Facial Rejuvenation with Concentrated Lipograft—A 12 Month Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Demographics Informed Consent
2.2. Liposuction Technique
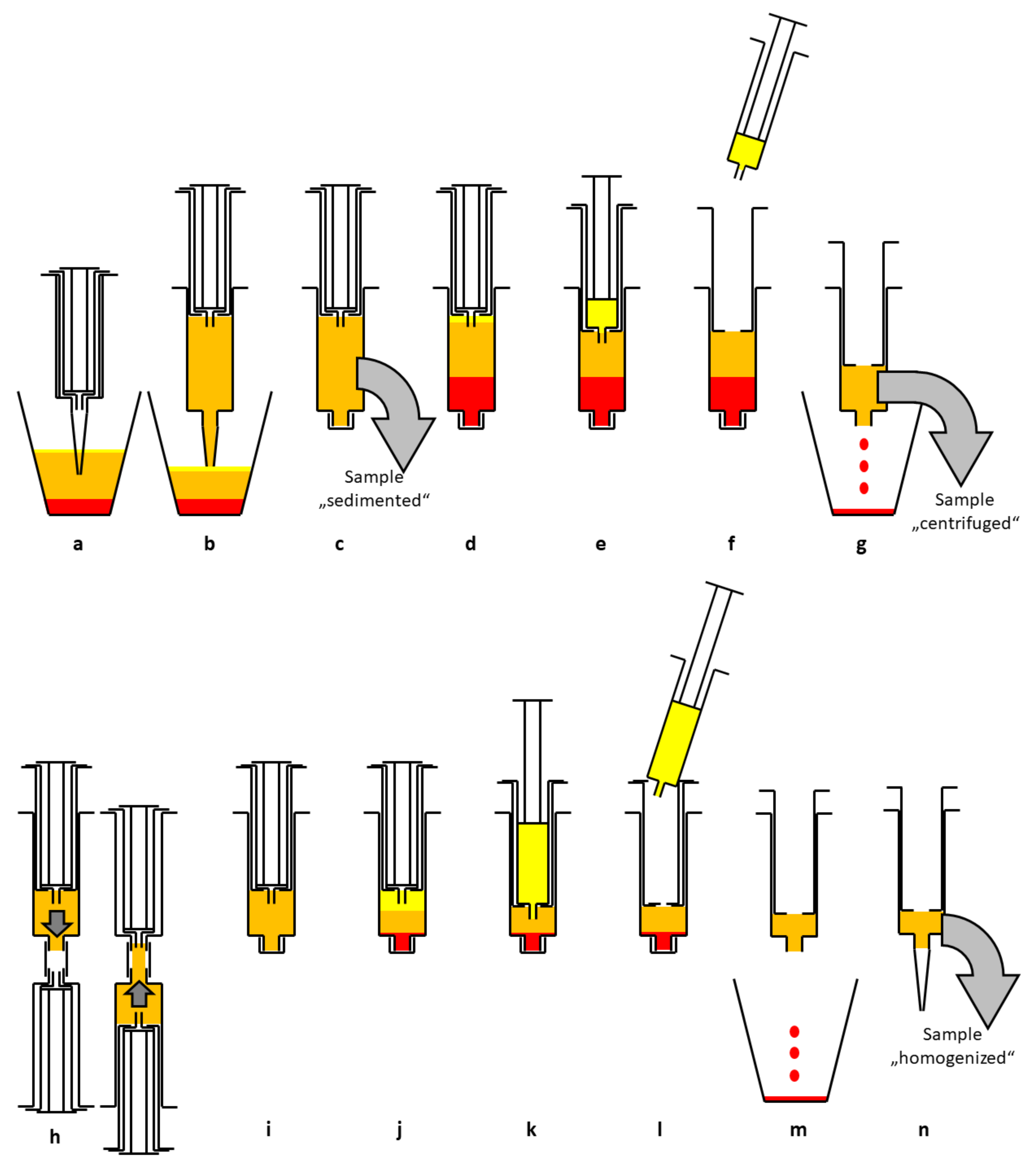
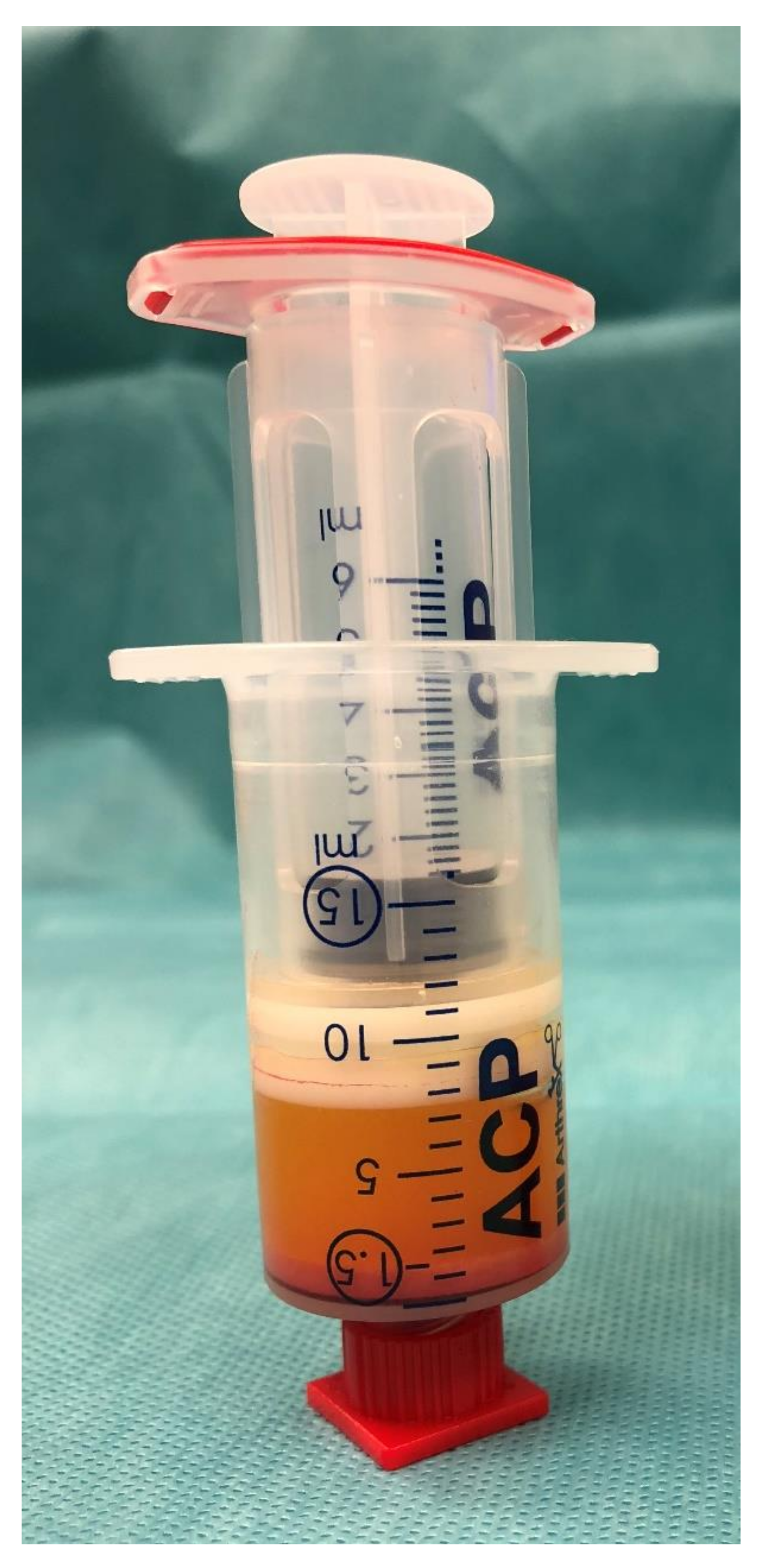
2.3. Processing of Lipoaspirate
2.4. Stem Cell Isolation and Counting
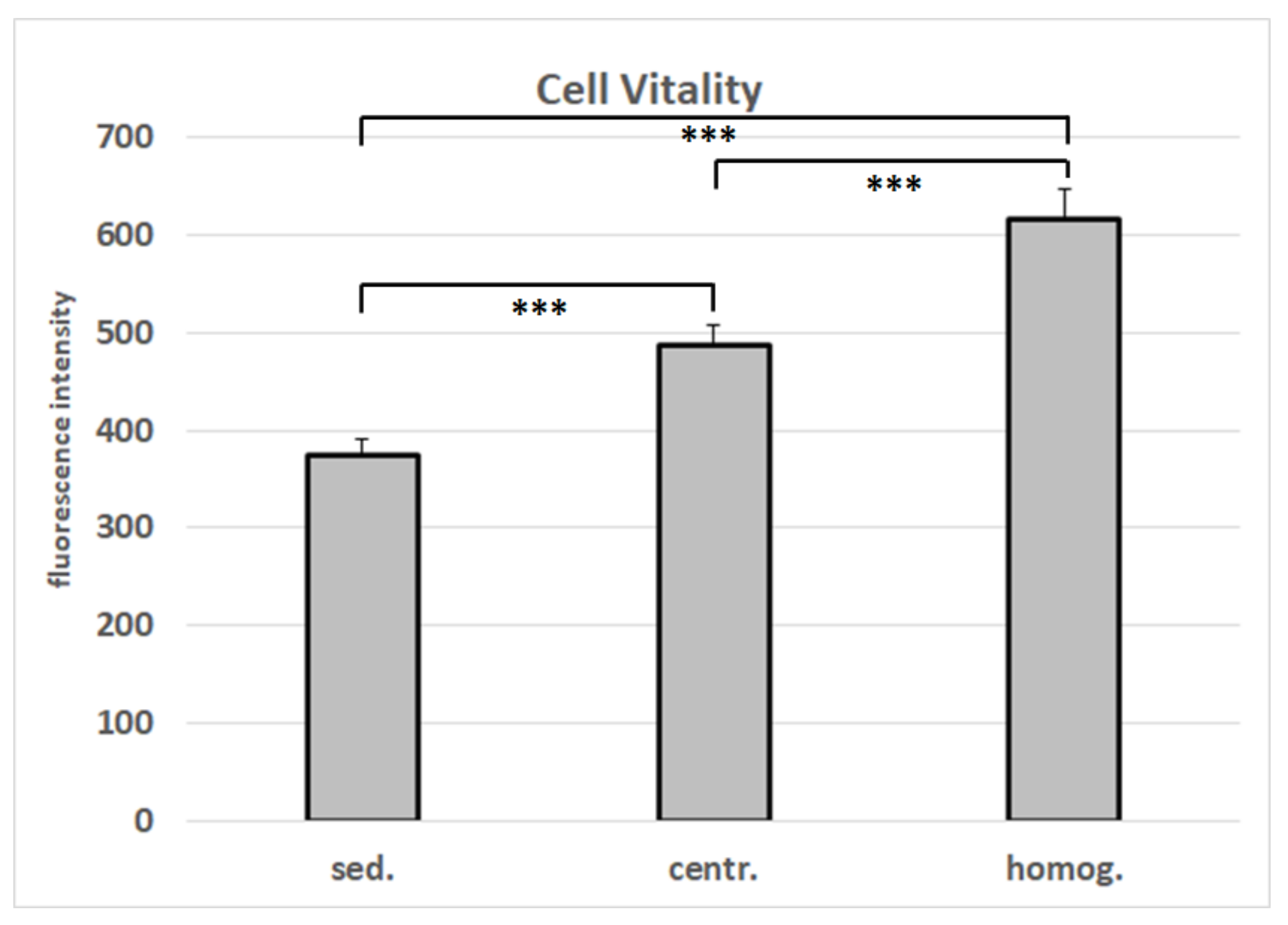
2.5. Cell Vitality Assay
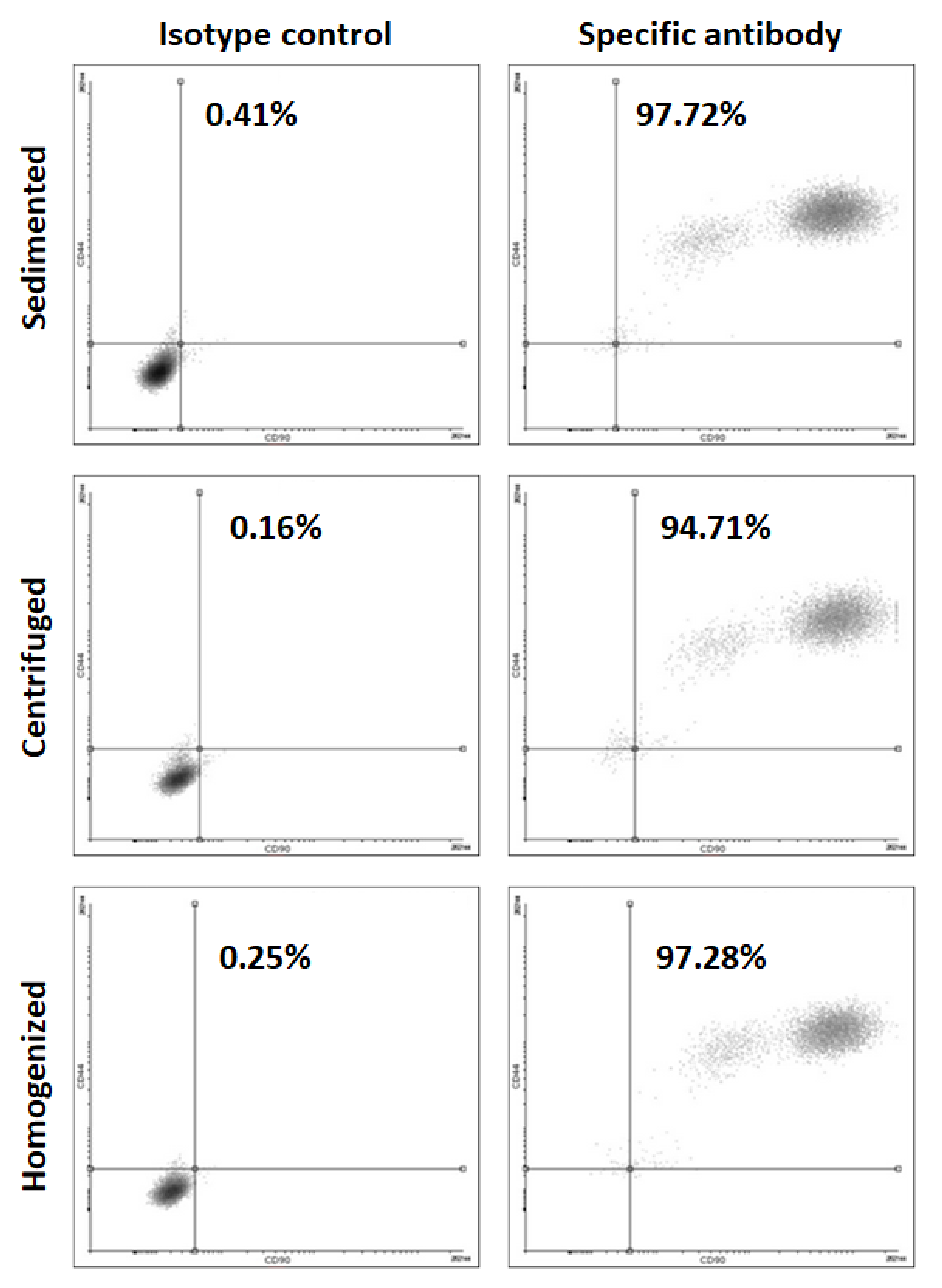
2.6. Flow Cytometry
2.7. Lipofilling Technique
2.8. Digital Photography
2.9. 3D Photography and Wrinkle Severity Rating Scale
2.10. Evaluation of Patient’s Satisfaction
2.11. Statistical Analysis
3. Results
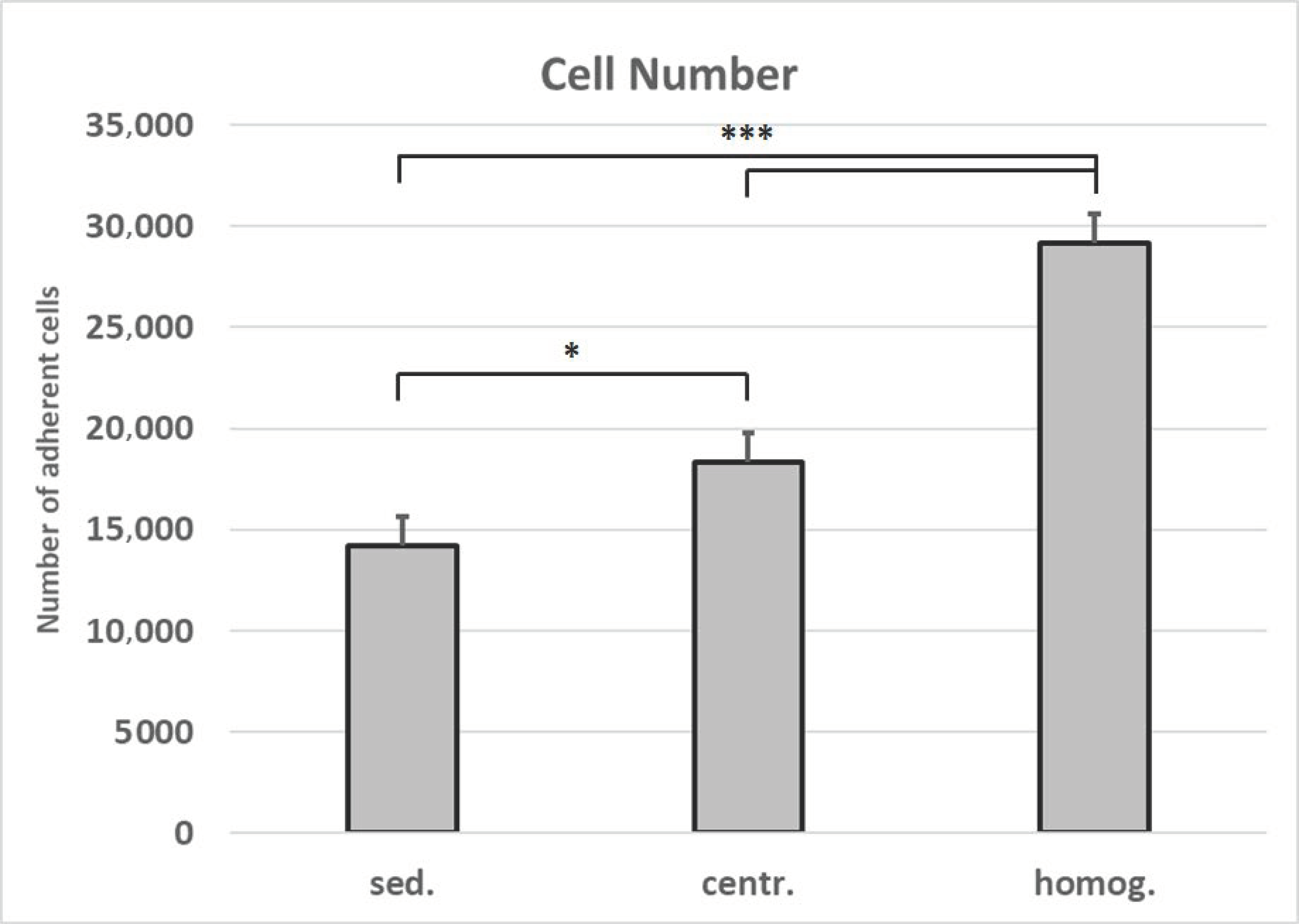
3.1. Cell Isolation
3.2. Lipofilling Volume
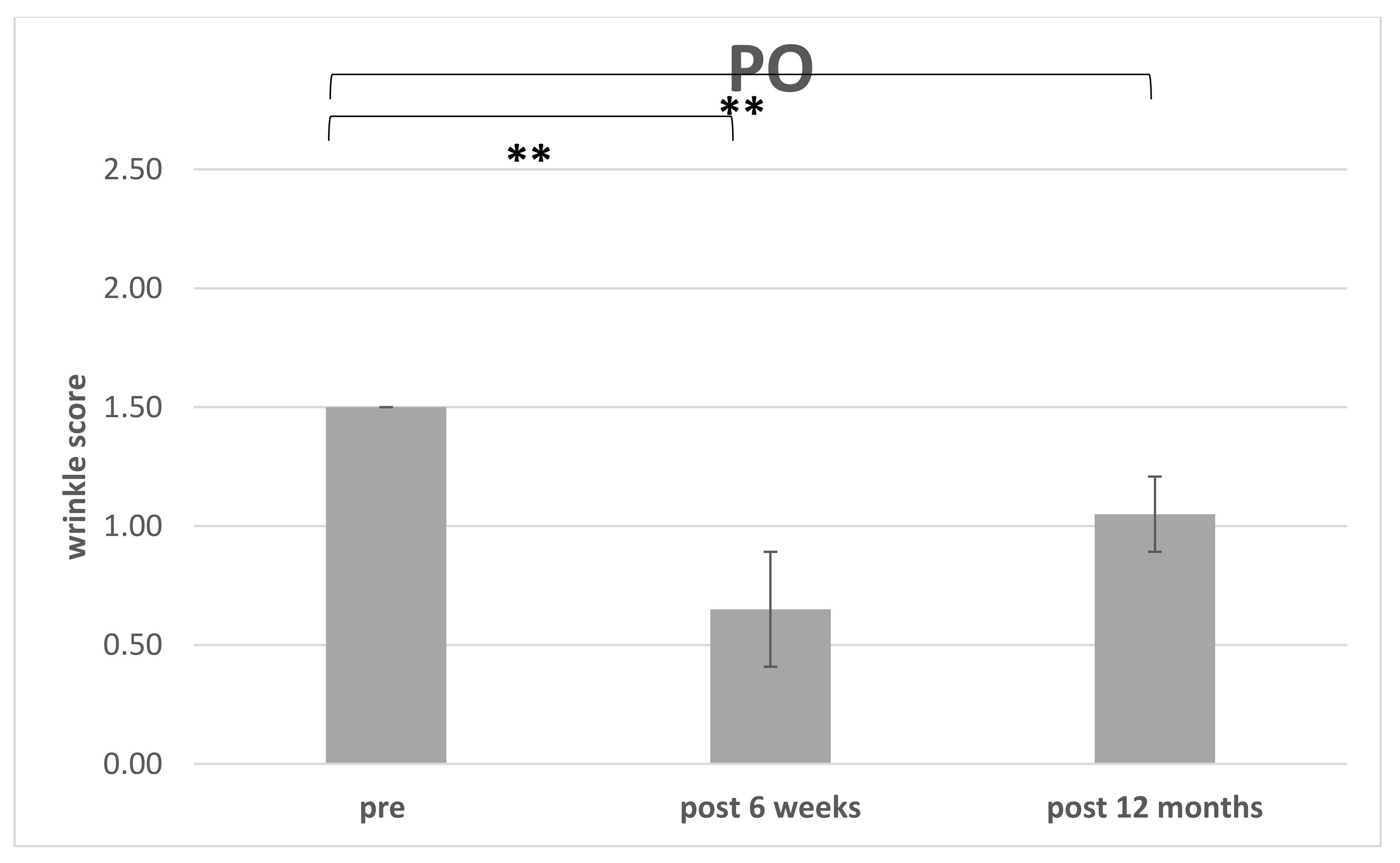
3.3. Improvement of Wrinkle Severity Scale (WSS)
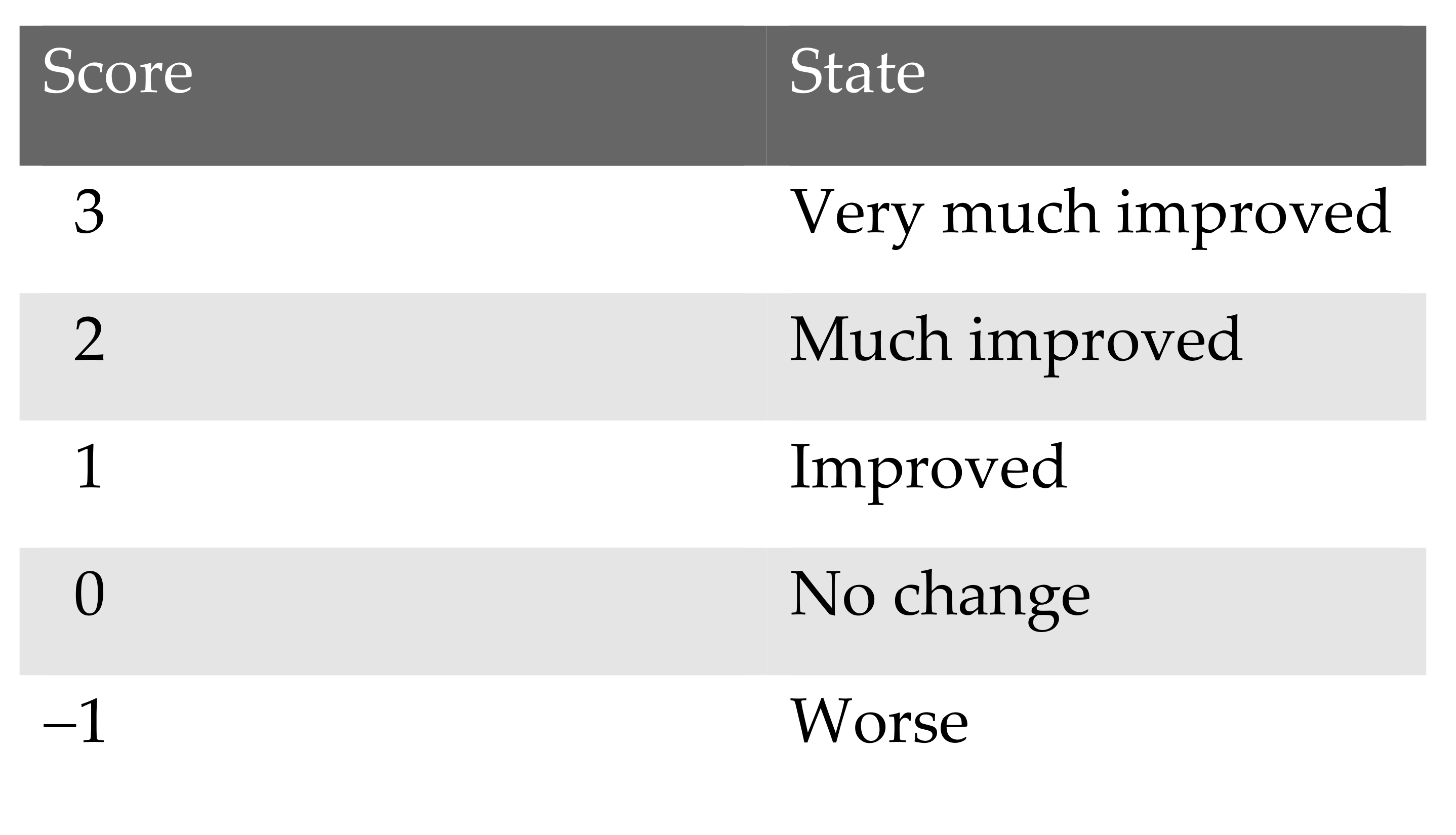
3.4. Patient’s Satisfaction with Surgical Intervention (Global Aesthetic Improvement Scale)
4. Discussion
4.1. Mechanical Processing of Lipoaspirate Versus Enzymatic Processing
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Coleman, S.R. Structural fat grafts: The ideal filler? Clin. Plast. Surg. 2001, 28, 111–119. [Google Scholar] [CrossRef]
- Coleman, S.R. Structural fat grafting: More than a permanent filler. Plast. Reconstr. Surg. 2006, 118 (Suppl. 3), 108S–120S. [Google Scholar] [CrossRef]
- Bucky, L.P.; Kanchwala, S.K. The role of autologous fat and alternative fillers in the aging face. Plast. Reconstr. Surg. 2007, 120 (Suppl. 6), 89S–97S. [Google Scholar] [CrossRef]
- Carraway, J.H.; Mellow, C.G. Syringe aspiration and fat concentration: A simple technique for autologous fat injection. Ann. Plast. Surg. 1990, 24, 293–296. [Google Scholar] [CrossRef]
- Prantl, L.; Schreml, J.; Gehmert, S.; Klein, S.; Bai, X.; Zeitler, K.; Stephan, S.; Eckhard, A.; Sanga, G.; Oliver, F. Transcription Profile in Sporadic Multiple Symmetric Lipomatosis Reveals Differential Expression at the Level of Adipose Tissue-Derived Stem Cells. Plast. Reconstr. Surg. 2016, 137, 1181–1190. [Google Scholar] [CrossRef]
- Schreml, S.; Babilas, P.; Fruth, S.; Orsó, E.; Schmitz, G.; Mueller, M.B.; Nerlich, M.; Prantl, L. Harvesting human adipose tissue-derived adult stem cells: Resection versus liposuction. Cytotherapy 2009, 11, 947–957. [Google Scholar] [CrossRef]
- Rauso, R.; Sesenna, E.; Fragola, R.; Zerbinati, N.; Nicoletti, G.F.; Tartaro, G. Skin Necrosis and Vision Loss or Impairment After Facial Filler Injection. J. Craniofac. Surg. 2020, 31, 2289–2293. [Google Scholar]
- Witmanowski, H.; Błochowiak, K. Another face of dermal fillers. Postepy. Derm. Alergol. 2020, 37, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Gal, S.; Pu, L.L.Q. An Update on Cryopreservation of Adipose Tissue. Plast. Reconstr. Surg. 2020, 145, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Hanke, A.; Prantl, L.; Wenzel, C.; Nerlich, M.; Brockhoff, G.; Loibl, M.; Sebastian, G. Semi-automated extraction and characterization of Stromal Vascular Fraction using a new medical device. Clin. Hemorheol. Microcirc. 2016, 64, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, S.H.; Lee, S.J.; Kim, S.H.; Suh, I.S.; Jeong, H.S. Clinical Impact of Highly Condensed Stromal Vascular Fraction Injection in Surgical Management of Depressed and Contracted Scars. Aesthetic Plast. Surg. 2018, 42, 1689–1698. [Google Scholar] [CrossRef]
- Gehmert, S.; Wenzel, C.; Loibl, M.; Brockhoff, G.; Huber, M.; Krutsch, W.; Nerlich, M.; Gosau, M.; Klein, S.; Schreml, S.; et al. Adipose tissue-derived stem cell secreted IGF-1 protects myoblasts from the negative effect of myostatin. BioMed Res. Int. 2014, 2014, 129048. [Google Scholar] [CrossRef]
- Pallua, N.; Grasys, J.; Kim, B.S. Enhancement of Progenitor Cells by Two-Step Centrifugation of Emulsified Lipoaspirates. Plast. Reconstr. Surg. 2018, 142, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Hashimoto, H.; Nakanishi, I.; Furukawa, M. Microvascular angiogenesis and apoptosis in the survival of free fat grafts. Laryngoscope 2000, 110, 1333–1338. [Google Scholar] [CrossRef]
- Leong, D.T.; Hutmacher, D.W.; Chew, F.T.; Lim, T.C. Viability and adipogenic potential of human adipose tissue processed cell population obtained from pump-assisted and syringe-assisted liposuction. J. Dermatol. Sci. 2005, 37, 169–176. [Google Scholar] [CrossRef] [PubMed]
- James, I.B.; Bourne, D.A.; DiBernardo, G.; Wang, S.S.; Gusenoff, J.A.; Marra, K.; Peter, R.J. The Architecture of Fat Grafting II: Impact of Cannula Diameter. Plast. Reconstr. Surg. 2018, 142, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Broelsch, G.F.; Konneker, S.; Ipaktchi, R.; Vogt, P.M. Current German and American guidelines for autologous fat grafting—A transatlantic comparison. Handchir. Mikrochir. Plast. Chir. Organ Der Dtsch. Arb. Fur Handchir. Organ Der Dtsch. Arb. Fur Mikrochir. Der Peripher. Nerven Und Gefasse 2017, 49, 408–414. [Google Scholar]
- Prantl, L.; Rennekampff, H.O.; Giunta, R.E.; Harder, Y.; von Heimburg, D.; Heine, N.; Herold, C.; Kneser, U.; Lampert, F.; Machens, H.G.; et al. Current Perceptions of Lipofilling on the Basis of the New Guideline on “Autologous Fat Grafting”. Handchir. Mikrochir. Plast. Chir. 2016, 48, 330–336. [Google Scholar] [PubMed]
- Klein, S.M.; Prantl, L.; Geis, S.; Eisenmann-Klein, M.; Dolderer, J.; Felthaus, O.; Loibl, M.; Heine, N. Pressure monitoring during lipofilling procedures. Clin. Hemorheol. Microcirc. 2014, 58, 9–17. [Google Scholar] [CrossRef]
- Ueberreiter, K.; von Finckenstein, J.G.; Cromme, F.; Herold, C.; Tanzella, U.; Vogt, P.M. BEAULI™--a new and easy method for large-volume fat grafts. Handchir. Mikrochir. Plast. Chir. Organ Der Dtsch. Arb. Fur Handchir. Organ Der Dtsch. Arb. Fur Mikrochir. Der Peripher. Nerven Und Gefasse 2010, 42, 379–385. [Google Scholar]
- Niechajev, I.; Sevćuk, O. Long-term results of fat transplantation: Clinical and histologic studies. Plast. Reconstr. Surg. 1994, 94, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, E.C.; Albano, N.J.; Hazen, A. Roll, Spin, Wash, or Filter? Processing of Lipoaspirate for Autologous Fat Grafting: An Updated, Evidence-Based Review of the Literature. Plast. Reconstr. Surg. 2015, 136, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Cederna, P.S.; Rubin, J.P.; Coleman, S.R.; Levi, B. The Current State of Fat Grafting: A Review of Harvesting, Processing, and Injection Techniques. Plast. Reconstr. Surg. 2015, 136, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Gir, P.; Brown, S.A.; Oni, G.; Kashefi, N.; Mojallal, A.; Rohrich, R.J. Fat grafting: Evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast. Reconstr. Surg. 2012, 130, 249–258. [Google Scholar] [CrossRef]
- Persichetti, P.; Simone, P.; Langella, M.; Marangi, G.F.; Carusi, C. Digital photography in plastic surgery: How to achieve reasonable standardization outside a photographic studio. Aesthetic Plast. Surg. 2007, 31, 194–200. [Google Scholar] [CrossRef]
- Prantl, L.; Brandl, D.; Ceballos, P. A Proposal for Updated Standards of Photographic Documentation in Aesthetic Medicine. Plast. Reconstr. Surg. Glob. Open. 2017, 5, e1389. [Google Scholar] [CrossRef]
- Yavuzer, R.; Smirnes, S.; Jackson, I.T. Guidelines for standard photography in plastic surgery. Ann Plast Surg. 2001, 46, 293–300. [Google Scholar] [CrossRef]
- Kamakura, T.; Kataoka, J.; Maeda, K.; Teramachi, H.; Mihara, H.; Miyata, K.; Kouichi, O.; Naomi, S.; Miyuki, K.; Kouhei, I. Platelet-Rich Plasma with Basic Fibroblast Growth Factor for Treatment of Wrinkles and Depressed Areas of the Skin. Plast. Reconstr. Surg. 2015, 136, 931–939. [Google Scholar] [CrossRef]
- Day, D.J.; Littler, C.M.; Swift, R.W.; Gottlieb, S. The wrinkle severity rating scale: A validation study. Am. J. Clin. Dermatol. 2004, 5, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Mailey, B.; Saba, S.; Baker, J.; Tokin, C.; Hickey, S.; Wong, R.; Anne, W.; Steven, C. A comparison of cell-enriched fat transfer to conventional fat grafting after aesthetic procedures using a patient satisfaction survey. Ann. Plast. Surg. 2013, 70, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xie, Y.; Huang, R.L.; Zhou, J.; Tanja, H.; Zhao, P.; Chen, C.; Sizheng, Z.; Lee, P.; Qingfeng, L. Facial Contouring by Targeted Restoration of Facial Fat Compartment Volume: The Midface. Plast. Reconstr. Surg. 2017, 139, 563–572. [Google Scholar] [CrossRef]
- Jiang, S.; Quan, Y.; Wang, J.; Cai, J.; Lu, F. Fat Grafting for Facial Rejuvenation Using Stromal Vascular Fraction Gel Injection. Clin. Plast. Surg. 2020, 47, 73–79. [Google Scholar] [CrossRef]
- Barone, M.; Cogliandro, A.; Salzillo, R.; Ciarrocchi, S.; Abu Hanna, A.; Russo, V.; Tenna, S.; Persichetti, P. Midface Lift Plus Lipofilling Preferential in Patients with Negative Lower Eyelid Vectors: A Randomized Controlled Trial. Aesthetic Plast. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Marten, T.; Elyassnia, D. Facial Fat Grafting: Why, Where, How, and How Much. Aesthetic Plast. Surg. 2018, 42, 1278–1297. [Google Scholar] [CrossRef] [PubMed]
- Olenczak, J.B.; Seaman, S.A.; Lin, K.Y.; Pineros-Fernandez, A.; Davis, C.E.; Salopek, L.S.; Shayn, P.M.; Patrick, C.S. Effects of Collagenase Digestion and Stromal Vascular Fraction Supplementation on Volume Retention of Fat Grafts. Ann. Plast. Surg. 2017, 78, S335–S342. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Mannucci, S.; Conti, G.; Dai Prè, E.; Sbarbati, A.; Riccio, M. A Non-Enzymatic Method to Obtain a Fat Tissue Derivative Highly Enriched in Adipose Stem Cells (ASCs) from Human Lipoaspirates: Preliminary Results. Int. J. Mol. Sci. 2018, 19, 2061. [Google Scholar] [CrossRef]
- Prantl, L.; Eigenberger, A.; Klein, S.; Limm, K.; Oefner, P.J.; Schratzenstaller, T.; Oliver, F. Shear Force Processing of Lipoaspirates for Stem Cell Enrichment Does Not Affect Secretome of Human Cells Detected by Mass Spectrometry In Vitro. Plast. Reconstr. Surg. 2020, 146, 749e–758e. [Google Scholar] [CrossRef]
- Ibatici, A.; Caviggioli, F.; Valeriano, V.; Quirici, N.; Sessarego, N.; Lisa, A.; Klinger, F.; Forcellini, D.; Maione, L.; Klinger, M. Comparison of cell number, viability, phenotypic profile, clonogenic, and proliferative potential of adipose-derived stem cell populations between centrifuged and noncentrifuged fat. Aesthetic Plast. Surg. 2014, 38, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Condé-Green, A.; de Amorim, N.F.; Pitanguy, I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: A comparative study. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Gontijo-de-Amorim, N.F.; Charles-de-Sá, L.; Rigotti, G. Fat Grafting for Facial Contouring Using Mechanically Stromal Vascular Fraction-Enriched Lipotransfer. Clin. Plast. Surg. 2020, 47, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [PubMed]
- Gimble, J.M.; Bunnell, B.A.; Chiu, E.S.; Guilak, F. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: Let’s not get lost in translation. Stem Cells 2011, 29, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, J.; Li, Q.; Zhang, A.; Jin, P. Autologous fat graft assisted by stromal vascular fraction improves facial skin quality: A randomized controlled trial. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat grafting: Basic research and clinical applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef]



| Patient | Gender/Age | Liposuction Area | PO/Volume (cc) Right/Left | NLF/Volume (Cc) Right/Left |
|---|---|---|---|---|
| 1 | w/49 | Flanks | 1/1 | 1/1 |
| 2 | w/42 | abdominal | 2/2 | 1/1 |
| 3 | w/60 | Abdominal | 2/2 | 2/2 |
| 4 | w/47 | Abdomen flanks | 1.5/1.5 | 1.5/1.5 |
| 5 | w/51 | abdomen | 2,5/2,5 | 2/2 |
| 6 | m/44 | abdomen | 1.5/1.5 | 1.5/1.5 |
| 7 | w/60 | abdomen | 3/3 | 3/3 |
| 8 | w/47 | abdomen | 3/3 | 2/2 |
| 9 | w/55 | legs | 5/5 | 2/2 |
| 10 | m/30 | Abdomen | 2,5/2,5 | / |
| 11 | w/72 | legs | 1/1 | 1.5/1.5 |
| 12 | w/56 | Flanks/axillary region | 2,5/2,5 | 3/3 |
| 13 | w/69 | abdomen | 5/5 | 5/5 |
| 14 | w/59 | abdomen | 3/3 | / |
| Patient | WSS NLF Pre OP | WSS NLF Post | WSS PO Pre OP | WSS PO Post OP | GAIS (1.5 Months) |
|---|---|---|---|---|---|
| 1 | 1.5 | 0.5 | 1.5 | 1 | 1 |
| 2 | 2 | 1 | 1.5 | 1 | 1 |
| 3 | 2 | 1 | 1.5 | 0.5 | 1 |
| 4 | 2 | 1 | 1.5 | 0.5 | 1 |
| 5 | 1.5 | 1 | 1.5 | 0.5 | 1 |
| 6 | 2 | 1 | 1.5 | 0.5 | 2 |
| 7 | 2 | 1 | 1.5 | 0.5 | 1 |
| 8 | 2 | 1 | 1.5 | 0.5 | 1 |
| 9 | 2 | 1 | 1.5 | 0.5 | 1 |
| 10 | / | / | 1.5 | 1 | 0 |
| 11 | 2 | 1 | 1.5 | 1 | 2 |
| 12 | 1.5 | 1 | 1.5 | 1 | 1 |
| 13 | 1.5 | 0.5 | 1.5 | 1 | 1 |
| 14 | / | / | 2 | 1.5 | 1 |
| Patient | WSS NLF Pre OP | WSS NLF Post OP | WSS PO Pre OP | WSS PO Post OP | GAIS (>6 Months) |
|---|---|---|---|---|---|
| 1 | 1.5 | 1 | 1.5 | 1 | 1 |
| 2 | 2 | 1.5 | 1.5 | 1.5 | 1 |
| 3 | 2 | 1 | 1.5 | 1 | 1 |
| 4 | 2 | 1 | 1.5 | 1 | 1 |
| 5 | 1.5 | 1 | 1.5 | 1 | 1 |
| 6 | 2 | 1.5 | 1.5 | 1 | 2 |
| 7 | 2 | 1.5 | 1.5 | 1 | 1 |
| 8 | 2 | 1.5 | 1.5 | 1 | 1 |
| 9 | 2 | 1.5 | 1.5 | 1 | 1 |
| 10 | / | / | 1.5 | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prantl, L.; Brix, E.; Kempa, S.; Felthaus, O.; Eigenberger, A.; Brébant, V.; Anker, A.; Strauss, C. Facial Rejuvenation with Concentrated Lipograft—A 12 Month Follow-Up Study. Cells 2021, 10, 594. https://doi.org/10.3390/cells10030594
Prantl L, Brix E, Kempa S, Felthaus O, Eigenberger A, Brébant V, Anker A, Strauss C. Facial Rejuvenation with Concentrated Lipograft—A 12 Month Follow-Up Study. Cells. 2021; 10(3):594. https://doi.org/10.3390/cells10030594
Chicago/Turabian StylePrantl, Lukas, Eva Brix, Sally Kempa, Oliver Felthaus, Andreas Eigenberger, Vanessa Brébant, Alexandra Anker, and Catharina Strauss. 2021. "Facial Rejuvenation with Concentrated Lipograft—A 12 Month Follow-Up Study" Cells 10, no. 3: 594. https://doi.org/10.3390/cells10030594
APA StylePrantl, L., Brix, E., Kempa, S., Felthaus, O., Eigenberger, A., Brébant, V., Anker, A., & Strauss, C. (2021). Facial Rejuvenation with Concentrated Lipograft—A 12 Month Follow-Up Study. Cells, 10(3), 594. https://doi.org/10.3390/cells10030594








