Tumor Necrosis Factor (TNF) Is Required for Spatial Learning and Memory in Male Mice under Physiological, but Not Immune-Challenged Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Assessment of Non-Cognitive Effects
2.2.1. Sickness Behavior
2.2.2. Open Field
2.3. Determination of TNF mRNA
2.4. Assessment of Cognitive Effects
2.5. Determination of Neurotransmitters
2.6. Statistical Analysis
3. Results
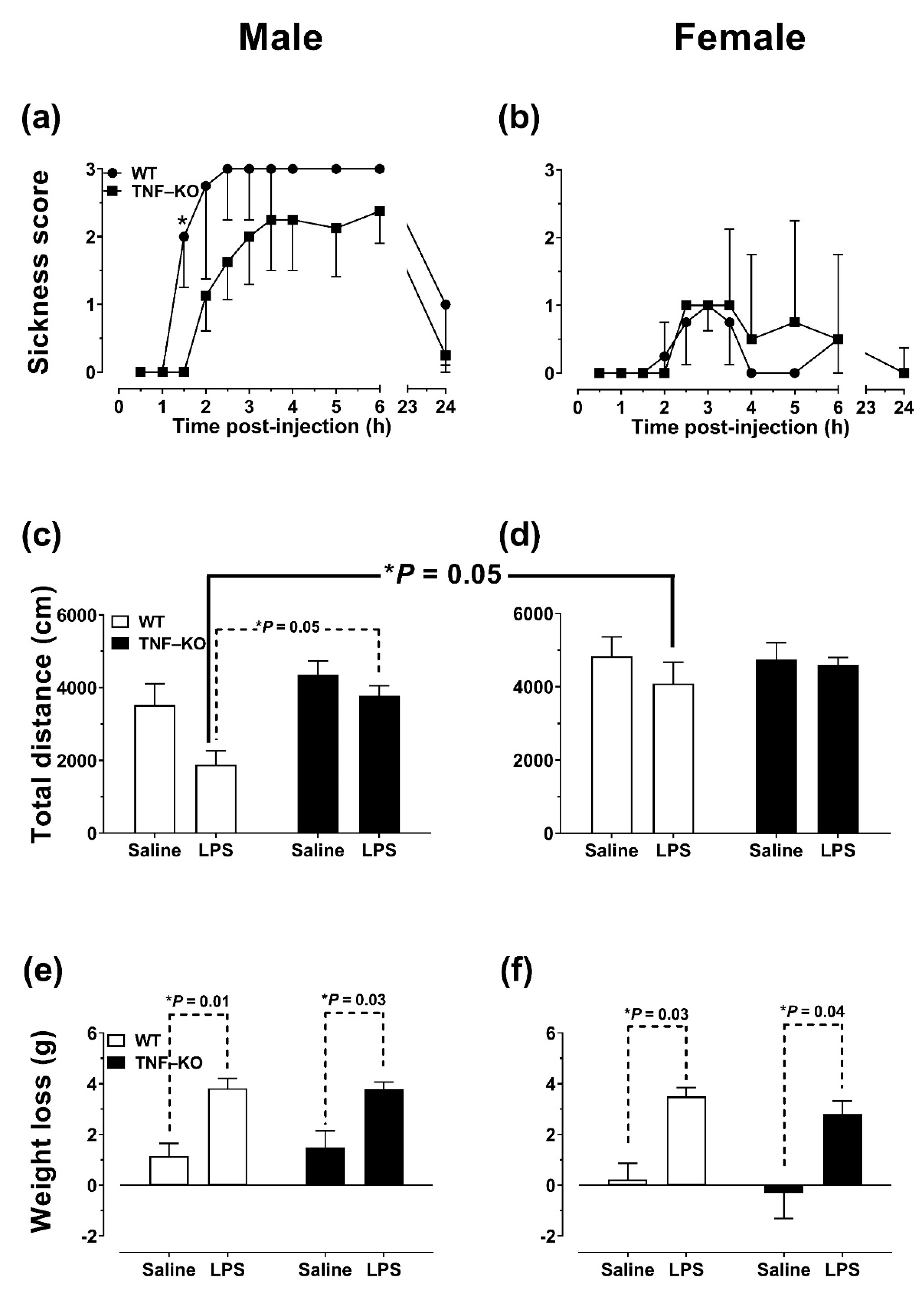
3.1. Assessment of Non-Cognitive Effects of LPS Administration
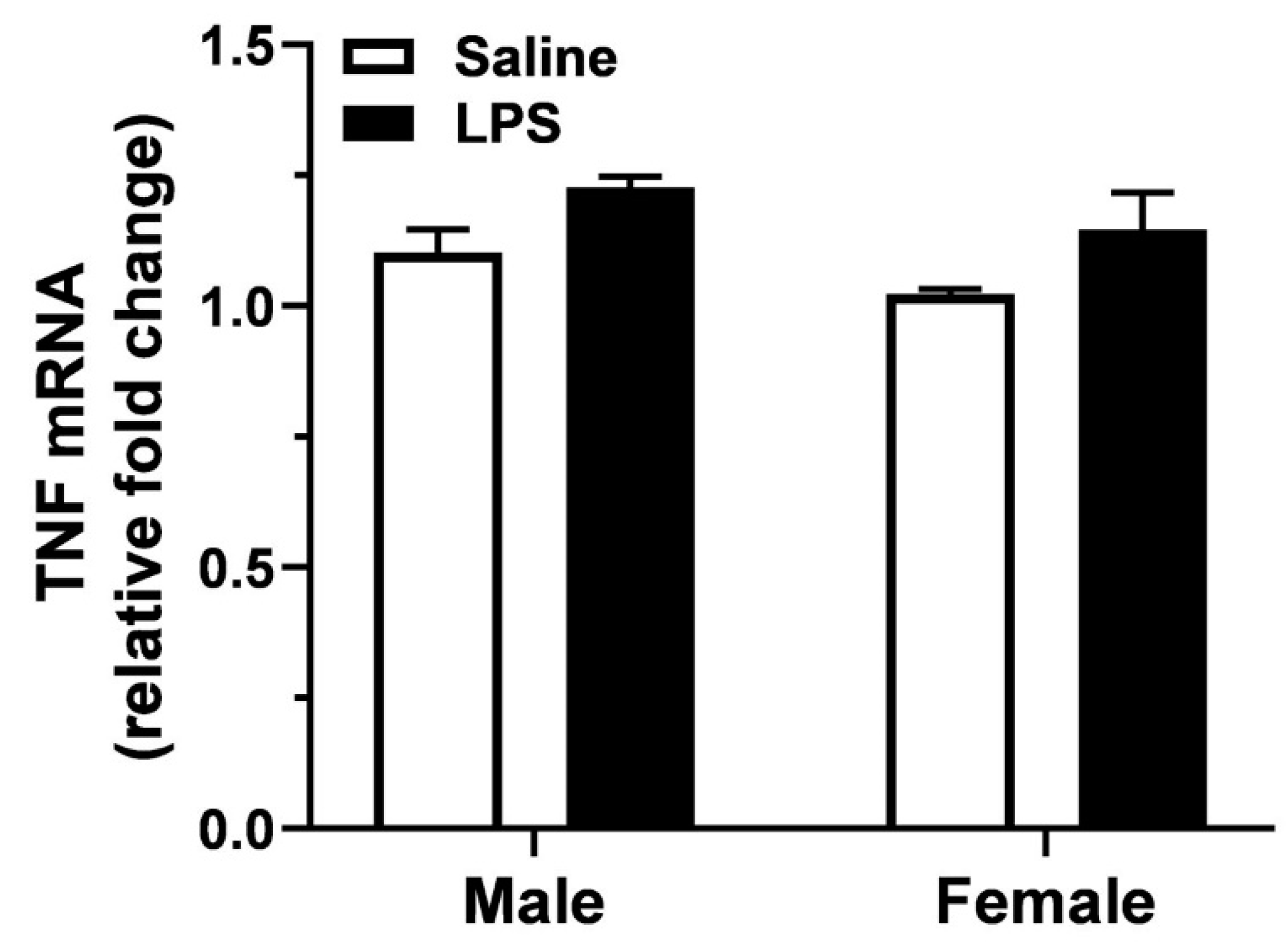
3.2. Hippocampal Levels of TNF mRNA
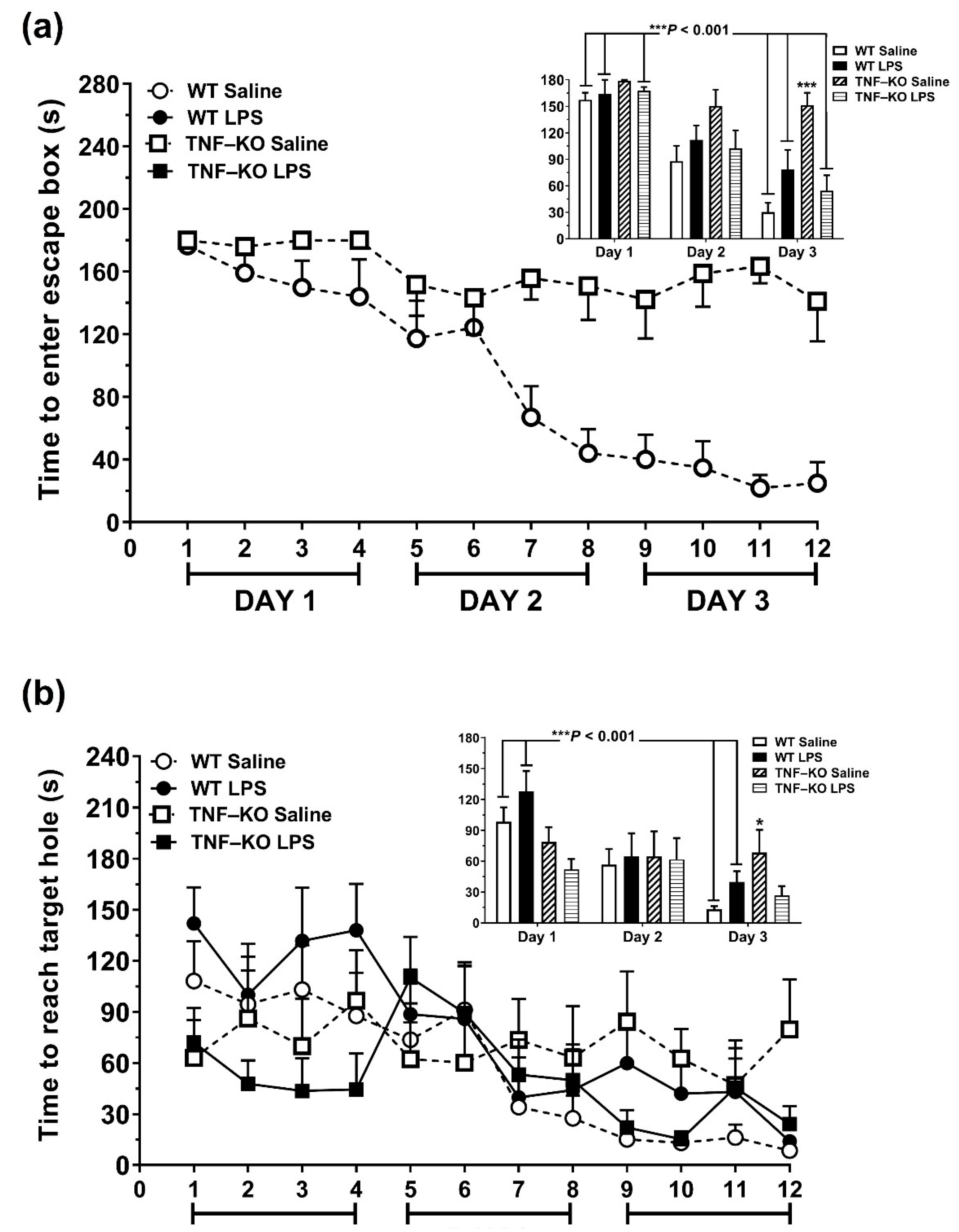
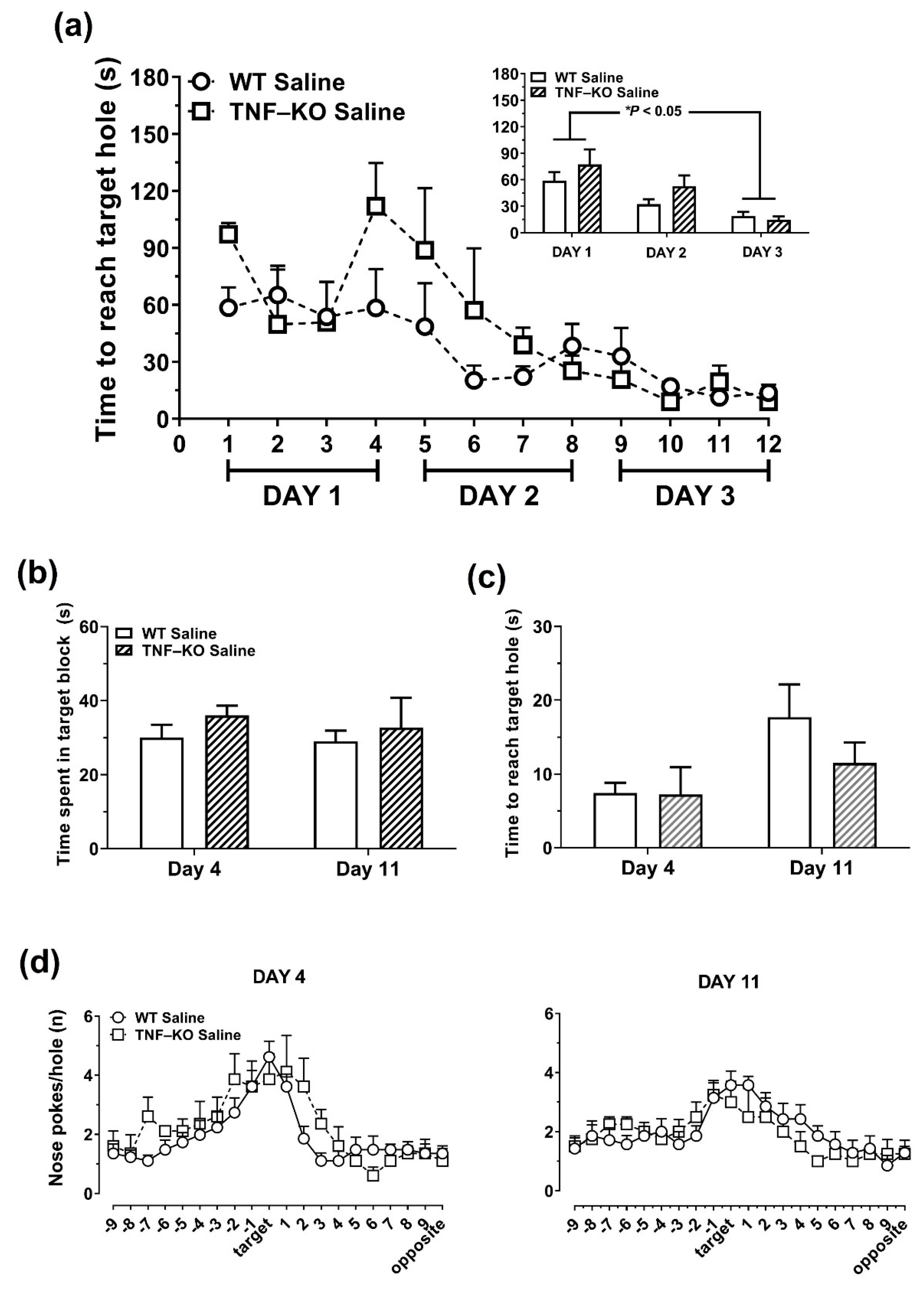
3.3. Impaired Learning in PBS-Treated Male TNF-KO Mice during the Acquisition Phase of the BMT
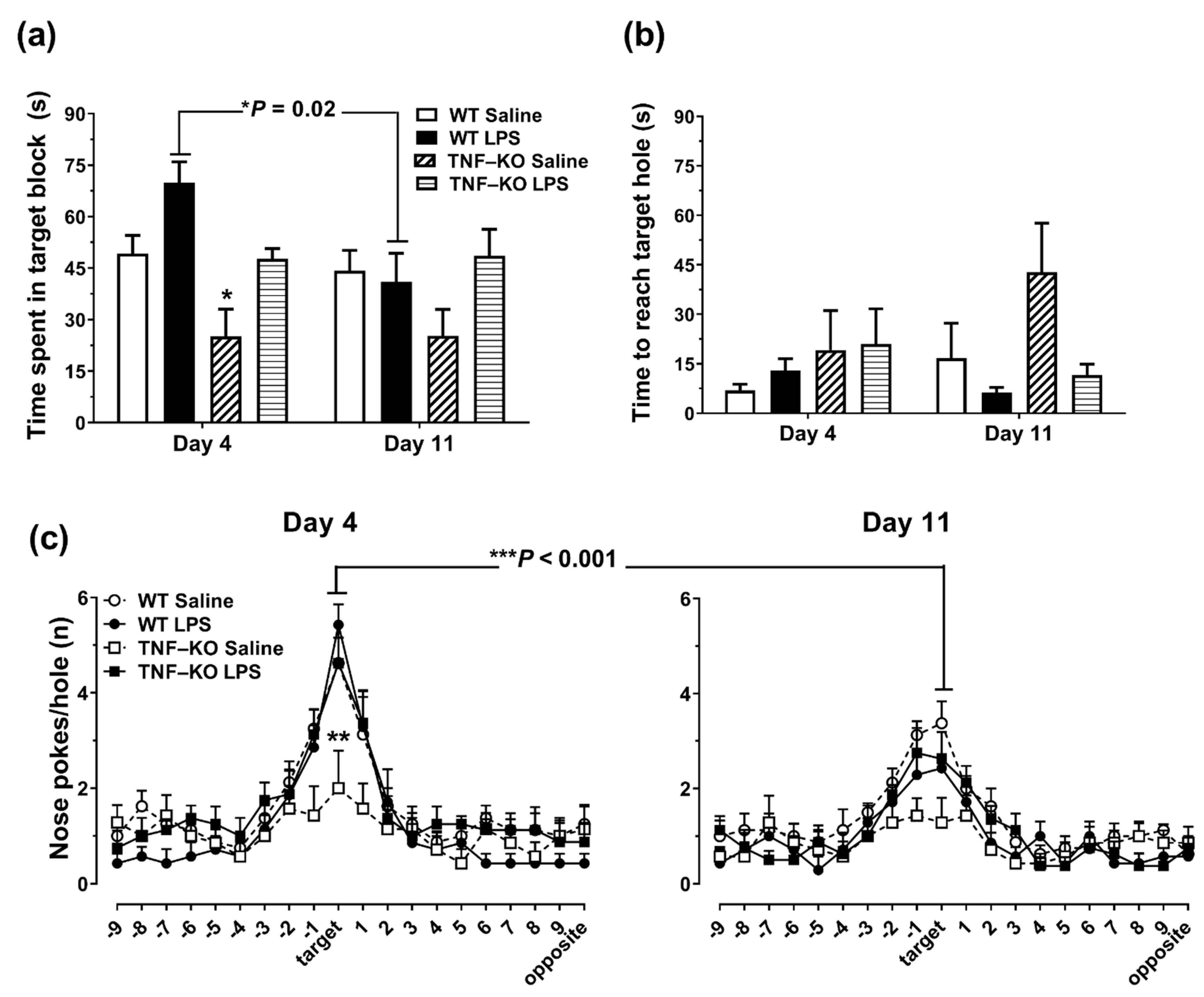
3.4. Memory Deficits in PBS-Treated Male TNF-KO Mice during the Probe Phase of the BMT
3.5. Female TNF-KO and WT Mice Are Not Different in the BMT
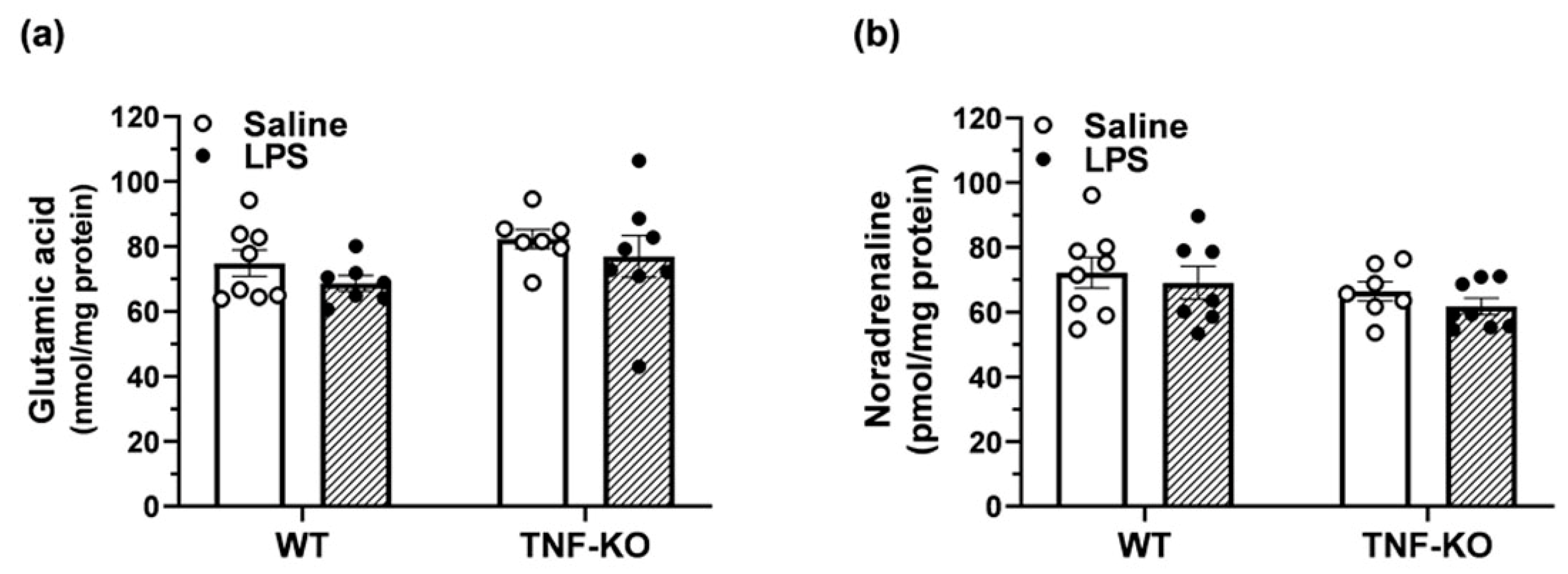
3.6. Neurotransmitter Levels in the Hippocampus of Male, PBS- and LPS-Treated Mice
4. Discussion
Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skelly, D.T.; Hennessy, M.A.E. Dansereau, and C. Cunningham. A Systematic Analysis of the Peripheral and Cns Effects of Systemic Lps, Il-1beta, [Corrected] Tnf-Alpha and Il-6 Challenges in C57bl/6 Mice. PLoS ONE 2013, 8, e69123. [Google Scholar] [CrossRef]
- Baune, B.T.; Wiede, F.; Braun, A.; Golledge, J.; Arolt, V.; Koerner, H. Cognitive dysfunction in mice deficient for TNF- and its receptors. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 147B, 1056–1064. [Google Scholar] [CrossRef]
- Doganavsargil-Baysal, O.; Cinemre, B.; Aksoy, U.M.; Akbas, H.; Metin, O.; Fettahoglu, C.; Gokmen, Z.; Davran, F. Levels of Tnf-Alpha, Soluble Tnf Receptors (Stnfr1, Stnfr2), and Cognition in Bipolar Disorder. Hum. Psychopharmacol. 2013, 28, 160–167. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, K.J.; Kim, H. Serum Tumour Necrosis Factor-Alpha and Interleukin-6 Levels in Alzheimer’s Disease and Mild Cognitive Impairment. Psychogeriatrics 2017, 17, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.H.; Tan, Y.L.; Yan, S.X.; Tian, L.; Chen, D.C.; Tan, S.P.; Wang, Z.R.; De Yang, F.; Yoon, J.H.; Zunta-Soares, G.B.; et al. Decreased serum TNF-alpha levels in chronic schizophrenia patients on long-term antipsychotics: Correlation with psychopathology and cognition. Psychopharmacology 2014, 232, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Motta, C.; Studer, V.; Barbieri, F.; Buttari, F.; Bergami, A.; Sancesario, G.; Bernardini, S.; de Angelis, G.; Martino, G.; et al. Tumor Necrosis Factor Is Elevated in Progressive Multiple Sclerosis and Causes Excitotoxic Neurodegeneration. Mult. Scler. 2014, 20, 304–312. [Google Scholar] [CrossRef]
- Camara, M.L.; Corrigan, F.; Jaehne, E.J.; Jawahar, M.C.; Anscomb, H.; Koerner, H.; Baune, B.T. Tnf-Alpha and Its Recep-tors Modulate Complex Behaviours and Neurotrophins in Transgenic Mice. Psychoneuroendocrinology 2013, 38, 3102–3114. [Google Scholar] [CrossRef]
- Donzis, E.J.; Tronson, N.C. Modulation of learning and memory by cytokines: Signaling mechanisms and long term consequences. Neurobiol. Learn. Mem. 2014, 115, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Lambertsen, K.L.; Clausen, B.H.; Babcock, A.A.; Gregersen, R.; Fenger, C.; Nielsen, H.H.; Haugaard, L.S.; Wirenfeldt, M.; Nielsen, M.; Dagnaes-Hansen, F.; et al. Microglia Protect Neurons against Ischemia by Synthesis of Tumor Necrosis Factor. J. Neurosci. 2009, 29, 1319–1330. [Google Scholar] [CrossRef]
- McAfoose, J.; Baune, B. Evidence for a cytokine model of cognitive function. Neurosci. Biobehav. Rev. 2009, 33, 355–366. [Google Scholar] [CrossRef]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Yli-Karjanmaa, M.; Larsen, K.S.; Fenger, C.D.; Kristensen, L.K.; Martin, N.A.; Jensen, P.T.; Breton, A.; Nathanson, L.; Nielsen, P.V.; Lund, M.C.; et al. TNF deficiency causes alterations in the spatial organization of neurogenic zones and alters the number of microglia and neurons in the cerebral cortex. Brain Behav. Immun. 2019, 82, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Babcock, A.A.; Ilkjær, L.; Clausen, B.H.; Villadsen, B.; Dissing-Olesen, L.; Bendixen, A.T.; Lyck, L.; Lambertsen, K.L.; Finsen, B. Cytokine-producing microglia have an altered beta-amyloid load in aged APP/PS1 Tg mice. Brain Behav. Immun. 2015, 48, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Clausen, B.H.; KLambertsen, L.; Babcock, A.A.; Holm, T.H.; Dagnaes-Hansen, F.; Finsen, B. Interleukin-1beta and Tumor Necrosis Factor-Alpha Are Expressed by Different Subsets of Microglia and Macrophages after Ischemic Stroke in Mice. J. Neuroinflammation 2008, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Galatro, T.F.; Holtman, I.R.; Lerario, A.M.; Vainchtein, I.D.; Brouwer, N.; Sola, P.R.; Veras, M.M.; Pereira, T.F.; Leite, R.E.P.; Moller, T.; et al. Transcriptomic Analysis of Purified Human Cortical Microglia Reveals Age-Associated Changes. Nat. Neurosci. 2017, 20, 1162–1171. [Google Scholar] [CrossRef]
- Gregersen, R.; Lambertsen, K.; Finsen, B. Microglia and Macrophages Are the Major Source of Tumor Necrosis Factor in Permanent Middle Cerebral Artery Occlusion in Mice. Br. J. Pharmacol. 2000, 20, 53–65. [Google Scholar] [CrossRef]
- Gerber, J.; Bottcher, T.; Hahn, M.; Siemer, A.; Bunkowski, S.; Nau, R. Increased Mortality and Spatial Memory Deficits in Tnf-Alpha-Deficient Mice in Ceftriaxone-Treated Experimental Pneumococcal Meningitis. Neurobiol. Dis. 2004, 16, 133–138. [Google Scholar] [CrossRef]
- Scherbel, U.; Raghupathi, R.; Nakamura, M.; Saatman, K.E.; Trojanowski, J.Q.; Neugebauer, E.; Marino, M.W.; McIntosh, T.K. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc. Natl. Acad. Sci. USA 1999, 96, 8721–8726. [Google Scholar] [CrossRef]
- McAfoose, J.; Koerner, H.; Baune, B. The effects of TNF deficiency on age-related cognitive performance. Psychoneuroendocrinology 2009, 34, 615–619. [Google Scholar] [CrossRef]
- Aloe, L.; Properzi, F.; Probert, L.; Akassoglou, K.; Kassiotis, G.; Micera, A.; Fiore, M. Learning Abilities, Ngf and Bdnf Brain Levels in Two Lines of Tnf-Alpha Transgenic Mice, One Characterized by Neurological Disorders, the Other Phenotypically Normal. Brain Res. 1999, 840, 125–137. [Google Scholar] [CrossRef]
- Mrak, R.E.; Griffin, W.S.T. Glia and their cytokines in progression of neurodegeneration. Neurobiol. Aging 2005, 26, 349–354. [Google Scholar] [CrossRef]
- Papageorgiou, I.E.; Lewen, A.; Galow, L.V.; Cesetti, T.; Scheffel, J.; Regen, T.; Hanisch, U.K.; Kann, O. Tlr4-Activated Mi-croglia Require Ifn-Gamma to Induce Severe Neuronal Dysfunction and Death in Situ. Proc. Natl. Acad. Sci. USA 2016, 113, 212–217. [Google Scholar] [CrossRef]
- Arai, K.; Matsuki, N.; Ikegaya, Y.; Nishiyama, N. Deterioration of Spatial Learning Performances in Lipopolysaccharide-Treated Mice. Jpn. J. Pharmacol. 2001, 87, 195–201. [Google Scholar] [CrossRef]
- Jensen, P.; Myhre, C.L.; Lassen, P.S.; Metaxas, A.; Khan, A.M.; Lambertsen, K.L.; Babcock, A.A.; Finsen, B.; Larsen, M.R.; Kempf, S.J. Tnfalpha Affects Creb-Mediated Neuroprotective Signaling Pathways of Synaptic Plasticity in Neurons as Re-vealed by Proteomics and Phospho-Proteomics. Oncotarget 2017, 8, 60223–60242. [Google Scholar] [CrossRef]
- Sparkman, N.L.; Martin, L.A.; Calvert, W.S.; Boehm, G.W. Effects of intraperitoneal lipopolysaccharide on Morris maze performance in year-old and 2-month-old female C57BL/6J mice. Behav. Brain Res. 2005, 159, 145–151. [Google Scholar] [CrossRef]
- Valero, J.; Mastrella, G.; Neiva, I.; Sanchez, S.; Malva, J.O. Long-Term Effects of an Acute and Systemic Administration of Lps on Adult Neurogenesis and Spatial Memory. Front. Neurosci. 2017, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.N.; Commins, S.; O’Mara, S.M. Lipopolysaccharide Causes Deficits in Spatial Learning in the Watermaze but Not in Bdnf Expression in the Rat Dentate Gyrus. Behav. Brain Res. 2001, 124, 47–54. [Google Scholar] [CrossRef]
- Cunningham, C.; Sanderson, D.J. Malaise in the Water Maze: Untangling the Effects of Lps and Il-1beta on Learning and Memory. Brain Behav. Immun. 2008, 22, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.C.; van Mil, S.; Murray, E.; Mallet, J.F.; Matar, C.; Ismail, N. Age and Sex Differences in Immune Response Following Lps Treatment in Mice. Brain Behav. Immun. 2016, 58, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Tronson, N.C.; Collette, K.M. (Putative) sex differences in neuroimmune modulation of memory. J. Neurosci. Res. 2017, 95, 472–486. [Google Scholar] [CrossRef]
- Pasparakis, M.; Alexopoulou, L.; Episkopou, V.; Kollias, G. Immune and inflammatory responses in TNF alpha-deficient mice: A critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 1996, 184, 1397–1411. [Google Scholar] [CrossRef]
- Gandhi, R.; Hayley, S.; Gibb, J.; Merali, Z.; Anisman, H. Influence of poly I:C on sickness behaviors, plasma cytokines, corticosterone and central monoamine activity: Moderation by social stressors. Brain Behav. Immun. 2007, 21, 477–489. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Gramsbergen, J.B.; Sivasaravanaparan, M.; Ditzel, N.; Sevelsted-Møller, L.M.; Olivan-Viguera, A.; Rabjerg, M.; Wulff, H.; Köhler, R. Genetic KCa3.1-Deficiency Produces Locomotor Hyperactivity and Alterations in Cerebral Monoamine Levels. PLoS ONE 2012, 7, e47744. [Google Scholar] [CrossRef]
- Madsen, P.M.; Clausen, B.H.; Degn, M.; Thyssen, S.; Kristensen, L.K.; Svensson, M.; Ditzel, N.; Finsen, B.; Deierborg, T.; Brambilla, R.; et al. Genetic Ablation of Soluble Tumor Necrosis Factor with Preservation of Membrane Tumor Necrosis Factor Is Associated with Neuroprotection after Focal Cerebral Ischemia. J. Cereb. Blood Flow. Metab. 2016, 36, 1553–1569. [Google Scholar] [CrossRef]
- Metaxas, A.; Vaitheeswaran, R.; Jensen, K.T.; Thygesen, C.; Ilkjaer, L.; Darvesh, S.; Finsen, B. Reduced Serotonin Transporter Levels and Inflammation in the Midbrain Raphe of 12 Month Old Appswe/Psen1de9 Mice. Curr. Alzheimer. Res. 2018, 15, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, B.; Patil, S.; Höger, H.; Luber, G. Barnes maze, a useful task to assess spatial reference memory in the mice. Protoc. Exch. 2007. [Google Scholar] [CrossRef]
- Bergh, M.S.-S.; Bogen, I.L.; Lundanes, E.; Øiestad, Å.M.L. Validated methods for determination of neurotransmitters and metabolites in rodent brain tissue and extracellular fluid by reversed phase UHPLC–MS/MS. J. Chromatogr. B 2016, 1028, 120–129. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Barter, J.; Kumar, A.; Stortz, J.A.; Hollen, M.; Nacionales, D.; Efron, P.A.; Moldawer, L.L.; Foster, T.C. Age and Sex Influ-ence the Hippocampal Response and Recovery Following Sepsis. Mol. Neurobiol. 2019, 56, 8557–8572. [Google Scholar] [CrossRef]
- Ruggieri, A.; Anticoli, S.; D’Ambrosio, A.; Giordani, L.; Viora, M. The influence of sex and gender on immunity, infection and vaccination. Ann. dell’Istituto Super. Sanità 2016, 52, 198–204. [Google Scholar]
- Asai, K.; Hiki, N.; Mimura, Y.; Ogawa, T.; Unou, K.; Kaminishi, M. Gender Differences in Cytokine Secretion by Human Peripheral Blood Mononuclear Cells: Role of Estrogen in Modulating Lps-Induced Cytokine Secretion in an ex vivo Septic Model. Shock 2001, 16, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Imahara, S.D.; Jelacic, S.; Junker, C.E.; O’Keefe, G.E. The influence of gender on human innate immunity. Surgery 2005, 138, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Moxley, G.; Posthuma, D.; Carlson, P.; Estrada, E.; Han, J.; Benson, L.L.; Neale, M.C. Sexual Dimorphism in Innate Immunity. Arthritis Rheum. 2002, 46, 250–258. [Google Scholar] [CrossRef]
- Aomatsu, M.; Kato, T.; Kasahara, E.; Kitagawa, S. Gender Difference in Tumor Necrosis Factor-Alpha Production in Hu-man Neutrophils Stimulated by Lipopolysaccharide and Interferon-Gamma. Biochem. Biophys. Res. Commun. 2013, 441, 220–225. [Google Scholar] [CrossRef]
- Guidet, B.; Maury, E. Sex and severe sepsis. Crit. Care 2013, 17, 1–2. [Google Scholar] [CrossRef]
- Nasir, N.; Jamil, B.; Siddiqui, S.; Talat, N.; Khan, F.A.; Hussain, R. Mortality in Sepsis and its relationship with Gender. Pak. J. Med. Sci. 2015, 31, 1201–1206. [Google Scholar] [CrossRef]
- Pietropaoli, A.P.; Glance, L.G.; Oakes, D.; Fisher, S.G. Gender differences in mortality in patients with severe sepsis or septic shock. Gend. Med. 2010, 7, 422–437. [Google Scholar] [CrossRef]
- Marriott, I.; Bost, K.L.; Huet-Hudson, Y.M. Sexual Dimorphism in Expression of Receptors for Bacterial Lipopolysaccharides in Murine Macrophages: A Possible Mechanism for Gender-Based Differences in Endotoxic Shock Susceptibility. J. Reprod. Immunol. 2006, 71, 12–27. [Google Scholar] [CrossRef]
- Merkel, S.M.; Alexander, S.; Zufall, E.; Oliver, J.D.; Huet-Hudson, Y.M. Essential Role for Estrogen in Protection againstVibrio vulnificus-Induced Endotoxic Shock. Infect. Immun. 2001, 69, 6119–6122. [Google Scholar] [CrossRef]
- Kuo, S.-M. Gender Difference in Bacteria Endotoxin-Induced Inflammatory and Anorexic Responses. PLoS ONE 2016, 11, e0162971. [Google Scholar] [CrossRef]
- Lasselin, J.; Schedlowski, M.; Karshikoff, B.; Engler, H.; Lekander, M.; Konsman, J.P. Comparison of Bacterial Lipopoly-saccharide-Induced Sickness Behavior in Rodents and Humans: Relevance for Symptoms of Anxiety and Depression. Neurosci. Biobehav. Rev. 2020, 115, 15–24. [Google Scholar] [CrossRef]
- Gvirtz, R.; Ogen-Shtern, N.; Cohen, G. Kinetic Cytokine Secretion Profile of LPS-Induced Inflammation in the Human Skin Organ Culture. Pharmaceutics 2020, 12, 299. [Google Scholar] [CrossRef]
- Chvatchko, Y.; Hoogewerf, A.J.; Meyer, A.; Alouani, S.; Juillard, P.; Buser, R.; Conquet, F.; Proudfoot, A.E.; Wells, T.N.; Power, C.A. A Key Role for Cc Chemokine Receptor 4 in Lipopolysaccharide-Induced Endotoxic Shock. J. Exp. Med. 2000, 191, 1755–1764. [Google Scholar] [CrossRef]
- Catorce, M.N.; Gevorkian, G. LPS-induced Murine Neuroinflammation Model: Main Features and Suitability for Pre-clinical Assessment of Nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef]
- Kaneko, Y.S.; Mori, K.; Nakashima, A.; Sawada, M.; Nagatsu, I.; Ota, A. Peripheral Injection of Lipopolysaccharide En-hances Expression of Inflammatory Cytokines in Murine Locus Coeruleus: Possible Role of Increased Norepinephrine Turnover. J. Neurochem. 2005, 94, 393–404. [Google Scholar] [CrossRef]
- Yamada, K.; Iida, R.; Miyamoto, Y.; Saito, K.; Sekikawa, K.; Seishima, M.; Nabeshima, T. Neurobehavioral Alterations in Mice with a Targeted Deletion of the Tumor Necrosis Factor-Alpha Gene: Implications for Emotional Behavior. J. Neuroimmunol. 2000, 111, 131–138. [Google Scholar] [CrossRef]
- Gawel, K.; Gibula, E.; Marszalek-Grabska, M.; Filarowska, J.; Kotlinska, J.H. Assessment of spatial learning and memory in the Barnes maze task in rodents—methodological consideration. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Myhre, C.L.; Thygesen, C.; Villadsen, B.; Vollerup, J.; Ilkjaer, L.; Krohn, K.T.; Grebing, M.; Zhao, S.; Khan, A.M.L.; Jensen, M.S.; et al. Microglia Express Insulin-Like Growth Factor-1 in the Hippocampus of Aged Appswe/Ps1deltae9 Transgenic Mice. Front. Cell Neurosci. 2019, 13, 308. [Google Scholar] [CrossRef]
- Metaxas, A.; Anzalone, M.; Vaitheeswaran, R.; Petersen, S.; Landau, A.M.; Finsen, B. Neuroinflammation and amyloid-beta 40 are associated with reduced serotonin transporter (SERT) activity in a transgenic model of familial Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 1–13. [Google Scholar] [CrossRef]
- Hickman, S.E.; Kingery, N.D.; Ohsumi, T.K.; Borowsky, M.L.; Wang, L.-C.; Means, T.K.; El Khoury, J. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 2013, 16, 1896–1905. [Google Scholar] [CrossRef]
- Pickering, M.; Cumiskey, D.; O’Connor, J.J. Actions of TNF-α on glutamatergic synaptic transmission in the central nervous system. Exp. Physiol. 2005, 90, 663–670. [Google Scholar] [CrossRef]
- Reynolds, J.L.; Ignatowski, T.A.; Spengler, R.N. Effect of Tumor Necrosis Factor-Alpha on the Reciprocal G-Protein-Induced Regulation of Norepinephrine Release by the Alpha2-Adrenergic Receptor. J. Neurosci. Res. 2005, 79, 779–787. [Google Scholar] [CrossRef]
- Mather, M.; Clewett, D.; Sakaki, M.; Harley, C.W. Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav. Brain Sci. 2016, 39, e200. [Google Scholar] [CrossRef] [PubMed]
- Myhrer, T. Neurotransmitter systems involved in learning and memory in the rat: A meta-analysis based on studies of four behavioral tasks. Brain Res. Rev. 2003, 41, 268–287. [Google Scholar] [CrossRef]
- Bluthe, R.-M.; Laye, S.; Michaud, B.; Combe, C.; Dantzer, R.; Parnet, P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: A study with interleukin-1 type I receptor-deficient mice. Eur. J. Neurosci. 2000, 12, 4447–4456. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.T.; Griffin, E.W.; Murray, C.L.; Harney, S.; O’Boyle, C.; Hennessy, E.; Dansereau, M.A.; Nazmi, A.; Tortorelli, L.; Rawlins, J.N.; et al. Acute Transient Cognitive Dysfunction and Acute Brain Injury Induced by Systemic Inflammation Occur by Dissociable Il-1-Dependent Mechanisms. Mol. Psychiatry 2019, 24, 1533–1548. [Google Scholar] [CrossRef] [PubMed]
- Hojgaard, P.; Ballegaard, C.; Cordtz, R.; Zobbe, K.; Clausen, M.; Glintborg, B.; Kristensen, L.E.; Dreyer, L. Gender Differ-ences in Biologic Treatment Outcomes-a Study of 1750 Patients with Psoriatic Arthritis Using Danish Health Care Registers. Rheumatology 2018, 57, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Jawaheer, D.; Olsen, J.; Hetland, M.L. Sex Differences in Response to Anti-Tumor Necrosis Factor Therapy in Early and Established Rheumatoid Arthritis—Results from the DANBIO Registry. J. Rheumatol. 2011, 39, 46–53. [Google Scholar] [CrossRef]
- Yagi, S.; Galea, L.A.M. Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 2019, 44, 200–213. [Google Scholar] [CrossRef]
- Castanon, N.; Bluthe, R.M.; Dantzer, R. Chronic Treatment with the Atypical Antidepressant Tianeptine Attenuates Sickness Behavior Induced by Peripheral but Not Central Lipopolysaccharide and Interleukin-1beta in the Rat. Psychopharmacology 2001, 154, 50–60. [Google Scholar] [CrossRef]
- Ismail, N.; Garas, P.; Blaustein, J.D. Long-Term Effects of Pubertal Stressors on Female Sexual Receptivity and Estrogen Receptor-Alpha Expression in Cd-1 Female Mice. Horm. Behav. 2011, 59, 565–571. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Lawson, M.A.; Andre, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-Induced Depressive-Like Behavior Is Mediated by Indoleamine 2,3-Dioxygenase Activation in Mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mygind, L.; Bergh, M.S.-S.; Tejsi, V.; Vaitheeswaran, R.; Lambertsen, K.L.; Finsen, B.; Metaxas, A. Tumor Necrosis Factor (TNF) Is Required for Spatial Learning and Memory in Male Mice under Physiological, but Not Immune-Challenged Conditions. Cells 2021, 10, 608. https://doi.org/10.3390/cells10030608
Mygind L, Bergh MS-S, Tejsi V, Vaitheeswaran R, Lambertsen KL, Finsen B, Metaxas A. Tumor Necrosis Factor (TNF) Is Required for Spatial Learning and Memory in Male Mice under Physiological, but Not Immune-Challenged Conditions. Cells. 2021; 10(3):608. https://doi.org/10.3390/cells10030608
Chicago/Turabian StyleMygind, Leda, Marianne Skov-Skov Bergh, Vivien Tejsi, Ramanan Vaitheeswaran, Kate L. Lambertsen, Bente Finsen, and Athanasios Metaxas. 2021. "Tumor Necrosis Factor (TNF) Is Required for Spatial Learning and Memory in Male Mice under Physiological, but Not Immune-Challenged Conditions" Cells 10, no. 3: 608. https://doi.org/10.3390/cells10030608
APA StyleMygind, L., Bergh, M. S.-S., Tejsi, V., Vaitheeswaran, R., Lambertsen, K. L., Finsen, B., & Metaxas, A. (2021). Tumor Necrosis Factor (TNF) Is Required for Spatial Learning and Memory in Male Mice under Physiological, but Not Immune-Challenged Conditions. Cells, 10(3), 608. https://doi.org/10.3390/cells10030608







