Combined Inactivation of Pocket Proteins and APC/CCdh1 by Cdk4/6 Controls Recovery from DNA Damage in G1 Phase
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Constructs
2.3. Antibodies and Reagents
2.4. siRNA Transfections and Automated Microscopy
2.5. Time-Lapse Microscopy
2.6. Immunofluorescence and Automated Image Analysis
2.7. Western Blot
3. Results
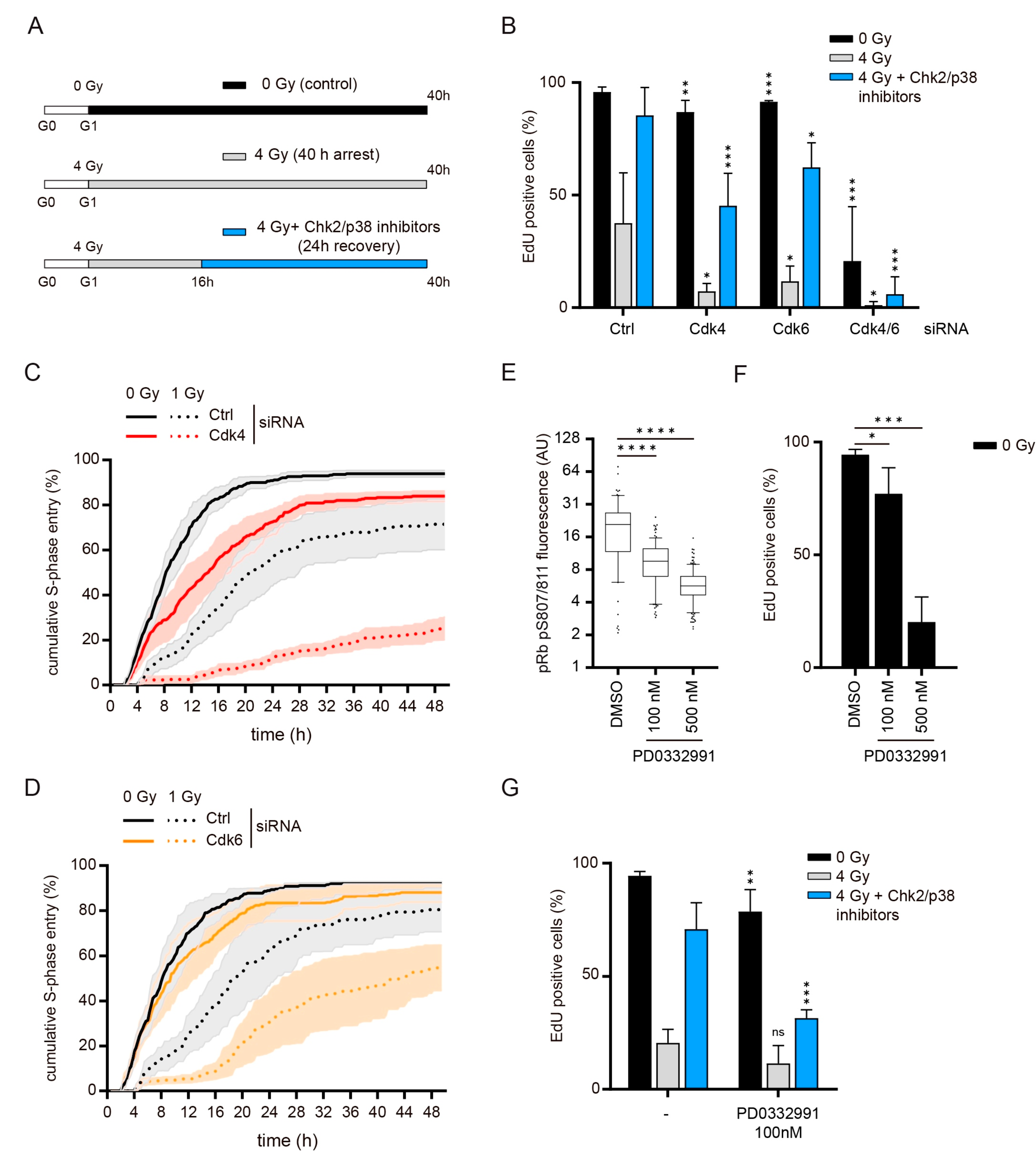
3.1. Recovery from a DNA Damage-Induced G1 Arrest Requires Cdk4 and Cdk6
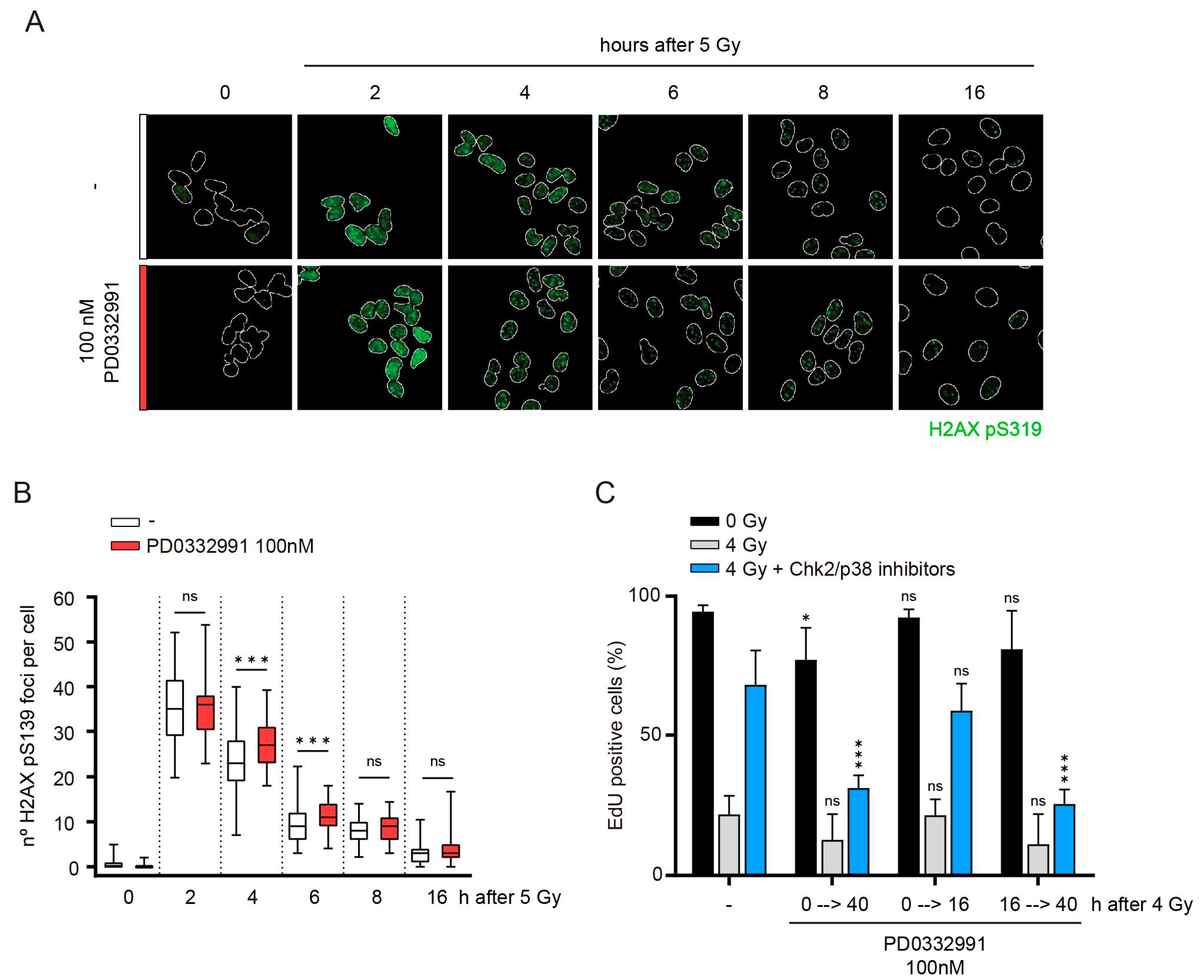
3.2. Cdk4/6 Activity Is Not Required during the Arrest
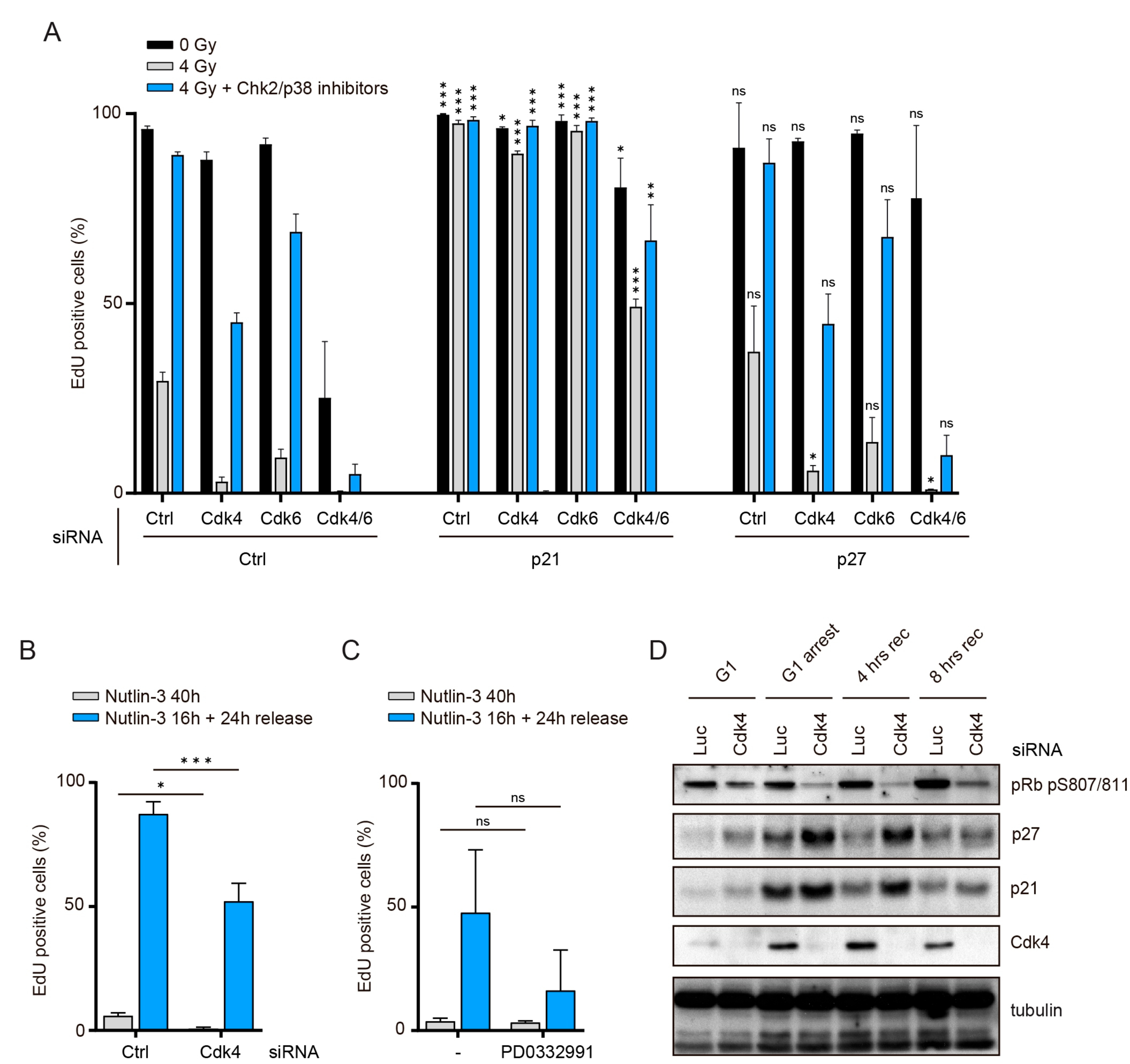
3.3. Cdk4/6 Activity Is Counteracted by p21 but not by p27 after DNA Damage
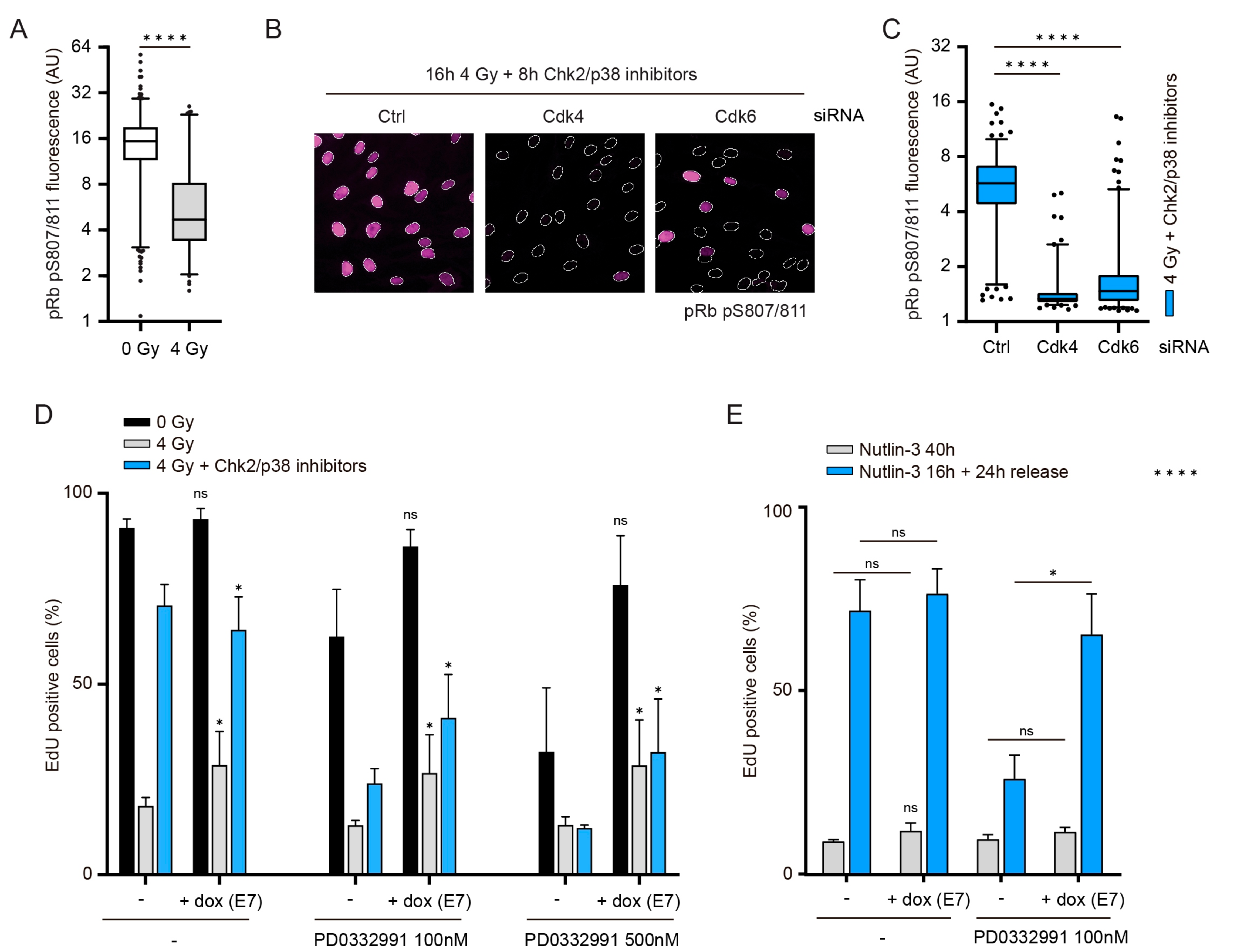
3.4. The Role of Cdk4/6 during Recovery Surpasses the Inactivation of Pocket Proteins
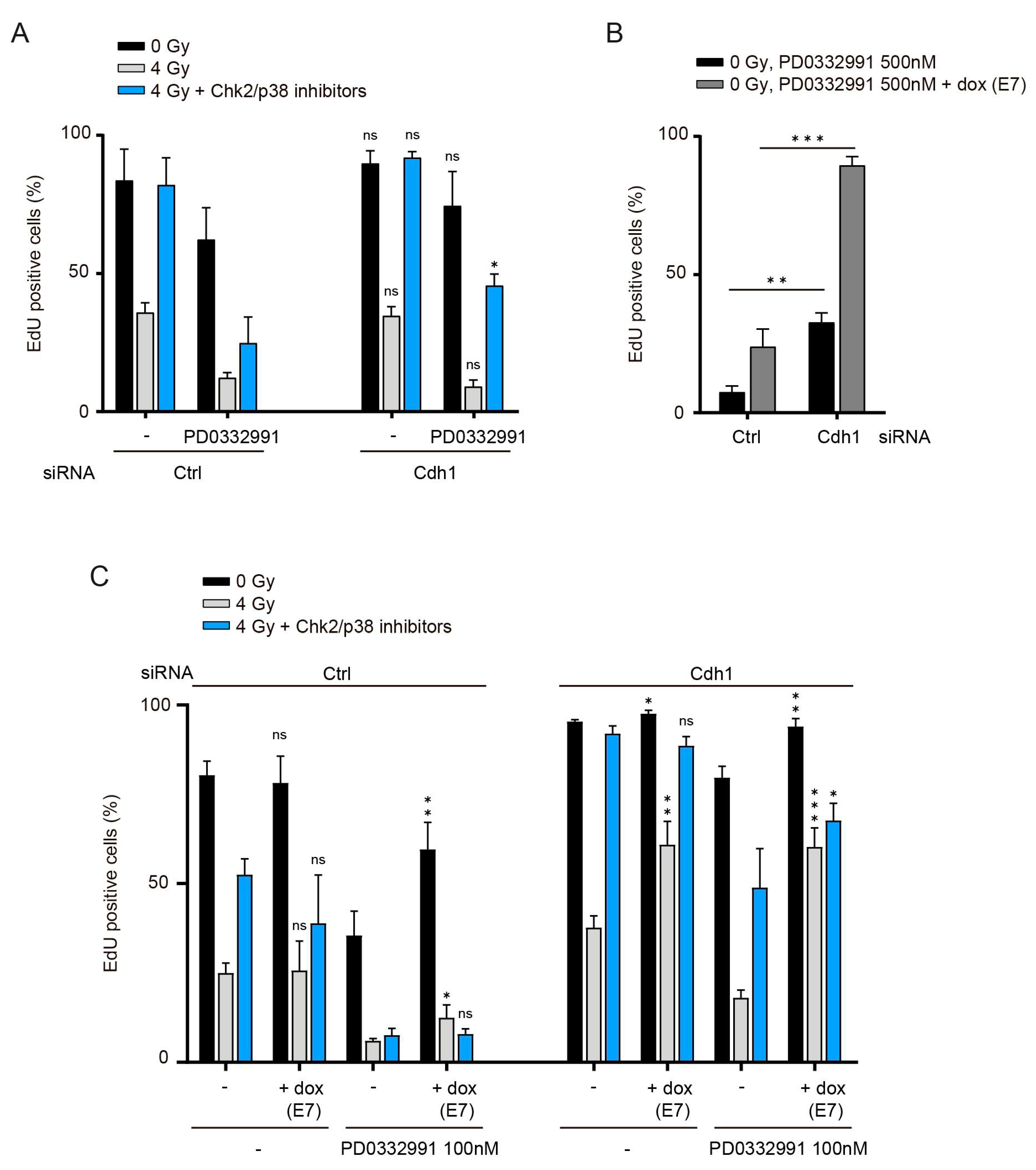
3.5. Combined Inactivation of Pocket Proteins and Cdh1 Enables Cdk4/6-Independent Recovery
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloom, J.; Cross, F.R. Multiple levels of cyclin specificity in cell-cycle control. Nat. Rev. Mol. Cell Biol. 2007, 8, 149–160. [Google Scholar] [CrossRef]
- Diffley, J.F.X. Regulation of Early Events in Chromosome Replication. Curr. Biol. 2004, 14, R778–R786. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.W. Recycling the Cell Cycle. Cell 2004, 116, 221–234. [Google Scholar] [CrossRef]
- Kuzminov, A. Recombinational Repair of DNA Damage inEscherichia coli and Bacteriophage λ. Microbiol. Mol. Biol. Rev. 1999, 63, 751–813. [Google Scholar] [CrossRef]
- Paulovich, A.G.; Toczyski, D.P.; Hartwell, L.H. When checkpoints fail. Cell 1997, 88, 315–321. [Google Scholar] [CrossRef]
- Tercero, J.A.; Diffley, J.F.X. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 2001, 412, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Bunz, F. Requirement for p53 and p21 to Sustain G2 Arrest After DNA Damage. Science 1998, 282, 1497–1501. [Google Scholar] [CrossRef]
- Shaltiel, I.A.; Krenning, L.; Bruinsma, W.; Medema, R.H. The same, only different—DNA damage checkpoints and their reversal throughout the cell cycle. J. Cell Sci. 2015, 128, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Bartek, J.; Lukas, J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr. Opin. Cell Biol. 2001, 13, 738–747. [Google Scholar] [CrossRef]
- Kastan, M.B.; Zhan, Q.; El-Deiry, W.S.; Carrier, F.; Jacks, T.; Walsh, W.V.; Plunkett, B.S.; Vogelstein, B.; Fornace, A.J. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 1992, 71, 587–597. [Google Scholar] [CrossRef]
- Manke, I.A.; Nguyen, A.; Lim, D.; Stewart, M.Q.; Elia, A.E.H.; Yaffe, M.B. MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G 2/M transition and S phase progression in response to UV irradiation. Mol. Cell 2005, 17, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Huang, M.; Elledge, S.J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 1998, 282, 1893–1897. [Google Scholar] [CrossRef]
- Kastan, M.B.; Onyekwere, O.; Sidransky, D.; Vogelstein, B.; Craig, R.W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991, 51, 6304–6311. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, P.; Wade Harper, J.; Elledge, S.J.; Leder, P. Mice Lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995, 82, 675–684. [Google Scholar] [CrossRef]
- Sugihara, E.; Kanai, M.; Saito, S.; Nitta, T.; Toyoshima, H.; Nakayama, K.; Nakayama, K.I.; Fukasawa, K.; Schwab, M.; Saya, H.; et al. Suppression of Centrosome Amplification after DNA Damage Depends on p27 Accumulation. Cancer Res. 2006, 66, 4020–4029. [Google Scholar] [CrossRef][Green Version]
- Vidal, A.; Koff, A. Cell-cycle inhibitors: Three families united by a common cause. Gene 2000, 247, 1–15. [Google Scholar] [CrossRef]
- Chen, H.-Z.; Tsai, S.-Y.; Leone, G. Emerging roles of E2Fs in cancer: An exit from cell cycle control. Nat. Rev. Cancer 2009, 9, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Barrière, C.; Santamaría, D.; Cerqueira, A.; Galán, J.; Martín, A.; Ortega, S.; Malumbres, M.; Dubus, P.; Barbacid, M. Mice thrive without Cdk4 and Cdk2. Mol. Oncol. 2007, 1, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, D.; Barrière, C.; Cerqueira, A.; Hunt, S.; Tardy, C.; Newton, K.; Cáceres, J.F.; Dubus, P.; Malumbres, M.; Barbacid, M. Cdk1 is sufficient to drive the mammalian cell cycle. Nature 2007, 448, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Yu, Q.; Sicinska, E.; Das, M.; Schneider, J.E.; Bhattacharya, S.; Rideout, W.M.; Bronson, R.T.; Gardner, H.; Sicinski, P. Cyclin E Ablation in the Mouse. Cell 2003, 114, 431–443. [Google Scholar] [CrossRef]
- Kozar, K.; Ciemerych, M.A.; Rebel, V.I.; Shigematsu, H.; Zagozdzon, A.; Sicinska, E.; Geng, Y.; Yu, Q.; Bhattacharya, S.; Bronson, R.T.; et al. Mouse Development and Cell Proliferation in the Absence of D-Cyclins. Cell 2004, 118, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Parisi, T. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO J. 2003, 22, 4794–4803. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Pacal, M.; Wenzel, P.; Knoepfler, P.S.; Leone, G.; Bremner, R. Division and apoptosis of E2f-deficient retinal progenitors. Nature 2009, 462, 925–929. [Google Scholar] [CrossRef]
- Timmers, C.; Sharma, N.; Opavsky, R.; Maiti, B.; Wu, L.; Wu, J.; Orringer, D.; Trikha, P.; Saavedra, H.I.; Leone, G. E2f1, E2f2, and E2f3 Control E2F Target Expression and Cellular Proliferation via a p53-Dependent Negative Feedback Loop. Mol. Cell. Biol. 2007, 27, 65–78. [Google Scholar] [CrossRef]
- Wenzel, P.L.; Chong, J.-L.; Sáenz-Robles, M.T.; Ferrey, A.; Hagan, J.P.; Gomez, Y.M.; Rajmohan, R.; Sharma, N.; Chen, H.-Z.; Pipas, J.M.; et al. Cell proliferation in the absence of E2F1-3. Dev. Biol. 2011, 351, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Shaltiel, I.A.; Aprelia, M.; Saurin, A.T.; Chowdhury, D.; Kops, G.J.P.L.; Voest, E.E.; Medema, R.H. Distinct phosphatases antagonize the p53 response in different phases of the cell cycle. Proc. Natl. Acad. Sci. USA 2014, 111, 7313–7318. [Google Scholar] [CrossRef]
- Salic, A.; Mitchison, T.J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 2415–2420. [Google Scholar] [CrossRef]
- Warmerdam, D.O.; Alonso-de Vega, I.; Wiegant, W.W.; van den Broek, B.; Rother, M.B.; Wolthuis, R.M.; Freire, R.; van Attikum, H.; Medema, R.H.; Smits, V.A. PHF6 promotes non-homologous end joining and G2 checkpoint recovery. EMBO Rep. 2019, e48460. [Google Scholar] [CrossRef]
- Kleiblova, P.; Shaltiel, I.A.; Benada, J.; Ševčík, J.; Pecháčková, S.; Pohlreich, P.; Voest, E.E.; Dundr, P.; Bartek, J.; Kleibl, Z.; et al. Gain-of-function mutations of PPM1D/Wip1 impair the p53-dependent G1 checkpoint. J. Cell Biol. 2013, 201, 511–521. [Google Scholar] [CrossRef]
- Chehab, N.H.; Malikzay, A.; Appel, M.; Halazonetis, T.D. Chk2/hCds1 functions as a DNA damage checkpoint in G1 by stabilizing p53. Genes Dev. 2000, 14, 278–288. [Google Scholar]
- Lafarga, V.; Cuadrado, A.; Lopez de Silanes, I.; Bengoechea, R.; Fernandez-Capetillo, O.; Nebreda, A.R. p38 Mitogen-Activated Protein Kinase- and HuR-Dependent Stabilization of p21Cip1 mRNA Mediates the G1/S Checkpoint. Mol. Cell. Biol. 2009, 29, 4341–4351. [Google Scholar] [CrossRef] [PubMed]
- Sakaue-Sawano, A.; Kurokawa, H.; Morimura, T.; Hanyu, A.; Hama, H.; Osawa, H.; Kashiwagi, S.; Fukami, K.; Miyata, T.; Miyoshi, H.; et al. Visualizing Spatiotemporal Dynamics of Multicellular Cell-Cycle Progression. Cell 2008, 132, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995, 9, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.L.; Cappell, S.D.; Tsai, F.; Overton, K.W.; Wang, C.L.; Meyer, T. The Proliferation-Quiescence Decision Is Controlled by a Bifurcation in CDK2 Activity at Mitotic Exit. Cell 2013, 155, 369–383. [Google Scholar] [CrossRef]
- Cuadrado, M.; Gutierrez-Martinez, P.; Swat, A.; Nebreda, A.R.; Fernandez-Capetillo, O. P27Kip1 stabilization is essential for the maintenance of cell cycle arrest in response to DNA damage. Cancer Res. 2009, 69, 8726–8732. [Google Scholar] [CrossRef]
- Burkhart, D.L.; Sage, J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat. Rev. Cancer 2008, 8, 671–682. [Google Scholar] [CrossRef]
- Cobrinik, D. Pocket proteins and cell cycle control. Oncogene 2005, 24, 2796–2809. [Google Scholar] [CrossRef]
- Harrington, E.A.; Bruce, J.L.; Harlow, E.; Dyson, N. pRB plays an essential role in cell cycle arrest induced by DNA damage. Proc. Natl. Acad. Sci. USA 1998, 95, 11945–11950. [Google Scholar] [CrossRef]
- Hu, T.; Ferril, S.C.; Snider, A.M.; Barbosa, M.S. In vivo analysis of HPV E7 protein association with pRb, p107 and p130. Int. J. Oncol. 1995, 6, 167–174. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, W.; Roman, A. The E7 proteins of low- and high-risk human papillomaviruses share the ability to target the pRB family member p130 for degradation. Proc. Natl. Acad. Sci. USA 2006, 103, 437–442. [Google Scholar] [CrossRef] [PubMed]
- The, I.; Ruijtenberg, S.; Bouchet, B.P.; Cristobal, A.; Prinsen, M.B.W.; Van Mourik, T.; Koreth, J.; Xu, H.; Heck, A.J.R.; Akhmanova, A.; et al. Rb and FZR1/Cdh1 determine CDK4/6-cyclin D requirement in C. elegans and human cancer cells. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Satyanarayana, A.; Kaldis, P. Mammalian cell-cycle regulation: Several cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 2009, 28, 2925–2939. [Google Scholar] [CrossRef]
- Yang, H.W.; Cappell, S.D.; Jaimovich, A.; Liu, C.; Chung, M.; Daigh, L.H.; Pack, L.R.; Fan, Y.; Regot, S.; Covert, M.; et al. Stress-mediated exit to quiescence restricted by increasing persistence in CDK4/6 activation. Elife 2020, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.C.; Meliton, L.; Ren, X.; Zhang, Y.; Balli, D.; Snyder, J.; Whitsett, J.A.; Kalinichenko, V.V.; Kalin, T.V. Deletion of forkhead box M1 transcription factor from respiratory epithelial cells inhibits pulmonary tumorigenesis. PLoS ONE 2009, 4, e6609. [Google Scholar] [CrossRef]
- Yu, Q.; Sicinska, E.; Geng, Y.; Ahnström, M.; Zagozdzon, A.; Kong, Y.; Gardner, H.; Kiyokawa, H.; Harris, L.N.; Stål, O.; et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 2006, 9, 23–32. [Google Scholar] [CrossRef]
- Ciznadija, D.; Liu, Y.; Pyonteck, S.M.; Holland, E.C.; Koff, A. Cyclin D1 and Cdk4 mediate development of neurologically destructive oligodendroglioma. Cancer Res. 2011, 71, 6174–6183. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Myant, K.; Reed, K.R.; Ridgway, R.A.; Athineos, D.; Van Den Brink, G.R.; Muncan, V.; Clevers, H.; Clarke, A.R.; Sicinski, P.; et al. Cyclin D2—Cyclin-dependent kinase 4/6 is required for efficient proliferation and tumorigenesis following Apc loss. Cancer Res. 2010, 70, 8149–8158. [Google Scholar] [CrossRef]
- Gillam, M.P.; Nimbalkar, D.; Sun, L.; Christov, K.; Ray, D.; Kaldis, P.; Liu, X.; Kiyokawa, H. MEN1 tumorigenesis in the pituitary and pancreatic islet requires CDK4 but not Cdk2. Oncogene 2015, 34, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Puebla, M.L.; Miliani de Marval, P.L.; LaCava, M.; Moons, D.S.; Kiyokawa, H.; Conti, C.J. Cdk4 Deficiency Inhibits Skin Tumor Development But Does Not Affect Normal Keratinocyte Proliferation. Am. J. Pathol. 2002, 161, 405–411. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [CrossRef]
- The Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [CrossRef] [PubMed]
- Padmakumar, V.C.; Aleem, E.; Berthet, C.; Hilton, M.B.; Kaldis, P. Cdk2 and Cdk4 Activities Are Dispensable for Tumorigenesis Caused by the Loss of p53. Mol. Cell. Biol. 2009, 29, 2582–2593. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaltiel, I.A.; Llopis, A.; Aprelia, M.; Klompmaker, R.; Menegakis, A.; Krenning, L.; Medema, R.H. Combined Inactivation of Pocket Proteins and APC/CCdh1 by Cdk4/6 Controls Recovery from DNA Damage in G1 Phase. Cells 2021, 10, 550. https://doi.org/10.3390/cells10030550
Shaltiel IA, Llopis A, Aprelia M, Klompmaker R, Menegakis A, Krenning L, Medema RH. Combined Inactivation of Pocket Proteins and APC/CCdh1 by Cdk4/6 Controls Recovery from DNA Damage in G1 Phase. Cells. 2021; 10(3):550. https://doi.org/10.3390/cells10030550
Chicago/Turabian StyleShaltiel, Indra A., Alba Llopis, Melinda Aprelia, Rob Klompmaker, Apostolos Menegakis, Lenno Krenning, and René H. Medema. 2021. "Combined Inactivation of Pocket Proteins and APC/CCdh1 by Cdk4/6 Controls Recovery from DNA Damage in G1 Phase" Cells 10, no. 3: 550. https://doi.org/10.3390/cells10030550
APA StyleShaltiel, I. A., Llopis, A., Aprelia, M., Klompmaker, R., Menegakis, A., Krenning, L., & Medema, R. H. (2021). Combined Inactivation of Pocket Proteins and APC/CCdh1 by Cdk4/6 Controls Recovery from DNA Damage in G1 Phase. Cells, 10(3), 550. https://doi.org/10.3390/cells10030550





