Identification of Ku70 Domain-Specific Interactors Using BioID2
Abstract
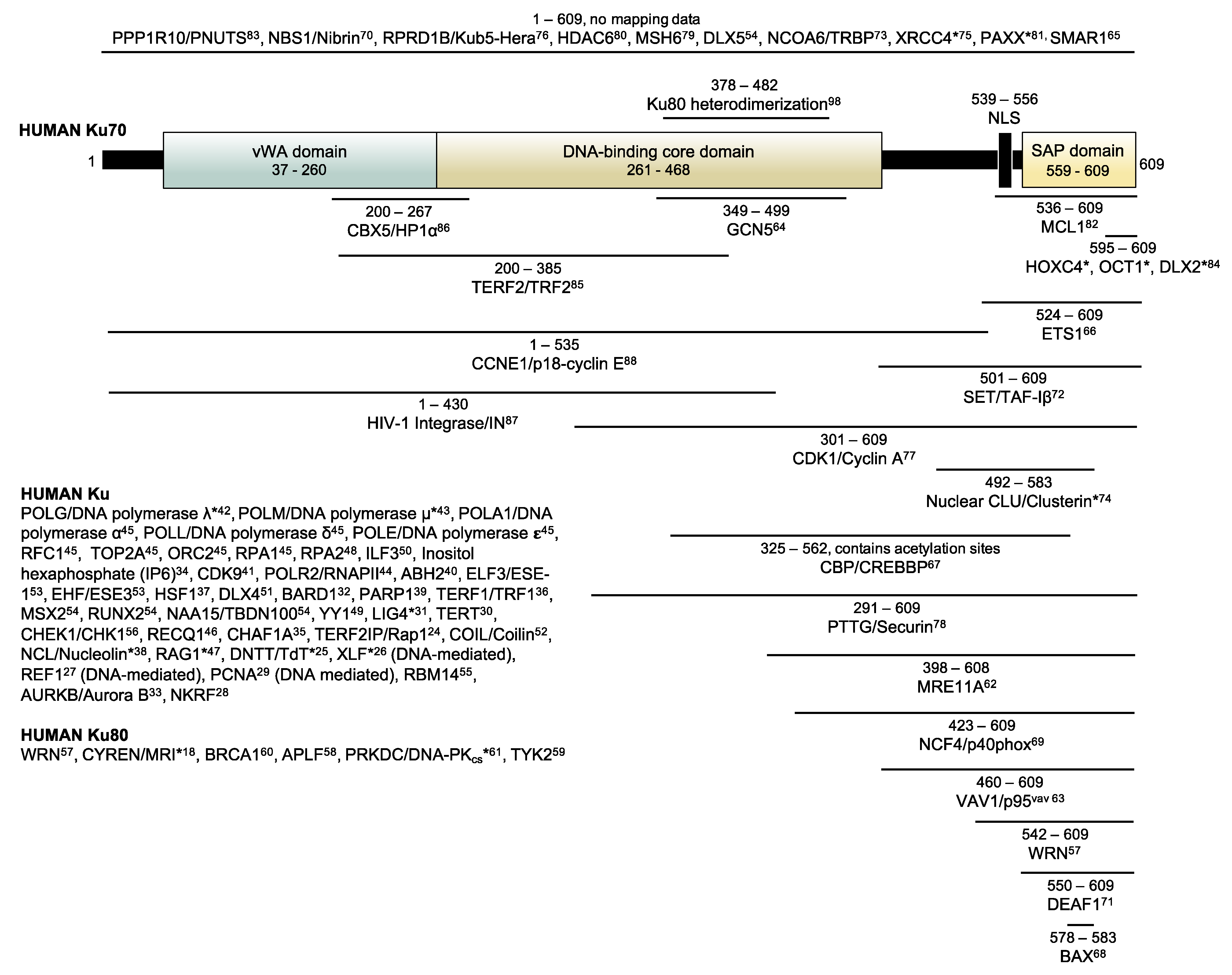
1. Introduction
2. Materials and Methods
2.1. Designing and Cloning Plasmid Expression Constructs
2.2. Cell Culturing, Transfections, Stable Cell Line Generation
2.3. Indirect Immunofluorescence
2.4. Preparation of Extracts and Immunoblotting
2.5. Co-Immunoprecipitation of Ku80, RNF113A, and Spindly
2.6. Double-Stranded DNA (dsDNA) Pull-Down Assay
2.7. Biotinylation and Streptavidin Pull-Down of Biotinylated Proteins
2.8. On-Bead Protein Digestion
2.9. Liquid Chromatography Electrospray Ionizing Tandem Mass Spectrometry (LC-ESI-MS/MS) and Data Analysis
2.10. SAINTexpress Analysis
2.11. Duolink® In situ Proximity Ligation Assay (PLA)
3. Results and Discussion
3.1. Designing the Ku70-BioID2 Constructs
3.2. Creating Ku70-BioID2 Polyclonal Stable Cell Lines in HEK293 Cells
3.3. Biotinylation and Expression of Stable Cell Lines
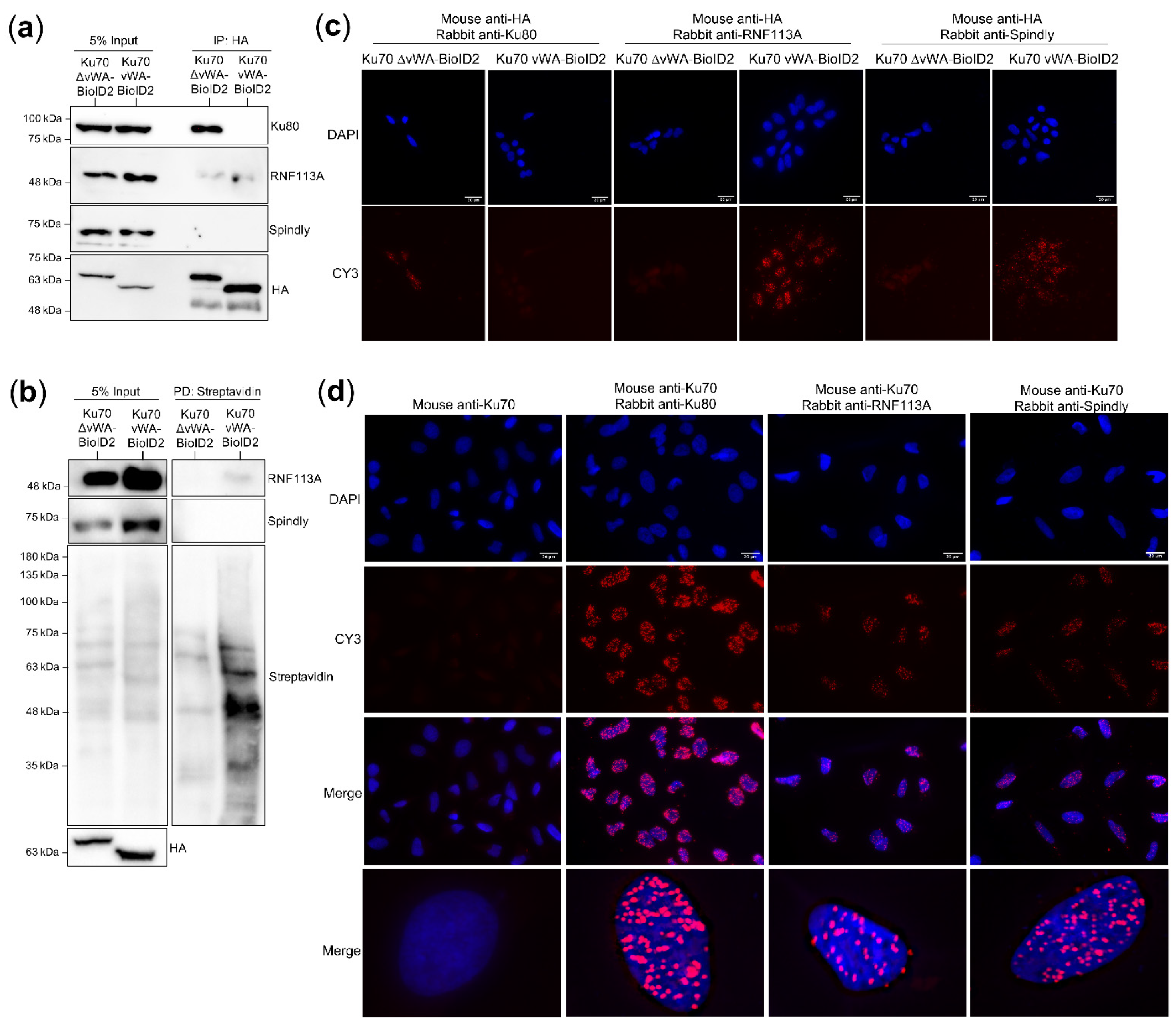
3.4. Co-Immunoprecipitation with Ku80 and Association with Broken Double-Stranded DNA
3.5. Conducting BioID2 and Preliminary Analysis
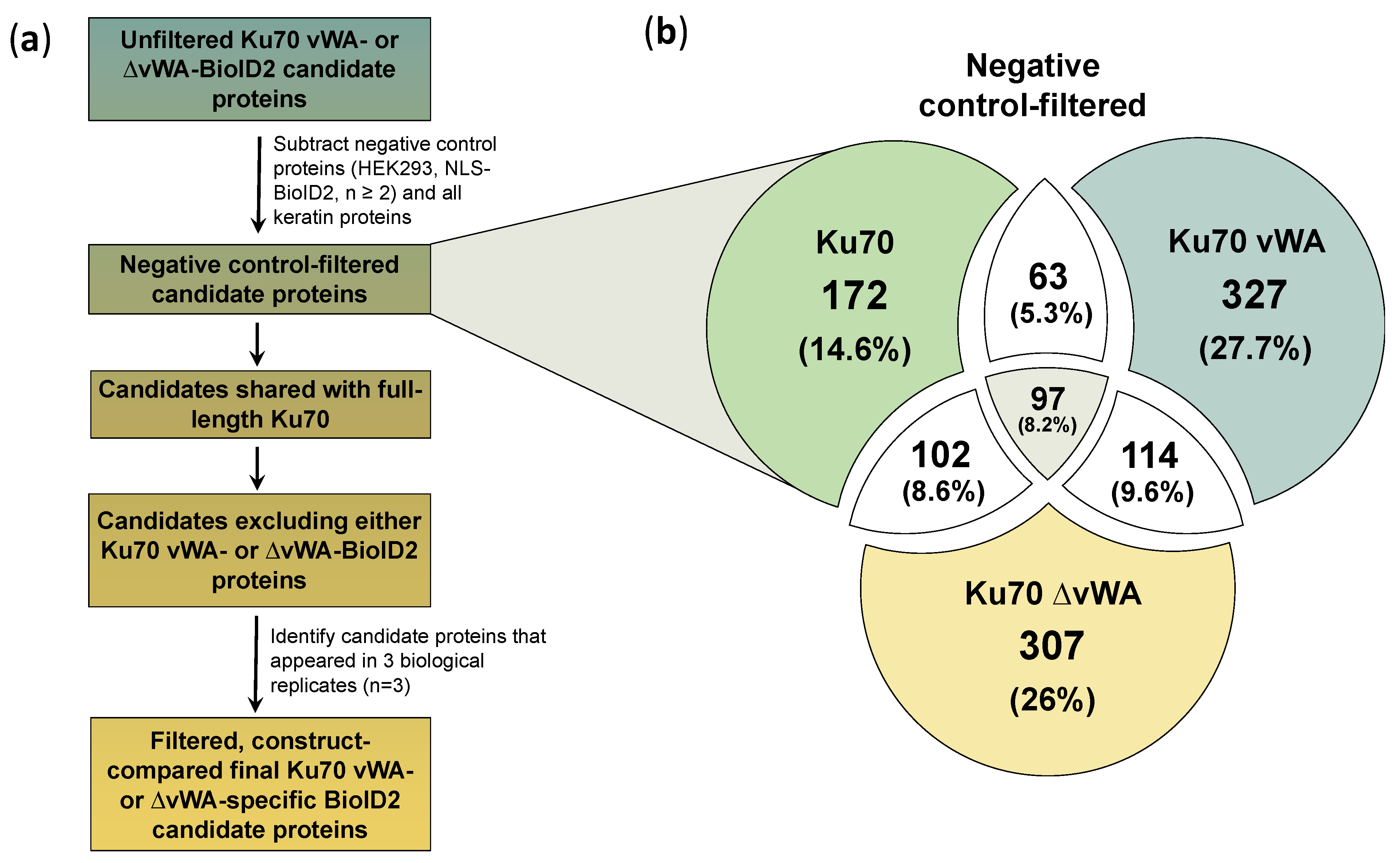
3.6. Filtering, Comparing, and Processing Ku70 vWA- and ∆vWA-Specific Protein Interactors
- Since Ku70 vWA and ∆vWA are mutually exclusive, no true BioID2 candidates would be shared, irrespective of the number of biological replicates in which the candidate appears;
- Both Ku70 vWA and ∆vWA candidates should be shared with full-length Ku70, meaning the candidate must be present in at least one biological replicate of Ku70-BioID2, which represents a positive control;
- All final candidates must appear in all three biological replicates of either Ku70 vWA- or Ku70 ∆vWA-BioID2 results.
3.7. Identifying and Analyzing Ku70 ∆vWA-Specific BioID2 Candidate Proteins
3.8. Identifying and Analyzing Ku70 vWA-Specific BioID2 Candidate Proteins
3.9. Validating RNF113A and Spindly as Ku70 and Ku70 vWA Proximal Interactors
3.10. Candidate Identification Using SAINTexpress Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roux, K.J.; Kim, D.I.; Raida, M.; Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012, 196, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Jensen, S.C.; Noble, K.A.; Kc, B.; Roux, K.H.; Motamedchaboki, K.; Roux, K.J. An improved smaller biotin ligase for BioID proximity labeling. Mol. Biol. Cell 2016, 27, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Ummethum, H.; Hamperl, S. Proximity labeling techniques to study chromatin. Front. Genet. 2020, 11, 450. [Google Scholar] [CrossRef]
- Uezu, A.; Kanak, D.J.; Bradshaw, T.W.A.; Soderblom, E.J.; Catavero, C.M.; Burette, A.C.; Weinberg, R.J.; Soderling, S.H. Identification of an elaborate complex mediating postsynaptic inhibition. Science 2016, 353, 1123–1129. [Google Scholar] [CrossRef]
- Schopp, I.M.; Amaya Ramirez, C.C.; Debeljak, J.; Kreibich, E.; Skribbe, M.; Wild, K.; Béthune, J. Split-BioID a conditional proteomics approach to monitor the composition of spatiotemporally defined protein complexes. Nat. Commun. 2017, 8, 15690. [Google Scholar] [CrossRef]
- Ramanathan, M.; Majzoub, K.; Rao, D.S.; Neela, P.H.; Zarnegar, B.J.; Mondal, S.; Roth, J.G.; Gai, H.; Kovalski, J.R.; Siprashvili, Z.; et al. RNA-protein interaction detection in living cells. Nat. Methods 2018, 15, 207–212. [Google Scholar] [CrossRef]
- Branon, T.C.; Bosch, J.A.; Sanchez, A.D.; Udeshi, N.D.; Svinkina, T.; Carr, S.A.; Feldman, J.L.; Perrimon, N.; Ting, A.Y. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 2018, 36, 880–887. [Google Scholar] [CrossRef]
- Chojnowski, A.; Sobota, R.M.; Ong, P.F.; Xie, W.; Wong, X.; Dreesen, O.; Burke, B.; Stewart, C.L. 2C-BioID: An advanced two component BioID system for precision mapping of protein interactomes. iScience 2018, 10, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Remnant, L.; Booth, D.G.; Vargiu, G.; Spanos, C.; Kerr, A.R.W.; Earnshaw, W.C. In vitro BioID: Mapping the CENP-A microenvironment with high temporal and spatial resolution. Mol. Biol. Cell 2019, 30, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.F.; Branon, T.C.; Rajeev, S.; Svinkina, T.; Udeshi, N.D.; Thoudam, T.; Kwak, C.; Rhee, H.-W.; Lee, I.-K.; Carr, S.A.; et al. Split-TurboID enables contact-dependent proximity labeling in cells. Proc. Natl. Acad. Sci. USA 2020, 117, 12143–12154. [Google Scholar] [CrossRef]
- Minde, D.-P.; Ramakrishna, M.; Lilley, K.S. Biotin proximity tagging favours unfolded proteins and enables the study of intrinsically disordered regions. Commun. Biol. 2020, 3, 38. [Google Scholar] [CrossRef]
- May, D.G.; Scott, K.L.; Campos, A.R.; Roux, K.J. Comparative application of BioID and TurboID for protein-proximity biotinylation. Cells 2020, 9, 1070. [Google Scholar] [CrossRef]
- Jäger, S.; Cimermancic, P.; Gulbahce, N.; Johnson, J.R.; McGovern, K.E.; Clarke, S.C.; Shales, M.; Mercenne, G.; Pache, L.; Li, K.; et al. Global landscape of HIV–human protein complexes. Nature 2011, 481, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Larsen, B.; Lin, Z.-Y.; Breitkreutz, A.; Mellacheruvu, D.; Fermin, D.; Qin, Z.S.; Tyers, M.; Gingras, A.-C.; Nesvizhskii, A.I. SAINT: Probabilistic scoring of affinity purification - mass spectrometry data. Nat. Methods 2011, 8, 70–73. [Google Scholar] [CrossRef]
- Teo, G.; Liu, G.; Zhang, J.; Nesvizhskii, A.I.; Gingras, A.-C.; Choi, H. SAINTexpress: Improvements and additional features in Significance Analysis of INTeractome software. J. Proteom. 2014, 100, 37–43. [Google Scholar] [CrossRef]
- Li, X.; Tran, K.M.; Aziz, K.E.; Sorokin, A.V.; Chen, J.; Wang, W. Defining the protein-protein interaction network of the human protein tyrosine phosphatase family. Mol. Cell. Proteom. 2016, 15, 3030–3044. [Google Scholar] [CrossRef] [PubMed]
- Prévost, M.; Chamousset, D.; Nasa, I.; Freele, E.; Morrice, N.; Moorhead, G.; Trinkle-Mulcahy, L. Quantitative fragmentome mapping reveals novel, domain-specific partners for the modular protein RepoMan (recruits PP1 onto mitotic chromatin at anaphase). Mol. Cell. Proteom. 2013, 12, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Grundy, G.J.; Rulten, S.L.; Arribas-Bosacoma, R.; Davidson, K.; Kozik, Z.; Oliver, A.W.; Pearl, L.H.; Caldecott, K.W. The Ku-binding motif is a conserved module for recruitment and stimulation of non-homologous end-joining proteins. Nat. Commun. 2016, 7, 11242. [Google Scholar] [CrossRef] [PubMed]
- Kalkat, M.; Resetca, D.; Lourenco, C.; Chan, P.-K.; Wei, Y.; Shiah, Y.-J.; Vitkin, N.; Tong, Y.; Sunnerhagen, M.; Done, S.J.; et al. MYC protein interactome profiling reveals functionally distinct regions that cooperate to drive tumorigenesis. Mol. Cell 2018, 72, 836–848.e7. [Google Scholar] [CrossRef] [PubMed]
- McClellan, D.; Casey, M.J.; Bareyan, D.; Lucente, H.; Ours, C.; Velinder, M.; Singer, J.; Lone, M.D.; Sun, W.; Coria, Y.; et al. Growth factor independence 1B-mediated transcriptional repression and lineage allocation require lysine-specific demethylase 1-dependent recruitment of the BHC complex. Mol. Cell. Biol. 2019, 39. [Google Scholar] [CrossRef]
- Abbasi, S.; Schild-Poulter, C. Mapping the Ku interactome using proximity-dependent biotin identification in human cells. J. Proteome Res. 2019, 18, 1064–1077. [Google Scholar] [CrossRef]
- Fell, V.L.; Schild-Poulter, C. The Ku heterodimer: Function in DNA repair and beyond. Mutat. Res. Rev. Mutat. Res. 2015, 763, 15–29. [Google Scholar] [CrossRef]
- Downs, J.A.; Jackson, S.P. A means to a DNA end: The many roles of Ku. Nat. Rev. Mol. Cell Biol. 2004, 5, 367–378. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.S.; Safari, A.; Liu, D.; Qin, J.; Songyang, Z. The human Rap1 protein complex and modulation of telomere length. J. Biol. Chem. 2004, 279, 28585–28591. [Google Scholar] [CrossRef] [PubMed]
- Purugganan, M.M.; Shah, S.; Kearney, J.F.; Roth, D.B. Ku80 is required for addition of N nucleotides to V(D)J recombination junctions by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 2001, 29, 1638–1646. [Google Scholar] [CrossRef]
- Nemoz, C.; Ropars, V.; Frit, P.; Gontier, A.; Drevet, P.; Yu, J.; Guerois, R.; Pitois, A.; Comte, A.; Delteil, C.; et al. XLF and APLF bind Ku80 at two remote sites to ensure DNA repair by non-homologous end joining. Nat. Struct. Mol. Biol. 2018, 25, 971–980. [Google Scholar] [CrossRef]
- Chung, U.; Igarashi, T.; Nishishita, T.; Iwanari, H.; Iwamatsu, A.; Suwa, A.; Mimori, T.; Hata, K.; Ebisu, S.; Ogata, E.; et al. The interaction between Ku antigen and REF1 protein mediates negative gene regulation by extracellular calcium. J. Biol. Chem. 1996, 271, 8593–8598. [Google Scholar] [CrossRef]
- Feldman, I.; Feldman, G.M.; Mobarak, C.; Dunkelberg, J.C.; Leslie, K.K. Identification of proteins within the nuclear factor-kappa B transcriptional complex including estrogen receptor-alpha. Am. J. Obstet. Gynecol. 2007, 196, 394-e1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Balajee, A.S.; Geard, C.R. Chromatin-bound PCNA complex formation triggered by DNA damage occurs independent of the ATM gene product in human cells. Nucleic Acids Res. 2001, 29, 1341–1351. [Google Scholar] [CrossRef][Green Version]
- Chai, W.; Ford, L.P.; Lenertz, L.; Wright, W.E.; Shay, J.W. Human Ku70/80 associates physically with telomerase through interaction with hTERT. J. Biol. Chem. 2002, 277, 47242–47247. [Google Scholar] [CrossRef]
- Costantini, S.; Woodbine, L.; Andreoli, L.; Jeggo, P.A.; Vindigni, A. Interaction of the Ku heterodimer with the DNA ligase IV/Xrcc4 complex and its regulation by DNA-PK. DNA Repair (Amst.) 2007, 6, 712–722. [Google Scholar] [CrossRef]
- Feki, A.; Jefford, C.E.; Berardi, P.; Wu, J.-Y.; Cartier, L.; Krause, K.-H.; Irminger-Finger, I. BARD1 induces apoptosis by catalysing phosphorylation of p53 by DNA-damage response kinase. Oncogene 2005, 24, 3726–3736. [Google Scholar] [CrossRef] [PubMed]
- Fell, V.L.; Walden, E.A.; Hoffer, S.M.; Rogers, S.R.; Aitken, A.S.; Salemi, L.M.; Schild-Poulter, C. Ku70 serine 155 mediates Aurora B inhibition and activation of the DNA damage response. Sci. Rep. 2016, 6, 37194. [Google Scholar] [CrossRef] [PubMed]
- Hanakahi, L.A.; West, S.C. Specific interaction of IP6 with human Ku70/80, the DNA-binding subunit of DNA-PK. EMBO J. 2002, 21, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Hoek, M.; Myers, M.P.; Stillman, B. An analysis of CAF-1-interacting proteins reveals dynamic and direct interactions with the KU complex and 14-3-3 proteins. J. Biol. Chem. 2011, 286, 10876–10887. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.L.; Gilley, D.; Galande, S.A.; Hande, M.P.; Allen, B.; Kim, S.H.; Li, G.C.; Campisi, J.; Kohwi-Shigematsu, T.; Chen, D.J. Ku acts in a unique way at the mammalian telomere to prevent end joining. Genes Dev. 2000, 14, 2807–2812. [Google Scholar] [CrossRef]
- Huang, J.; Nueda, A.; Yoo, S.; Dynan, W.S. Heat shock transcription factor 1 binds selectively in vitro to Ku protein and the catalytic subunit of the DNA-dependent protein kinase. J. Biol. Chem. 1997, 272, 26009–26016. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Fujimoto, H.; Sato, J.; Hayashi, I.; Burma, S.; Matsuura, S.; Chen, D.J.; Komatsu, K. Nucleolin participates in DNA double-strand break-induced damage response through MDC1-dependent pathway. PLoS ONE 2012, 7, e49245. [Google Scholar] [CrossRef]
- Li, B.; Navarro, S.; Kasahara, N.; Comai, L. Identification and biochemical characterization of a Werner’s syndrome protein complex with Ku70/80 and poly(ADP-ribose) polymerase-1. J. Biol. Chem. 2004, 279, 13659–13667. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gao, S.; Wang, L.; Yu, F.; Li, J.; Wang, C.; Li, J.; Wong, J. ABH2 couples regulation of ribosomal DNA transcription with DNA alkylation repair. Cell Rep. 2013, 4, 817–829. [Google Scholar] [CrossRef]
- Liu, H.; Herrmann, C.H.; Chiang, K.; Sung, T.-L.; Moon, S.-H.; Donehower, L.A.; Rice, A.P. 55K isoform of CDK9 associates with Ku70 and is involved in DNA repair. Biochem. Biophys. Res. Commun. 2010, 397, 245–250. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, H.; Tippin, B.; Goodman, M.F.; Shimazaki, N.; Koiwai, O.; Hsieh, C.-L.; Schwarz, K.; Lieber, M.R. A biochemically defined system for mammalian nonhomologous DNA end joining. Mol. Cell 2004, 16, 701–713. [Google Scholar] [CrossRef]
- Mahajan, K.N.; Nick McElhinny, S.A.; Mitchell, B.S.; Ramsden, D.A. Association of DNA polymerase mu (Pol mu) with Ku and ligase IV: Role for Pol mu in end-joining double-strand break repair. Mol. Cell. Biol. 2002, 22, 5194–5202. [Google Scholar] [CrossRef]
- Maldonado, E.; Shiekhattar, R.; Sheldon, M.; Cho, H.; Drapkin, R.; Rickert, P.; Lees, E.; Anderson, C.W.; Linn, S.; Reinberg, D. A human RNA polymerase II complex associated with SRB and DNA-repair proteins. Nature 1996, 381, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Matheos, D.; Ruiz, M.T.; Price, G.B.; Zannis-Hadjopoulos, M. Ku antigen, an origin-specific binding protein that associates with replication proteins, is required for mammalian DNA replication. Biochim. Biophys. Acta 2002, 1578, 59–72. [Google Scholar] [CrossRef]
- Parvathaneni, S.; Stortchevoi, A.; Sommers, J.A.; Brosh, R.M.; Sharma, S. Human RECQ1 interacts with Ku70/80 and modulates DNA end-joining of double-strand breaks. PLoS ONE 2013, 8, e62481. [Google Scholar] [CrossRef] [PubMed]
- Raval, P.; Kriatchko, A.N.; Kumar, S.; Swanson, P.C. Evidence for Ku70/Ku80 association with full-length RAG1. Nucleic Acids Res. 2008, 36, 2060–2072. [Google Scholar] [CrossRef]
- Shao, R.G.; Cao, C.X.; Zhang, H.; Kohn, K.W.; Wold, M.S.; Pommier, Y. Replication-mediated DNA damage by camptothecin induces phosphorylation of RPA by DNA-dependent protein kinase and dissociates RPA:DNA-PK complexes. EMBO J. 1999, 18, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Sucharov, C.C.; Helmke, S.M.; Langer, S.J.; Perryman, M.B.; Bristow, M.; Leinwand, L. The Ku protein complex interacts with YY1, is up-regulated in human heart failure, and represses alpha myosin heavy-chain gene expression. Mol. Cell. Biol. 2004, 24, 8705–8715. [Google Scholar] [CrossRef]
- Ting, N.S.; Kao, P.N.; Chan, D.W.; Lintott, L.G.; Lees-Miller, S.P. DNA-dependent protein kinase interacts with antigen receptor response element binding proteins NF90 and NF45. J. Biol. Chem. 1998, 273, 2136–2145. [Google Scholar] [CrossRef]
- Trinh, B.Q.; Ko, S.Y.; Barengo, N.; Lin, S.-Y.; Naora, H. Dual functions of the homeoprotein DLX4 in modulating responsiveness of tumor cells to topoisomerase II-targeting drugs. Cancer Res. 2013, 73, 1000–1010. [Google Scholar] [CrossRef]
- Velma, V.; Carrero, Z.I.; Cosman, A.M.; Hebert, M.D. Coilin interacts with Ku proteins and inhibits in vitro non-homologous DNA end joining. FEBS Lett. 2010, 584, 4735–4739. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fang, R.; Cho, J.-Y.; Libermann, T.A.; Oettgen, P. Positive and negative modulation of the transcriptional activity of the ETS factor ESE-1 through interaction with p300, CREB-binding protein, and Ku 70/86. J. Biol. Chem. 2004, 279, 25241–25250. [Google Scholar] [CrossRef]
- Willis, D.M.; Loewy, A.P.; Charlton-Kachigian, N.; Shao, J.-S.; Ornitz, D.M.; Towler, D.A. Regulation of osteocalcin gene expression by a novel Ku antigen transcription factor complex. J. Biol. Chem. 2002, 277, 37280–37291. [Google Scholar] [CrossRef]
- Yuan, M.; Eberhart, C.G.; Kai, M. RNA binding protein RBM14 promotes radio-resistance in glioblastoma by regulating DNA repair and cell differentiation. Oncotarget 2014, 5, 2820–2826. [Google Scholar] [CrossRef] [PubMed]
- Goudelock, D.M.; Jiang, K.; Pereira, E.; Russell, B.; Sanchez, Y. Regulatory interactions between the checkpoint kinase Chk1 and the proteins of the DNA-dependent protein kinase complex. J. Biol. Chem. 2003, 278, 29940–29947. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, P.; Snowden, C.M.; Ramsden, D.A.; Bohr, V.A. Ku heterodimer binds to both ends of the Werner protein and functional interaction occurs at the Werner N-terminus. Nucleic Acids Res. 2002, 30, 3583–3591. [Google Scholar] [CrossRef] [PubMed]
- Shirodkar, P.; Fenton, A.L.; Meng, L.; Koch, C.A. Identification and functional characterization of a Ku-binding motif in Aprataxin Polynucleotide Kinase/Phosphatase-like Factor (APLF). J. Biol. Chem. 2013, 288, 19604–19613. [Google Scholar] [CrossRef]
- Adam, L.; Bandyopadhyay, D.; Kumar, R. Interferon-alpha signaling promotes nucleus-to-cytoplasmic redistribution of p95Vav, and formation of a multisubunit complex involving Vav, Ku80, and Tyk2. Biochem. Biophys. Res. Commun. 2000, 267, 692–696. [Google Scholar] [CrossRef]
- Jiang, G.; Plo, I.; Wang, T.; Rahman, M.; Cho, J.H.; Yang, E.; Lopez, B.S.; Xia, F. BRCA1-Ku80 protein interaction enhances end-joining fidelity of chromosomal double-strand breaks in the G1 phase of the cell cycle. J. Biol. Chem. 2013, 288, 8966–8976. [Google Scholar] [CrossRef] [PubMed]
- Singleton, B.K.; Torres-Arzayus, M.I.; Rottinghaus, S.T.; Taccioli, G.E.; Jeggo, P.A. The C terminus of Ku80 activates the DNA-dependent protein kinase catalytic subunit. Mol. Cell. Biol. 1999, 19, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Goedecke, W.; Eijpe, M.; Offenberg, H.H.; van Aalderen, M.; Heyting, C. Mre11 and Ku70 interact in somatic cells, but are differentially expressed in early meiosis. Nat. Genet. 1999, 23, 194–198. [Google Scholar] [CrossRef]
- Romero, F.; Dargemont, C.; Pozo, F.; Reeves, W.H.; Camonis, J.; Gisselbrecht, S.; Fischer, S. p95vav associates with the nuclear protein Ku-70. Mol. Cell. Biol. 1996, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Barlev, N.A.; Poltoratsky, V.; Owen-Hughes, T.; Ying, C.; Liu, L.; Workman, J.L.; Berger, S.L. Repression of GCN5 histone acetyltransferase activity via bromodomain-mediated binding and phosphorylation by the Ku-DNA-dependent protein kinase complex. Mol. Cell. Biol. 1998, 18, 1349–1358. [Google Scholar] [CrossRef]
- Chaudhary, N.; Nakka, K.K.; Chavali, P.L.; Bhat, J.; Chatterjee, S.; Chattopadhyay, S. SMAR1 coordinates HDAC6-induced deacetylation of Ku70 and dictates cell fate upon irradiation. Cell Death Dis. 2014, 5, e1447. [Google Scholar] [CrossRef] [PubMed]
- Choul-Li, S.; Legrand, A.J.; Bidon, B.; Vicogne, D.; Villeret, V.; Aumercier, M. Ets-1 interacts through a similar binding interface with Ku70 and poly (ADP-ribose) polymerase-1. Biosci. Biotechnol. Biochem. 2018, 82, 1753–1759. [Google Scholar] [CrossRef]
- Cohen, H.Y.; Lavu, S.; Bitterman, K.J.; Hekking, B.; Imahiyerobo, T.A.; Miller, C.; Frye, R.; Ploegh, H.; Kessler, B.M.; Sinclair, D.A. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol. Cell 2004, 13, 627–638. [Google Scholar] [CrossRef]
- Gomez, J.A.; Gama, V.; Yoshida, T.; Sun, W.; Hayes, P.; Leskov, K.; Boothman, D.; Matsuyama, S. Bax-inhibiting peptides derived from Ku70 and cell-penetrating pentapeptides. Biochem. Soc. Trans. 2007, 35, 797–801. [Google Scholar] [CrossRef]
- Grandvaux, N.; Grizot, S.; Vignais, P.V.; Dagher, M.C. The Ku70 autoantigen interacts with p40phox in B lymphocytes. J. Cell. Sci. 1999, 112, 503–513. [Google Scholar]
- Iijima, K.; Muranaka, C.; Kobayashi, J.; Sakamoto, S.; Komatsu, K.; Matsuura, S.; Kubota, N.; Tauchi, H. NBS1 regulates a novel apoptotic pathway through Bax activation. DNA Repair (Amst.) 2008, 7, 1705–1716. [Google Scholar] [CrossRef]
- Jensik, P.J.; Huggenvik, J.I.; Collard, M.W. Deformed Epidermal Autoregulatory Factor-1 (DEAF1) interacts with the Ku70 subunit of the DNA-dependent protein kinase complex. PLoS ONE 2012, 7, e33404. [Google Scholar] [CrossRef][Green Version]
- Kim, K.-B.; Kim, D.-W.; Park, J.W.; Jeon, Y.-J.; Kim, D.; Rhee, S.; Chae, J.-I.; Seo, S.-B. Inhibition of Ku70 acetylation by INHAT subunit SET/TAF-Iβ regulates Ku70-mediated DNA damage response. Cell. Mol. Life Sci. 2014, 71, 2731–2745. [Google Scholar] [CrossRef]
- Ko, L.; Chin, W.W. Nuclear receptor coactivator thyroid hormone receptor-binding protein (TRBP) interacts with and stimulates its associated DNA-dependent protein kinase. J. Biol. Chem. 2003, 278, 11471–11479. [Google Scholar] [CrossRef]
- Leskov, K.S.; Klokov, D.Y.; Li, J.; Kinsella, T.J.; Boothman, D.A. Synthesis and functional analyses of nuclear Clusterin, a cell death protein. J. Biol. Chem. 2003, 278, 11590–11600. [Google Scholar] [CrossRef] [PubMed]
- Mari, P.-O.; Florea, B.I.; Persengiev, S.P.; Verkaik, N.S.; Brüggenwirth, H.T.; Modesti, M.; Giglia-Mari, G.; Bezstarosti, K.; Demmers, J.A.A.; Luider, T.M.; et al. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc. Natl. Acad. Sci. USA 2006, 103, 18597–18602. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.C.; Richard, P.; Rommel, A.; Fattah, F.J.; Motea, E.A.; Patidar, P.L.; Xiao, L.; Leskov, K.; Wu, S.-Y.; Hittelman, W.N.; et al. Kub5-Hera, the human Rtt103 homolog, plays dual functional roles in transcription termination and DNA repair. Nucleic Acids Res. 2014, 42, 4996–5006. [Google Scholar] [CrossRef] [PubMed]
- Müller-Tidow, C.; Ji, P.; Diederichs, S.; Potratz, J.; Bäumer, N.; Köhler, G.; Cauvet, T.; Choudary, C.; van der Meer, T.; Chan, W.-Y.I.; et al. The cyclin A1-CDK2 complex regulates DNA double-strand break repair. Mol. Cell. Biol. 2004, 24, 8917–8928. [Google Scholar] [CrossRef]
- Romero, F.; Multon, M.C.; Ramos-Morales, F.; Domínguez, A.; Bernal, J.A.; Pintor-Toro, J.A.; Tortolero, M. Human Securin, hPTTG, is associated with Ku heterodimer, the regulatory subunit of the DNA-dependent protein kinase. Nucleic Acids Res. 2001, 29, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Shahi, A.; Lee, J.-H.; Kang, Y.; Lee, S.H.; Hyun, J.-W.; Chang, I.-Y.; Jun, J.-Y.; You, H.J. Mismatch-repair protein MSH6 is associated with Ku70 and regulates DNA double-strand break repair. Nucleic Acids Res. 2011, 39, 2130–2143. [Google Scholar] [CrossRef]
- Subramanian, C.; Jarzembowski, J.A.; Opipari, A.W.; Castle, V.P.; Kwok, R.P.S. HDAC6 deacetylates Ku70 and regulates Ku70-Bax binding in neuroblastoma. Neoplasia 2011, 13, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Tadi, S.K.; Tellier-Lebègue, C.; Nemoz, C.; Drevet, P.; Audebert, S.; Roy, S.; Meek, K.; Charbonnier, J.-B.; Modesti, M. PAXX is an accessory c-NHEJ factor that associates with Ku70 and has overlapping functions with XLF. Cell Rep. 2016, 17, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xie, M.; Li, R.; Owonikoko, T.K.; Ramalingam, S.S.; Khuri, F.R.; Curran, W.J.; Wang, Y.; Deng, X. Role of Ku70 in deubiquitination of Mcl-1 and suppression of apoptosis. Cell Death Differ. 2014, 21, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Fisher, L.A.; Bessho, T.; Peng, A. Protein phosphatase 1 and phosphatase 1 nuclear targeting subunit-dependent regulation of DNA-dependent protein kinase and non-homologous end joining. Nucleic Acids Res. 2017, 45, 10583–10594. [Google Scholar] [CrossRef]
- Schild-Poulter, C.; Pope, L.; Giffin, W.; Kochan, J.C.; Ngsee, J.K.; Traykova-Andonova, M.; Haché, R.J. The binding of Ku antigen to homeodomain proteins promotes their phosphorylation by DNA-dependent protein kinase. J Biol Chem 2001, 276, 16848–16856. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Jung, D.; Jung, Y.; Lee, S.G.; Lee, I. Interaction of human Ku70 with TRF2. FEBS Lett. 2000, 481, 81–85. [Google Scholar] [CrossRef]
- Song, K.; Jung, Y.; Jung, D.; Lee, I. Human Ku70 interacts with heterochromatin protein 1alpha. J. Biol. Chem. 2001, 276, 8321–8327. [Google Scholar] [CrossRef]
- Zheng, Y.; Ao, Z.; Wang, B.; Jayappa, K.D.; Yao, X. Host protein Ku70 binds and protects HIV-1 integrase from proteasomal degradation and is required for HIV replication. J. Biol. Chem. 2011, 286, 17722–17735. [Google Scholar] [CrossRef]
- Mazumder, S.; Plesca, D.; Kinter, M.; Almasan, A. Interaction of a cyclin E fragment with Ku70 regulates Bax-mediated apoptosis. Mol. Cell. Biol. 2007, 27, 3511–3520. [Google Scholar] [CrossRef]
- Fell, V.L.; Schild-Poulter, C. Ku regulates signaling to DNA damage response pathways through the Ku70 von Willebrand A domain. Mol. Cell. Biol. 2012, 32, 76–87. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Bandeira, N.; Sharma, V.; Perez-Riverol, Y.; Carver, J.J.; Kundu, D.J.; García-Seisdedos, D.; Jarnuczak, A.F.; Hewapathirana, S.; Pullman, B.S.; et al. The ProteomeXchange consortium in 2020: Enabling “big data” approaches in proteomics. Nucleic Acids Res. 2020, 48, D1145–D1152. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem 2003, 75, 4646–4658. [Google Scholar] [CrossRef]
- Koike, M.; Ikuta, T.; Miyasaka, T.; Shiomi, T. The nuclear localization signal of the human Ku70 is a variant bipartite type recognized by the two components of nuclear pore-targeting complex. Exp. Cell Res. 1999, 250, 401–413. [Google Scholar] [CrossRef]
- Koike, M.; Shiomi, T.; Koike, A. Ku70 can translocate to the nucleus independent of Ku80 translocation and DNA-PK autophosphorylation. Biochem. Biophys. Res. Commun. 2000, 276, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Bertinato, J.; Schild-Poulter, C.; Haché, R.J. Nuclear localization of Ku antigen is promoted independently by basic motifs in the Ku70 and Ku80 subunits. J. Cell. Sci. 2001, 114, 89–99. [Google Scholar] [PubMed]
- Görlich, D.; Prehn, S.; Laskey, R.A.; Hartmann, E. Isolation of a protein that is essential for the first step of nuclear protein import. Cell 1994, 79, 767–778. [Google Scholar] [CrossRef]
- Koike, M.; Miyasaka, T.; Mimori, T.; Shiomi, T. Subcellular localization and protein-protein interaction regions of Ku proteins. Biochem. Biophys. Res. Commun. 1998, 252, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Batey, S.; Nickson, A.A.; Clarke, J. Studying the folding of multidomain proteins. HFSP J. 2008, 2, 365–377. [Google Scholar] [CrossRef]
- Gaudet, R. Divide and conquer: High resolution structural information on TRP channel fragments. J. Gen. Physiol. 2009, 133, 231–237. [Google Scholar] [CrossRef]
- Bhaskara, R.M.; Srinivasan, N. Stability of domain structures in multi-domain proteins. Sci. Rep. 2011, 1, 40. [Google Scholar] [CrossRef]
- Walker, J.R.; Corpina, R.A.; Goldberg, J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature 2001, 412, 607–614. [Google Scholar] [CrossRef]
- Samavarchi-Tehrani, P.; Samson, R.; Gingras, A.-C. Proximity dependent biotinylation: Key enzymes and adaptation to proteomics approaches. Mol. Cell. Proteom. 2020, 19, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Mykkänen, O.M.; Grönholm, M.; Rönty, M.; Lalowski, M.; Salmikangas, P.; Suila, H.; Carpén, O. Characterization of human Palladin, a microfilament-associated protein. Mol. Biol. Cell 2001, 12, 3060–3073. [Google Scholar] [CrossRef]
- Peng, X.; Wang, J.; Peng, W.; Wu, F.-X.; Pan, Y. Protein-protein interactions: Detection, reliability assessment and applications. Brief. Bioinform. 2017, 18, 798–819. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.; Lako, M.; van Herpe, I.; Evans, J.; Saretzki, G.; Hole, N. A role for nucleoprotein Zap3 in the reduction of telomerase activity during embryonic stem cell differentiation. Mech. Dev. 2004, 121, 1509–1522. [Google Scholar] [CrossRef]
- Houlard, M.; Artus, J.; Léguillier, T.; Vandormael-Pournin, S.; Cohen-Tannoudji, M. DNA-RNA hybrids contribute to the replication dependent genomic instability induced by Omcg1 deficiency. Cell Cycle 2011, 10, 108–117. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Qiao, Y.; Shi, Y.; Liu, W.; Zeng, Q.; Xie, H.; Shi, X.; Sun, Y.; Liu, X.; et al. ZNF830 mediates cancer chemoresistance through promoting homologous-recombination repair. Nucleic Acids Res. 2018, 46, 1266–1279. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.W.; Fava, L.L.; Uldschmid, A.; Schmitz, M.H.A.; Gerlich, D.W.; Nigg, E.A.; Santamaria, A. Mitotic control of kinetochore-associated dynein and spindle orientation by human Spindly. J. Cell Biol. 2009, 185, 859–874. [Google Scholar] [CrossRef]
- Gatti da Silva, G.H.; Jurica, M.S.; Chagas da Cunha, J.P.; Oliveira, C.C.; Coltri, P.P. Human RNF113A participates of pre-mRNA splicing in vitro. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef]
- Kim, K.; Min, J.; Kirby, T.W.; Gabel, S.A.; Pedersen, L.C.; London, R.E. Ligand binding characteristics of the Ku80 von Willebrand domain. DNA Repair (Amst.) 2020, 85, 102739. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, S.; Gullberg, M.; Jarvius, J.; Olsson, C.; Pietras, K.; Gústafsdóttir, S.M.; Ostman, A.; Landegren, U. Protein detection using proximity-dependent DNA ligation assays. Nat. Biotechnol. 2002, 20, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Dingar, D.; Kalkat, M.; Chan, P.-K.; Srikumar, T.; Bailey, S.D.; Tu, W.B.; Coyaud, E.; Ponzielli, R.; Kolyar, M.; Jurisica, I.; et al. BioID identifies novel c-MYC interacting partners in cultured cells and xenograft tumors. J. Proteom. 2015, 118, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Schweingruber, C.; Soffientini, P.; Ruepp, M.-D.; Bachi, A.; Mühlemann, O. Identification of interactions in the NMD complex using proximity-dependent biotinylation (BioID). PLoS ONE 2016, 11, e0150239. [Google Scholar] [CrossRef]
- Shostak, K.; Jiang, Z.; Charloteaux, B.; Mayer, A.; Habraken, Y.; Tharun, L.; Klein, S.; Xu, X.; Duong, H.Q.; Vislovukh, A.; et al. The X-linked trichothiodystrophy-causing gene RNF113A links the spliceosome to cell survival upon DNA damage. Nat. Commun. 2020, 11, 1270. [Google Scholar] [CrossRef]
- Koike, M.; Awaji, T.; Kataoka, M.; Tsujimoto, G.; Kartasova, T.; Koike, A.; Shiomi, T. Differential subcellular localization of DNA-dependent protein kinase components Ku and DNA-PKcs during mitosis. J. Cell Sci. 1999, 112, 4031–4039. [Google Scholar] [PubMed]



| Construct Name | Residues (aa) | Length (bp) | Fusion Protein Name | Size (kDa) |
|---|---|---|---|---|
| NLS | 539–556 | 54 | NLS-BioID2-HA | 30 |
| Ku70 | 1–609 | 1827 | Ku70-BioID2-HA | 96 |
| Ku70 vWA | 1–250 | 750 | Ku70 vWA-NLS-BioID2-HA | 60 |
| Ku70 ∆vWA | 251–609 | 1077 | Ku70 ∆vWA-BioID2-HA | 68 |
| Gene Name | Full Name |
|---|---|
| WRN 1 | Werner syndrome ATP-dependent helicase |
| NKRF 1 | NF-kappa-B-repressing factor |
| APLF 1 | Aprataxin and PNK-like factor |
| ZFR | Zinc finger RNA-binding protein |
| RPL35A | 60S ribosomal protein L35a |
| HSPE1 | 10 kDa heat shock protein |
| ASPM | Abnormal spindle-like microcephaly associated protein |
| Gene Name | Full Name |
|---|---|
| YLPM1 | YLP motif-containing protein 1 |
| WAC | WW domain-containing adapter protein with coiled-coil |
| FAM192A | Protein FAM192A |
| NFATC2IP | NFATC2-interacting protein |
| ZNF830 | Zinc finger protein 830 |
| ALKBH5 | RNA demethylase ALKBH5 |
| API5 | Apoptosis inhibitor 5 |
| SPDL1 | Protein Spindly |
| RFX5 | DNA-binding protein RFX5 |
| RNF113A | RING finger protein 113A |
| SHOX2 | Short stature homeobox protein 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi, S.; Schild-Poulter, C. Identification of Ku70 Domain-Specific Interactors Using BioID2. Cells 2021, 10, 646. https://doi.org/10.3390/cells10030646
Abbasi S, Schild-Poulter C. Identification of Ku70 Domain-Specific Interactors Using BioID2. Cells. 2021; 10(3):646. https://doi.org/10.3390/cells10030646
Chicago/Turabian StyleAbbasi, Sanna, and Caroline Schild-Poulter. 2021. "Identification of Ku70 Domain-Specific Interactors Using BioID2" Cells 10, no. 3: 646. https://doi.org/10.3390/cells10030646
APA StyleAbbasi, S., & Schild-Poulter, C. (2021). Identification of Ku70 Domain-Specific Interactors Using BioID2. Cells, 10(3), 646. https://doi.org/10.3390/cells10030646





