z.; Qin, Z.; et al. Aescin-induced reactive oxygen species play a pro-survival role in human cancer cells via ATM/AMPK/ULK1-mediated autophagy. Acta Pharmacol. Sin. 2018, 39, 1874–1884. [Google Scholar] [CrossRef]
- Yang, S.; Long, L.; Li, D.; Zhang, J.; Jin, S.; Wang, F.; Chen, J. β-Guanidinopropionic acid extends the lifespan of Drosophila melanogaster via an AMP-activated protein kinase-dependent increase in autophagy. Aging Cell 2015, 14, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Billin, A.; Campbell, M.; Russell, A.; Huffman, K.; Kraus, W. The AMPK/p27 Axis Regulates Autophagy/Apoptosis Decisions in Aged Skeletal Muscle Stem Cells. Stem Cell Rep. 2018, 11, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. The mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (SIRT1): Oversight for neurodegenerative disorders. Biochem. Soc. Trans. 2018, 46, 351–360. [Google Scholar] [CrossRef]
- Kosztelnik, M.; Kurucz, A.; Papp, D.; Jones, E.; Sigmond, T.; Barna, J.; Traka, M.; Lorincz, T.; Szarka, A.; Banhegyi, G.; et al. Suppression of AMPK/aak-2 by NRF2/SKN-1 down-regulates autophagy during prolonged oxidative stress. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 2372–2387. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Lee, H.; Min, K. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, J.; Liao, M.; Hu, M.; Li, W.; Ouyang, H.; Wang, X.; Ye, T.; Zhang, Y.; Ouyang, L. An overview of Sirtuins as potential therapeutic target: Structure, function and modulators. Eur. J. Med. Chem. 2019, 161, 48–77. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Ali, Z.; Suri, M.; Kim, S.; Shatla, A.; Ringer, A.; Lopes, D.; Guterman, L.; Hopkins, L. Intra-arterial third-generation recombinant tissue plasminogen activator (reteplase) for acute ischemic stroke. Neurosurgery 2001, 49, 41–48, discussion 48–50. [Google Scholar] [CrossRef] [PubMed]
- Broderick, J.; Lu, M.; Jackson, C.; Pancioli, A.; Tilley, B.; Fagan, S.; Kothari, R.; Levine, S.; Marler, J.; Lyden, P.; et al. Apolipoprotein E phenotype and the efficacy of intravenous tissue plasminogen activator in acute ischemic stroke. Ann. Neurol. 2001, 49, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.Y.; Miller, C.; Bitterman, K.J.; Wall, N.R.; Hekking, B.; Kessler, B.; Howitz, K.T.; Gorospe, M.; De Cabo, R.; Sinclair, D.A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004, 305, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Bernier, M.; Paul, R.K.; Martin-Montalvo, A.; Scheibye-Knudsen, M.; Song, S.; He, H.-J.; Armour, S.M.; Hubbard, B.P.; Bohr, V.A.; Wang, L.; et al. Negative regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein. J. Biol. Chem. 2011, 286, 19270–19279. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Weng, W.; Gao, R.; Liu, Y. New Insights for Cellular and Molecular Mechanisms of Aging and Aging-Related Diseases: Herbal Medicine as Potential Therapeutic Approach. Oxidative Med. Cell. Longev. 2019, 2019, 4598167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, J.; Hu, K.; He, X.; Yun, D.; Tong, T.; Han, L. Sirtuins and their Biological Relevance in Aging and Age-Related Diseases. Aging Dis. 2020, 11, 927–945. [Google Scholar] [CrossRef] [PubMed]
- Tissenbaum, H.A.; Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H. The sirtuin SIRT6 regulates lifespan in male mice. Nature 2012, 483, 218–221. [Google Scholar] [CrossRef]
- Rogina, B.; Helfand, S. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA 2004, 101, 15998–16003. [Google Scholar] [CrossRef]
- Kaeberlein, M.; McVey, M.; Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999, 13, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Brace, C.; Rensing, N.; Cliften, P.; Wozniak, D.; Herzog, E.; Yamada, K.; Imai, S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef]
- Kim, M.; An, H.; Kim, D.; Lee, B.; Lee, H.; Ullah, S.; Kim, S.; Jeong, H.; Moon, K.; Lee, E.; et al. Novel SIRT1 activator MHY2233 improves glucose tolerance and reduces hepatic lipid accumulation in db/db mice. Bioorg. Med. Chem. Lett. 2018, 28, 684–688. [Google Scholar] [CrossRef]
- Kayashima, Y.; Katayanagi, Y.; Tanaka, K.; Fukutomi, R.; Hiramoto, S.; Imai, S. Alkylresorcinols activate SIRT1 and delay ageing in Drosophila melanogaster. Sci. Rep. 2017, 7, 43679. [Google Scholar] [CrossRef]
- Bakhtiari, N.; Mirzaie, S.; Hemmati, R.; Moslemee-Jalalvand, E.; Noori, A.; Kazemi, J. Mounting evidence validates Ursolic Acid directly activates SIRT1: A powerful STAC which mimic endogenous activator of SIRT1. Arch. Biochem. Biophys. 2018, 650, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, Y.; Lee, J.; Choi, D.; Cho, Y.; Shin, J.; Park, J.; Lee, J.; Kim, W.; Seo, D.; et al. The natural phytochemical dehydroabietic acid is an anti-aging reagent that mediates the direct activation of SIRT1. Mol. Cell. Endocrinol. 2015, 412, 216–225. [Google Scholar] [CrossRef]
- Pereira-Simon, S.; Rubio, G.A.; Xia, X.; Cai, W.; Choi, R.; Striker, G.E.; Elliot, S.J. Inhibition of Advanced Glycation End Products (AGEs) Accumulation by Pyridoxamine Modulates Glomerular and Mesangial Cell Estrogen Receptor α Expression in Aged Female Mice. PLoS ONE 2016, 11, e0159666. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ullah, R.; Rehman, S.U.; Shah, S.A.; Saeed, K.; Muhammad, T.; Park, H.Y.; Jo, M.H.; Choe, K.; Rutten, B.P.F.; et al. 17β-Estradiol Modulates SIRT1 and Halts Oxidative Stress-Mediated Cognitive Impairment in a Male Aging Mouse Model. Cells 2019, 8, 928. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, P.; Zhao, G.; Wang, H.; Wang, M.; Chen, J.; Tong, T. Upregulation of SIRT1 by 17β-estradiol depends on ubiquitin-proteasome degradation of PPAR-γ mediated by NEDD4-1. Protein Cell 2013, 4, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Baur, J.A.; Imai, S.-I. NAD Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, J.; Liu, G.H.; Belmonte, J.C.I. The ageing epigenome and its rejuvenation. Nat. Rev. Mol. Cell Biol. 2020. [Google Scholar] [CrossRef]
- Braidy, N.; Liu, Y. NAD+ therapy in age-related degenerative disorders: A benefit/risk analysis. Exp. Gerontol. 2020, 110831. [Google Scholar] [CrossRef]
- Cantó, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef]
- Gariani, K.; Menzies, K.J.; Ryu, D.; Wegner, C.J.; Wang, X.; Ropelle, E.R.; Moullan, N.; Zhang, H.; Perino, A.; Lemos, V.; et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology 2016, 63, 1190–1204. [Google Scholar] [CrossRef]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, S.; Huang, X.; Xie, Q.; Xu, Y.; Shang, D.; Hao, C. Nicotinamide Mononucleotide, an NAD Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1-Dependent Manner. J. Am. Soc. Nephrol. JASN 2017, 28, 2337–2352. [Google Scholar] [CrossRef]
- De Picciotto, N.E.; Gano, L.B.; Johnson, L.C.; Martens, C.R.; Sindler, A.L.; Mills, K.F.; Imai, S.; Seals, D.R. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 2016, 15, 522–530. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Yang, F.; Ning, G.; Zhen, L.; Wu, L.; Zheng, Y.; Zhang, Q.; Lin, D.; Xie, C.; et al. Nicotinamide mononucleotide promotes osteogenesis and reduces adipogenesis by regulating mesenchymal stromal cells via the SIRT1 pathway in aged bone marrow. Cell Death Dis. 2019, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Chaudhury, A.; Rodriguez-Aguayo, C.; Lu, L.; Akhanov, V.; Catic, A.; Popov, Y.V.; Verdin, E.; Johnson, H.; Stossi, F.; et al. Telomere Dysfunction Induces Sirtuin Repression that Drives Telomere-Dependent Disease. Cell Metab. 2019, 29, 1274–1290.e9. [Google Scholar] [CrossRef]
- Pi, C.; Yang, Y.; Sun, Y.; Wang, H.; Sun, H.; Ma, M.; Lin, L.; Shi, Y.; Li, Y.; Li, Y.; et al. Nicotinamide phosphoribosyltransferase postpones rat bone marrow mesenchymal stem cell senescence by mediating NAD-Sirt1 signaling. Aging 2019, 11, 3505–3522. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Nyúl-Tóth, Á.; Balasubramanian, P.; Tarantini, S.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Farkas, E.; Wren, J.; Garman, L.; et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: Transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. GeroScience 2020, 42, 527–546. [Google Scholar] [CrossRef]
- Mendelsohn, A.; Larrick, J. The NAD+/PARP1/SIRT1 Axis in Aging. Rejuvenation Res. 2017, 20, 244–247. [Google Scholar] [CrossRef]
- Li, D.; Huang, F.; Ni, M.; Fu, H.; Zhang, L.; Shen, F. α7 Nicotinic Acetylcholine Receptor Relieves Angiotensin II-Induced Senescence in Vascular Smooth Muscle Cells by Raising Nicotinamide Adenine Dinucleotide-Dependent SIRT1 Activity. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Anwar, S. Sirtuins in Brain Aging and Neurological Disorders. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 321–329. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarragó, M.; Chini, C.; Nin, V.; Escande, C.; Warner, G.; Puranik, A.; Schoon, R.; Reid, J.; Galina, A.; et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Escande, C.; Nin, V.; Price, N.; Capellini, V.; Gomes, A.; Barbosa, M.; O’Neil, L.; White, T.; Sinclair, D.; Chini, E. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: Implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 2013, 62, 1084–1093. [Google Scholar] [CrossRef]
- Haffner, C.; Becherer, J.; Boros, E.; Cadilla, R.; Carpenter, T.; Cowan, D.; Deaton, D.; Guo, Y.; Harrington, W.; Henke, B.; et al. Discovery, Synthesis, and Biological Evaluation of Thiazoloquin(az)olin(on)es as Potent CD38 Inhibitors. J. Med. Chem. 2015, 58, 3548–3571. [Google Scholar] [CrossRef]
- Sepehri, B.; Ghavami, R. Molecular docking and CoMFA studies of thiazoloquin(az)olin(on)es as CD38 inhibitors: Determination of inhibitory mechanism, pharmacophore interactions, and design of new inhibitors. J. Biomol. Struct. Dyn. 2017, 35, 1890–1898. [Google Scholar] [CrossRef]
- Rascón, B.; Hubbard, B.; Sinclair, D.; Amdam, G. The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging 2012, 4, 499–508. [Google Scholar] [CrossRef]
- Da Luz, P.; Tanaka, L.; Brum, P.; Dourado, P.; Favarato, D.; Krieger, J.; Laurindo, F. Red wine and equivalent oral pharmacological doses of resveratrol delay vascular aging but do not extend life span in rats. Atherosclerosis 2012, 224, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Strong, R.; Miller, R.; Astle, C.; Baur, J.; de Cabo, R.; Fernandez, E.; Guo, W.; Javors, M.; Kirkland, J.; Nelson, J.; et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wheeler, C.; Alberico, T.; Sun, X.; Seeberger, J.; Laslo, M.; Spangler, E.; Kern, B.; de Cabo, R.; Zou, S. The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. Age 2013, 35, 69–81. [Google Scholar] [CrossRef]
- Pang, J.; Xiong, H.; Ou, Y.; Yang, H.; Xu, Y.; Chen, S.; Lai, L.; Ye, Y.; Su, Z.; Lin, H.; et al. SIRT1 protects cochlear hair cell and delays age-related hearing loss via autophagy. Neurobiol. Aging 2019, 80, 127–137. [Google Scholar] [CrossRef]
- Sin, T.; Yu, A.; Yung, B.; Yip, S.; Chan, L.; Wong, C.; Rudd, J.; Siu, P. Effects of long-term resveratrol-induced SIRT1 activation on insulin and apoptotic signalling in aged skeletal muscle. Acta Diabetol. 2015, 52, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ko, S.Y.; Garrett, I.R.; Mundy, G.R.; Gutierrez, G.E.; Edwards, J.R. The polyphenol resveratrol promotes skeletal growth in mice through a sirtuin 1-bone morphogenic protein 2 longevity axis. Br. J. Pharm. 2018, 175, 4183–4192. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, Z.; Ji, S.; Liu, T.; Hou, Y.; Li, S.; Li, G. Resveratrol reduces senescence-associated secretory phenotype by SIRT1/NF-κB pathway in gut of the annual fish Nothobranchius guentheri. Fish Shellfish Immunol. 2018, 80, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Ginés, C.; Cuesta, S.; Kireev, R.; García, C.; Rancan, L.; Paredes, S.D.; Vara, E.; Tresguerres, J.A.F. Protective effect of resveratrol against inflammation, oxidative stress and apoptosis in pancreas of aged SAMP8 mice. Exp. Gerontol. 2017, 90, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Lin, Y.; Zhang, C.; Sun, H.; Zhou, L.; Schatten, H.; Sun, Q.; Qian, W. In vivo Resveratrol increases resistance of mouse oocytes to postovulatory aging. Aging 2018, 10, 1586–1596. [Google Scholar] [CrossRef]
- Ham, H.J.; Park, J.W.; Bae, Y.S. Defect of SIRT1-FoxO3a axis is associated with the production of reactive oxygen species during protein kinase CK2 downregulation-mediated cellular senescence and nematode aging. BMB Rep. 2019, 52, 265–270. [Google Scholar] [CrossRef]
- Huang, J.; Hsu, S.; Li, D.; Chen, K.; Kuo, C.; Hung, L. Resveratrol Mitigates High-Fat Diet-Induced Vascular Dysfunction by Activating the Akt/eNOS/NO and Sirt1/ER Pathway. J. Cardiovasc. Pharmacol. 2018, 72, 231–241. [Google Scholar] [CrossRef]
- Kumar, V.; Pandey, A.; Jahan, S.; Shukla, R.K.; Kumar, D.; Srivastava, A.; Singh, S.; Rajpurohit, C.S.; Yadav, S.; Khanna, V.K.; et al. Differential responses of Trans-Resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci. Rep. 2016, 6, 28142. [Google Scholar] [CrossRef]
- Locatelli, F.M.; Kawano, T.; Iwata, H.; Aoyama, B.; Eguchi, S.; Nishigaki, A.; Yamanaka, D.; Tateiwa, H.; Shigematsu-Locatelli, M.; Yokoyama, M. Resveratrol-loaded nanoemulsion prevents cognitive decline after abdominal surgery in aged rats. J. Pharm. Sci. 2018, 137, 395–402. [Google Scholar] [CrossRef]
- Muñoz, A.; Corrêa, C.; Lopez-Lopez, A.; Costa-Besada, M.; Diaz-Ruiz, C.; Labandeira-Garcia, J. Physical Exercise Improves Aging-Related Changes in Angiotensin, IGF-1, SIRT1, SIRT3, and VEGF in the Substantia Nigra. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Kuo, W.; Baskaran, R.; Kuo, C.; Chen, Y.; Chen, W.; Ho, T.; Day, C.; Mahalakshmi, B.; Huang, C. Swimming exercise stimulates IGF1/ PI3K/Akt and AMPK/SIRT1/PGC1α survival signaling to suppress apoptosis and inflammation in aging hippocampus. Aging 2020, 12, 6852–6864. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, T.; Tung, Y.; Lin, W. Effect of Exercise Training on Skeletal Muscle SIRT1 and PGC-1α Expression Levels in Rats of Different Age. Int. J. Med. Sci. 2016, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Rinaldi, B.; Corbi, G.; Conti, V.; Stiuso, P.; Boccuti, S.; Rengo, G.; Rossi, F.; Filippelli, A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008, 11, 139–150. [Google Scholar] [CrossRef]
- Chen, W.K.; Tsai, Y.L.; Shibu, M.A.; Shen, C.Y.; Chang-Lee, S.N.; Chen, R.J.; Yao, C.H.; Ban, B.; Kuo, W.W.; Huang, C.Y. Exercise training augments Sirt1-signaling and attenuates cardiac inflammation in d-galactose induced-aging rats. Aging 2018, 10, 4166–4174. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Zhu, Y.; Li, Z.; Zhu, Y.; Wu, J.; Qin, Z.; Xiang, M.; Lin, F. Exercise activates lysosomal function in the brain through AMPK-SIRT1-TFEB pathway. CNS Neurosci. Ther. 2019, 25, 796–807. [Google Scholar] [CrossRef]
- Kim, J.S.; Jeon, J.; An, J.J.; Yi, H.K. Interval running training improves age-related skeletal muscle wasting and bone loss: Experiments with ovariectomized rats. Exp. Physiol. 2019, 104, 691–703. [Google Scholar] [CrossRef]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.; Kroemer, G. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef] [PubMed]
- Parikh, I.; Guo, J.; Chuang, K.; Zhong, Y.; Rempe, R.; Hoffman, J.; Armstrong, R.; Bauer, B.; Hartz, A.; Lin, A. Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions. Aging 2016, 8, 2814–2826. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol. Metab. TEM 2009, 20, 325–331. [Google Scholar] [CrossRef]
- Kaeberlein, M.; Kirkland, K.T.; Fields, S.; Kennedy, B.K. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004, 2, E296. [Google Scholar] [CrossRef]
- Zainabadi, K.; Liu, C.J.; Caldwell, A.L.M.; Guarente, L. SIRT1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis. PLoS ONE 2017, 12, e0185236. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Qin, J.; Chen, C.; Fu, Y.; Wang, W. Moderate calorie restriction attenuates age-associated alterations and improves cardiac function by increasing SIRT1 and SIRT3 expression. Mol. Med. Rep. 2018, 18, 4087–4094. [Google Scholar] [CrossRef]
- Albert, V.; Hall, M.N. mTOR signaling in cellular and organismal energetics. Curr. Opin. Cell Biol. 2015, 33, 55–66. [Google Scholar] [CrossRef]
- Di Francesco, A.; Diaz-Ruiz, A.; De Cabo, R.; Bernier, M. Intermittent mTOR Inhibition Reverses Kidney Aging in Old Rats. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 843–844. [Google Scholar] [CrossRef] [PubMed]
- Apelo, S.I.A.; Lamming, D.W. Rapamycin: An InhibiTOR of Aging Emerges from the Soil of Easter Island. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 841–849. [Google Scholar] [CrossRef]
- Peterson, T.R.; Laplante, M.; Thoreen, C.C.; Sancak, Y.; Kang, S.A.; Kuehl, W.M.; Gray, N.S.; Sabatini, D.M. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 2009, 137, 873–886. [Google Scholar] [CrossRef]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef]
- Papadopoli, D.; Boulay, K.; Kazak, L.; Pollak, M.; Mallette, F.; Topisirovic, I.; Hulea, L. mTOR as a central regulator of lifespan and aging. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, L.; Zhang, X.; Shi, C.; Qiao, S.; Ma, Z.; Yuan, J. Snapshot: Implications for mTOR in Aging-related Ischemia/Reperfusion Injury. Aging Dis. 2019, 10, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Vellai, T.; Takacs-Vellai, K.; Zhang, Y.; Kovacs, A.L.; Orosz, L.; Müller, F. Genetics: Influence of TOR kinase on lifespan in C. elegans. Nature 2003, 426, 620. [Google Scholar] [CrossRef] [PubMed]
- Kapahi, P.; Zid, B.M.; Harper, T.; Koslover, D.; Sapin, V.; Benzer, S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004, 14, 885–890. [Google Scholar] [CrossRef]
- Kaeberlein, M.; Powers, R.W., 3rd; Steffen, K.K.; Westman, E.A.; Hu, D.; Dang, N.; Kerr, E.O.; Kirkland, K.T.; Fields, S.; Kennedy, B.K. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 2005, 310, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Guertin, D.A.; Stevens, D.M.; Thoreen, C.C.; Burds, A.A.; Kalaany, N.Y.; Moffat, J.; Brown, M.; Fitzgerald, K.J.; Sabatini, D.M. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell 2006, 11, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Widlund, A.; Baur, J.; Vang, O. mTOR: More targets of resveratrol? Expert Rev. Mol. Med. 2013, 15, e10. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Ali, S.M.; Sengupta, S.; Sheen, J.-H.; Hsu, P.P.; Bagley, A.F.; Markhard, A.L.; Sabatini, D.M. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 2006, 22, 159–168. [Google Scholar] [CrossRef]
- Yang, F.; Chu, X.; Yin, M.; Liu, X.; Yuan, H.; Niu, Y.; Fu, L. mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behav. Brain Res. 2014, 264, 82–90. [Google Scholar] [CrossRef]
- Chellappa, K.; Brinkman, J.A.; Mukherjee, S.; Morrison, M.; Alotaibi, M.I.; Carbajal, K.A.; Alhadeff, A.L.; Perron, I.J.; Yao, R.; Purdy, C.S.; et al. Hypothalamic mTORC2 is essential for metabolic health and longevity. Aging Cell 2019, 18, e13014. [Google Scholar] [CrossRef]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef] [PubMed]
- Vézina, C.; Kudelski, A.; Sehgal, S.N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. 1975, 28, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Boutouja, F.; Stiehm, C.M.; Platta, H.W. mTOR: A Cellular Regulator Interface in Health and Disease. Cells 2019, 8, 18. [Google Scholar] [CrossRef]
- Chen, X.; Liu, M.; Tian, Y.; Li, J.; Qi, Y.; Zhao, D.; Wu, Z.; Huang, M.; Wong, C.C.L.; Wang, H.-W.; et al. Cryo-EM structure of human mTOR complex 2. Cell Res. 2018, 28, 518–528. [Google Scholar] [CrossRef]
- Chung, C.L.; Lawrence, I.; Hoffman, M.; Elgindi, D.; Nadhan, K.; Potnis, M.; Jin, A.; Sershon, C.; Binnebose, R.; Lorenzini, A.; et al. Topical rapamycin reduces markers of senescence and aging in human skin: An exploratory, prospective, randomized trial. GeroScience 2019, 41, 861–869. [Google Scholar] [CrossRef]
- Lesniewski, L.A.; Seals, D.R.; Walker, A.E.; Henson, G.D.; Blimline, M.W.; Trott, D.W.; Bosshardt, G.C.; LaRocca, T.J.; Lawson, B.R.; Zigler, M.C.; et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell 2017, 16, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Tan, W.; Ji, J.; Feng, G.; Meng, Y.; Da, Z.; Guo, G.; Xia, Y.; Zhu, X.; Shi, G.; et al. Rapamycin reverses the senescent phenotype and improves immunoregulation of mesenchymal stem cells from MRL/lpr mice and systemic lupus erythematosus patients through inhibition of the mTOR signaling pathway. Aging 2016, 8, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yue, Q.; Xie, J.; Zhang, S.; He, W.; Bai, S.; Tian, S.; Zhang, Y.; Xiong, M.; Sun, Z.; et al. Rapamycin-mediated mTOR inhibition impairs silencing of sex chromosomes and the pachytene piRNA pathway in the mouse testis. Aging 2019, 11, 185–208. [Google Scholar] [CrossRef]
- Palomera-Ávalos, V.; Griñán-Ferré, C.; Izquierdo, V.; Camins, A.; Sanfeliu, C.; Canudas, A.M.; Pallàs, M. Resveratrol modulates response against acute inflammatory stimuli in aged mouse brain. Exp. Gerontol. 2018, 102, 3–11. [Google Scholar] [CrossRef]
- Wang, N.; Luo, Z.; Jin, M.; Sheng, W.; Wang, H.; Long, X.; Wu, Y.; Hu, P.; Xu, H.; Zhang, X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging 2019, 11, 3117–3137. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shang, X.; Wu, H.; Gautam, S.C.; Al-Holou, S.; Li, C.; Kuo, J.; Zhang, L.; Chopp, M. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J. Exp. Oncol. 2009, 8, 25–33. [Google Scholar]
- Gurusamy, N.; Lekli, I.; Mukherjee, S.; Ray, D.; Ahsan, M.K.; Gherghiceanu, M.; Popescu, L.M.; Das, D.K. Cardioprotection by resveratrol: A novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc. Res. 2010, 86, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yao, H.; Chen, X.; Wang, Z.; Xiang, Y.; Xia, J.; Liu, Y.; Wang, Y. Ginsenoside Rg1 Decreases Oxidative Stress and Down-Regulates Akt/mTOR Signalling to Attenuate Cognitive Impairment in Mice and Senescence of Neural Stem Cells Induced by d-Galactose. Neurochem. Res. 2018, 43, 430–440. [Google Scholar] [CrossRef]
- Victorino, A.B.; Serra, F.T.; Piñero, P.P.; de Almeida, A.A.; Lopim, G.M.; Matias Junior, I.; Machado, H.R.; Lent, R.; Cabral, F.R.; Gomez-Pinilla, F.; et al. Aerobic exercise in adolescence results in an increase of neuronal and non-neuronal cells and in mTOR overexpression in the cerebral cortex of rats. Neuroscience 2017, 361, 108–115. [Google Scholar] [CrossRef]
- Bao, C.; Yang, Z.; Li, Q.; Cai, Q.; Li, H.; Shu, B. Aerobic Endurance Exercise Ameliorates Renal Vascular Sclerosis in Aged Mice by Regulating PI3K/AKT/mTOR Signaling Pathway. DNA Cell Biol. 2020, 39, 310–320. [Google Scholar] [CrossRef]
- Zid, B.M.; Rogers, A.N.; Katewa, S.D.; Vargas, M.A.; Kolipinski, M.C.; Lu, T.A.; Benzer, S.; Kapahi, P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell 2009, 139, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.; Felix, D.; Gutiérrez-Gutiérrez, Ó.; De Miguel-Bonet, M.; Sahu, S.; Fernández-Varas, B.; Perona, R.; Aboobaker, A.; Flores, I.; González-Estévez, C. Downregulation of mTOR Signaling Increases Stem Cell Population Telomere Length during Starvation of Immortal Planarians. Stem Cell Rep. 2019, 13, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Tulsian, R.; Velingkaar, N.; Kondratov, R. Caloric restriction effects on liver mTOR signaling are time-of-day dependent. Aging 2018, 10, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liao, Y.; Tsai, S.; Thompson, L. Age-dependent effects of caloric restriction on mTOR and ubiquitin-proteasome pathways in skeletal muscles. GeroScience 2019, 41, 871–880. [Google Scholar] [CrossRef]
- Dong, W.; Wang, R.; Ma, L.; Xu, B.; Zhang, J.; Zhao, Z.; Wang, Y.; Zhang, X. Influence of age-related learning and memory capacity of mice: Different effects of a high and low caloric diet. Aging Clin. Exp. Res. 2016, 28, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, H.; Zhang, Q.; Feng, M.; Zhang, L. The low protein diet affects the nonspecific inflammatory response of middle-aged and old mice through mTOR. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7551–7561. [Google Scholar] [CrossRef]
- Hansen, M.; Taubert, S.; Crawford, D.; Libina, N.; Lee, S.J.; Kenyon, C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 2007, 6, 95–110. [Google Scholar] [CrossRef]
- Chun, Y.; Kim, J. Autophagy: An Essential Degradation Program for Cellular Homeostasis and Life. Cells 2018, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Kawakami, Y.; Lavasani, M.; Mu, X.; Cummins, J.H.; Yurube, T.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Robbins, P.D.; et al. mTOR signaling plays a critical role in the defects observed in muscle-derived stem/progenitor cells isolated from a murine model of accelerated aging. J. Orthop. Res. 2017, 35, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.J.; Chen, S.C.; Garelick, M.G.; Dai, D.F.; Liao, C.Y.; Schreiber, K.H.; MacKay, V.L.; An, E.H.; Strong, R.; Ladiges, W.C.; et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci. Transl. Med. 2012, 4, 144ra103. [Google Scholar] [CrossRef] [PubMed]
- Escobar, K.; Cole, N.; Mermier, C.; Van Dusseldorp, T. Autophagy and aging: Maintaining the proteome through exercise and caloric restriction. Aging Cell 2019, 18, e12876. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Lee, K.; Kim, J.; Choi, H. Interaction between mTOR pathway inhibition and autophagy induction attenuates adriamycin-induced vascular smooth muscle cell senescence through decreased expressions of p53/p21/p16. Exp. Gerontol. 2018, 109, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Khayati, K.; Antikainen, H.; Bonder, E.; Weber, G.; Kruger, W.; Jakubowski, H.; Dobrowolski, R. The amino acid metabolite homocysteine activates mTORC1 to inhibit autophagy and form abnormal proteins in human neurons and mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cao, J.; Yang, E.; Liang, B.; Ding, J.; Liang, J.; Xu, J. Curcumin improves age-related and surgically induced osteoarthritis by promoting autophagy in mice. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Wang, L.; Du, J.; Zhao, F.; Chen, Z.; Chang, J.; Qin, F.; Wang, Z.; Wang, F.; Chen, X.; Chen, N. Trillium tschonoskii maxim saponin mitigates d-galactose-induced brain aging of rats through rescuing dysfunctional autophagy mediated by Rheb-mTOR signal pathway. Biomed. Pharm. 2018, 98, 516–522. [Google Scholar] [CrossRef]
- Shi, P.; Liu, W.; Wang, H.; Li, F.; Zhang, H.; Wu, Y.; Kong, Y.; Zhou, Z.; Wang, C.; Chen, W.; et al. Metformin suppresses triple-negative breast cancer stem cells by targeting KLF5 for degradation. Cell Discov. 2017, 3, 17010. [Google Scholar] [CrossRef]
- Price, N.; Gomes, A.; Ling, A.; Duarte, F.; Martin-Montalvo, A.; North, B.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lim, J.; Kim, M.; Ban, T.; Jang, I.; Yoon, H.; Park, C.; Chang, Y.; Choi, B. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chen, J.; Xiao, M.; Sun, Y.; Zhao, Y.; Pu, D.; Lv, A.; Wang, M.; Zhou, J.; Zhu, S.; et al. The effect of exercise, resveratrol or their combination on Sarcopenia in aged rats via regulation of AMPK/Sirt1 pathway. Exp. Gerontol. 2017, 98, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Bayod, S.; Guzmán-Brambila, C.; Sanchez-Roige, S.; Lalanza, J.; Kaliman, P.; Ortuño-Sahagun, D.; Escorihuela, R.; Pallàs, M. Voluntary exercise promotes beneficial anti-aging mechanisms in SAMP8 female brain. J. Mol. Neurosci. MN 2015, 55, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, H.; Jin, Q.; You, W.; Cheng, H.; Liu, Y.; Song, E.; Liu, G.; Tan, X.; Zhang, X.; et al. Resveratrol induces apoptosis and inhibits adipogenesis by stimulating the SIRT1-AMPKα-FOXO1 signalling pathway in bovine intramuscular adipocytes. Mol. Cell. Biochem. 2018, 439, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Fulco, M.; Cen, Y.; Zhao, P.; Hoffman, E.P.; McBurney, M.W.; Sauve, A.A.; Sartorelli, V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell 2008, 14, 661–673. [Google Scholar] [CrossRef]
- Jung, C.; Ro, S.; Cao, J.; Otto, N.; Kim, D. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, R.; Zhao, H.; Chen, G.; Jiang, Y.; Lyu, X.; Wu, T. Reduction of Aging-Induced Oxidative Stress and Activation of Autophagy by Bilberry Anthocyanin Supplementation via the AMPK-mTOR Signaling Pathway in Aged Female Rats. J. Agric. Food Chem. 2019, 67, 7832–7843. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J.; Choi, H. Genistein-induced LKB1-AMPK activation inhibits senescence of VSMC through autophagy induction. Vasc. Pharmacol. 2016, 81, 75–82. [Google Scholar] [CrossRef]
- Dodds, S.G.; Parihar, M.; Javors, M.; Nie, J.; Musi, N.; Sharp, Z.D.; Hasty, P. Acarbose improved survival for Apc mice. Aging Cell 2020, e13088. [Google Scholar] [CrossRef]
- Feng, X.; Pan, J.; Li, J.; Zeng, C.; Qi, W.; Shao, Y.; Liu, X.; Liu, L.; Xiao, G.; Zhang, H.; et al. Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR. Aging 2020, 12, 1087–1103. [Google Scholar] [CrossRef]
- Qing, L.; Fu, J.; Wu, P.; Zhou, Z.; Yu, F.; Tang, J. Metformin induces the M2 macrophage polarization to accelerate the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome singling pathway. Am. J. Transl. Res. 2019, 11, 655–668. [Google Scholar] [PubMed]
- Qin, N.; Wei, L.; Li, W.; Yang, W.; Cai, L.; Qian, Z.; Wu, S. Local intra-articular injection of resveratrol delays cartilage degeneration in C57BL/6 mice by inducing autophagy via AMPK/mTOR pathway. J. Pharmacol. Sci. 2017, 134, 166–174. [Google Scholar] [CrossRef]
- Chen, J.; Gao, J.; Sun, W.; Li, L.; Wang, Y.; Bai, S.; Li, X.; Wang, R.; Wu, L.; Li, H.; et al. Involvement of exogenous H2S in recovery of cardioprotection from ischemic post-conditioning via increase of autophagy in the aged hearts. Int. J. Cardiol. 2016, 220, 681–692. [Google Scholar] [CrossRef]
- Rigacci, S.; Miceli, C.; Nediani, C.; Berti, A.; Cascella, R.; Pantano, D.; Nardiello, P.; Luccarini, I.; Casamenti, F.; Stefani, M. Oleuropein aglycone induces autophagy via the AMPK/mTOR signalling pathway: A mechanistic insight. Oncotarget 2015, 6, 35344–35357. [Google Scholar] [CrossRef]
- Dong, D.; Cai, G.; Ning, Y.; Wang, J.; Lv, Y.; Hong, Q.; Cui, S.; Fu, B.; Guo, Y.; Chen, X. Alleviation of senescence and epithelial-mesenchymal transition in aging kidney by short-term caloric restriction and caloric restriction mimetics via modulation of AMPK/mTOR signaling. Oncotarget 2017, 8, 16109–16121. [Google Scholar] [CrossRef]
- Rühlmann, C.; Wölk, T.; Blümel, T.; Stahn, L.; Vollmar, B.; Kuhla, A. ApoELong-term caloric restriction in -deficient mice results in neuroprotection via Fgf21-induced AMPK/mTOR pathway. Aging 2016, 8, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, H.S.; McBurney, M.; Robbins, P.D. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS ONE 2010, 5, e9199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cai, G.; Fu, B.; Feng, Z.; Ding, R.; Bai, X.; Liu, W.; Zhuo, L.; Sun, L.; Liu, F.; et al. SIRT1 is required for the effects of rapamycin on high glucose-inducing mesangial cells senescence. Mech. Ageing Dev. 2012, 133, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; He, Z.; Chen, W.; Lu, J. Rapamycin attenuates palmitate-induced lipid aggregation by up-regulating sirt-1 signaling in AML12 hepatocytes. Pharmazie 2016, 71, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Guarente, L. mTORC1 and SIRT1 Cooperate to Foster Expansion of Gut Adult Stem Cells during Calorie Restriction. Cell 2016, 166, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Lee, M.R.; Huang, X.; Messina-Graham, S.; Broxmeyer, H.E. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells 2014, 32, 1183–1194. [Google Scholar] [CrossRef]
- Chen, H.; Shen, F.; Sherban, A.; Nocon, A.; Li, Y.; Wang, H.; Xu, M.J.; Rui, X.; Han, J.; Jiang, B.; et al. DEP domain-containing mTOR-interacting protein suppresses lipogenesis and ameliorates hepatic steatosis and acute-on-chronic liver injury in alcoholic liver disease. Hepatology 2018, 68, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.J.; Pamplona, R.; Ramirez-Tortosa, M.C.; Granados-Principal, S.; Perez-Lopez, P.; Naudí, A.; Portero-Otin, M.; López-Frías, M.; Battino, M.; Quiles, J.L. Age-related changes in brain mitochondrial DNA deletion and oxidative stress are differentially modulated by dietary fat type and coenzyme Q10. Free Radic. Biol. Med. 2011, 50, 1053–1064. [Google Scholar] [CrossRef]
- Jin, K. A Microcirculatory Theory of Aging. Aging Dis. 2019, 10, 676–683. [Google Scholar] [CrossRef] [PubMed]
| Disease | Signaling Pathways | Cell Type/Model | Reference |
|---|---|---|---|
| Hutchinson-Gilford progeria syndrome (HGPS) | AMPK | HGPS cells | [30] |
| Muscle atrophy | protein kinase A (PKA)/LKB1/AMPK | High-fat diet-induced muscle atrophy in aged animal model | [39] |
| Parkinson’s disease (PD) | AMPK, brain-derived neurotrophic factor (BDNF) | 6-OHDA-induced PD animal model; MPTP-treated and haloperidol-induced catalepsy animal models | [37,38] |
| Alzheimer’s disease (AD) | SIRT1, mTOR | 5xFAD-ApoE4 (E4FAD) AD mouse model; Htau mice; adeno-associated viral vector-based mouse model of early-stage AD-type tauopathy; APP/PS1 mice | [40,41,42,43] |
| Diabetic encephalopathy (DE) | phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mTOR | Streptozotocin (STZ)-induced rat model | [44] |
| Osteoarthritis (OA) | PI3K/Akt/mTOR | Human OA chondrocytes | [45] |
| Machado-Joseph disease (MJD) | SIRT1 | Machado-Joseph disease mouse model | [46] |
| Diabetes | SIRT1/LKB1/AMPK | Type-2 diabetic KKA mice | [47] |
| Sarcopenia | SIRT1/PGC1-α | Aged rats | [48] |
| Chronic obstructive pulmonary disease (COPD) | mTOR | Lung tissue and derived cultured cells from patients with COPD | [49] |
| Ovarian cancer | Akt/mTOR | Human ovarian cancer cells | [50] |
| Breast cancer | mTOR | Xenograft mouse model | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Zhang, H.; Wang, B.; Zhang, Y.; Zheng, X.; Shao, B.; Zhuge, Q.; Jin, K. Key Signaling Pathways in Aging and Potential Interventions for Healthy Aging. Cells 2021, 10, 660. https://doi.org/10.3390/cells10030660
Yu M, Zhang H, Wang B, Zhang Y, Zheng X, Shao B, Zhuge Q, Jin K. Key Signaling Pathways in Aging and Potential Interventions for Healthy Aging. Cells. 2021; 10(3):660. https://doi.org/10.3390/cells10030660
Chicago/Turabian StyleYu, Mengdi, Hongxia Zhang, Brian Wang, Yinuo Zhang, Xiaoying Zheng, Bei Shao, Qichuan Zhuge, and Kunlin Jin. 2021. "Key Signaling Pathways in Aging and Potential Interventions for Healthy Aging" Cells 10, no. 3: 660. https://doi.org/10.3390/cells10030660
APA StyleYu, M., Zhang, H., Wang, B., Zhang, Y., Zheng, X., Shao, B., Zhuge, Q., & Jin, K. (2021). Key Signaling Pathways in Aging and Potential Interventions for Healthy Aging. Cells, 10(3), 660. https://doi.org/10.3390/cells10030660





